Abstract
The human X-linked zinc finger MYM-type protein 3 (ZMYM3) contains the longest GA-STR identified across protein-coding gene 5′ UTR sequences, at 32-repeats. This exceptionally long GA-STR is located at a complex string of GA-STRs with a human-specific formula across the complex as follows: (GA)8-(GA)4-(GA)6-(GA)32 (ZMYM3-207 ENST00000373998.5). ZMYM3 was previously reported among the top three genes involved in the progression of late-onset Alzheimer’s disease. Here we sequenced the ZMYM3 GA-STR complex in 750 human male subjects, consisting of late-onset neurocognitive disorder (NCD) as a clinical entity (n = 268) and matched controls (n = 482). We detected strict monomorphism of the GA-STR complex, except of the exceptionally long STR, which was architecturally skewed in respect of allele distribution between the NCD cases and controls [F (1, 50) = 12.283; p = 0.001]. Moreover, extreme alleles of this STR at 17, 20, 42, and 43 repeats were detected in seven NCD patients and not in the control group (Mid-P exact = 0.0003). A number of these alleles overlapped with alleles previously found in schizophrenia and bipolar disorder patients. In conclusion, we propose selective advantage for the exceptional length of the ZMYM3 GA-STR in human, and its link to a spectrum of diseases in which major cognition impairment is a predominant phenotype.
Subject terms: Evolution, Genetics, Molecular biology, Neuroscience
Introduction
Human-specific characteristics and phenotypes such as late-onset neurocognitive disorder (NCD) (also known as dementia) are likely to be the consequence or by-product of human-specific evolutionary events. In agreement with the above model, recent emerging evidence indicates that signals of brain evolution in anatomically modern humans are strongly related to the Alzheimer disease (AD) pathways1. Remarkably, certain human-specific derived alleles protect against post-reproductive cognitive decline2.
In comparison to single nucleotide substitutions, short tandem repeats (STRs) offer a significantly more versatile reservoir of genetic variations that may be necessary for speciation and species-specific phenotypes3. Following a genome-scale analysis of all human protein- coding genes annotated in the GeneCards database, we previously reported a catalog of genes containing “exceptionally long” STRs (> 5 repeats) in their core promoters4,5 and the 5′ untranslated region (UTR)6. The emerging comparative and functional analyses of a number of the identified STRs support adaptive evolutionary patterns for the expansion of a number of these STRs3,7, and the co-occurrence of alleles at the extreme ends of these STRs with major human cognitive disorders, including schizophrenia (SCZ), bipolar disorder (BPD) and late-onset NCD8–12 .In line with the above findings, recent reports indicate that STR length influences expression quantitative trait loci (eQTL) associations13.
In the category of GA-STRs, the zinc finger MYM-type containing 3 (ZMYM3) gene contains the longest annotated 5′ UTR GA-STR at 32-repeats6, which is part of a complex of four consecutive GA-STRs of human-specific formula across the complex (ZMYM3-207 ENST00000373998.5) (Table 1)8. ZMYM3 is located at Xq13.1, and encodes a zinc-finger protein, which is a component of histone deacetylase-containing multiprotein complexes that function through modifying chromatin structure to keep genes silent14.
Table 1.
Across-species landscape of the ZMYM3 GA-STR complex.
| Species | GA-STR complex formula |
|---|---|
| Human | 8-4-6-32 |
| Bonobo | 5-4-6-11 |
| Chimpanzee | 5-4-6-12-16 |
| Orangutan | 4-4-4-13 |
| Drill | 5 |
| Olive baboon | 5-4-4 |
| Macaque | 5-4 |
| Golden snub-nosed monkey | 5-4-5 |
| Marmoset | 4-4-4-18 |
| Bushbaby | – |
| Capuchin | 4 |
| Black snub-nosed monkey | – |
| Tarsier | 4 |
| Mouse Lemur | 4-4 |
| Chinese hamster | – |
| Ferret | 8-4-15 |
| Guinea pig | – |
| Platypus | – |
| Panda | 11-4-4 |
| Goat | – |
| Lion | 8-5-5 |
| Elephant | – |
| Mouse | – |
| Arabian camel | – |
| Armadillo | – |
| Cat | 6-4-6 |
| Dog | 20 |
| Mega bat | 10-5 |
| Rabbit | 6 |
| Cow | – |
| Chicken | – |
ZMYM3 was previously reported among the top three genes involved in the progression of late-onset AD15. Several alternatively spliced transcript variants have been found for this gene, of which the variant containing the exon 1 5′UTR is specifically expressed in the brain16, and spans the GA-STR complex6. Disruption of this GA-STR complex was reported in a X:13 translocation in a case of X-linked mental retardation17. More recently, deleterious mutations in the coding sequence of this gene were reported in conjunction with X-linked intellectual disability in a Finish family by X-exome sequencing18.
Here we sequenced the ZMYM3 GA-STR complex in late-onset neurocognitive disorder (NCD) patients and matched controls. This investigation was founded on the following facts: the role of ZMYM3 as one of the top three genes involved in the progression of late-onset AD, exceptional length of the STR in human and human-specificity of the STR complex formula in which this STR is located, a link between this STR and instances of cognition deficit (a property that is severely compromised in NCD), its predominant expression in the human brain, and proximity to the + 1 TSS.
Materials and methods
Subjects
Seven hundred fifty unrelated Iranian male subjects (age ≥ 60 years), consisting of late-onset NCD patients (n = 268) and controls (n = 482) were recruited from the provinces of Qazvin and Rasht. All patients were included based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) for NCD. In each participant, the Persian version of the Abbreviated Mental Test Score (AMTS) was implemented (inclusion criteria: AMTS < 7), medical history was taken, complemented by CT-scans in a number of instances (Suppl. 1). The control group was selected based on AMTS of ≥ 8, and history in all subjects, and normal CT-scan where possible. The AMTS is currently one of the most accurate primary screening instruments to increase the probability of NCD20. The Persian version of the AMTS is a valid cognitive assessment tool for older Iranian adults and can be reliably used for NCD screening in Iran, with an over 90% sensitivity19. The cases and controls were matched based on age and residential district. Informed consent was obtained from the subjects (informed consent was obtained from the guardians of all the subjects where necessary) and their identities remained confidential throughout the study. This research was approved by the Ethics Committee of the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran, and was consistent with the principles outlined in an internationally recognized standard for the ethical con duct of human research.
Statistical analysis
The chi-squared test was used to compare the distribution of each allele between the control and NCD groups. The Mid-P exact test was used for the alleles detected at the extreme ends of the allele distribution curve, which were detected in the NCD patients, and not in the controls in this study and two previous studies of SCZ and BPD8,9. Levene's test was used to assess the equality of variances of allelic distribution for the two groups.
Allele/genotype analysis of the ZMYM3 gene GA-STR complex
Genomic DNA was obtained from peripheral blood using a standard precipitation method, and PCR was carried out as previously described8. Briefly, PCRs were performed in a thermocycler (peqSTAR) under the following conditions: 94 °C for 4 min, followed by 40 cycles including denaturing at 94 °C for 30 s, annealing for 30 s at 63 °C, and extension at 72 °C for 30 s. A final extension was conducted at 72 °C for 5 min. All samples were sequenced for the ZMYM3 GA-complex using an ABI PRISM 377 DNA sequencer.
Results
Variation status of the ZMYM3 GA-STR complex in the human subjects studied
The complex in which the exceptionally long GA-STR (32-repeat) is located consists of four consecutive GA-STRs with the 8-4-6-32 formula in human8. The 8-4-6 formula was found to be monomorphic across the 750 human subjects studied. The exceptionally long GA-STR, however, was polymorphic in the human subjects studied. The 8-4-6-32 formula was human-specific when screened in 31 species encompassing various orders, including Primates, Rodents, Laurasiatheria, Scandentia, and Afrotheria (Table 1).
Alteration of the overall allele/genotype architecture at the exceptionally long GA-STR in late-onset NCD patients vs. controls
The overall distribution of the alleles was compared between the NCD cases and controls, which revealed inequality of the population variances between the two groups [F (1, 50) = 12.283; p = 0.001] (Fig. 1).
Figure 1.
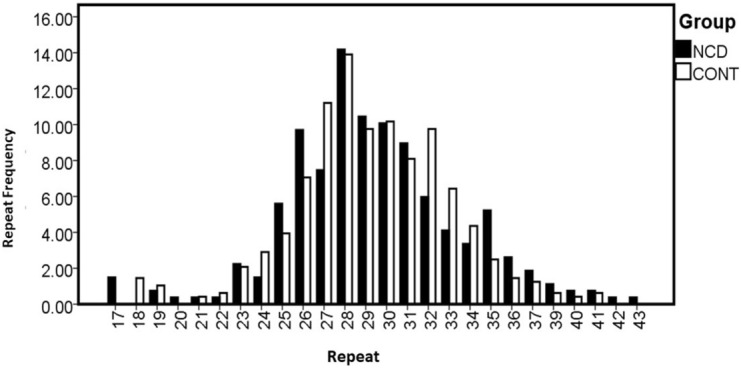
Allele range of the ZMYM3 GA-repeat in the human subjects studied (cases and controls included).
Allele range of the ZMYM3 GA-repeat in the late-onset NCD patients and controls
The allele range of the GA-repeat was between 18 and 41-repeats across the control subjects, and 17 and 43-repeats in the late-onset NCD patients (Fig. 1, Table 2).
Table 2.
Allele/genotype distribution of the ZMYM3 exceptionally long STR in NCD patients and controls.
| Repeat size | NCD | CONT | Chi square | P-value |
|---|---|---|---|---|
| 17 | 4 | 0 | 7.233** | 0.00715755 |
| 18 | 0 | 7 | 3.929* | 0.04746016 |
| 19 | 2 | 5 | 0.158 | 0.69100458 |
| 20 | 1 | 0 | 1.801 | 0.17959165 |
| 21 | 1 | 2 | 0.008 | 0.92873007 |
| 22 | 1 | 3 | 0.202 | 0.65311132 |
| 23 | 6 | 10 | 0.022 | 0.8820871 |
| 24 | 4 | 14 | 1.466 | 0.22597787 |
| 25 | 15 | 19 | 0.374 | 0.54083196 |
| 26 | 26 | 34 | 0.901 | 0.34251372 |
| 27 | 20 | 54 | 2.71 | 0.09972099 |
| 28 | 38 | 67 | 0.011 | 0.91647033 |
| 29 | 28 | 47 | 0.093 | 0.76039737 |
| 30 | 27 | 49 | 0.002 | 0.96432941 |
| 31 | 24 | 39 | 0.167 | 0.68279188 |
| 32 | 16 | 47 | 3.2 | 0.07363827 |
| 33 | 11 | 31 | 1.764 | 0.18412637 |
| 34 | 9 | 21 | 0.447 | 0.50376306 |
| 35 | 14 | 12 | 3.848* | 0.04980535 |
| 36 | 7 | 7 | 1.264 | 0.2608953 |
| 37 | 5 | 6 | 0.459 | 0.4980917 |
| 39 | 3 | 3 | 0.536 | 0.4640952 |
| 40 | 2 | 2 | 0.356 | 0.55073617 |
| 41 | 2 | 3 | 0.04 | 0.84148058 |
| 42 | 1 | 0 | 1.801 | 0.17959165 |
| 43 | 1 | 0 | 1.801 | 0.17959165 |
| Total | 268 | 482 |
NCD neurocognitive disorder, CONT control.
**p < 0.01, *p < 0.05.
Disease-only alleles across the ZMYM3 exceptionally long GA-STR in late-onset NCD patients
Alleles were detected at 17, 20, 42, and 43-repeats in seven NCD patients (2.61% of the NCD cases) that were not detected in the control individuals (Mid-P exact = 0.0003) (Fig. 2). On the other hand, all alleles that were detected in the controls were also detected in the NCD patients. The 17-repeat is the shortest allele detected in our human samples to date. The longest allele detected in human to date is at 45-repeats, detected in a case of SCZ8.
Figure 2.
Electropherogarm of the ZMYM3 GA-repeat extreme alleles in the NCD patients.
Clinical characteristics of the patients harboring disease-only alleles
The seven patients harboring disease-only alleles (Table 3) revealed extensive abnormalities in the available CT-scan records (Fig. 3). The observed lesions included extensive hypodense areas, calcifications, cortical atrophy, and ventricular enlargement. In patients, 2, 3, and 4, bilateral periventricular hypodense areas were detected, which indicated possible chronic microvascular changes and vascular dementia. The remaining four patients may be having AD based on the gradual deterioration of cognition in clinical examination and extensive temporal and cortical atrophy. CT-scans of a number of control individuals are also included for comparison (Fig. 4).
Table 3.
NCD patients harboring alleles at the extreme ends of the ZMYM3 exceptionally long GA-STR*.
| Patient no. | Age | STR repeat | AMTS** |
|---|---|---|---|
| 1 | 93 | 17 | 5 |
| 2 | 64 | 17 | 4 |
| 3 | 65 | 17 | 1 |
| 4 | 65 | 17 | 5 |
| 5 | 83 | 20 | 5 |
| 6 | 80 | 42 | 6 |
| 7 | 78 | 43 | 5 |
*Those alleles were not detected in our NCD cohort and two cohorts previously studied, including schizophrenia and bipolar disorder.
**Abbreviated mental test score.
Figure 3.

CT-scan of the patients with the extreme alleles. (A) 17-repeat, (B) 17-repeat, (C) 42-repeat, (D) 43-repeat. Extensive hypodense areas and calcification were detected in various brain sections.
Figure 4.

CT-scan of a number of control individuals.
Discussion
The ZMYM3 GA-STR complex is human-specific in formula, the exceptionally long STR within this complex reaches maximum length in human, and the transcript encompassing this STR complex is specifically expressed in the brain (Table 1). ZMYM3 was previously reported among the top three regulators of AD progression15. This gene may also link to other major disorders that are associated with major cognition impairment in human, such as SCZ, BPD, and intellectual disability8,9,17,18. The above findings raise the possibility that ZMYM3 may be a master gene in the evolution of human cognition.
We investigated the ZMYM3 GA-STR complex in late-onset NCD as a clinical entity, without differentiating the subtypes of NCD. The advantage of this novel approach was to eliminate the often-ambiguous diagnoses made for the NCD subtypes, which frequently co-occur and overlap in respect of the clinical and pathophysiological manifestations9,21–25.
We found a significant skewing of the genetic architecture at the exceptionally long STR in the NCD patients vs. controls. Moreover, in seven NCD patients, we detected alleles that were not detected in the controls. A number of the disease-only alleles overlapped with alleles detected previously by our group in SCZ and BPD9. The 17 and 43-repeat alleles overlapped in NCD, SCZ and BPD, whereas, the 20-repeat allele overlapped in NCD and SCZ. It is possible that these alleles coincide with the overlapping cognition impairment component across the studied disorders. In line with the above findings, several other genes of overlapping nature have been reported by other groups across late-onset NCD and psychiatric disorders26–28. The 42-repeat allele detected in the NCD patients was not detected in any human subjects studied to date.
Although the disease-only alleles detected by our group are at frequencies of < 0.02 in late-onset NCD, and encompass 2.61% of the patients, it is conceivable that fractional numbers of the remaining majority harbor alleles at unknown STR loci yet to be identified in the future studies. It is often claimed that genes affecting health in older age are beyond the reach of natural selection. However, findings on the APOE alleles and several other NCD susceptibility loci indicate that natural selection indeed happens in such alleles29,30.
The reason we chose only male subjects is that ZMYM3 is X-linked, and therefore, it is expected that there are significant phenotypic differences as a result of gender. A future study is warranted to explore the significance of this STR in female subjects. Expansion of certain STR classes, especially trinucleotide repeats, can cause a range of neurological disorders, including Huntington disease, various ataxias, motor neuron disease, frontotemporal dementia, and fragile X syndrome31. It remains to be clarified how the ZMYM3 GA-STR complex functions in the human brain. From what we know so far, GA-STRs of the range observed in the ZMYM3 complex can dramatically alter gene expression32,33. ZMYM3 is among the top three master regulators causally responsible for regulating the transcriptional signature of AD progression15. While GWAS approaches employ single nucleotide polymorphisms rather than STRs, and therefore can fail to detect instances of association with STRs, they have linked ZMYM3 to a number of neurological disorders of major cognitive impairment, including prion disease and multiple sclerosis34,35.
Considering that the ZMYM3 GA-complex contains three of the longest GA-STRs identified in a human protein-coding gene 5′ UTR, this GA-rich region may also function as a X chromosome dosage compensation mechanism as described in model organisms such as Drosophila36. In human, the GAGA-binding c-Krox/Th/POK protein specifically binds to (GA)837, and can modulate chromatin remodeling and gene expression activity. Of note, (GA)8 is one of the human-specific length STRs across the ZMYM3 STR complex.
Conclusion
In conclusion, the ZMYM3 GA-STR is a prime example in which alleles at the extreme short and long ends of exceptionally long STRs may be associated with a spectrum of major human disorders in which cognition impairment is the predominant phenotype. Independent studies of various neurocognitive disorders are warranted to confirm the significance of our findings.
Supplementary information
Acknowledgements
This research was funded by the University of Social Welfare and Rehabilitation Sciences.
Abbreviations
- AD
Alzheimer’s disease
- BPD
Bipolar disorder
- NCD
Neurocognitive disorder
- SCZ
Schizophrenia
- STR
Short tandem repeat
- TSS
Transcription start site
- UTR
Untranslated region
- ZMYM3
Zinc finger MYM-type protein 3
Author contributions
H.A. collected the samples and the clinical data from those samples, and performed experiments. S.K. performed experiments. F.A. prepared Figs. 1 and 2. A.D. and R.N. contributed to data acquisition and coordination. A.B. prepared Table 2. MK performed some of the experiments. A.A. contributed to coordination. A.K. extracted some of the DNA samples. F.A. contributed to data acquisition. M.O. conceived, designed, supervised the project, and wrote the manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76461-z.
References
- 1.Zhou H, et al. A chronological atlas of natural selection in the human genome during the past half-million years. BioRxiv. 2015 doi: 10.1101/018929. [DOI] [Google Scholar]
- 2.Schwarz F, Springer SA, Altheide TK, Varki NM, Gagneux P, Varki A. Human-specific derived alleles of CD33 and other genes protect against postreproductive cognitive decline. Proc. Natl. Acad. Sci. 2016;113(1):74–79. doi: 10.1073/pnas.1517951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammadparast S, Bayat H, Biglarian A, Ohadi M. Exceptional expansion and conservation of a CT-repeat complex in the core promoter of PAXBP1 in primates. Am. J. Primatol. 2014;76(8):747–756. doi: 10.1002/ajp.22266. [DOI] [PubMed] [Google Scholar]
- 4.Ohadi M, Mohammadparast S, Darvish H. Evolutionary trend of exceptionally long human core promoter short tandem repeats. Gene. 2012;507(1):61–67. doi: 10.1016/j.gene.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Darvish H, et al. Exceptional human core promoter nucleotide compositions. Gene. 2011;475(2):79–86. doi: 10.1016/j.gene.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Namdar-Aligoodarzi P, Mohammadparast S, Zaker-Kandjani B, Kakroodi ST, Vesiehsari MJ, Ohadi M. Exceptionally long 5′ UTR short tandem repeats specifically linked to primates. Gene. 2015;569(1):88–94. doi: 10.1016/j.gene.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Nikkhah M, Rezazadeh M, Khorshid HK, Biglarian A, Ohadi M. An exceptionally long CA-repeat in the core promoter of SCGB2B2 links with the evolution of apes and Old World monkeys. Gene. 2016;576(1):109–114. doi: 10.1016/j.gene.2015.09.070. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh F, Bozorgmehr A, Tavakkoly-Bazzaz J, Ohadi M. Skewing of the genetic architecture at the ZMYM3 human-specific 5′ UTR short tandem repeat in schizophrenia. Mol. Genet. Genomics. 2018;293(3):747–752. doi: 10.1007/s00438-018-1415-8. [DOI] [PubMed] [Google Scholar]
- 9.Alizadeh F, et al. Disease-only alleles at the extreme ends of the human ZMYM3 exceptionally long 5′ UTR short tandem repeat in bipolar disorder: a pilot study. J. Affect. Disord. 2019;251:86–90. doi: 10.1016/j.jad.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 10.Emamalizadeh B, et al. The human RIT2 core promoter short tandem repeat predominant allele is species-specific in length: a selective advantage for human evolution? Mol. Genet. Genomics. 2017;292(3):611–617. doi: 10.1007/s00438-017-1294-4. [DOI] [PubMed] [Google Scholar]
- 11.Khademi E, et al. Support for “disease-only” genotypes and excess of homozygosity at the CYTH4 primate-specific GTTT-repeat in schizophrenia. Genet. Test. Mol. Biomark. 2017;21(8):485–490. doi: 10.1089/gtmb.2016.0422. [DOI] [PubMed] [Google Scholar]
- 12.Afshar H, et al. Natural selection at the NHLH2 core promoter exceptionally long CA-repeat in human and disease-only genotypes in late-onset neurocognitive disorder. Gerontology. 2020;66(5):514–522. doi: 10.1159/000509471. [DOI] [PubMed] [Google Scholar]
- 13.Jakubosky D, et al. Properties of structural variants and short tandem repeats associated with gene expression and complex traits. Nat. Commun. 2020;11(1):1–15. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakimi M-A, Dong Y, Lane WS, Speicher DW, Shiekhattar R. A candidate X-linked mental retardation gene is a component of a new family of histone deacetylase-containing complexes. J. Biol. Chem. 2003;278(9):7234–7239. doi: 10.1074/jbc.M208992200. [DOI] [PubMed] [Google Scholar]
- 15.Aubry S, et al. Assembly and interrogation of Alzheimer’s disease genetic networks reveal novel regulators of progression. PLoS ONE. 2015;10(3):e0120352. doi: 10.1371/journal.pone.0120352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheer MP, et al. DXS6673E encodes a predominantly nuclear protein, and its mouse ortholog DXHXS6673E is alternatively spliced in a developmental-and tissue-specific manner. Genomics. 2000;63(1):123–132. doi: 10.1006/geno.1999.6027. [DOI] [PubMed] [Google Scholar]
- 17.van der Maarel SM, et al. Cloning and characterization of DXS6673E, a candidate gene for X-linked mental retardation in Xq13. 1. Hum. Mol. Genet. 1996;5(7):887–897. doi: 10.1093/hmg/5.7.887. [DOI] [PubMed] [Google Scholar]
- 18.Philips AK, et al. X-exome sequencing in Finnish families with Intellectual Disability-four novel mutations and two novel syndromic phenotypes. Orphanet. J. Rare Dis. 2014;9(1):49. doi: 10.1186/1750-1172-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedayati M, Sum S, Hosseini SR, Faramarzi M, Pourhadi S. Investigating the effect of physical games on the memory and attention of the elderly in adult day-care centers in Babol and Amol. Clin. Interv. Aging. 2019;14:859. doi: 10.2147/CIA.S196148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter CR, et al. Accuracy of dementia screening instruments in emergency medicine: a diagnostic meta-analysis. Acad. Emerg. Med. 2019;26(2):226–245. doi: 10.1111/acem.13573. [DOI] [PubMed] [Google Scholar]
- 21.Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke. 2010;41(10_suppl_1):S144–S146. doi: 10.1161/STROKEAHA.110.598326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karantzoulis S, et al. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev. Neurother. 2011;11(11):1579–1591. doi: 10.1586/ern.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caulkins J. Commentary on Cunningham et al. (2015): essential chemical controls-miracle from a black box? Addiction. 2015;110(5):821–822. doi: 10.1111/add.12864. [DOI] [PubMed] [Google Scholar]
- 24.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension: epidemiology, pathobiology, and treatment. Circ. Res. 2019;124(7):1025–1044. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y-F, et al. Genetic overlap between vascular pathologies and Alzheimer's dementia and potential causal mechanisms. Alzheimer's Dementia. 2019;15(1):65–75. doi: 10.1016/j.jalz.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drange OK, et al. Genetic overlap between Alzheimer’s disease and bipolar disorder implicates the MARK2 and VAC14 genes. Front. Neurosci. 2019;13:220. doi: 10.3389/fnins.2019.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamatsu Y, et al. Transgenerational interaction of Alzheimer’s disease with schizophrenia through amyloid evolvability. J. Alzheimers Dis. 2019;68(2):473–481. doi: 10.3233/JAD-180986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesdorffer DC. Comorbidity between neurological illness and psychiatric disorders. CNS Spectr. 2016;21(3):230–238. doi: 10.1017/S1092852915000929. [DOI] [PubMed] [Google Scholar]
- 29.Raj T, et al. Alzheimer disease susceptibility loci: evidence for a protein network under natural selection. Am. J. Hum. Genet. 2012;90(4):720–726. doi: 10.1016/j.ajhg.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drenos F, Kirkwood TB. Selection on alleles affecting human longevity and late-life disease: the example of apolipoprotein E. PLoS ONE. 2010;5(4):e10022. doi: 10.1371/journal.pone.0010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannan AJ. Tandem repeats mediating genetic plasticity in health and disease. Nat. Rev. Genet. 2018;19(5):286. doi: 10.1038/nrg.2017.115. [DOI] [PubMed] [Google Scholar]
- 32.Corney B, Widnall C, Rees D, Davies J, Crunelli V, Carter D. Regulatory architecture of the neuronal Cacng2/Tarpγ2 gene promoter: multiple repressive domains, a polymorphic regulatory short tandem repeat, and bidirectional organization with co-regulated lncRNAs. J. Mol. Neurosci. 2019;67(2):282–294. doi: 10.1007/s12031-018-1208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valipour E, et al. Polymorphic core promoter GA-repeats alter gene expression of the early embryonic developmental genes. Gene. 2013;531(2):175–179. doi: 10.1016/j.gene.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 34.Baranzini SE, et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain. 2010;133(9):2603–2611. doi: 10.1093/brain/awq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mead S, et al. Genome-wide association study in multiple human prion diseases suggests genetic risk factors additional to PRNP. Hum. Mol. Genet. 2012;21(8):1897–1906. doi: 10.1093/hmg/ddr607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuzu G, et al. Expansion of GA dinucleotide repeats increases the density of CLAMP binding sites on the X-chromosome to promote Drosophila dosage compensation. PLoS Genet. 2016;12(7):e1006120. doi: 10.1371/journal.pgen.1006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger N, Dubreucq B. Evolution goes GAGA: GAGA binding proteins across kingdoms. Biochim. Biophys. Acta Gene Regul. Mech. 2012;1819(8):863–868. doi: 10.1016/j.bbagrm.2012.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



