Abstract
Background:
A newly recognized Multisystem Inflammatory Syndrome in Children (MIS-C) has had a paradigm-shifting effect on the perception of SARS-CoV-2 illness severity in children. We report the clinical and biochemical features of liver involvement, and the comorbidities that present with hepatitis, in a substantial cohort of patients.
Methods & Results:
This is a retrospective cohort study of 44 patients with MIS-C admitted at Morgan Stanley Children’s Hospital of New York-Presbyterian during April and May 2020. We evaluated the number of patients who developed hepatitis and examined both demographics and inflammatory laboratory values to ascertain those that were at higher risk for liver involvement and more severe disease. Hepatitis was present in 19 subjects (43%) and was associated with more severe disease. Individuals with hepatitis had significantly higher rates of shock at presentation (21.1% vs. 0%, p=0.008), greater respiratory support requirement (42.1% vs. 12%, p=0.005), and longer hospitalization times (median 7-days [IQR5,10] vs 4-days [IQR3.5,6.5], p<0.05). Patients with hepatitis also had significantly higher levels of ferritin (706.9 vs. 334.2 mg/mL, p<0.01), Interleukin-6 (233.9 vs. 174.7 pg/mL, p<0.05), troponin (83 vs. 28.5 ng/L, p<0.05) and B-type Natriuretic peptide (7424.5 vs. 3209.5 pg/mL, p<0.05). The single patient with liver failure also developed multiorgan failure requiring vasopressors, hemodialysis, and mechanical ventilation. All patients were discharged, though more than 50% had persistent hepatitis up to one month after discharge.
Conclusion:
Hepatitis is common in children with MIS-C and is associated with a more severe presentation and persistent elevation of LFTs in many. Despite the positive outcomes reported here, close follow-up is warranted given the limited knowledge of the long-term impact of SARS-CoV-2 on the liver.
Keywords: Multisystem Inflammatory Syndrome in children, SARS-CoV-2 hepatitis, SARS-CoV-2 in children, pediatrics
Introduction:
In the context of the rapidly evolving pandemic of the severe acute respiratory syndrome, coronavirus-2(SARS-CoV-2), the majority of observations describe a benign clinical course in children(1, 2). As of May 16, 2020, New York City reported that children <18 years of age represented only 9% of hospitalized cases and 0.06% of the 15,888 fatalities(3). The recognition of a new clinical entity associated with SARS-CoV-2 exposure, described as a multisystem inflammatory syndrome in children (MIS-C) has emerged(4, 5) and has had a paradigm-shifting effect on the perception of SARS-CoV-2 illness severity in children. Although the condition is rare, affected children may become critically ill(2, 6).
Individuals with MIS-C present with fever, often accompanied by severe abdominal pain, progressive respiratory failure, and myocardial dysfunction with varying degrees of cardiogenic shock and circulatory failure(5, 7). They can further deteriorate to the point of requiring organ replacement therapies including hemodialysis, extracorporeal membrane oxygenation, or mechanical ventilation(7). Thus, the morbidity of this syndrome is vastly different from the benign course initially reported of SARS-CoV-2 infection in most pediatric patients(7).
A key target organ in MIS-C that has yet to be extensively described is the liver. Up to 60% of patients with SARS-CoV-2 infection have elevated liver enzymes, and these elevations are higher in more severe cases(8, 9). Interestingly, obesity, which is also highly associated with hepatitis, has been reported as the most frequent comorbidity among children hospitalized with SARS-CoV-2 infection(10). In the interest of rapidly disseminating information, we previously reported on the gastrointestinal manifestations of this cohort(11). We expand here on the clinical and biochemical features of liver involvement, and the associated comorbidities that present with hepatitis, in a substantial cohort of patients diagnosed with MIS-C.
Methods:
A retrospective chart review of 44 cases admitted to the Morgan Stanley Children’s Hospital of New York-Presbyterian that fulfill criteria for MIS-C was completed. As defined by the U.S. Centers for Disease Control, the criteria include persistent fever >38 degrees Celsius for >24 hours, laboratory evidence of inflammation, and severe clinical illness involving >2 systems with end-organ damage. Additionally, patients had evidence of SARS-CoV-2 infection determined by a positive reverse-transcriptase quantitative Polymerase Chain Reaction (RT-PCR) from oropharyngeal or nasopharyngeal swabs (NPS), SARS-CoV-2 IgM and/or IgG antibodies by a qualitative serological test, or a strong epidemiological history of SARS-CoV-2 exposure(5). Full criteria are detailed in the supplement table 1.
Hepatitis was defined as an elevation of alanine aminotransferase (ALT) >40 and aspartate aminotransferase (AST) > 50, as these values fall above the 97%ile for all ages and both sexes, as defined by Bussler et al (12). Three patients with elevated AST and normal ALT were not included in the hepatitis cohort since exclusive AST elevation can originate from other sources. Further collected data included demographics, presenting symptoms, history of contact with confirmed or suspected cases of SARS-CoV-2 infection, vital signs, laboratory data (white blood cell count, platelet count, transaminases, albumin, international normalized ratio (INR), gamma-glutamyltransferase (GGT), bilirubin, Erythrocyte sedimentation rate, C-Reactive Protein, procalcitonin, ferritin, fibrinogen, B-type Natriuretic Peptide [BNP]), abdominal imaging and echocardiogram findings. Student’s t-test and Mann-Whitney U test were performed to identify variables associated with liver test abnormalities, where appropriate. A p-value of <0.05 was chosen as the cutoff for significance. The Institutional Review Board of Columbia University Irving Medical Center approved this study with a waiver of informed consent.
Results:
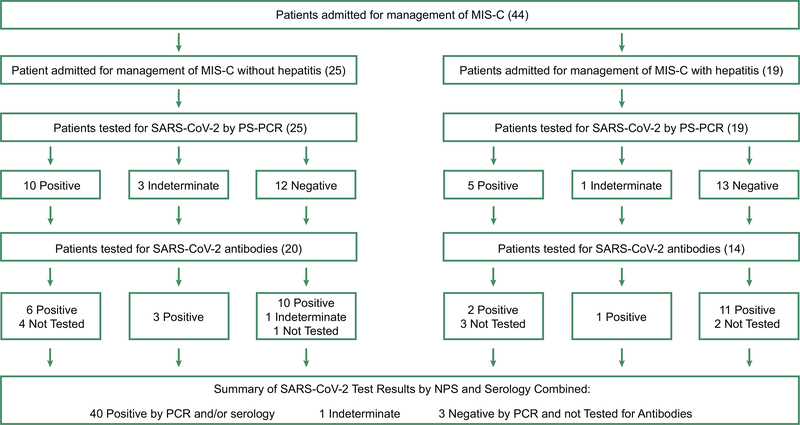
Among the 44 children admitted between April 18 and May 22, 2020 for the management of MIS-C, 19 subjects (43%) had hepatitis. Clinical and laboratory criteria supporting the diagnosis of MIS-C for this cohort are provided in supplemental table 2. Demographic and presenting symptoms were reported previously(11). Forty-one (93.2%) of the patients tested positive or indeterminate for at least one modality of SARS-CoV-2. Interestingly, only 34% of the entire cohort and 26.3 % of subjects with hepatitis were positive for SARS-CoV-2 by RT-PCR (Figure 1), while all patients tested for SARS-CoV-2 antibodies resulted positive except one patient who was indeterminate. Three patients that tested negative for SARS-CoV-2 by RT-PCR, and did not get tested for antibodies, had a strong epidemiological history of exposure to a SARS-CoV-2 infection.
Figure 1:
Results of SARS-CoV-2 RT-PCR were defined as positive (both viral gene detected), indeterminate (one of the two targets detected, reflecting low level detection) and negative (no viral gene detected). SARS-CoV-2 antibody testing was done using a New York-State Department of Health–approved assay for IgM and IgG antibodies (96% specificity, 93% sensitivity). Test results were defined based on whether SARS-CoV-2-antibodies were present to both the N and S protein (positive), indeterminate (only to the N protein) or negative (absent). MIS-C: multisystem inflammatory syndrome in children; RT-PCR: reverse transcriptase Polymerase Chain Reaction; SARS-CoV-2: Severe acute respiratory syndrome-coronavirus 2.
Of the 44 patients, 39% had a body mass index (BMI)> 85%ile for age, and 24% had a BMI >95%ile. In patients with hepatitis, 52% had BMI >85%ile for age, and 37% had a BMI >95%ile. In those without hepatitis, only 28% had BMI>85%ile and 16% had BMI>95%ile. There was a trend toward higher BMI in patients with hepatitis; however, the difference did not reach statistical significance. Only 7/44 (16%) had documented comorbidities (asthma:3, seizure disorder:1, microcephaly:1, ex-premature:2) and comorbidities were not associated with presence of hepatitis.
Of the 19 patients with hepatitis, seven had an ALT >100 U/L, and one had an ALT >4000. The ALT was higher than AST in 18/19 subjects. The only instance where AST was higher than ALT was in one patient with multiorgan failure. Total and direct bilirubin levels were significantly higher (p<0.01), and albumin was lower (p<0.05) in patients with hepatitis (Table 1), suggesting that the liver enzyme elevations were related to liver disease in these patients as opposed to dysfunction in other organs, such as heart or muscle. Liver enzymes peaked in 63% (12/19) at admission and in the remainder, peaked at one to four days after admission. INR was similar in both groups, and only one patient had an INR>2. GGT, CPK and aldolase were not tested in enough subjects to assess relevance. White blood cell counts were similar for both groups throughout admissions. Platelet counts, however, were significantly lower at admission and at their nadir in patients with hepatitis (p<0.05).
Table 1:
Clinically relevant characteristics supporting multisystem involvement and disease severity differences between subject with and without hepatitis. CPAP: Continuous Positive Airway Pressure; BPAP: Bilevel positive airway pressure; IQR: interquartile range.
| Variables (n [%]) | Hepatitis n:19 | No Hepatitis n:25 | P value |
|---|---|---|---|
| Shock any time (22 [50%]) | 10 | 12 | n.s. |
| Shock at presentation (4 [11%]) | 4 | 0 | <0.01 |
| Oxygen requirements (11[25%]) | 8 | 3 | <0.05 |
| Nasal canula | 3 | 3 | |
| CPAP/BPAP | 3 | 0 | |
| Non rebreather | 1 | 0 | |
| Mechanical ventilation | 1 | 0 | |
| Acute Kidney Injury (7 [15.9%]) | 4 | 3 | n.s. |
| Renal replacement therapy | 1 | 0 | |
| Coronary aneurism in ECHO (6 [13.6]) | 3 | 3 | ns |
| Length hospitalization (median, IQR) | 7(5,10) | 4(3.5–6.5) | <0.05 |
| Intensive care admission (29 [66%]) | 13 (68.4%) | 16 (64%) | n.s. |
More severe disease was seen in the cohort with hepatitis, compared to those without, based on laboratory values, clinical presentation and length of hospitalization. Of the multiple inflammatory laboratory values evaluated in patients with MIS-C and hepatitis (Table 1), the levels of BNP, troponin, interleukin-6, and ferritin were significantly higher in patients with hepatitis (p<0.05). More severe disease was also indicated by the clinical presentations: Four patients in the hepatitis group required vasopressors at admission compared to none in the other group (p<0.05; Table 2). Further, significantly more patients in the hepatitis group (8 vs. 3) required respiratory support, and the level of support required was also higher. Length of hospitalization was significantly longer in the hepatitis group with a median of 7-days (IQR:5–10) days compared with 4-days (IQR:3.5–6.5) in patients without hepatitis.
Table 2:
Comparison of clinically relevant laboratory values between the patients with and without hepatitis. IQR: interquartile range, mg/L: milligrams per liter, mg/dL: milligrams per deciliters, mg/mL: milligram per milliliter, pg/mL: picograms per milliliter, n: number, U/L: unit per liters
| Variables (normal values) | Abnormal liver test n; 19 Median (IQR) | Normal liver test n:25 Median (IQR) | P values |
|---|---|---|---|
| ALT (9–40 U/L) | 74(52–146) | 19(15–26) | <0.01 |
| AST (15–50 U?L) | 62(52–97) | 19(15–26) | <0.01 |
| Total Bilirubin mg/dL | 0.7(0.4–1.9) | 0.4(0.2–0.5) | <0.05 |
| Direct Bilirubin mg/dL | 0.3(0.2–0.8) | 0.1(0.1–0.2) | <0.05 |
| International Normalized Ratio (0.8–1.2) | 1.25(12.−1.43) | 1.2(1.1–1.4) | n.s. |
| Albumin (Lowest level) (3.9–5.2g/dL) | 2.8 (2.2–3.4) | 3.3 (2.55–3.95) | <0.05 |
| Admission Platelet count (194–345x10(3)/uL | 163 (124–281) | 216 (156.5–310.3) | <0.05 |
| Lowest Platelet count (194–345x10(3)/uL | 142(97–221) | 188(127–305) | <0.05 |
| Highest Platelet count (194–345x10(3)/uL | 426(235–547) | 342(274–445) | n.s. |
| Highest White blood cell (4.2–9.4x10(3)/uL) | 16.4(11.3–26.7) | 11.7(8.94–21.2) | n.s. |
| C-reactive protein (0–10mg/L) | 178.85(29.3–300) | 140.44(25.84–252.1) | n.s |
| Erythrocyte sedimentation rate (0–20mm/h) | 69 (44–79) | 64 (47–89.5) | n.s. |
| Lactate dehydrogenase (140–280 U/L) (n=39) | 333 (288–444) | 310 (261.25–409.75) | n.s. |
| Interleukin-6 (<5pg/mL) (n=31) | 233.9 (80–315) | 174.65 (25.18–315) | <0.05 |
| Ferritin(13–150mg/mL) | 706.9(454.5–1121) | 334.2 (169.63–518.13) | <0.01 |
| Weight Body Mass Index %tile | 84.79(58–96-59) | 66.37(29.95–90.12) | n.s. |
| B-type Natriuretic Peptide | 7424–5(3356–31995.3) | 3209.5 (467.98–13243) | <0.05 |
| Troponin | 83(37.75–241.5) | 28.5(14–64.5) | <0.05 |
Abdominal imaging was performed on eight (42.1%) patients with hepatitis. Four of the six abdominal ultrasounds had abnormal liver-associated findings: two ultrasounds showed significant ascites (one individual also had gallbladder wall thickening), one each demonstrated hepatomegaly or a thick-walled gallbladder. One of the two magnetic resonance imaging studies showed ascites. Some individuals with hepatitis underwent evaluation for other viral etiologies (n= 15), autoimmune (n=1) and/or metabolic disorders (n=1), without any abnormal results. All 19 patients in the hepatitis cohort had their liver tests re-checked at the end of admission and/or upon follow-up (2–4 weeks). Hepatitis had improved in 17 (89.5%) and normalized in 9 (47.4%) of the patients by one month. However, hepatitis persisted in 53.6% of the subjects during the last follow-up, underscoring the importance of post-discharge follow up.
Of the 44 patients in this study, only one patient required mechanical ventilation, renal replacement therapy, and vasopressor support. This patient also developed severe hepatitis and coagulopathy. Six (13.6%) patients developed coronary dilation detected either during hospitalization or on follow-up echocardiogram. This difference was not statistically significant (hepatitis 15.8% vs. those without 12%; p=0.4). There were no fatalities, and all patients were discharged as of May 31, 2020. Longer follow-up is needed to evaluate for permanent organ damage.
Discussion:
MIS-C is a severe condition, emphasizing the broad clinical spectrum of pediatric SARS-CoV-2 infection(2, 5). The multisystemic inflammation and presence of coronary dilation in 13.6% of the patients support clinical similarities to Kawasaki Disease(4), but are significantly different from the clinical manifestations of SARS-CoV-2 infection seen in children and adults(1, 10, 13).
Analysis of BMI demonstrated that 39% of the entire cohort was overweight, and 24% was obese. Elevated BMI in association with hepatitis could support the diagnosis of underlying nonalcoholic liver disease (NAFLD). Several studies have suggested that NAFLD can have a synergistic effect on viral oxidative stress in hepatocytes(14), predisposing children to higher liver enzyme elevations during a viral infection. The only patient who developed multiorgan failure and severe hepatitis was obese. This observation may also raise the concern that obesity could be an epidemiological factor influencing disease behavior for MIS-C. In our cohort, however, few patients had imaging and those that were imaged did not demonstrate classic findings of NAFLD.
Similar to adults with hepatitis(8), the data show that children with hepatitis may have more significant disease. This was demonstrated by significantly higher proinflammatory cytokine levels (i.e., IL-6, ferritin, troponin, and BNP), higher vasopressor support requirement at admission, more frequent and advanced modality of respiratory support, and longer hospitalization times in this population.
Similar to prior reports (4, 6), only 16% of our MIS-C cohort had comorbid conditions. To compare this cohort to pediatric patients admitted with SARS-Cov-2 infection but without MIS-C, we have done a preliminary evaluation of 88 age- and sex-matched subjects admitted to our hospital around the same time with SARS-CoV-2 infection, but whom did not fulfill criteria for MIS-C (unpublished data). The clinical presentation of this cohort was quite different from those patients with MIS-C; 60% of patients admitted with SARS-Cov-2 but not MIS-C had comorbidities, compared to only 16% of the patients with MIS-C. Despite the increased presence of comorbidities, the clinical course was milder in the non-MIS-C group with only 16% of this population requiring intensive care and only 6.8% requiring vasopressor support. This non-MIS-C cohort is now undergoing further analyses.
Notably, the majority of patients with MIS-C, including those with hepatitis, were negative for SARS-CoV-2 RT-PCR (56.8%) but positive for SARS-CoV-2 antibodies (92.3%). This observation supports the idea that the mechanism of SARS-CoV-2-induced hepatitis may be, in part, secondary to an immune-mediated response(15, 16). Because, however, some patients with hepatitis were also SARS-CoV-2 PCR positive, there may also be a viral-induced cytopathogenic effect in the liver(17). It is also important to consider the possibility of ischemia and drug-induced liver injury acting as modifiers on these clinical presentations since all patients received multiple drugs for MIS-C treatment and clinical support and several patients had evidence of poor organ perfusion. Further studies, with larger numbers of patients, are necessary to confirm these possibilities.
Clinical reports regarding the clinical presentation of MIS-C have focused on the inflammatory syndrome and cardiovascular decompensation, but there is a lack of published data regarding the details of hepatic involvement in this syndrome. We report here on a cohort of patients with MIS-C and that those with hepatitis present with more severe disease. Although the clinical presentation was severe, hepatitis improved in 89.5% of the subjects and resolved in 47.4% by one-month post-discharge. An extensive workup to evaluate the etiology of hepatitis during the acute phase of this illness may not thus be necessary in mild cases, especially if the history or physical examination do not suggest underlying liver disease. We do suggest, however, that close follow-up of all patients with hepatitis be conducted and further investigation may be warranted if resolution is not achieved within a reasonable time frame, so underlying liver diseases or persistent liver damage are not missed.
Conclusions:
The emergence of MIS-C has altered the perception that SARS-CoV-2 infection is benign in children. This syndrome is complex and presents with prominent gastrointestinal and hepatic involvement. These data suggest an association between MIS-C and hepatitis, thus supporting the notion that evaluation and trending of liver enzymes during and after hospitalization is indicated. Hepatitis was also associated with more severe clinical manifestations of MIS-C and even liver failure in one patient. Longer follow up and prospective studies are needed to determine the real impact of SARS-CoV-2 virus in the liver and to identify the risk factors that contribute to disease severity.
Supplementary Material
Acknowledgments
Financial Support:
AC: None
JM: None
PZ: None
BDS: None
KGM: Advisory board Takeda
MM: Advisory board Gilead
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- INR
international normalized ratio
- GGT
gamma-glutamyltransferase
- SARS-CoV-2
severe acute respiratory syndrome, coronavirus 2
- RT-PCR
reverse-transcriptase quantitative Polymerase Chain Reaction
- COVID-19
coronavirus disease 19
- CRP
C-reactive protein
- HS troponin
high sensitivity troponin
- ICU
intensive care unit
- IL-6
interleukin-6
- NAFLD
nonalcoholic fatty liver disease
Footnotes
Conflicts of Interest: None declared related to this manuscript
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/HEP.31526
Bibliography:
- 1.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.New York city COVID cases on March 17th 2020. In: New City Department of health COVID-19; 2020. [Google Scholar]
- 4.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 In: Emergency Response and Preparedness website. CDC web; page; 2020. [Google Scholar]
- 6.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinz N, Griesemer A, Kinney J, Vittorio J, Lagana SM, Goldner D, Velasco M, et al. A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transplant 2020:e13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, Banker SL, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr 2020:e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussler S, Vogel M, Pietzner D, Harms K, Buzek T, Penke M, Handel N, et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase): Effects of age, sex, body mass index, and pubertal stage. Hepatology 2018;68:1319–1330. [DOI] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Fan C, Chen Y, Liu H, Wang S, Dong P, Li L, et al. Effect of hepatic steatosis on the progression of chronic hepatitis B: A prospective cohort and in vitro study. Oncotarget 2017;8:58601–58610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int 2020;40:1278–1281. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagana SM, De Michele S, Lee MJ, Emond JC, Griesemer AD, Tulin-Silver SA, Verna EC, et al. COVID-19 Associated Hepatitis Complicating Recent Living Donor Liver Transplantation. Arch Pathol Lab Med 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.