Abstract
The cannabinoid signaling system regulates intraocular pressure (IOP) in the mouse via a complex system that includes three receptors: CB1, GPR18 and GPR119. In each case, activating the receptor lowers IOP, but CB1 receptors are found both at sites of aqueous humor inflow and outflow. As such, knockout mice for any of these receptors would be expected to have higher-than average, or at least unchanged, intraocular pressure. The current study investigates the unexpected observation that CB1 knockout mice have lower pressure than wild type counterparts by testing various regulators of cannabinoid signaling in murine models of IOP. We now report that a CB1 antagonist has differential effects on IOP: SR141716 raises IOP in standard light cycle (SLC) but lowers IOP in reverse light cycle (RLC). This is mimicked by ABD1085, a negative allosteric modulator of CB1. CB1 inhibitors lower IOP in both normotensive and hypertensive mouse eyes. The pressure-lowering effect is absent in CB1 knockout mice. IOP rebounds after the end of treatment but shows no sign of desensitization with daily treatment for a week. Unlike the positive cannabinoid effect, antagonist effects are not sex-dependent. We propose that there are two mechanisms of action for CB1, one that lowers IOP upon activation and a second with inverse sign that lowers IOP when CB1 is antagonized. The relatively lower pressure in CB1 knockout mouse eyes suggests that this second negative regulation of IOP is dominant.
Keywords: Cannabinoid, CB1, ocular pressure, glaucoma, antagonist
1. INTRODUCTION
In 1971, Hepler and Frank (Hepler and Frank, 1971) reported that smoked cannabis can lower intraocular pressure (IOP). Subsequent studies showed that the phytocannabinoid tetrahydrocannabinol (THC) is the active ingredient of cannabis that lowers IOP (Purnell and Gregg, 1975), likely by acting at both sites of aqueous humor inflow and outflow (Beilin et al., 2000; Green and Kim, 1976) though the details of this regulation remain to be determined. Scientific interest in the nature of THC regulation of IOP waned well before the receptor-based cannabinoid signaling system was described (Matsuda et al., 1990; Munro et al., 1993). It is now understood that THC acts on a complex cannabinoid signaling system that includes a stable of receptors (CB1, CB2, GPR18, GPR119 and likely others), lipid messengers known as endocannabinoids, and the enzymatic machinery to synthesize, transport and metabolize these messengers (reviewed in (Kano et al., 2009)). We have shown that three cannabinoid-related receptors – CB1, GPR18, and GPR119 -- can lower IOP when activated, each through distinct mechanisms (Caldwell et al., 2013; Hudson et al., 2011; Miller et al., 2017; Miller et al., 2016b). Building on this, we have recently shown that THC lowers IOP by activating the CB1 and GPR18 receptors in combination (Miller et al., 2018).
In the course of our studies of cannabinoid regulation of IOP we noted that CB1 knockout mice have significantly lower IOP than their wild type counterparts (Figure 1). The following explores the underpinnings of this unexpected finding, including evidence for a second site of action for CB1.
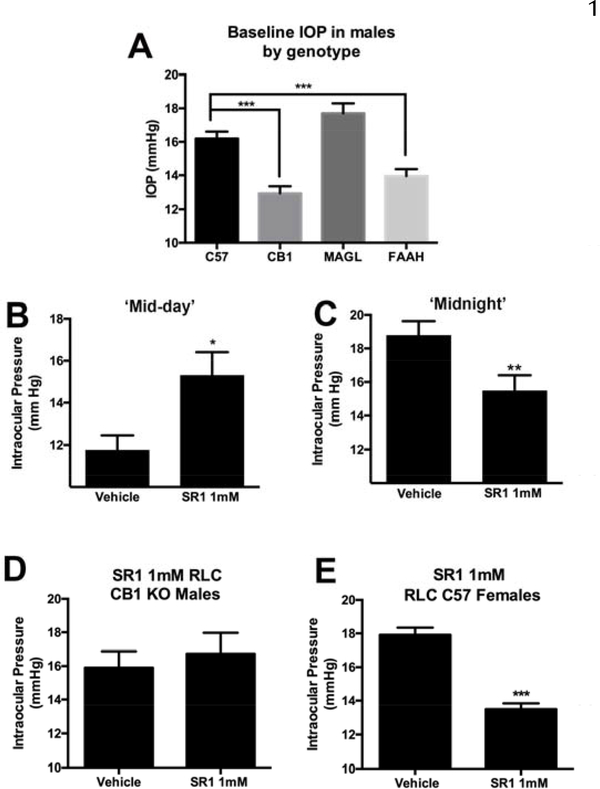
Figure 1. Evidence for a second, diurnally expressed, site of action for CB1 in regulation of OP.
A) CB1 knockouts have lower baseline ocular pressure relative to WT controls. B) CB1 antagonist SR141716 (SR1) raises pressure at mid-day as expected in mice maintained in standard light cycle, but C) lowers IOP in reverse light cycle. D) SR1 has no effect in CB1 knockout males, but does lower IOP in females (E). A,E ***, p<0.001, unpaired t-test. B-C) *, p<0.05, p<0.01, paired t-test.
2. MATERIAL AND METHODS
2.1. Animals
Experiments were conducted at the Indiana University Bloomington campus or at the Indiana University School of Medicine Indianapolis campus. All mice used for IOP experiments were handled according to the guidelines of the Indiana University/Indiana University School of Medicine animal care committees and in accordance with the ARVO animal statement. Mice (age 3–8 months) were kept on a 12 h (06:00–18:00) standard light dark cycle (SLC) or on a reverse light cycle (RLC) to assess diurnal impact on drug effects, and fed ad libitum. CB1 knockouts are on a CD1 background strain. The remaining mice were on a C57BL/6J (C57) background. We have previously shown that mice on a CD1 background see reductions in ocular pressure upon topical treatment with CB1 cannabinoid agonists WIN55212 and CP55940 that are absent in CB1 knockouts (Hudson et al., 2011). Experiments made use of males but we explicitly tested for sex-dependence of the primary effect (Expt 3.2, Figure 1E). Non-transgenic mice were obtained from Jackson Labs (Bar Harbor, ME) or were kindly provided by Dr. Ken Mackie (Indiana University, Bloomington IN). Mice were allowed to acclimatize to the animal care facility for at least a week prior to their use in experiments. CB1−/−, MAGL−/−, and FAAH−/− mice were kindly provided by Dr. Ken Mackie. The knockouts were both global knockouts. CB1−/− animals were originally received from Dr. Catherine Ledent (Catholic University, Leuven) as heterozygotes. The FAAH−/− and MAGL−/− mice were developed in the laboratory of Ben Cravatt (Scripps Research Institute, La Jolla, CA).
2.2. The ocular hypertension (OHT) model
To determine the effect of a CB1 negative allosteric modulator on ocular hypertensive eyes, we injected 2.5×10^7 pfu of Ad5-hΔTGFβ2 viruses (Vector Biolabs, Malvern, PA) into one of the mouse eyes intravitreally using published approaches (Mao et al., 2012; Millar et al., 2008). Briefly, female C57BL/6J mice (Jackson Laboratory) aged at around 3 months were housed at Indiana University School of Medicine animal facility in a reversed light cycle as described before. After baseline IOP establishment, the mice were anesthetized using 3% isoflurane with SomnoSuite (Kent Scientific, Torrington, CT). A Hamilton syringe with a 33 gauge needle was used to inject 2μl viral particles into the vitreous chamber over 2 minutes (Millar et al., 2008). After injection IOP was monitored once a week as described under Intraocular Pressure Measurements.
After development of OHT, the mice were used for ABD1085 treatment. On the day of treatment, baseline IOP was measured for the OHT eyes at 12pm followed by topical treatment with 5μl vehicle (Tocrisolve 100 with 6% ethanol) Tocrisolve 100 is a soya-based solvent (Tocris Biosciences, Minneapolis, MN) (Oltmanns et al., 2008)). IOP was monitored 1 hour and 1 day after treatment. After a 2-day washout, the mice were treated with 3mM ABD1085 in a similar manner. ABD1085 was first prepared in 100% ethanol as 50mM stock and then diluted to 3mM using Tocrisolve 100. This study was conducted in a masked manner. Data were analyzed using paired t-tests comparing drug vs. vehicle treatment in the same animal.
2.3. Intraocular pressure measurements
IOP was measured in mice by rebound tonometry, using a Tonolab (Icare Finland Oy, Helsinki, Finland) (Wang et al., 2005). This instrument uses a light plastic-tipped probe to briefly make contact with the cornea; after the probe encounters the eye the instrument measures the speed at which the probe rebounds in order to calculate IOP. To obtain reproducible IOP measurements, mice were anesthetized with isoflurane (3% induction). The anesthetized mouse was then placed on a platform in a prone position, where anesthesia was maintained with 2% isoflurane. Baseline IOP measurements are taken in both eyes. A ‘measurement’ consisted of the average value of six readings. One eye was then treated with drug (dissolved in Tocrisolve, 5μL final volume applied topically) while the other eye was treated with vehicle. Some drugs (E.g. ABD1085) required an additional presolubilization step in ethanol, resulting in a net concentration of 3–6% ethanol in the Tocrisolve. The Tocrisolve vehicle control was adjusted accordingly. The animal was then allowed to recover. After an hour the animal was again anesthetized as above. IOP was then measured in the drug-treated and vehicle-treated contralateral eye. In principle, anesthesia may alter IOP, however our tests of IOP in contralateral eyes of the same animal means that observed effects are independent of hypothesized anesthetic effects on IOP.
For normotensive mice, IOP measurements following drug administration were analyzed by paired t-tests comparing drug-treated eyes to vehicle-treated contralateral eyes.
For injection of PSNCBAM1, the drug was dissolved in a 18:1:1 saline, cremophor, ethanol mixture and injected intraperitoneally. IOP was measured before injection and again 1 hour after treatment and the results analyzed using a paired t-test comparing treated vs. baseline condition for a given animal.
2.4. Drugs
SR141716 was obtained through the NIDA drug supply program. Topically applied drugs were prepared by dilution in Tocrisolve. ABD1085 was synthesized in the laboratory of Dr. Iain Greig.
3. RESULTS
3.1. CB1 knockout mice have a lower baseline IOP than wild type (WT) mice
In the course of our investigations of cannabinoid regulation of IOP we noted that some of the knockout strains had a consistently low IOP. This was not MAGL, as one might have predicted since blocking MAGL substantially lowers IOP. Instead we found that the genotype that exhibited the lowest IOP was the CB1 knockout line, though FAAH knockouts also had lower pressures (Fig. 1A, IOP in WT (C57, mmHg): 16.2 ± 0.4 n=104; CB1: 13.0 ± 0.4 n=88; FAAH: 13.9 ± 0.4, n=98; MAGL: 17.7 ± 0.6, n=30; ***, p<0.001 for FAAH and CB1 vs. WT by one-way ANOVA with Dunnett’s post hoc test vs. WT).
3.2. A second form of IOP regulation by CB1
To explore this further, we tested the effect of the CB1 antagonist SR141716 (SR1) on IOP. When applied topically in a mouse during mid-day (standard light cycle, SLC), we found that SR141716 raised IOP. This is presumed to be due to reversal of tonic reduction of IOP by CB1(Hudson et al., 2011) (Fig 1B, 1Hr (control): 15.1 ± 0.9, (SR1, 1mM): 17.6 ± 0.9, n=14; p<0.05 by paired t-test vs. contralateral eye).
However the opposite proved to be the case in reverse light cycle (RLC, mid-night equivalent) because SR141716 lowered IOP (Fig 1C, 1Hr (control): 18.8 ± 0.9, (SR1, 1mM): 15.5 ± 0.9, n=15; p<0.005 by paired t-test vs. contralateral eye).
We tested SR141716 in male CB1 KO mice in RLC but saw no change in IOP after SR1 treatment (Fig. 1D, 1Hr (control): 15.9 ± 1.0; (SR1, 1mM): 16.7 ± 1.2, n=6; NS by paired t-test vs. contralateral eye). This suggests that the effect is CB1-dependent.
We have previously found that conventional CB1 regulation of IOP is sex-dependent (Miller et al., 2018), with stronger effects of THC in males. We therefore tested for the effect of SR1 on females, finding that in females under RLC, pressure also declined substantially and was, if anything, more pronounced than in males (Fig. 1C: 1Hr (control): 17.9 ± 0.4, (SR1, 1mM): 13.5 ± 0.3, n=7; p<0.0001 by paired t-test vs. contralateral eye).
3.3. CB1 antagonist effects do not desensitize after 1 week but do result in a rebound after cessation
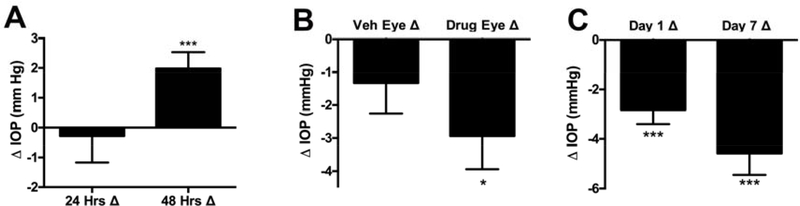
We tested the duration of the effect of SR1 with the finding that SR1 effects are absent at 24 hours after a single treatment but that IOP rebounded above baseline at 48 hours (Fig. 2A: SR141716 1mM effect at 24 hrs (Δ IOP ± SEM): −0.3 ± 0.3; at 48 hrs: 2.0 ± 0.6, n=16; ***, p<0.005 by 1-sample t-test vs. 0 (0 = no effect)).
Figure 2. Multi-day treatment with SR141716.
A) A single treatment with SR141716 (SR1, 1mM) does not last 24 hours but does result in a rebound at 48 hrs. B) A 7-day daily treatment results in a persistent lowering of IOP (24 hrs after 6th treatment) though there may be some cross-over into vehicle treated eye. C) SR141716 effects do not show evidence of desensitization with repeated 7-day treatments. *, p<0.05, ***, p<0.005 by 1-sample t test vs. 0 (0 = no change).
We also tested for the consequence of repeated daily treatment with SR1. We found that seven days of daily treatment had several effects. First we found that measuring IOP on the 7th day before treatment (i.e. 24 hours after the 6th daily treatment) IOP was still lowered in the drug-treated eye (Fig. 2B: SR141716 1mM, 7 days in vehicle-treated eye, before 7th treatment (Δ IOP ± SEM): −1.3 ± 0.9; drug-treated eye: −2.9 ± 1.0, n=8; *, p<0.05 by 1 sample t-test vs. 0 (0 = no effect)). The IOP was also lower in the vehicle treated eye but this difference was not statistically significant. Seven days of treatment did not result in desensitization of the IOP response (Fig. 2C: SR141716 1mM effect at 1 day in drug-treated eye (Δ IOP ± SEM): −2.8 ± 0.6; drug-treated eye: −4.6 ± 0.9, n=8; ***, p<0.005 by 1 sample t-test vs. 0 (0 = no effect)) and ended with a drop of 4.6 mm Hg in IOP. No outward signs of irritation were noted after the 7 days of daily treatment.
3.4. Negative allosteric modulation of CB1 can lower IOP
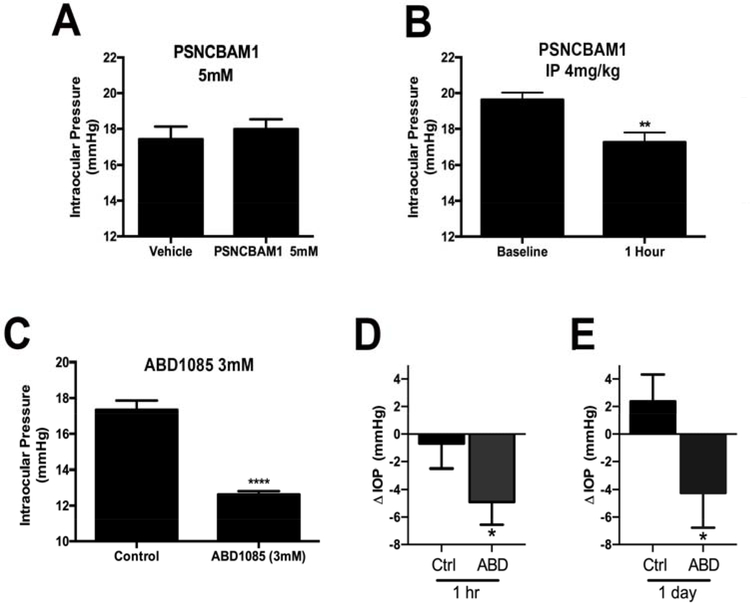
One area of recent interest in the cannabinoid field has been the development and application of allosteric modulators. It is supposed that allosteric modulation occurs via binding at a second “allosteric” site of action on the receptor to modify signaling at the main “orthosteric” site, thus offering a potential advantage over orthosteric antagonists. In principle this may limit modulation to receptors that are being activated by the endogenous ligand. Both negative and positive allosteric modulators of CB1 have been described (Mitjavila et al., 2018; Straiker et al., 2015) and we have tested positive allosteric modulators for the potential ability to enhance endogenous cannabinoid signaling in the eye and thereby lower ocular pressure (Cairns et al., 2017) but find that they have only limited effects. Our findings for SR141716 indicate that a negative allosteric modulator of CB1 may offer some advantages in lowering IOP. We have previously tested a panel of first generation negative allosteric modulators (NAMs) in a neuronal model and found PSNCBAM1 to be an efficacious blocker of cannabinoid CB1 signaling (Straiker et al., 2015). We therefore tested PSNCBAM1 topically but found it to be without effect (Fig 3A, vehicle-treated eye (mm Hg ± SEM): 17.4 ± 0.7, PSNCBAM1 (5mM): 18.0 ± 0.6, n=8, not significant by paired t-test). To rule out the possibility of a false-negative due to poor corneal penetration for PSNCBAM1, we also tested IOP after injection with the compound finding that this yielded a significant drop in IOP (Fig 3B, baseline IOP (mm Hg ± SEM): 19.6 ± 0.4; 1 hour after PSNCBAM1 (4mg/kg IP): 17.3 ± 0.5; n=16; p<0.005 by paired t-test vs. baseline in a given animal). We also tested a 2nd generation NAM ABD1085 (Greig et al., 2016) that proved highly effective at lowering IOP. ABD1085 lowered pressure by nearly 4.5 mmHg (Fig. 3C: 1Hr (control): 17.4 ± 0.5, (ABD1085, 3mM): 12.6 ± 0.2, n=8; p<0.0001 by paired t-test vs. contralateral eye).
Figure 3. Negative allosteric modulators of CB1 lower IOP.
A) The negative allosteric modulator (NAM) PSNCBAM1 does not lower IOP relative to the contralateral eye when applied topically. B) However PSNCBAM1 does lower IOP when injected. B: **, p<0.01, paired t-test vs. baseline. C) ABD1085 substantially lowers IOP when applied topically in a normotensive model. ****, p<0.0001, paired t-test vs. contralateral eye. **, p<0.05, unpaired t-test vs. Vehicle-treated animals. D-E) ABD1085 lowers IOP at 1hr and a day in a TGFβ2-induced hypertensive mouse model. ΔIOP = post-treatment IOP- pre-treatment IOP. *, p<0.05, n=9, paired t-test.
After studying ABD1085 in normotensive mouse eyes, we then tested it using a well-characterized ocular hypertensive (OHT) model. It is known that TGFβ2 is elevated in many POAG eyes (Tripathi et al., 1994). Experimentally, high TGFβ2 induces glaucomatous changes in the trabecular meshwork (TM) and elevates IOP in human and mouse eyes (Fleenor et al., 2006; Shepard et al., 2010). Therefore, the TGFβ2-induced OHT mouse model is suitable to mimic OHT conditions in POAG patients and has been widely used (McDowell et al., 2015; McDowell et al., 2013; Raychaudhuri et al., 2018). We injected serum type 5 adenovirus (Ad5) which overexpressed a constitutive actively form of human TGFβ2 into one of the mouse eyes (Shepard et al., 2010). Previous studies have shown that the Ad5 virus has a tropism for the mouse TM (Millar et al., 2008). OHT was successfully induced in injected eyes with an averaged IOP elevation of 13.4mmHg (mean ± SD: 18.0 ± 2.0 vs. 31.4 ± 8.9 mmHg; N=9, and p<0.01; see supplemental data for details).
After OHT induction, we treated OHT eyes once with 5ul vehicle or 3mM ABD1085 once topically. We measured IOP before and after treatment, finding that the ocular hypotensive effect of ABD1085 lasted for a day (Fig. 3D–E: Δ IOP at 1Hr: 0.69 ± 1.80 (vehicle) vs. −4.91 ± 1.64 mmHg (ABD1085) and at 1 day: 2.38 ± 1.95 (vehicle) vs. −4.24 ± 2.52 mmHg (ABD1085); P<0.05 by paired t-test for the same eyes). Individual mouse IOP values are listed in supplemental data.
4. DISCUSSION
We are gradually approaching a more complete picture of how the cannabinoid signaling system regulates intraocular pressure. This regulation is complex, involving no less than three related receptors – CB1, GPR18 and GPR119 – and both diurnal regulation and sex-dependent effects. We now report evidence for an additional mode of action for cannabinoid CB1 receptors involving inverse regulation of IOP: Inhibition of CB1 receptors lowers IOP, an effect that is effectively mimicked by negative allosteric modulators of CB1 and absent in CB1 knockout mice. The NAM ABD1085 was effective for a full day in a hypertensive mouse model. In contrast to the previously described effect of CB1 activation, this mechanism does not appear to be sex-dependent. Also, the effect doesn’t desensitize after 1 week of treatment. CB1 receptors therefore serve dual, opposing roles in regulating IOP.
The location of this effect, at inflow vs. outflow sites, remains undetermined, but there are reasons to consider outflow as the more likely site of action for inverse CB1 regulation of IOP. It is likely that the component whereby CB1 activation lowers IOP is due to activity in the ciliary body (i.e. inflow) since proteins for both CB1 and the 2-AG metabolizing enzyme MAGL are found in the pigmented ciliary epithelium (Miller et al., 2016a; Straiker et al., 1999). Blocking MAGL elevates 2-AG levels and lowers IOP in a CB1-dependent manner, presumably by reducing the rate of aqueous humor formation. The second inverse-sign CB1 regulation is presumably localized elsewhere, most likely at a site of outflow, and so altering the outflow resistance, however this has not been determined explicitly. We have shown that CB1 receptors are found in many structures of the human eye including the TM (Straiker et al., 1999) and an early study found evidence for action of THC at both inflow and outflow sites (Green and Kim, 1976). Since THC is presumably acting as an agonist, the effect of antagonists may have been overlooked until now. A separate study examining the effects of HU211 also found evidence for a separate site of action but this compound is not considered a cannabinoid receptor agonist so the described effects may not be cannabinoid-dependent (Beilin et al., 2000). Since THC can also lower IOP via GPR18 (Miller et al., 2018) this may be explained as a consequence of GPR18 activation, though the site of action for GPR18 also remains undetermined.
Not only did we not see signs of desensitization with a week of daily treatments, if anything the net effect at the end of 7 days was a greater inhibition than after a single treatment, lowering IOP by more than 4.5 mmHg in a normotensive model. In addition, the 6th treatment lowered IOP 24 hours later, indicating that daily treatments are sufficient to maintain these salutary effects on IOP. Results with the NAM ABD1085 were consistent with this.
The negative CB1 IOP regulation has a diurnal aspect. The CB1 agonist lowers IOP in both RLC and SLC while the antagonist only lowers IOP in RLC. We have previously shown that mRNA levels of the anandamide-metabolizing enzyme fatty amide hydrolase (FAAH) change diurnally (Miller et al., 2016b). Because of the broad distribution of CB1 receptors in various ocular structures, it may be difficult to distinguish diurnal changes in CB1 receptor expression that occur in a subset of those receptors.
It is also notable that the sex-dependence that we have seen for standard CB1 inhibition of IOP is not seen here. Reduction in IOP was if anything greater in females than in males.
In summary, we have determined that cannabinoid CB1 receptors, known to be expressed at both sites of aqueous humor inflow and outflow, regulate IOP via dual, opposing mechanisms. In contrast to the conventional mechanism whereby activation of CB1 lowers IOP, this second form – which may be dominant - is inversely signed such that antagonizing CB1 receptors lowers IOP. The action is diurnally regulated but not sex-dependent.
Supplementary Material
Highlights.
Cannabinoid CB1 receptor activation and antagonism can each lower ocular pressure (i.e. CB1 agonists and antagonists can lower pressure).
CB1 antagonism appears to be dominant in terms of its effects on ocular pressure.
Both CB1 antagonists and negative allosteric modulators can lower ocular pressure.
Unlike CB1 agonist effects, CB1 antagonist action is not sex-dependent.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01EY024625 (A.S.), R01EY026962 (W.M.))
Abbreviations
- C57
C57BL/6J
- FAAH
fatty acid amide hydrolase
- IOP
intraocular pressure
- MAGL
monoacylglycerol lipase
- NAM
negative allosteric modulators
- NAPE-PLD
N-acyl phosphatidylethanolamine-specific phospholipase D
- OHT
ocular hypertension
- RLC
reversed light dark cycle
- SLC
standard light dark cycle
- THC
tetrahydrocannabinol
- TM
trabecular meshwork
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Beilin M, Neumann R, Belkin M, Green K, Bar-Ilan A, 2000. Pharmacology of the intraocular pressure (IOP) lowering effect of systemic dexanabinol (HU-211), a non-psychotropic cannabinoid. J Ocul Pharmacol Ther 16, 217–230. [DOI] [PubMed] [Google Scholar]
- Cairns EA, Szczesniak AM, Straiker AJ, Kulkarni PM, Pertwee RG, Thakur GA, Baldridge WH, Kelly MEM, 2017. The In Vivo Effects of the CB1-Positive Allosteric Modulator GAT229 on Intraocular Pressure in Ocular Normotensive and Hypertensive Mice. J Ocul Pharmacol Ther 33, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M, Hu S, Viswanathan S, Kelly ME, Straiker A, 2013. A GPR18-based signaling system regulates IOP in murine eye. British Journal of Pharmacology 169, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF, 2006. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci 47, 226–234. [DOI] [PubMed] [Google Scholar]
- Green K, Kim K, 1976. Interaction of adrenergic antagonists with prostaglandin E2 and tetrahydrocannabinol in the eye. Invest Ophthalmol 15, 102–111. [PubMed] [Google Scholar]
- Greig IR, Baillie GL, Abdelrahman M, Trembleau L, Ross RA, 2016. Development of indole sulfonamides as cannabinoid receptor negative allosteric modulators. Bioorg Med Chem Lett 26, 4403–4407. [DOI] [PubMed] [Google Scholar]
- Hepler RS, Frank IR, 1971. Marihuana smoking and intraocular pressure. Jama 217, 1392. [PubMed] [Google Scholar]
- Hudson BD, Beazley M, Szczesniak AM, Straiker A, Kelly ME, 2011. Indirect sympatholytic actions at beta-adrenoceptors account for the ocular hypotensive actions of cannabinoid receptor agonists. J Pharmacol Exp Ther 339, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M, 2009. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Mao W, Millar JC, Wang WH, Silverman SM, Liu Y, Wordinger RJ, Rubin JS, Pang IH, Clark AF, 2012. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci 53, 7043–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI, 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. [DOI] [PubMed] [Google Scholar]
- McDowell CM, Hernandez H, Mao W, Clark AF, 2015. Gremlin Induces Ocular Hypertension in Mice Through Smad3-Dependent Signaling. Invest Ophthalmol Vis Sci 56, 5485–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CM, Tebow HE, Wordinger RJ, Clark AF, 2013. Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice. Exp Eye Res 116, 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JC, Pang IH, Wang WH, Wang Y, Clark AF, 2008. Effect of immunomodulation with anti-CD40L antibody on adenoviral-mediated transgene expression in mouse anterior segment. Mol Vis 14, 10–19. [PMC free article] [PubMed] [Google Scholar]
- Miller S, Daily L, Leishman E, Bradshaw H, Straiker A, 2018. Delta9-Tetrahydrocannabinol and Cannabidiol Differentially Regulate Intraocular Pressure. Invest Ophthalmol Vis Sci 59, 5904–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Hu SS, Leishman E, Morgan D, Wager-Miller J, Mackie K, Bradshaw HB, Straiker A, 2017. A GPR119 signaling system in the murine eye regulates intraocular pressure in a sex-dependent manner. Invest Ophthalmol Vis Sci 58, 2930–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Leishman E, Hu SS, Elghouche A, Daily L, Murataeva N, Bradshaw H, Straiker A, 2016a. Harnessing the endocannabinoid 2-arachidonoylglycerol to lower intraocular pressure in a murine model. Invest Ophthalmol Vis Sci 57, 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Leishman E, Oehler O, Daily L, Murataeva N, Wager-Miller J, Bradshaw H, Straiker A, 2016b. Evidence for a GPR18 role in diurnal regulation of intraocular pressure. Invest Ophthalmol Vis Sci 57, 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjavila J, Yin D, Kulkarni PM, Zanato C, Thakur GA, Ross R, Greig I, Mackie K, Straiker A, 2018. Enantiomer-specific positive allosteric modulation of CB1 signaling in autaptic hippocampal neurons. Pharmacol Res 129, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M, 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Oltmanns MH, Samudre SS, Castillo IG, Hosseini A, Lichtman AH, Allen RC, Lattanzio FA, Williams PB, 2008. Topical WIN55212–2 alleviates intraocular hypertension in rats through a CB1 receptor mediated mechanism of action. J Ocul Pharmacol Ther 24, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell WD, Gregg JM, 1975. Delta(9)-tetrahydrocannabinol,, euphoria and intraocular pressure in man. Ann Ophthalmol 7, 921–923. [PubMed] [Google Scholar]
- Raychaudhuri U, Millar JC, Clark AF, 2018. Knockout of tissue transglutaminase ameliorates TGFbeta2-induced ocular hypertension: A novel therapeutic target for glaucoma? Exp Eye Res 171, 106–110. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF, 2010. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci 51, 2067–2076. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mitjavila J, Yin D, Gibson A, Mackie K, 2015. Aiming for allosterism: Evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacol Res 99, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker AJ, Maguire G, Mackie K, Lindsey J, 1999. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci 40, 2442–2448. [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ, 1994. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res 59, 723–727. [DOI] [PubMed] [Google Scholar]
- Wang WH, Millar JC, Pang IH, Wax MB, Clark AF, 2005. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci 46, 4617–4621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.