Abstract
Autism spectrum disorder (ASD), is a neurodevelopmental disorder characterized by social deficits, communication impairments, restrictive behaviors, and cognitive flexibility deficits. The valproic acid (VPA) model of autism has been widely used to examine changes in rodent behavior and neurobiology to better understand ASD. This study examined social and anxiety behavior as well as cognitive flexibility in VPA and control offspring. Results for social behavior were consistent with prior studies showing reduced sociability in VPA rats and increased self-grooming, which may be viewed as a repetitive behavior. VPA rats also had deficits in performing the set-shifting task, with female VPA rats demonstrating greater impairment compared to female control rats and male VPA rats. These results support the hypothesis that females diagnosed with ASD may suffer from different symptoms and present a unique behavioral profile compared to males with ASD. Female VPA rats were also less likely to form an attentional set; offering evidence that the VPA model of autism is encompassing executive function deficits similar to those observed in humans with ASD.
Keywords: autism models, cognitive flexibility, attention, sociability, executive functions
1.1. Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social interactions, communication, and increases in repetitive behavior. Another common symptom of ASD is problems with executive functions 1-4. Executive functions encompass working memory, response inhibition but also include cognitive flexibility or attentional switching and are impaired in ASD 5. Cognitive flexibility is the ability to shift your behavior when environmental circumstances change or when the current rules guiding your behavior change. People with ASD demonstrate impairments in cognitive flexibility 6,7, including when they have to shift their attention to an external rule change 3,8. They also make more errors on switch trials 9, indicating they may have difficulty with disengagement. For this study, cognitive flexibility within the valproic acid (VPA) model of autism was tested.
The VPA model is widely recognized as having both construct and face validity 10. It was developed after realizing that women who were prescribed medications containing VPA, to treat seizures and bi-polar disorder, had an increased risk between 4-8% of their children developing ASD compared to the regular population 11-13. VPA can cause hyperacetylation in the embryonic brain 14 and this can lead to loss of neurons in development 10. Other possible mechanisms include excitatory and inhibitory imbalances related to altered GABA function15, dopaminergic expression 16, and changes in fatty acid synthase expression17. Past research has provided support for the model demonstrating that VPA treated offspring exhibit symptoms of ASD-like behavior including reduced social interactions 18-21 and increased repetitive behavior 22. However, the model has not been extensively studied to examine if VPA animals exhibit other symptoms frequently seen within ASD, such as altered cognitive function. A few research studies have found behavioral flexibility impairments in mice strains when using probabilistic feedback 22. In addition, use of the 5-choice serial reaction time task (5-CSRT), a behavioral task that examines components of attention, has found VPA treated rats were slower to acquire training levels compared to control rats and were impaired on accuracy when stimulus durations were varied 23. These results suggest that the VPA model is capturing some of the executive function deficits observed in people with ASD.
A common task to test cognitive flexibility in humans is the Wisconsin Card Sorting task (WCST) and is frequently used to assess executive function deficits 24. In the WCST, humans are asked to sort a deck of cards by one feature on those cards but are only give yes/no feedback. The cards have different shapes, colors, and a different number of items on each card. Cards can be sorted by one dimension at a time (i.e., color or shape). The rule for sorting is changed after several completed trials and participants must find the new sorting rule through trial and error. The attentional set-shifting task (ASST) was created to examine formation of attentional sets in animals 25. Rats were trained to dig in different media types with various scented flowerpots. The digging media and the odors are cues that inform the rat where to dig for reward. The specific digging medium (i.e., sawdust) or the odor (i.e., vanilla) can serve as the rewarded cue. After reaching criterion on one rule, the rule is changed, and rats must learn to dig to the newly rewarded cue. The task has several rule changes, which assess how well rats can recognize the rule has changed and they need to choose a different cue to find the reward. As a rat learns to choose the correct odor from an odor pair it is establishing a learning set. When the rule is changed the animals must attend to a new domain, and now dig in the location marked by a specific digging media and ignore the odor. The switch between odor pairs is called an intra-dimensional shift (IDS), the rat learns new odor discriminations, but it is the same rule, follow your nose. When the rule switches to the digging media, it is an extra-dimensional shift (EDS), as now the rat ignores the odor cue and attends to the digging media. This task also has two reversals that immediately follow the IDS and EDS stages, which examine an animal’s ability to learn when the contingency between a stimulus and reward has changed.
The VPA model has been used to study some aspects of cognition but, this is the first study examine IDS and EDS performance during set-shifting of VPA animals. Humans with ASD have executive function deficits and in particular have issues with the EDS 4,8,26,27. Based on these results in humans it was hypothesized that VPA animals would be impaired on the EDS phases of the task 4,8,26,27.
Regarding sex differences of executive functions in humans with ASD the literature is mixed. Therefore, it was especially important to study differences between female and male rats. Females with ASD have more extreme executive function impairments compared to males with ASD 6,28-32, but for certain executive components related to timing and visuospatial responses females are better than males 33,34. Due to the deficits in human females on executive components it was hypothesized VPA female rats would perform worse compared to VPA male rats. Likewise, humans with ASD do not show reversal learning deficits, or impairment in learning the basic rules of these executive tasks. Therefore, it was hypothesized that VPA rats would not be impaired on the reversal phases or the simple discrimination (SD) or compound discrimination (CD) phases of the set-shifting task. The primary interest was to assess cognitive performance in the set-shifting task, and additional behavioral tasks were run to assess if the VPA offspring demonstrated similar social and anxiety-like behaviors found by other research groups. These included the 3-chamber task, social pair interactions, and a marble burying task. The 3-chamber task is used to assess social novelty. The social dyad task and 3-chamber task were conducted in adolescence when rats demonstrate increased social play behaviors. Based on prior studies it was predicted that VPA rats would have decreases in social exploration behaviors such as sniffing and increased time investigating a novel object in the 3-chamber task 21,35,36. Social play behaviors were examined in dyads of control-control or VPA-VPA matched by weight and sex. It was predicted that VPA animals would demonstrate fewer play behaviors compared to controls. The marble burying task is used to study both repetitive behavior and anxiety in obsessive compulsive animal models. These experiments were part of a larger set of studies and therefore the same animals were examined across a wide array of tasks. Each rat pup was run through each task in the same order, see the method for a timeline. One aspect of interest was examining similar behaviors across different tasks, in the same animals, it was not possible to counterbalance task order due to possible age effects in the social tasks. Future analyses are currently examining brain changes in this same cohort of animals to examine how changes in grey matter volume impacts different behavioral outcomes.
2.1. Methods
Subjects
Sixteen pregnant dams (Long-Evans) arrived from Charles River on gestational day 6. On gestational day 12 they were injected with a single dose of saline (5 dams) or VPA (11 dams) (sodium valproate (Sigma), 250mg/ml, mixed in saline, 600 mg/kg). More VPA dams were included because prior work has demonstrated that VPA litters are sometimes smaller than control litters. Dams were briefly anesthetized on isoflurane gas to administer a less stressful I.P. injection. The isoflurane administration (required by the IACUC) was very brief lasting only a few minutes and should not have adversely effected the pups, as longer exposure is required to see adverse effects in offspring 37. All procedures were conducted in accordance with the Kansas State University IACUC and the NIH guide for the care and use of laboratory animals. Rats were given free access to food except for when undergoing preparation for the set-shifting task. Lights were on starting at 7 am- 7 pm. Rats were reared with litter mates until weaning, then were pair housed with a same sex littermate.
2.2. Timeline of events
Three basic behavioral tasks were run to examine autism like symptoms in VPA treated offspring. A social interaction task similar to 19 on postnatal days (PND 30-35), the 3-chamber task (PND 35-45) and marble burying (PND 52-65). For the set-shifting task rats were trained after reaching adulthood starting around PND 70. The social interaction dyads and 3-chamber task was conducted in adolescence because that is when there is a greater likelihood of observing social and play behaviors 18,38. All rats went through the same behavioral tests, this was done to ensure a robust total number of animals for each task.
2.3. Video equipment
All videos were collected and analyzed with the Limelight software camera system (Actimetrics, IL).
2.4. Social interaction
Rats were weighed and separated overnight from their littermate to increase sociability 39. Rats were then paired with a stranger rat that was within 15 grams (VPA-VPA or Control-Control) for 20 minutes 19,20. The testing apparatus was a plexiglas box (35 cm long, 25 cm wide, and 37 cm high). Rats were videotaped from above and behaviors were scored by an independent observer, blind to condition and each behavior was assigned to one reviewer. Play behaviors included pinning (one rat is rotated onto its dorsal surface, being pinned by other rat), boxing (animals facing upright toward one another pawing at one other) and wrestling (animals roll over each other in play). Social exploration behaviors included climbing (on or over), following, touching, sniffing, and anogenital sniffing, for further definitions see 38. Self-grooming was considered a repetitive behavior. For the social interaction behaviors, both the duration and number of bouts were scored during the first 10 minutes as bin 1 and the second 10 minutes as bin 2.
2.5. 3 chamber task
Rats were placed in the middle chamber for 5 minutes to habituate to the chamber (16 W x 31.5 L x 10 D cm). Then a novel rat was placed in a side chamber (side counterbalanced) and the experimental rat explored all three rooms of the chamber for 10 minutes. On one side room there was a novel object, an empty plastic cage, and on the other side was an identical plastic cage which was placed over a novel rat, this was called the novel room. After 10 minutes, a second novel rat was introduced into the opposite side chamber (former object room becomes second novel room) and the experimental rat explored for a further 10 minutes. Behavior was videotaped and time spent in each chamber was recorded. The time spent investigating the empty cage (novel object), or where the novel rats resided, was also coded by a blind reviewer. Distance traveled was recorded in cm. The ‘novel’ rats were a set of 6 control rats that were used on a rotational basis to prevent fatigue and counterbalanced for side placement. This ensured that every experimental rat was naïve to the novel rats in the side chambers.
2.6. Marble burying
Rats (PND 52-65) were placed in a doublewide home cage (40 W x 43 L x 20 H cm) filled 3 cm deep with regular pine bedding. They were allowed to habituate for 5 minutes then placed in their home cage briefly while an arrangement of 20 marbles (2.5 cm diameter of the marble) in a 4 x 5 pattern were placed on the bedding in the double wide cage. Rats were then placed back in the double wide cage and had 20 minutes to bury marbles. Rats were videotaped and a picture was taken at the end of the session. The container was emptied, wiped down with 70% ethanol, dried and then refilled for the next session. Marbles buried by greater than 2/3 were considered buried. Additional behaviors coded by blind reviewers were self-grooming, digging, and inactive using definitions from 40. Behaviors were coded for bouts and duration.
2.7. Set-shifting task
Apparatus and stimuli
A Plexiglas box (50 x 37.5 x 25 cm) with a black divider was used as the testing arena. The sliding door allowed access to the flowerpots (which were held to the floor of the chamber with Velcro to prevent tipping). All media stimuli were mixed and recycled between rats to allow any odor cues to be disseminated. However, once a pot had been scented with the blotting paper it was only ever scented with the same scent. Crushed Honey Nut Cheerio powder was applied to all digging media to prevent reward odor from serving as a digging cue. Media were shredded manila folders, aspen shavings, foam rubber, felt, burlap ribbon, and silk ribbon. Odors were rum, vanilla, lemon, almond, cinnamon, and anise. A pilot study demonstrated that rats could discriminate these odors.
Training.
Rats were trained in the set-shifting task with 6 stages (simple discrimination (SD), compound discrimination (CD), intra-dimensional shift (IDS), reversal 1 (R1), extra-dimensional shift (EDS) and reversal 2 (R2) to test cognitive flexibility. Rats underwent food restriction for between 7-10 days, obtaining around 90% of their body weight and then underwent training. Honey Nut Cheerios (General Mills, MN) and a flowerpot were introduced into the home cage the night before training began. Basic training consisted of shaping digging behavior in the testing arena. Rats were allowed to obtain a small 1/3 piece of cereal on top of the bedding of the flowerpots. During successive trials the cereal was buried deeper until the rat was successfully digging to retrieve it at about 3.5 cm deep. Day 2 of training consisted of an odor discrimination (tea tree oil vs lavender) with plain pine bedding, and a medium discrimination (shredded paper vs pine shavings) on unscented pots. Pots themselves were not scented rather blotting paper was taped to the inner lip of the ceramic pot and scented with a pipettor (5 microliters). The paper was re-scented before every training session. In all sessions the first four trials were discovery trials and were not scored, the rat could sample the other pot in these trials only. After these trials, the undug pot was lifted out immediately after the rat initiated a dig (defined as vigorously moving the medium with nose or paw). After a dig (successful or unsuccessful) the opposite pot was removed to prevent digging in the other flowerpot, which occurred after the first 4 discovery trials. This was done to ensure once a choice was made an animal could not return to the other pot. Rats were removed from the task if they became inactive. They were given two 10-minute breaks and if continued to not dig for three trials in a row then were removed from the task. Training criteria for basic training (day 1) was 10 consecutive digs, whereas for all other training phases it was 6 correct consecutive trials to move to next phase of training or next phase of the task. Day 3 of training was the day of the set-shifting task the SD, CD, IDS, R1, EDS and R2 were administered. All stimuli were counterbalanced with a Latin square design and half of the rats were trained odor to medium shifts, half medium to odor shifts, Table 1.
Table 1: Set-shifting task.
These are the phases of the task and the examples of the media and odor combinations for one rat. (SD- Simple Discrimination, CD-Compound Discrimination, IDS-Intradimensional shift, EDS-Extradimensional shift, REV-Reversal).
| Phase | SD | CD | IDS | REV1 | EDS | REV2 |
|---|---|---|---|---|---|---|
| Example Rat | Aspen* v. Manila Folders | Aspen*/Rum v. Manila Folders/Vanilla | Foam Rubber*/Cinnamon v. Felt/Anise | Felt*/Cinnamon v. Foam Rubber/Anise | Almond*/Burlap v. Lemon/Ribbon | Lemon*/Burlap v. Almond/Ribbon |
Indicates rewarded pot & correct choice
(Note: For any phase there is another pairing of pots so that across trials all combinations occur, for example for the CD (Aspen*/Vanilla v Manilla folders/Rum) would be offered.
2.8. Data analysis
2.8.1. Social interaction
Each social behavior duration and bouts were analyzed with a mixed ANOVA (time bin as the repeated measure and between-subject factors of sex and condition (control vs. VPA), with LSD posthocs.
2.8.2. 3 Chamber task
Time spent data for the three-chamber task were analyzed with 2-way ANOVA with sex and condition as factors, Tukey post hocs were applied where appropriate. The time spent interacting (defined as touching the object/ rat cage) was also coded from the video, and the number of bouts of interacting were calculated. In addition, the sociability index (SI) from 35 was calculated defined as: SI= (time interacting novel rat 1 – time interacting object/ time interacting novel rat 1 + time interacting object). It ranges from −1 to 1 and higher scores indicate more sociability. Lastly, the distance traveled in each room and total distance were tracked in cm.
2.8.3. Marble Burying
Number of buried marbles were analyzed with a 2-way ANOVA (sex, condition). Bouts and durations for digging, inactivity, and grooming were analyzed with a 2-way ANOVA (sex, condition).
2.8.4. Set-shifting
An attentional set is defined as requiring more trials to complete the EDS compared to the IDS, due than the to the difficulty of the EDS compared to the IDS 41. This is characterized by the rodent not focusing attention onto the proper stimulus dimension during learning the different phases, which causes the rat to fail at forming a proper attentional set, therefore resulting in the EDS not being a true test of their ability to shift the attentional set. Group means were examined to determine in an attentional set was formed for trials to criterion, if the EDS was greater than their trials to criterion for the IDS, the a set was formed 42. For trials to criterion and errors different ANOVAs were run (between-subject factors sex and condition) examining performance across the phases CD, SD, IDS, and EDS and reversal stages of the attentional set-shifting task. All statistics were conducted in JMP analysis software (Statistical Discovery. From SAS, NC, USA) and graphs were made with Prism 8, (GraphPad, San Diego, CA) software.
3.0. Results
Sixteen pregnant dams were injected (5 with saline and 11 with VPA), 2 VPA dams did not deliver. There were 25 male control pups weaned and 23 female controls weaned. There were 29 VPA male pups weaned and 29 female pups, after weaning 7 VPA rats were lost to sickness.
Twelve videos were corrupted before data could be scored, which were unfortunately mostly VPA pairings, leaving data from 10 male control pairs, 10 female control pairs, 7 VPA male pairs and 6 VPA female pairs. For bouts of social behavior, female VPA rats spent significantly less time sniffing, with significant interactions of bin by condition (F5,62=4.97; p=0.02), and condition by sex (F5,62=4.42; p=0.03), Figure 1A. LSD post hocs demonstrated that female VPA animals had significantly fewer bouts of sniffing compared to controls, p=0.02. For self-grooming bouts there was a main effect of bin (F5, 61=26.64, p=0.001) and condition (F5, 61=4.25, p=0.04), demonstrating more grooming for VPA animals, Figure 1B. Touching bouts had a significant main effect of bin (F5,62=2.26, p=0.003), demonstrating controls and VPA animals decreased touching in bin 2, Figure 1C.
Figure 1: Bouts of social behaviors.
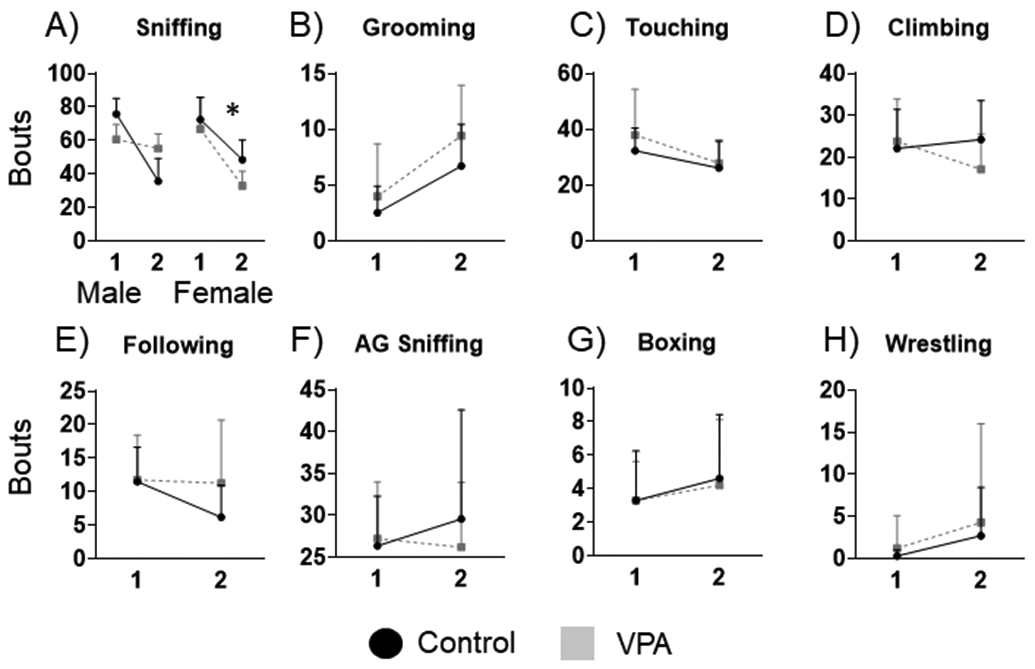
A) Female VPA rats had significantly less sniffing compared to control females. B) All animals had more bouts of grooming during bin 2 and VPA rats had more grooming than control rats. C) Touching behavior decreased for both VPA and control animals during bin 2. D), E), F), G), H) No significant differences were found for bouts of climbing, following, AG sniffing, boxing or wrestling. *= significant group differences
For self-grooming duration there were main effects of bin (F5, 61=44.96 p=0.001) and condition (F5, 61=16.46, p=0.001), and an interaction between bin and condition, (F5, 61=11.20, p=0.001). The LSD post hoc demonstrated that VPA animals spent significantly more time grooming than control animals, especially during bin 2, Figure 2A. Touching duration had a significant main effect of bin (F5,62=16.26, p=0.001), Figure 2B. Durations of climbing behavior had a significant interaction of bin by condition, (F5,62=4.17, p=0.003), the LSD post hoc (p=0.04) demonstrated that VPA animals decreased climbing during the second bin compared to control animals, Figure 2C. Durations of following had a significant interaction of bin by condition (F5,62=5.76, p=0.01), and condition by sex (F5,62=5.03, p=0.02), the LSD post hoc found that during bin 2 female VPA rats spent more time following than control females, Figure 2C. Sniffing duration had a main effect of bin (F5,62=7.84, p=0.001), where both VPA and control rats decrease sniffing durations in bin 2, Figure 2E. Durations of boxing had a significant effect of bin (F5,62=6.18, p=0.007), and a condition by sex interaction (F5,62=6.18, p=00.01), and the LSD post hoc (p=0.04) found that female VPA rats decreased boxing compared to control females, Figure 2G. Durations of wrestling had a significant effect of bin (F5,62=2.04, p=0.04) where both VPA and control rats had a slight increase in wrestling duration for bin 2. Pinning occurred so rarely that is was unable to be scored and may have been due to the lighting conditions of the recording room being a bit too bright.
Figure 2: Durations of social behaviors.
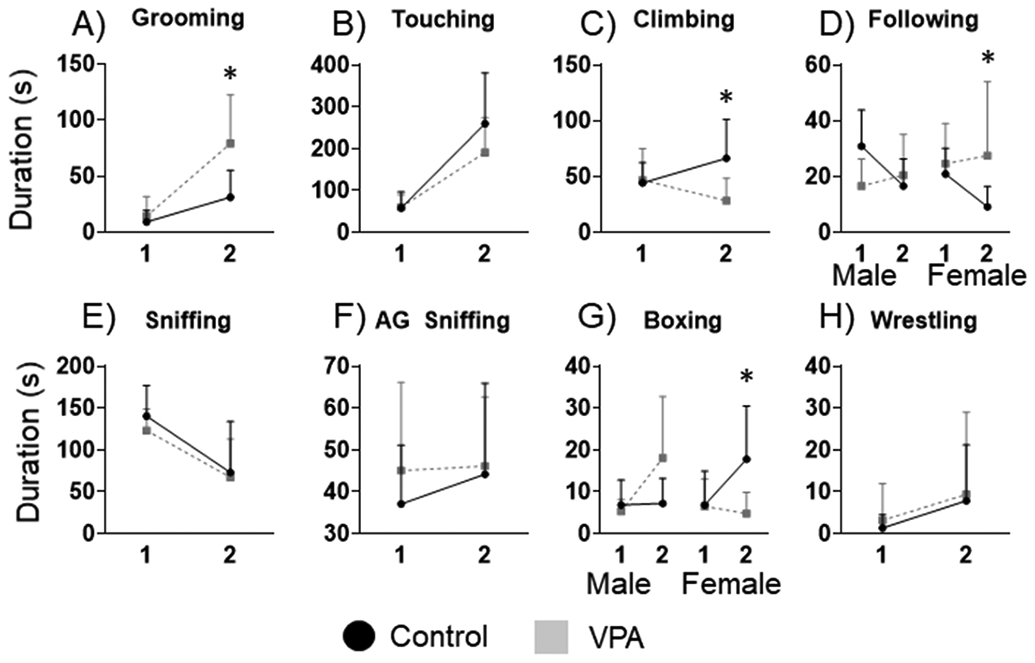
A) VPA animals spent significantly more time than control animals grooming during bin 2. B) Both control and VPA rats increased touching durations during bin 2. C) VPA rats had shorter durations of climbing during bin 2 compared to control rats. D) Female VPA rats spent significantly more time following during bin 2 compared to control females. E) All rats decreased sniffing durations during bin 2. F) There were no group effects for AG sniffing. G) Female VPA rats spent significantly less time boxing compared to control females. H) There were no significant differences for durations of wrestling. *= significant group differences
3.1. 3 chamber task
Time spent
There were significant main effects of condition (F3, 94=9.13, p=0.0008) and sex (p=0.0014) for time spent in the room with the object, Figure 3. There was also a significant interaction (p=0.02). Tukey post hocs revealed that VPA males spent significantly more time with the object compared to control males (p=.0006). Control males spent less time in the object room than female rats (control or VPA), (p=0.001;0.0001). There were also significant main effects of condition (F1, 94=19.6, p=0.0001), sex (F1, 94=6.4, p=0.01) and an interaction between condition and sex (F1, 94=4.07, p=0.04) for time spent in the middle chamber during the first 10 minutes. Tukey post hocs revealed that control males spent significantly more time in the middle chamber than VPA males (p=.0001); or control females (p=0.01) or VPA females (p=0.001). There were no significant main effects or an interaction for time spent in the novel animal room.
Figure 3: Time spent during bin 1 of 3 chamber task.
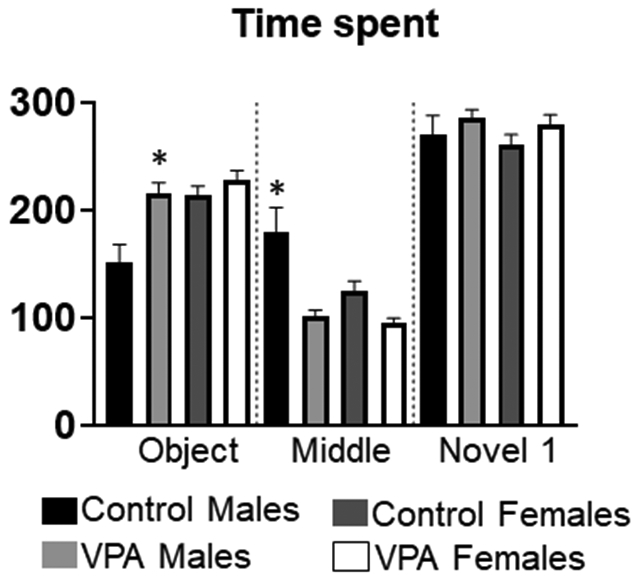
VPA males spent significantly more time with the object than control rats. Control males spent significantly more time in the middle chamber than any other group. There were no group differences for time spent in the novel 1 room. *= significant group differences
For the second 10 minutes, there was a significant main effect of condition (F4, 93=2.13, p=.02) for the middle chamber, demonstrating that control animals spent more time in the middle chamber than VPA animals. There were no other significant effects found for time spent in the novel 2 room, middle or familiar room.
3.1.2. Interactions with the rat cage
When a rat was interacting via touching an object within the cage (novel object or cage with another rat in it) this was scored as an interaction. During the first 10 minutes of the test, for the percent of interaction time with the novel rat cage, there was no effect of condition but there was a significant effect of sex (F3,95=11.2, p=0.001), Figure 4. For the number of bouts of interaction there was a significant effect of sex (F3,95=4.56, p=0.003). This indicated that males had a higher number of bouts than female rats regardless of condition group. The sociability index was calculated and there were significant effects of condition (F3,95= 5.39, p=0.02) and sex (F3,95= 6.85, p=0.01) of this value, indicating that VPA rats were less social than controls, and females displayed less social behavior than males.
Figure 4. Percent of time interacting with novel cage during first 10 minutes of 3 chamber task.
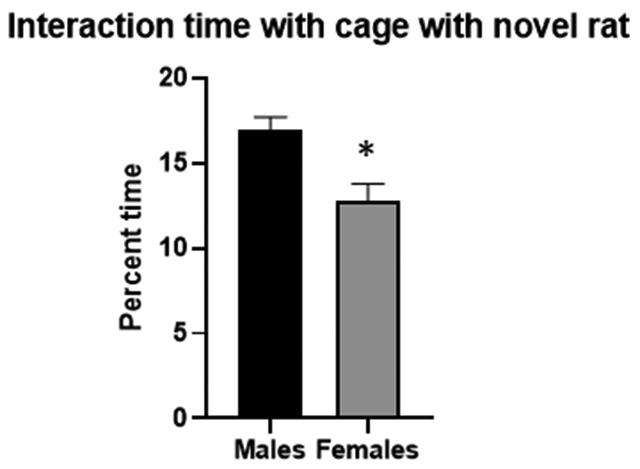
During bin 1 there was a main sex effect demonstrating that female rats, regardless of condition, interacted less than male rats with the novel rat. *= significant group differences
3.1.3. Distance traveled
During the first 10 minutes, within the object room there was a significant interaction (F3,89=7.97, p=0.01) and a main effect of sex (p<0.001). Tukey post hoc tests demonstrated differences between the control males and the VPA males (p=0.022), VPA females (p<0.001) and control females (p<0.001). Where control males traveled significantly less within the object room compared to all other groups. In the middle room, VPA animals traveled less demonstrated by a main effect of condition (F3,89=2.53, p=0.01). There was a main effect of sex for total distance traveled, (F3,89=4.70, p=0.001). For the second 10 minutes, there was a main effect of sex in the familiar room F3,91=4.20 (p=0.002). In the middle room there were significant effects of condition and sex (F3,91=3.19. p=0.01, 0.05). For total distance traveled there was a significant interaction and main effect of sex (F3,91=6.46, p=0.02; 0.000). Tukey post hocs found differences between male controls and female controls (p=0.0004) and female controls and VPA males (p=0.009), Figure 5.
Figure 5: Total distance traveled during the 3-chamber task.
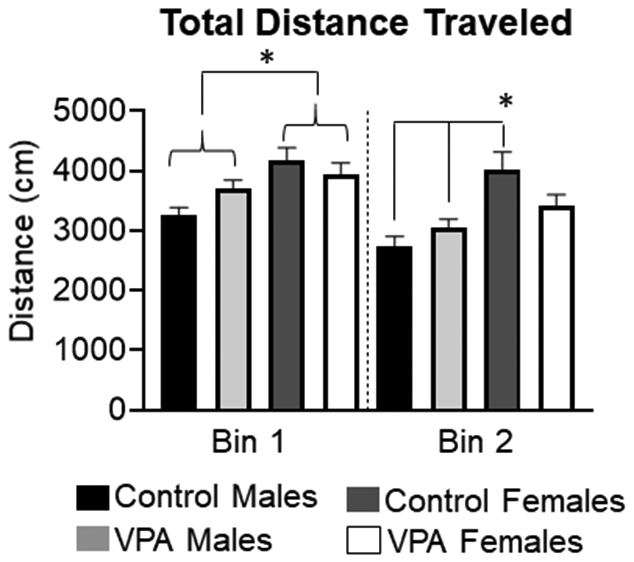
During bin 1 there was a main sex effect demonstrating that female rats, regardless of condition, traveled more than male rats. During bin 2 control females traveled more than male rats. *= significant group differences
3.2. Marble burying
A main effect of condition for digging bouts was found (F 1, 100=17.30, p < 0.001), such that VPA rats exhibited fewer bouts of digging behavior when compared to control rats regardless of sex. A main effect for condition was found for digging duration,( F 1, 100=7.35, p= 0.008), such that VPA rats exhibited digging behavior for shorter periods of time when compared to control rats regardless of sex, Figure 6A.
Figure 6: Marble burying task.
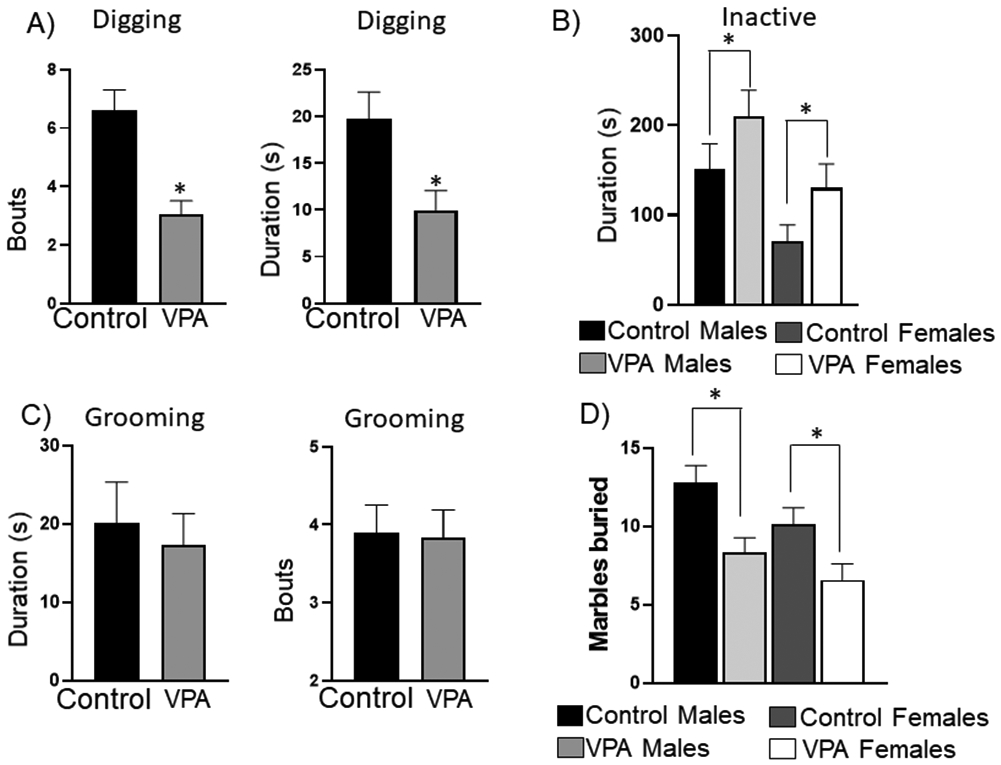
A) VPA animals spent significantly fewer bouts with shorter durations of digging compared to control animals. B) VPA animals spent significantly longer durations being inactive compared to controls, and male rats were significantly more inactive than female rats. C) There were no significant group differences for grooming during the marble task. D) VPA rats buried significantly fewer marbles than controls and females buried significantly fewer marbles than male rats. *= significant group differences
A significant main of sex was identified for inactive duration, (F 1, 100= 9.16, p=0.003), such that male rats were inactive for more total time during the task when compared to females regardless of condition, 6B. Another significant main effect of condition was also identified, (F 1, 100= 4.96, p=0.028), such that VPA rats were inactive for a greater amount of time during the task when compared to control rats regardless of sex, Figure 6B. The effect for condition for inactive bouts was close to significance (F1,100 =2.9, p=0.08). No significant effects were identified for grooming duration or grooming bouts, Figure 6C. There was a significant main effect of condition (F 1, 100 = 14.63, p=0.0002), and sex (F 1, 100 =4.46, p=0.03) for marbles buried. Males buried significantly more marbles than females and VPA animals buried significantly fewer marbles than controls, Figure 6D. Although this test has been reported as a measure of repetitive behavior, it can also be used to study anxiety in animal models 43. Scored behaviors demonstrated VPA rats sat still in the corner of the arena, spending significantly less time digging and more time inactive than controls. These behavioral differences indicated that VPA animals were less interested in digging than controls. The sitting still and increased inactive time could represent signs of anxiety.
3.3. Set-shifting task
A total of 99 rats were trained to perform set-shifting, however 4 rats failed to learn to dig and another 14 failed to complete the phases of the task, (12/14 of these were VPA). This left N=81 for set-shifting completion. Three rats were excluded from analysis for being extreme outliers, which was determined with the interquartile method, leaving N=78.
Rats were classified as set formers if they required more trials to criterion on the EDS compared to the IDS. This was determined using group means, in which VPA rats required more trials to complete the IDS (M = 10.56) than the EDS (M = 9.95). Control rats did not show this effect and required more trials to complete the EDS (M = 9.95) than the IDS (M = 8.95).
Examining the trials to criterion for the IDS phase of the task there was a significant main effect of sex (F1,74 =8.32, p=0.005), where females performed worse than males. There was also a significant main effect of condition (F1,74 =4.26, p=0.042), demonstrating VPA animals performed worse than control animals, Figure 7. Due to the significant sex difference and impact on IDS in particular, data was also separated by sex and an ANOVA was run to examine only female performance on the IDS. Female VPA rats were significantly worse on the IDS for trials to criterion compared to female controls (F1,36 =3.91, p=0.05), Figure 8. For errors on the IDS phase of the task there was a significant main effect of sex (F1,74 =4.13, p=0.028), and a significant main effect of condition (F1,73 =4.96, p=0.028), illustrating VPA animals made more errors than controls, Figure 9. There were no significant differences for SD, CD, EDS or Reversal phases of the task.
Figure 7: Set-shifting task performance, trials to criterion.
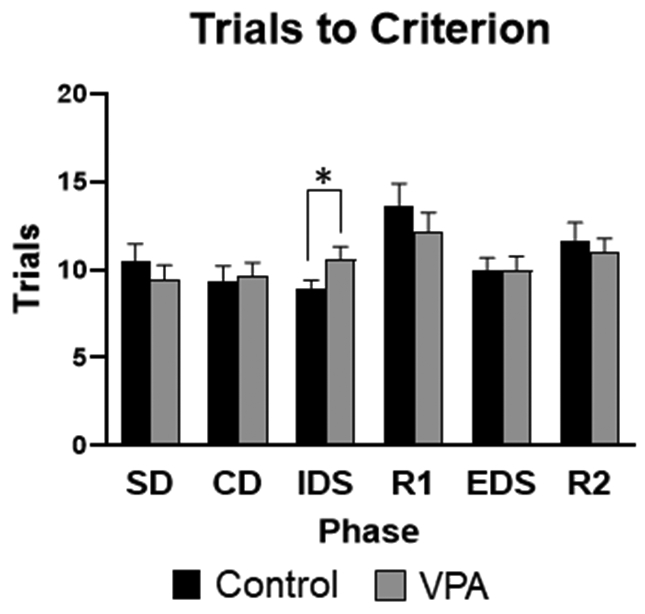
VPA animals were significantly worse on the IDS phase compared to control animals. *= significant group difference.
Figure 8: Set-shifting task performance, trials to criterion.
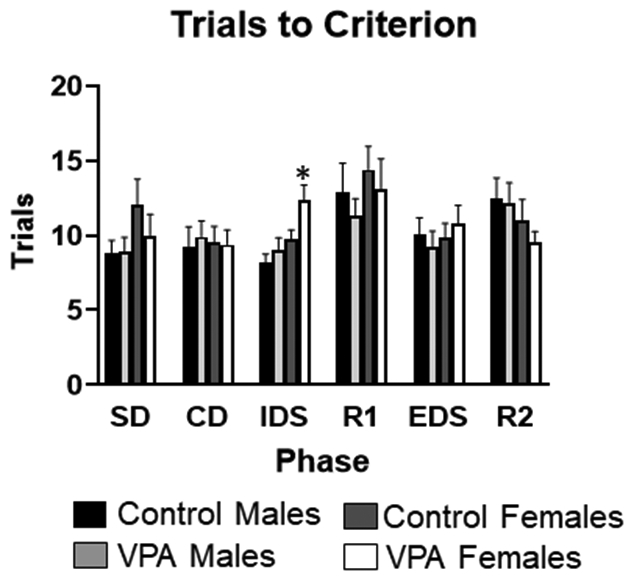
VPA females were significantly worse on the IDS phase compared to control females. *= significant group difference.
Figure 9: Set-shifting task, error trials.
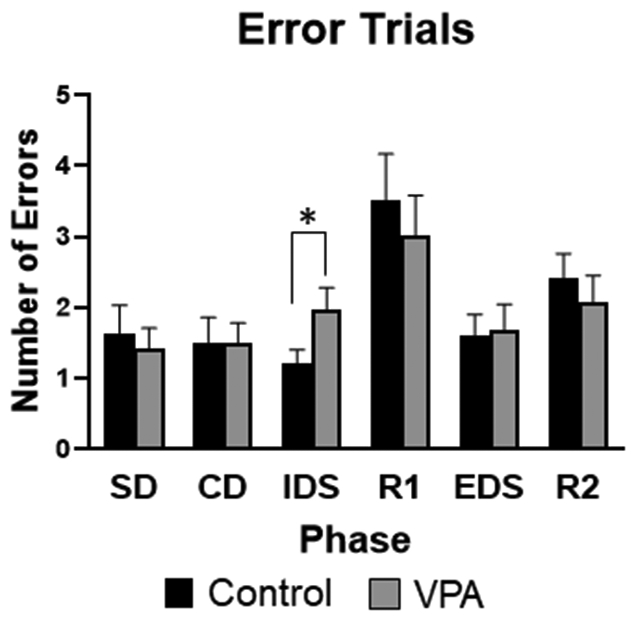
VPA rats made significantly more errors on the IDS phase compared to control animals. *= significant group differences
4.0. Discussion
The major result from these experiments was that female VPA rats were impaired on IDS trials and were also unable to form an attentional set. The main sex effect in the set-shifting task was primarily driven by deficits in the VPA females. This was supported by the fact that VPA females were worse than control females. Taken together these results suggest that female VPA animals have impaired learning at higher levels, when a shift of attention was involved, but not for basic learning such as during the simple or compound discriminations. These deficits also mirror some of the results from the human literature. For example, girls with ASD perform worse on WCST and the Tower of London task compared to boys with ASD 6,28. In another task, even when participants were able to learn the rules of the task and apply them, females had worse inhibition compared to males 29. Another recent study found females with ASD were impaired on the WCST task compared to males with ASD, and had more perseverative errors 30. A study conducted in young adults found that females with ASD had impaired executive functioning compared to males with ASD and that this contributed to real life social issues 32. These studies were behavioral but fMRI studies in humans suggest that for people with ASD there is altered frontal lobe anatomy and functionality during executive function tasks 44-47. This aligns with what is known from the animal literature where frontal areas such as the medial prefrontal cortex and the anterior cingulate mediate EDS and IDS performance 25,48. It is possible that there is altered frontal cortical structure in VPA animals that impairs performance and future studies will examine this relationship.
One result that was not statistically significant effect at the group level was for EDS deficits in VPA animals, even though VPA females did perform worse compared to VPA males. This may in part be due to lack of set-formation. Future studies will attempt to drive better learning by use another variant of the SST with multiple IDS stages. Overall, these results support some of the findings in humans, where female VPA rats were worse compared to male VPA rats on the set-shifting task, which provides evidence that the VPA model is capturing some of the cognitive deficits observed in humans with ASD.
In addition, fewer VPA animals (males and females) formed an attentional set. Forming an attentional set is usually provided as one way to assess how well an individual understands the current rules that are being implemented within the context of the task. There were also significantly more VPA rats that failed to complete the phases of the task. These results suggest a further impairment within VPA rats. Future studies will probe set formation by using a variant of the task with multiple IDS phases, to examine whether IDS deficits are different from set formation 49.
The additional behavioral tasks implicated some similar results to the prior literature examining VPA exposure. Decreases in VPA rats sniffing and climbing behavior is consistent with prior research 18-20,50,51. However, there were sex differences for a couple of behaviors including increased following, and decreased boxing in female VPA rats. Tentatively the increase in following behavior could be evidence of an altered social profile in female VPA rats. Another group has found differences in female VPA social behaviors, with more females hiding compared to males 20. Since hiding was not an option in this experiment, it is difficult to know how that would change the ratio of other behaviors. In human females with ASD, it has been suggested that they have a different social profile compared to males with ASD 52. In some instances, it has been described, as not a decrease in sociability, but an alteration in social interactions where they mimic social norms 34. Perhaps, the increase in following behavior is an aspect related to the different ASD symptomology of female VPA rats. Although, special caution is warranted as not all of the all of the female VPA social interactions were scored.
Lastly, there was a significant increase in self-grooming during social interactions of VPA treated rats. This suggests that for these Long-Evans rats the VPA exposure may have exacerbated a normal behavior making it into a repetitive behavior during social interactions. Perhaps in some situations excessive self-grooming is similar to repetitive behaviors that occur in ASD (i.e., hand flapping). Grooming also happens more often in Long-Evans rats compared to Sprague Dawley rats 40, which are typically studied in the VPA literature. The differences observed in this study could in part be due to strain differences interacting with VPA exposure.
Data from the three-chamber task suggests that VPA male rats exhibited some similarities to prior studies, where they spent more time than control rats in the room with the object. The index examining social behavior, which measured the time spent interacting with the cages, indicated that in general VPA rats were less social than controls. Whereas VPA males traveled frequently between rooms, the control male rats spent a significant amount of time in the middle chamber, avoiding both side rooms, and traveled the least compared to all groups. This data suggests that VPA males were interesting in exploring more than control animals and were more active, they traveled the most distance out of all of the groups. It also suggests there may be a floor effect, because the control males were not very social and preferred to sit in the middle room. For this task the VPA females were less affected and were not different from female controls in the time spent measures or in the distance traveled.
In the marble burying task, VPA animals buried significantly fewer marbles than controls, and females buried fewer than males. Additional scored behaviors during this task categorized VPA treated animals as having spent more time inactive or sitting in the corner of the apparatus. VPA rats also demonstrated less time digging. These results are different from previous studies where VPA rats exhibited more marble burying behavior 53 but the lack of digging behavior indicated that VPA rats were different from control animals.
Across the tasks control males less interested in exploration behaviors. During social interaction there was more of an impact of VPA exposure in female rats, in the 3-chamber task there appeared to be more impact on the VPA males. VPA exposure may cause different responses in male and female rats and these tasks test different behaviors. For instance, males interacted more with the novel rat cage in the first bin of the 3-chamber task but during the second bin sat in the middle chamber the longest. Future studies examining how social dynamic change across time in different environments may help elucidate sex and treatment differences. The 3-chamber task examines social novelty, but actual touching is not allowed, whereas the dyad interactions assess how rats physical touch, sniff and play. Just as humans have different social behaviors in different environments, rats exhibit different behavioral profiles when directly interacting with a rat versus exploring a novel environment.
In humans, the behavioral profile of females with ASD can differ from males, especially in regard to social behaviors 54. There were sex differences observed in the social interaction task, three chamber task, marble task, and the set-shifting task. Most of these differences indicated that the female VPA treated animals were different from male VPA or control animals. The altered social interaction and decreased ability to perform the set-shifting task suggest that in this strain of rat, female VPA treated rats may differ from male VPA treated rats and exhibit a different behavioral profile, with some shared characteristics (reduced sniffing for both). Taken together these results suggest that VPA females have a different phenotype compared to male VPA animals. Clinicians have suggested that there is a bias for diagnosing females with ASD 55 and that differences between the sexes need to be addressed 52. This work highlights the importance of understanding sex differences within VPA animals and provides a platform to further study brain differences associated with these cognitive changes that may underlie some of the sex differences.
One limitation of using the 6 phases of the set-shifting task was that it may have hampered the ability of rats to form an attentional set. Future research projects will adapt the multiple IDS version of the task 56 to attempt to strengthen the formation of an attentional set. Another limitation is that estrus phases were not tracked, which can also be addressed in future studies. Lastly, litter effects can influence data when multiple pups are used from the same litter. This study used many litters to increase power and decrease the likelihood of a specific litter driving an effect. In addition, examination of the residuals by litter demonstrated that the litters had equivalent variance outside of three litters that only had one pup complete set-shifting, which suggests that a litter effect had little impact on the results. Future studies will assign one male and one female pup to different experimental conditions to control for the litter effect.
4.1. Conclusions
Overall, these data suggest that VPA animals, especially the female VPA rats are impaired on the set-shifting task and that this animal model is capturing some of the cognitive deficits observed in humans. The VPA females exhibited impaired performance on the intra-dimensional shifts, impaired set formation and the worst performance on the extra-dimensional shift. In addition, female VPA rats exhibited altered social behaviors such as increased following, which aligns with humans with ASD having different social behavioral deficits compared to males with ASD. Future research will determine the effect that VPA condition has on areas of the brain important for cognitive flexibility such as the prefrontal cortex. Structural differences could account for the sex differences between male and female VPA treated rats. It will be important to assess neural changes between the VPA and control animals and to determine if any neural markers of VPA exposure relate to the cognitive changes observed here.
Acknowledgments
We would like to thank Adam Foxe and William DeCoteau for their mentorship and guidance and Parker Beer, Konnor Cook, and Alexa Pritchard for assistance in data collection.
Funding:
This project was supported by a grant from the National Institute of General Medical Science GM113109 of the National Institutes of Health NIGMS- GM113109 NIH for providing core lab support at the Cognitive and Neurobiological approaches to Plasticity (CNAP) Center. Start-up funds from Kansas State University and a USRG from KSU to BP.
References
- 1.Geurts HM, Corbett B & Solomon M The paradox of cognitive flexibility in autism. Trends Cogn. Sci 13, 74–82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demetriou EA et al. Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatry (2017) doi: 10.1038/mp.2017.75 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozonoff S, Pennington BF & Rogers SJ Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J. Child Psychol. Psychiatry 32, 1081–1106 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Hughes C, Russell J & Robbins TW Evidence for executive dysfunction in autism. Neuropsychologia 32, 477–493 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Merchan-Naranjo J et al. Executive function is affected in autism spectrum disorder, but does not correlate with intelligence. Rev. Psiquiatr. Salud Ment 9, 39–50 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Memari AH et al. Cognitive flexibility impairments in children with autism spectrum disorders: links to age, gender and child outcomes. Res. Dev. Disabil 34, 3218–3226 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Miller HL, Ragozzino ME, Cook EH, Sweeney JA & Mosconi MW Cognitive set shifting deficits and their relationship to repetitive behaviors in autism spectrum disorder. J. Autism Dev. Disord 45, 805–816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozonoff S et al. Performance on Cambridge neuropsychological test automated battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the Collaborative Programs of Excellence in Autism network. J. Autism Dev. Disord 34, 139–150 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Van Eylen L et al. Cognitive flexibility in autism spectrum disorder: Explaining the inconsistencies? Res. Autism Spectr. Disord 5, 1390–1402 (2011). [Google Scholar]
- 10.Mabunga DF, Gonzales EL, Kim JW, Kim KC & Shin CY Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol 24, 285–301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean JC et al. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J. Med. Genet 39, 251–260 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomiak T, Turner N & Hu B What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Patholog. Res. Int 2013, 712758 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen J et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. Jama 309, 1696–1704 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phiel CJ et al. Histone Deacetylase is a Direct Target of Valproic Acid, a Potent Anticonvulsant, Mood Stabilizer, and Teratogen. J. Biol. Chem 276, 36734–36741 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Hou Q et al. A developmental study of abnormal behaviors and altered GABAergic signaling in the VPA-treated rat model of autism. Front. Behav. Neurosci 12, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiavi S et al. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci 13, 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J et al. Increased expression of fatty acid synthase and acetyl-CoA carboxylase in the prefrontal cortex and cerebellum in the valproic acid model of autism. Exp. Ther. Med 12, 1293–1298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider T & Przewlocki R Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30, 80–90 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Markram K, Rinaldi T, La Mendola D, Sandi C & Markram H Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology 33, 901–913 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Edalatmanesh MA, Nikfarjam H, Vafaee F & Moghadas M Increased hippocampal cell density and enhanced spatial memory in the valproic acid rat model of autism. Brain Res. 1526, 15–26 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Kerr DM, Downey L, Conboy M, Finn DP & Roche M Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav. Brain Res 249, 124–133 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Amodeo DA, Jones JH, Sweeney JA & Ragozzino ME Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav. Brain Res 227, 64–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anshu K et al. Altered attentional processing in male and female rats in a prenatal valproic acid exposure model of autism spectrum disorder. Autism Res. (2017) doi: 10.1002/aur.1852 [doi]. [DOI] [PubMed] [Google Scholar]
- 24.Owen Adrian M, Roberts AC, Poley CE, Sahakian BJ, R. T. VERSUS INTRA-DIMENSIONAL SET SHIFTING PERFORMANCE FOLLOWING FRONTAL LOBE EXCISIONS , TEMPORAL LOBE EXCISIONS OR AMYGDALO- HIPPOCAMPECTOMY IN MAN. Neuropsychologia 29, 993–1006 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Birrell JM & Brown VJ Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci 20, 4320–4325 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yerys BE et al. Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism 13, 523–539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill EL & Bird CM Executive processes in Asperger syndrome: Patterns of performance in a multiple case series. Neuropsychologia 44, 2822–2835 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Hjelmquist E Nydn2000. 185, 180–185 (2000). [Google Scholar]
- 29.Lemon JM, Gargaro B, Enticott PG & Rinehart NJ Brief report: Executive functioning in autism spectrum disorders: A gender comparison of response inhibition. J. Autism Dev. Disord 41, 352–356 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Kiep M & Spek AA Executive functioning in men and women with an autism spectrum disorder. Autism Res. 10, 940–948 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Baio J et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morb. Mortal. Wkly. report.Surveillance Summ. (Washington, D.C. 2002) 67, 1–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White EI et al. Sex differences in parent-reported executive functioning and adaptive behavior in children and young adults with autism spectrum disorder. Autism Res. 10, 1653–1662 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bölte S, Duketis E, Poustka F & Holtmann M Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism 15, 497–511 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Lehnhardt FG et al. Sex-Related Cognitive Profile in Autism Spectrum Disorders Diagnosed Late in Life: Implications for the Female Autistic Phenotype. J. Autism Dev. Disord 46, 139–154 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Bambini-Junior V et al. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci. Lett 583, 176–182 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Bambini-Junior V et al. Animal model of autism induced by prenatal exposure to valproate: behavioral changes and liver parameters. Brain Res. 1408, 8–17 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Andropoulos DB Effect of Anesthesia on the Developing Brain : Infant and Fetus. 77030, 1–11 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Mikulecká A, Šubrt M, Parȓízková M, Mareš P & Kubová H Consequences of early postnatal benzodiazepines exposure in rats. II. social behavior. Front. Behav. Neurosci 8, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinaldi T, Silberberg G & Markram H Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb. cortex (New York, N.Y. 1991) 18, 763–771 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Ku KM, Weir RK, Silverman JL, Berman RF & Bauman MD Behavioral phenotyping of juvenile long-evans and sprague-dawley rats: Implications for preclinical models of autism spectrum disorders. PLoS One 11, 1–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tait DS & Brown VJ Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav. Brain Res 187, 100–109 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Bradshaw SE, Agster KL, Waterhouse BD & McGaughy JA Age-related changes in prefrontal norepinephrine transporter density: The basis for improved cognitive flexibility after low doses of atomoxetine in adolescent rats. Brain Res. 1641, 245–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olexová L, Štefánik P & Kršková L Increased anxiety-like behaviour and altered GABAergic system in the amygdala and cerebellum of VPA rats — An animal model of autism. Neurosci. Lett 629, 9–14 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Agam Y, Joseph RM, Barton JJ & Manoach DS Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage 52, 336–348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barttfeld P et al. State-dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder. Neuropsychologia 50, 3653–3662 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Gilbert SJ, Bird G, Brindley R, Frith CD & Burgess PW Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia 46, 2281–2292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitz N et al. Neural correlates of executive function in autistic spectrum disorders. Biol. Psychiatry 59, 7–17 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Ng CW, Noblejas MI, Rodefer JS, Smith CB & Poremba A Double dissociation of attentional resources: prefrontal versus cingulate cortices. J. Neurosci 27, 12123–12132 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tait DS, Bowman EM, Neuwirth LS & Brown VJ Assessment of intradimensional/extradimensional attentional set-shifting in rats. Neurosci. Biobehav. Rev 89, 72–85 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Morakotsriwan N, Wattanathorn J, Kirisattayakul W & Chaisiwamongkol K Autistic-Like Behaviors, Oxidative Stress Status, and Histopathological Changes in Cerebellum of Valproic Acid Rat Model of Autism Are Improved by the Combined Extract of Purple Rice and Silkworm Pupae. Oxid. Med. Cell. Longev 2016, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuevas-Olguin R et al. Cerebrolysin prevents deficits in social behavior, repetitive conduct, and synaptic inhibition in a rat model of autism. J. Neurosci. Res 95, 2456–2469 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Halladay AK et al. Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 6, 1–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi CS et al. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci. Rep 6, 36250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai MC et al. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One 6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loomes R, Hull L & Mandy WPL What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Chase EA, Tait DS & Brown VJ Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur. J. Neurosci 36, 2368–2376 (2012). [DOI] [PubMed] [Google Scholar]


