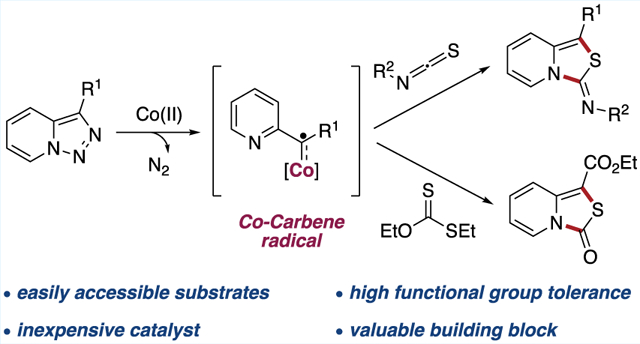
Abstract
An efficient radical transannulation reaction of pyridotriazoles with isothiocyanates and xanthate esters was developed. This method features conversion of pyridotriazoles into two N-fused heterocyclic aromatic systems—imino-thiazolopyridines and oxo-thiazolopyridine derivatives—via one-step Co(II)-catalyzed transannulation reaction proceeding via a radical mechanism. The synthetic usefulness of the developed method was illustrated in the synthesis of amino acid derivatives and further transformations of obtained reaction products.
Graphical Abstract
Heteroaromatic rings are paramount motifs of drugs and bioactive molecules.1 Thus, not surprisingly that, in drug discovery research, the development of novel synthetic approaches for the rapid construction of new heterocyclic ring systems from easily available precursors is of central importance.2 Recently, transition-metal-catalyzed denitrogenative transformations of pyridotriazoles have been emerging as a powerful tool for the synthesis of diverse N-heterocyclic frameworks.3,4 These protocols take advantage of the well-known ring-chain tautomerism of the pyridotriazole core in solution into the corresponding diazo tautomer A, which then can be trapped by a transition-metal catalyst to form the reactive pyridyl metal carbene intermediate B (see Scheme 1a). First reported in 2007,5 the transannulation reaction of pyridotriazoles was then quickly extended to other transannulations,6 as well as X–H insertions,7 cyclopropanation,8 and other reactions.9 These protocols operate through an ionic pathway, involving transition-metal carbene intermediate B.
Scheme 1.

Concepts for Transannulation of Pyridotriazoles
Recently, metalloradical catalysis employing open-shell metalloradical complexes has been introduced as a conceptually new strategy featuring alternative trends in reactivity and selectivity. Of particular importance are stable metalloradicals of cobalt(II) porphyrins ([Co(Por)]), which form strong metal-carbon single bonds, thus behaving as carbene radical surrogates.10 Lately, a rich chemistry of Co(II)-based metalloradicals toward a variety of useful synthetic transformations has been developed.11,12 Surprisingly, engagement of this strategy in denitrogenative transannulation reaction is scarce.13 In continuation of our studies on application of pyridotriazoles in synthesis of nitrogen-containing heterocycles,5a–e herein, we report a Co-catalyzed denitrogenative transannulation of pyridotriazoles with isothiocyanates and xanthate esters toward the synthesis of two N-heterocyclic aromatic systems proceeding via carbene radical intermediate C (see Scheme 1b).
First, transannulation of pyridotriazole 1a and t-butyl isothiocyanate 2a14 en route to imino-thiazolopyridine 3a was examined in the presence of several synthesized metal-porphyrin-based catalysts (Table 1). It was found that Co(TPP) (TPP = tetraphenyl porphyrin) was a superior catalyst delivering 3 in 72% isolated yield (Table 1, entry 1). Employment of other Co-porphyrin-based catalysts was less efficient (Table 1, entries 2 and 3), whereas use of other Fe- and Ru-porphyrin-based catalysts was inefficient (Table 1, entries 4–6).
Table 1.
Optimization of Reaction Conditionsa
 | ||
|---|---|---|
| entry | deviation from standard conditions | yield of 3ab (%) |
| 1 | none | 72c |
| 2 | Co(F20TPP) | 64 |
| 3 | Co[(p-OMe)4TPP] | 48 |
| 4 | Fe(TPP)Cl | 0d |
| 5 | Fe(F20TPP)Cl | 0d |
| 6 | Ru(TPP)CO | 0d |
0.05 mmol scale; 1:2 = 1:5.
Determined by gas chromatography/mass spectroscopy (GC/MS), using pentadecane as an internal standard with a catalyst loading of 8 mol %.
0.2 mmol scale, isolated yields.
Pyridotriazole 1a was mostly recovered.
Next, the generality of this methodology was examined. With regard to the pyridotriazole component, several differently substituted heterocycles at C5 and C6 turned out to be capable substrates for transannulation with t-butyl isothiocyanate (2a) (Scheme 2). Thus, halogenated and methylated pyridotriazoles reacted smoothly to give the corresponding imino-thiazolopyridine products 3b–3f in good to excellent yields. Employment of methyl ester-containing substrate 3g and thiophene-containing pyridotriazole 3i posed no problem. Notably, this reaction was equally efficient with N-fused pyrazinotriazole to deliver imino-thiazolopyrazine derivative 3h in high yield. This reaction also appeared to be very general for isothiocyanates (see Scheme 3). A variety of functional groups was tolerated at the para-position of arylisothiocyanates, providing the corresponding products 3k–3p in good to excellent yields. Similarly, transannulation of isothiocyanates possessing substituents at the meta and ortho positions of the arene proceeded uneventfully (3q–3u). Benzodioxolyl- and naphthyl-containing isothiocyanates smoothly underwent transannulation, affording 3v and 3w in good yields. In addition, it was found that alkyl isothiocyanates also are capable partners for this transformation. Thus, isothiocyanates possessing various benzyl and alkyl groups all reacted well, providing products 3x–3z in good to high yields. Moreover, this reaction chemoselectively gave transannulation products 3aa with allyl-containing isothiocyanate, the double bond moiety of which was not compromised. Notably, chiral isothiocyanates could also be efficiently employed in this reaction. Thus, isothiocyanates derived from amino acid esters smoothly underwent transannulation reaction to produce derivatives 3ab–3ad in moderate yields.
Scheme 2.

Scope of Pyridotriazolesa
a0.2 mmol scale, isolated yields. See the Supporting Information for experimental details. bReaction was performed in 1 mmol scale. cToluene (0.5 M) used as a solvent.
Scheme 3.

Scope of Isothiocyanatesa
a0.2 mmol scale, isolated yields. See Supporting Information for experimental details. bToluene (0.5 M) used as a solvent.
Encouraged by the successful transannulation of pyridotriazoles with isothiocyanates, we examined reactivity of carbonyl sulfide (OCS) in this transformation (Scheme 4). Because of its gaseous nature, carbonyl sulfides are experimentally difficult to handle. Hence, xanthate ester, which is a stable precursor of carbonyl sulfide that is produced via Chugaev elimination, was examined.15 Gratifyingly, xanthate ester successfully underwent transannulation reactions with pyridotriazoles in the presence of 12 mol % Co(TPP) catalyst, providing oxo-thiazolopyridine derivatives 5a–5f in moderate yields.
Scheme 4.

Scope of Transannulation with Xanthate Estera a0.2 mmol scale, isolated yields.
It was also shown that the tert-butyl group at imino-thiazolopyridine 3a can easily be removed (under nonoptimized conditions) to access 6 possessing an N–H moiety, which can routinely be further functionalized, for instance, into benzoylated derivative 7 (see Scheme 5). Note that the latter cannot be accessed directly via the transannulation reaction of acylated isothiocyanates under the reaction conditions tested. It is believed that 6 can serve as a convenient synthon for a modular one-step synthesis of library of N-substituted imino-thiazolopyridines.
Scheme 6.

Radical Scavenging Experiment Used to Trap the Co(III)-Carbene Radical Intermediate
Scheme 5.

Product Transformations
The radical nature of this transformation was validated by the following radical trapping experiment (see Scheme 6). The reaction in the presence of dibenzoyl peroxide 8 resulted in the formation of benzoyloxy-containing product 9, thus supporting involvement of the carbene radical intermediate C.
On the basis of the above study and literature reports, the following plausible mechanism for this transannulation reaction is proposed. Xanthate ester 4, which is obtained via Chugaev elimination, acts as a carbonyl sulfide (12) surrogate in this transformation (see Scheme 7a). Pyridotriazole 1 exist in equilibrium with its open diazo tautomer A, which, upon denitrogenative reaction with Co(II)-based metalloradical catalyst D, produces a key α-Co(III)-pyridyl radical intermediate C (Scheme 7b). A subsequent trapping of this eletrophilc radical with isothiocyanate 216 or the in-situ-generated carbonyl sulfide 12 produces radical intermediate E, which, upon radical cyclization, leads to imino-thiazolopyridine 3 or oxo-thiazolopyridines 5 and regenerates the Co catalyst.
Scheme 7.

Proposed Radical Activation Mechanism
In summary, we have developed general and efficient Co(II)-catalyzed radical transannulation reactions of pyridotriazoles with isothiocyanates and carbonyl sulfide. This operationally simple protocol exhibits wide functional-group tolerance, efficiently producing N-fused imino-thiazolopyridines and oxothiazolopyridines.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institute of Health (No. GM120281), National Science Foundation (No. CHE-1955663), and Welch Foundation (Chair, No. AT-0041) for financial support. We are also thankful to Prof. Sheena D’Arcy for help with HRMS analysis.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c03099.
Experimental procedures, optimization process, characterization data, and 1H and 13C NMR spectra of all new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Pozharskii AF; Soldatenkov AT; Katritzky AR Heterocycles in Life and Society; Wiley: New York, 1997. [Google Scholar]; (b) Balaban AT; Oniciu DC; Katritzky AR Chem. Rev 2004, 104, 2777–2812. [DOI] [PubMed] [Google Scholar]
- (2).For the importance of new heterocyclic scaffolds for medicinal chemistry, see:; Pitt WR; Parry DM; Perry BG; Groom CR J. Med. Chem 2009, 52, 2952–2963. [DOI] [PubMed] [Google Scholar]
- (3).For reviews on reactivity of pyridotriazoles, see:; (a) Chattopadhyay B; Gevorgyan V Angew. Chem., Int. Ed 2012, 51, 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anbarasan P; Yadagiri D; Rajasekar S Synthesis 2014, 46, 3004–3023. [Google Scholar]; (c) Filippov IP; Titov GD; Rostovskii NV Synthesis 2020, DOI: 10.1055/s-0040-1707254. [DOI] [Google Scholar]; (d) Yadagiri D; Rivas M; Gevorgyan VJ Org. Chem 2020, 85, 11030–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For reviews on reactivity of N-sulfonyl triazoles, see:; (a) Gulevich AV; Gevorgyan V Angew. Chem., Int. Ed 2013, 52, 1371–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Davies HML; Alford JS Chem. Soc. Rev 2014, 43, 5151–5162. [DOI] [PubMed] [Google Scholar]; (c) Jiang Y; Sun R; Tang X-Y; Shi M Chem. - Eur. J 2016, 22, 17910–17924. [DOI] [PubMed] [Google Scholar]; (d) Li W; Zhang J Chem. - Eur. J 2020, 26, DOI: 10.1002/chem.202085262. [DOI] [Google Scholar]
- (5).For the first report on transannulation of pyridotriazoles, see:; (a) Chuprakov S; Hwang FW; Gevorgyan V Angew. Chem., Int. Ed 2007, 46, 4757–4759. Also see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chuprakov S; Gevorgyan V Org. Lett 2007, 9, 4463–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shi Y; Gulevich AV; Gevorgyan V Angew. Chem., Int. Ed 2014, 53, 14191–14195. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Helan V; Gulevich AV; Gevorgyan V Chem. Sci 2015, 6, 1928–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shi Y; Gevorgyan V Chem. Commun 2015, 51, 17166–17169. [DOI] [PubMed] [Google Scholar]; (f) Kim JH; Gensch T; Zhao D; Stegemann L; Strassert CA; Glorius F Angew. Chem., Int. Ed 2015, 54, 10975–10979. [DOI] [PubMed] [Google Scholar]; (g) Adam R; Alom S; Abarca B; Ballesteros R Tetrahedron 2016, 72, 8436–8441. [Google Scholar]; (h) Joshi A; Chandra Mohan D; Adimurthy S Org. Lett 2016, 18, 464–467. [DOI] [PubMed] [Google Scholar]; (i) Jeon WH; Son J-Y; Kim JE; Lee PH Org. Lett 2016, 18, 3498–3501. [DOI] [PubMed] [Google Scholar]; (j) Joshi A; Mohan DC; Adimurthy SJ Org. Chem 2016, 81, 9461–9469. [DOI] [PubMed] [Google Scholar]; (k) Kim H; Kim S; Kim J; Son J-Y; Baek Y; Um K; Lee PH Org. Lett 2017, 19, 5677–5680. [DOI] [PubMed] [Google Scholar]; (l) Zhang G-T; Zhang J; Xu Y-J; Dong L Eur. J. Org. Chem 2018, 2018, 4197–4201. [Google Scholar]; (m) Zhang Z; Yadagiri D; Gevorgyan V Chem. Sci 2019, 10, 8399–8404. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Joshi A; Semwal R; Suresh E; Adimurthy S Chem. Commun 2019, 55, 10888–10891. [DOI] [PubMed] [Google Scholar]; (o) Xu H-B; Zhu Y-Y; Dong L J. Org. Chem 2019, 84, 16286–16292. [DOI] [PubMed] [Google Scholar]; (p) Dong C; Wang X; Pei Z; Shen R Org. Lett 2019, 21, 4148–4152. [DOI] [PubMed] [Google Scholar]; (q) Dong Y; Chen J; Cui Y; Bao L; Xu H Org. Lett 2020, 22, 772–775. [DOI] [PubMed] [Google Scholar]; (r) Wang H; Cai S; Ai W; Xu X; Li B; Wang B Org. Lett 2020, 22, 7255–7260. [DOI] [PubMed] [Google Scholar]
- (6).For selected reports on transannulation of N-sulfonyl triazoles, see:; (a) Horneff T; Chuprakov S; Chernyak N; Gevorgyan V; Fokin VV J. Am. Chem. Soc 2008, 130, 14972–14974. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miura T; Yamauchi M; Murakami M Chem. Commun 2009, 1470–1471. [DOI] [PubMed] [Google Scholar]; (c) Chattopadhyay B; Gevorgyan V Org. Lett 2011, 13, 3746–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Alford JS; Spangler JE; Davies HML J. Am. Chem. Soc 2013, 135, 11712–11715. [DOI] [PubMed] [Google Scholar]; (e) Miura T; Hiraga K; Biyajima T; Nakamuro T; Murakami M Org. Lett 2013, 15, 3298–3301. [DOI] [PubMed] [Google Scholar]; (f) Miura T; Tanaka T; Hiraga K; Stewart SG; Murakami MJ Am. Chem. Soc 2013, 135, 13652–13655. [DOI] [PubMed] [Google Scholar]; (g) Parr BT; Davies HML Angew. Chem., Int. Ed 2013, 52, 10044–10047. [DOI] [PubMed] [Google Scholar]; (h) Parr BT; Green SA; Davies HML J. Am. Chem. Soc 2013, 135, 4716–4718. [DOI] [PubMed] [Google Scholar]; (i) Schultz EE; Sarpong RJ Am. Chem. Soc 2013, 135, 4696–4699. [DOI] [PubMed] [Google Scholar]; (j) Shi Y; Gevorgyan V Org. Lett 2013, 15, 5394–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Spangler JE; Davies HML J. Am. Chem. Soc 2013, 135, 6802–6805. [DOI] [PubMed] [Google Scholar]; (l) Zibinsky M; Fokin VV Angew. Chem., Int. Ed 2013, 52, 1507–1510. [DOI] [PubMed] [Google Scholar]; (m) Chen K; Zhu Z-Z; Zhang Y-S; Tang X-Y; Shi M Angew. Chem., Int. Ed 2014, 53, 6645–6649. [DOI] [PubMed] [Google Scholar]; (n) Kim C-E; Park S; Eom D; Seo B; Lee PH Org. Lett 2014, 16, 1900–1903. [DOI] [PubMed] [Google Scholar]; (o) Medina F; Besnard C; Lacour J Org. Lett 2014, 16, 3232–3235. [DOI] [PubMed] [Google Scholar]; (p) Miura T; Funakoshi Y; Murakami MJ Am. Chem. Soc 2014, 136, 2272–2275. [DOI] [PubMed] [Google Scholar]; (q) Shang H; Wang Y; Tian Y; Feng J; Tang Y Angew. Chem., Int. Ed 2014, 53, 5662–5666. [DOI] [PubMed] [Google Scholar]; (r) Yang J-M; Zhu C-Z; Tang X-Y; Shi M Angew. Chem., Int. Ed 2014, 53, 5142–5146. [DOI] [PubMed] [Google Scholar]; (s) Lindsay VNG; Viart HMF; Sarpong RJ Am. Chem. Soc 2015, 137, 8368–8371. [DOI] [PubMed] [Google Scholar]; (t) Cheng W; Tang Y; Xu Z-F; Li C-Y Org. Lett 2016, 18, 6168–6171. [DOI] [PubMed] [Google Scholar]; (u) Chen W; Bai Y-L; Luo Y-C; Xu P-F Org. Lett 2017, 19, 364–367. [DOI] [PubMed] [Google Scholar]; (v) Fu L; Davies HML Org. Lett 2017, 19, 1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]; (w) Li Y; Zhang R; Ali A; Zhang J; Bi X; Fu J Org. Lett 2017, 19, 3087–3090. [DOI] [PubMed] [Google Scholar]; (x) Yadagiri D; Chaitanya M; Reddy ACS; Anbarasan P Org. Lett 2018, 20, 3762–3765. [DOI] [PubMed] [Google Scholar]
- (7).For selected reports on X–H insertions of N-sulfonyl triazoles, see:; (a) Chuprakov S; Malik JA; Zibinsky M; Fokin VV J. Am. Chem. Soc 2011, 133, 10352–10355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miura T; Biyajima T; Fujii T; Murakami MJ Am. Chem. Soc 2012, 134, 194–196. [DOI] [PubMed] [Google Scholar]; (c) Miura T; Tanaka T; Biyajima T; Yada A; Murakami M Angew. Chem., Int. Ed 2013, 52, 3883–3886. [DOI] [PubMed] [Google Scholar]; (d) Chuprakov S; Worrell BT; Selander N; Sit RK; Fokin VV J. Am. Chem. Soc 2014, 136, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Miura T; Zhao Q; Murakami M Angew. Chem., Int. Ed 2017, 56, 16645–16649. [DOI] [PubMed] [Google Scholar]
- (8).For selected reports on cyclopropanation of N-sulfonyl triazoles, see:; (a) Chuprakov S; Kwok SW; Zhang L; Lercher L; Fokin VV J. Am. Chem. Soc 2009, 131, 18034–18035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Grimster N; Zhang L; Fokin VV J. Am. Chem. Soc 2010, 132, 2510–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Culhane JC; Fokin VV Org. Lett 2011, 13, 4578–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Alford JS; Davies HML Org. Lett 2012, 14, 6020–6023. [DOI] [PubMed] [Google Scholar]
- (9).For selected transformations on N-sulfonyl triazoles, see:; (a) Selander N; Worrell BT; Chuprakov S; Velaparthi S; Fokin VV J. Am. Chem. Soc 2012, 134, 14670–14673. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yadagiri D; Reddy ACS; Anbarasan P Chem. Sci 2016, 7, 5934–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bosmani A; Guarnieri-Ibáñez A; Goudedranche S; Besnard C; Lacour J Angew. Chem., Int. Ed 2018, 57, 7151–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li F; Pei C; Koenigs RM Org. Lett 2020, 22, 6816–6821. [DOI] [PubMed] [Google Scholar]
- (10).(a) Ikeno T; Iwakura I; Yabushita S; Yamada T Org. Lett 2002, 4, 517–520. [DOI] [PubMed] [Google Scholar]; (b) Ikeno T; Iwakura I; Yamada TJ Am. Chem. Soc 2002, 124, 15152–15153. [DOI] [PubMed] [Google Scholar]; (c) Dzik WI; Xu X; Zhang XP; Reek JNH; de Bruin BJ Am. Chem. Soc 2010, 132, 10891–10902. [DOI] [PubMed] [Google Scholar]; (d) Belof JL; Cioce CR; Xu X; Zhang XP; Space B; Woodcock HL Organometallics 2011, 30, 2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Dzik WI; Zhang XP; de Bruin B Inorg. Chem 2011, 50, 9896–9903. [DOI] [PubMed] [Google Scholar]; (f) Lu H; Dzik WI; Xu X; Wojtas L; de Bruin B; Zhang XP J. Am. Chem. Soc 2011, 133, 8518–8521. [DOI] [PubMed] [Google Scholar]
- (11).(a) Huang L; Chen Y; Gao G-Y; Zhang XP J. Org. Chem 2003, 68, 8179–8184. [DOI] [PubMed] [Google Scholar]; (b) Chen Y; Fields KB; Zhang XP J. Am. Chem. Soc 2004, 126, 14718–14719. [DOI] [PubMed] [Google Scholar]; (c) Chen Y; Zhang XP J. Org. Chem 2004, 69, 2431–2435. [DOI] [PubMed] [Google Scholar]; (d) Chen Y; Ruppel JV; Zhang XP J. Am. Chem. Soc 2007, 129, 12074–12075. [DOI] [PubMed] [Google Scholar]; (e) Zhu S; Perman JA; Zhang XP Angew. Chem., Int. Ed 2008, 47, 8460–8463. [DOI] [PubMed] [Google Scholar]; (f) Zhu S; Ruppel JV; Lu H; Wojtas L; Zhang XP J. Am. Chem. Soc 2008, 130, 5042–5043. [DOI] [PubMed] [Google Scholar]; (g) Ruppel JV; Gauthier TJ; Snyder NL; Perman JA; Zhang XP Org. Lett 2009, 11, 2273–2276. [DOI] [PubMed] [Google Scholar]; (h) Zhu S; Xu X; Perman JA; Zhang XP J. Am. Chem. Soc 2010, 132, 12796–12799. [DOI] [PubMed] [Google Scholar]; (i) Xu X; Lu H; Ruppel JV; Cui X; Lopez de Mesa S; Wojtas L; Zhang XP J. Am. Chem. Soc 2011, 133, 15292–15295. [DOI] [PubMed] [Google Scholar]; (j) Cui X; Xu X; Wojtas L; Kim MM; Zhang XP J. Am. Chem. Soc 2012, 134, 19981–19984. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Jin L-M; Xu X; Lu H; Cui X; Wojtas L; Zhang XP Angew. Chem., Int. Ed 2013, 52, 5309–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Ruppel JV; Cui X; Xu X; Zhang XP Org. Chem. Front 2014, 1, 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Cui X; Xu X; Jin L-M; Wojtas L; Zhang XP Chem. Sci 2015, 6, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Jiang H; Lang K; Lu H; Wojtas L; Zhang XP J. Am. Chem. Soc 2017, 139, 9164–9167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Wang Y; Wen X; Cui X; Wojtas L; Zhang XP J. Am. Chem. Soc 2017, 139, 1049–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Xu X; Wang Y; Cui X; Wojtas L; Zhang XP Chem. Sci 2017, 8, 4347–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Wang Y; Wen X; Cui X; Zhang XP J. Am. Chem. Soc 2018, 140, 4792–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Wen X; Wang Y; Zhang XP Chem. Sci 2018, 9, 5082–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Hu Y; Lang K; Tao J; Marshall MK; Cheng Q; Cui X; Wojtas L; Zhang XP Angew. Chem., Int. Ed 2019, 58, 2670–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Paul ND; Chirila A; Lu H; Zhang XP; de Bruin B Chem. - Eur. J 2013, 19, 12953–12958. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Paul ND; Mandal S; Otte M; Cui X; Zhang XP; de Bruin BJ Am. Chem. Soc 2014, 136, 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Das BG; Chirila A; Tromp M; Reek JNH; de Bruin BJ Am. Chem. Soc 2016, 138, 8968–8975. [DOI] [PubMed] [Google Scholar]; (d) te Grotenhuis C; Das BG; Kuijpers PF; Hageman W; Trouwborst M; de Bruin B Chem. Sci 2017, 8, 8221–8230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) te Grotenhuis C; van den Heuvel N; van der Vlugt JI; de Bruin B Angew. Chem., Int. Ed 2018, 57, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chirila A; van Vliet KM; Paul ND; de Bruin B Eur. J. Inorg. Chem 2018, 2018, 2251–2258. [Google Scholar]; (g) Karns AS; Goswami M; de Bruin B Chem. - Eur. J 2018, 24, 5253–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lankelma M; Olivares AM; de Bruin B Chem. - Eur. J 2019, 25, 5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zhou M; Lankelma M; van der Vlugt JI; de Bruin B Angew. Chem., Int. Ed 2020, 59, 11073–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Roy S; Das SK; Chattopadhyay B Angew. Chem., Int. Ed 2018, 57, 2238–2243. [DOI] [PubMed] [Google Scholar]
- (14).For Rh-catalyzed transannulation of N-sulfonyl triazoles with heterocumulenes by Fokin and co-workers, see:; Chuprakov S; Kwok SW; Fokin VV J. Am. Chem. Soc 2013, 135, 4652–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Tschugaeff L Ber. Dtsch. Chem. Ges 1899, 32, 3332–3335. [Google Scholar]
- (16).For selectivity of addition of electrophilic and nucleophilic radicals to isothiocyanates, see:; Weragoda GK; Pilkington RL; Polyzos A; O’Hair RAJ Org. Biomol. Chem 2018, 16, 9011–9020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


