Abstract
Powder based additive manufacturing (AM) technology of Ti and its alloys has received great attention in biomedical applications owing to its advantages such as customized fabrication, potential to be cost-, time-, and resource-saving. The performance of additive manufactured implants or scaffolds strongly depends on various kinds of AM technique and the quality of Ti and its alloy powders. This paper has specifically covered the process of commonly used powder-based AM technique and the powder production of Ti and its alloy. The selected techniques include laser-based powder bed fusion of metals (PBF-LB/M), electron beam powder bed fusion of metals (PBF-EB/M), and directed energy deposition utilized in the production of the biomaterials are discussed as well as the powder fed system of binder jetting. Moreover, titanium based powder production methods such as gas atomization, plasma atomization, and plasma rotating electrode process are also discussed.
Keywords: Additive manufacturing, Titanium (Ti) and its alloy powder, Biomaterials, 3D printing
Introduction
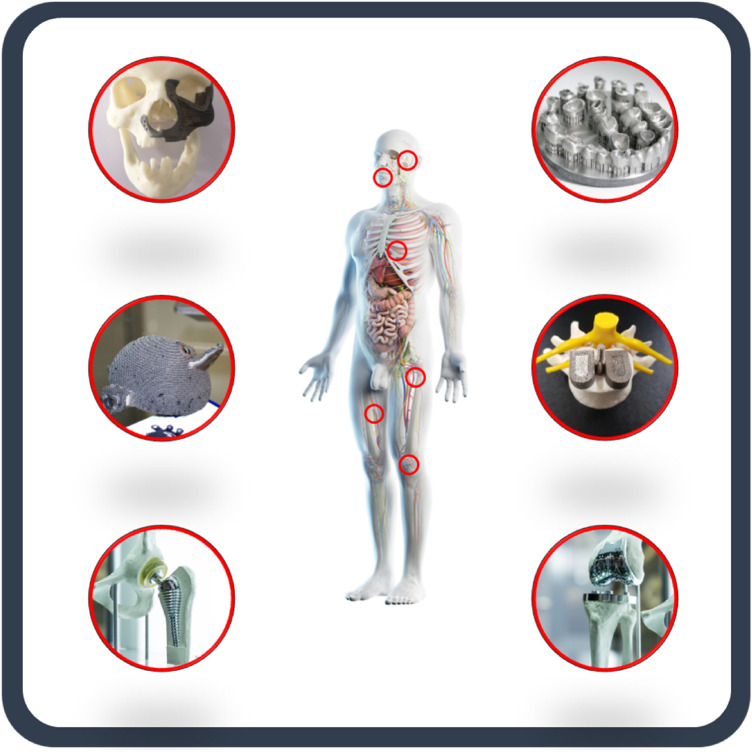
Additive manufacturing (AM) is a powerful tool to fabricate complex geometries layer-by-layer with computer-aided design (CAD) [1–5]. In the recent decades, it has received a great attention globally among diverse fields and has the field has developed rapidly with its advantages. It is capable of fabricating complex parts that are not possible through other methods, and its potential to be cost-effective, time-saving, and resource-saving has attracted both the academic and industry researchers [6–8]. It is widely used in biomedical applications such as orthopedic implants, dental implants, and cardiovascular systems (Fig. 1) [9–13].
Fig. 1.
Schematic diagram of the biomedical application of additive manufacturing of biometals includes cranial prosthesis, dental implants, acetabular cup, interbody fusion cage, hip prosthesis and knee prosthesis
Metallic implants are widely used in load-bearing orthopedic field [14–21]. Some of the commonly used metals include titanium, stainless steels, and cobalt-chromium alloys. Ti6Al4V alloy, also known as Ti64, has been one of the most widely accepted as a reliable material in the biomedical field with its unique combination of mechanical properties, corrosion resistance and biocompatibility [22–25]. Initially, the alloy was designed for use in the aerospace industries back in the 1950s [26]. Ti64 has an α-β structure with high strength, low density, and corrosion resistance thus it was well suited for its use as jet engines, gas turbines and other aircraft structural parts [27–30]. Furthermore, its’ corrosion resistance to most corrosive acids and alkalis further spanned its uses in other fields [31, 32]. Today, Ti64 is still extensively used in aerospace applications but also used in numerous non aerospace fields such as marine, automobile, energy, chemical, and biomedical industries [33, 34]. The Ti6Al4V alloy takes about a half of the market share of Ti uses globally, being the most widely used Ti alloy. In the medical field, the material properties of Ti64 such as its high strength and fracture toughness, low density, and excellent corrosion resistance and biocompatibility made it an attractive biomaterial candidate for uses in dental and orthopedic applications [35–37].
Modern manufacturing of medical implants mostly used austenitic stainless steel, cobalt-chromium-based and titanium-based materials. In order to obtain customized properties that meets the structural requirements, advanced fabricating techniques such as additive manufacturing (metal-AM) are used [38]. The AM methods can be categorized into powder bed and powder-fed systems. In this review of some common powder bed systems which include laser-based powder bed fusion of metals (PBF-LB/M), electron beam powder bed fusion of metals (PBF-EB/M), and binder jetting (BJ) are discussed as well as the powder fed system of directed energy deposition (DED) [39–41]. These technologies have become a powerful tool in a myriad of applications in orthopedics, dental, and cardiovascular fields. For example, to fabricate an implant that would replace a bone defect, it should replicate the intricate structure and provide mechanical support to mimic the function of the defect site [42]. With the use of X-ray computed tomography or magnetic resonance imaging data of individual patients, 3D modeling can be done with CAD (computer-aided design) to define the specific geometry. The metal-AM techniques have been selected for use in additive manufactured implants and other biomedical applications with varying material and structural properties with advantages in terms of cost, manufacturing time, and repeatability [43].
The performance of these additive manufactured implants strongly depends on the quality of Ti and its alloy powders. Before the development of AM technology, powder metallurgy (PM) process such as metal injection molding and hot isostatic pressing also used Ti and its alloy powders to fabricate near-net-shape bulk products. The powders for AM have stringent physical and chemical demands. Generally, the powders in powder bed and powder-fed systems must have good flowability, and excellent tap density for uniformity of additive manufactured products [44–46]. Consequently, homogeneous spherical shaped powders are preferred. Furthermore, low oxygen contents in Ti and its alloy powders are needed to ensure sufficient ductility and fracture toughness [6, 47]. Gas atomization (GA), plasma atomization (PA) and plasma rotating electrode process (PREP) are conventional method to produce spherical powders with high purity for various PM processes [48, 49]. And as the portion of AM increases, the importance of powder production processes also increases.
This paper provides a general review of powder processing of Ti and its alloy for biomedical application using four metal-AM methods (PBF-LB/M, PBF-EB/M, BJ, and DED) and also covers the production process (GA, PA, and PREP) of Ti and its alloys for AM.
Powder-based additive manufacturing processes
Laser-based powder bed fusion of metals (PBF-LB/M)
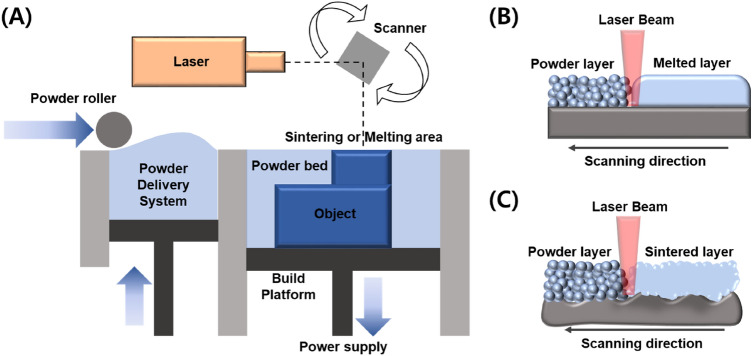
Laser-based powder bed fusion methods can be divided into complete melting and partially sintering of powder. As shown in Fig. 2a, b, high-power lasers that completely melt the powder layer-by-layer is the method that involves melting of the powder. It was first introduced in mid-1990s [50] which was built on the concept of selectively laser sintering powder that was developed in the late 1980s which only partially melts the powder while retaining its solid core as shown in Fig. 2a, c [51]. Powder-based metallic manufacturing involves polymer serving as a binding agent to hold the metallic powder together at relatively low temperature, and it is later removed through a burn-out process which results in a porous structure. As the schematic shows, the two method generally have a powder dispensing platform with a roller and a build platform both on supporting pistons. Roller layers a powder evenly and laser beam is used to either melts or selectively sinters powder according to a CAD model. Once the layer is complete the working piston is lowered and the sequential layers are repeatedly fabricated layer-by-layer. Previous works include working on decreasing the porosity as well as adding a post-processing step following selective laser sintering to produce fuller density products for use as biomaterials [52, 53]. Studies on porous materials have extensively focused on tuning the geometry of porous structure and biocompatibility to meet the needs for use in orthopedic implants that can interlock the implant in defect site and simultaneously allow better cell-to-implant interaction [54].
Fig. 2.
Schematic diagram of the a PBF-LB/M, b the working principles of complete melting of powder layer and c selective sintering of powder, respectively
The starting material for PBF-LB/M can be a single or multi-component and PBF-LB/M is performed in vacuum or inert-gas protected state to prevent the metallic powders from reacting with other gases [55]. In particular, Ti and its alloys are easily oxidized thus it is crucial to use inert gas or use in vacuum state to minimize the oxygen exposure. Depending on the starting powder, the consolidation process has two binding mechanisms [56]. First mechanism involves liquid phase sintering in which polymer is used to act as a binder for metal particles with a laser beam energy density of 1 J mm−3, and additional thermal treatment is needed to burn out the binder and sinter the green body followed by an infiltration method to obtain higher density product. The other mechanism is of direct melting of metallic powders (e.g. Ti6Al4V and CoCr alloy) with laser beam energy density of 200 J mm−3 and no further process is required [57]. The advantages of using PBF-LB/M include near-net-shape fabrication of complex shapes, high mechanical properties, high dimensional accuracy, better surface finish, and high material recycling rate [43]. Generally, maximum beam power, beam spot size, scan speed, and layer thickness are 120 W, 30–250 μm, 0.3–1 m/s, and 20–100 μm, respectively [26]. With successful advances in machine development and efforts to make it more productive and cost-effective, it is the one of the popular powder bed technology today in fabricating metallic materials for biomedical application using AM technique. The involvement of globally leading AM companies in the PBF-LB/M technology development speeded up fthe advancement in machines such as enlarging building envelope size up to 500 × 280 × 365 mm3, changing the laser type to Quad IPG laser, adjusting the substrate plate, and altering the powder removal process [41]. Current usage of PBF-LB/M includes printing implants with complex-geometry for orthopedic and dental uses. Complex structures such as gyroid scaffolds haven been fabricated and studied for suitability as biomaterials [58, 59]. Moreover, studies on post processing of the additively manufactured samples to enhance the mechanical and biological properties for use as biomaterials are ongoing such as HIP, machining and polishing [60–62]. Studies have further studied surface modification of the products for enhancing biocompatibility and reducing side effects [63]. The limitations of the techniques include time consuming process and acute size restriction difficulty in scaling-up and high residual stress [64].
Electron beam powder bed fusion of metals (PBF-EB/M)
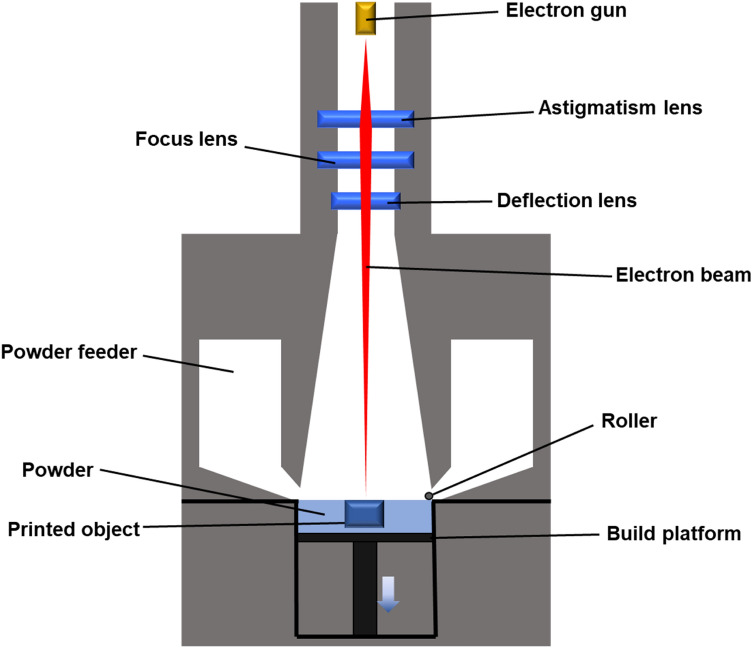
Electron beam powder bed fusion of metals (PBF-EB/M) technique, developed in mid-2010 by Arcam AB, is an powder fed fusion AM method for metals that uses high-speed electron beam energy under high vacuum (10−4–10−5 mbar) [65, 66]. The metal powders are selectively bombarded and melted to fabricate samples as shown in Fig. 3. Today, metal-AM for use as biomaterials mainly use laser and electron beam sources. As Fig. 3 shows, the components that make up the PBF-EB/M technique are very similar to PBF-LB/M technique in that rake, build platform, powder hopper, and energy source are involved [66]. Powder bed is first prepared on the build platform then electron beam is selectively aligned based on the CAD model and melts the powder. The electromagnetic lens focuses the accelerated electrons into energy beam [67]. The process is repeated layer-by-layer building onto previously fabricated layer. When the manufacturing process is over the residual powder is removed [68]. Generally, the maximum beam power, beam spot size, scan speed, and layer thickness are approximately 3500 W, 200–1000 μm, > 1000 m/s, 50–200 μm, respectively [26]. The energy density of electron beam above 100 kW cm−2 can be obtained at a power of 60 kW [69].
Fig. 3.
Schematic diagram of the electron beam powder bed fusion of metals (PBF-EB/M)
The manipulation of the electron beam is capable by varying the parameters of the magnetic field. The chamber atmosphere along with manufacturing parameters such as the electron and focusing current, action time, powder properties, acceleration voltage, and scanning mode are important factors in PBF-EB/M technique [4, 70]. These can have effects on the size, roughness and porosity [71]. The processing and the final product quality depends largely on the powder properties. It is important for powder used in PBF-EB/M to have high flowability. Generally, sphere powder fabricated through gas atomization with a target size of 40–105 μm is used. However, working with larger mean diameter powder is easier in terms of process stability for which powder produced by plasma rotation electrode process (PREP) is preferred with target size of 50–150 μm [72]. Another important powder properties to take into consideration for EBM is repulsion of charges on powders which can result in smoke events [73]. Pre-treatment such as heating the plate prior to processing to slightly sinter the powder is used which also enhances the conductivity that can help processing stability. The powder used for PBF-EB/M can be reused with sieving [44].
The products fabricated through PBF-EB/M technique in comparison to PBF-LB/M show decreased residual stress but results in a rougher surface since the PBF-LB/M technique can work with smaller particles [74, 75]. In addition it has a higher energy utilization rate, higher material absorption rate, and lower operating cost. PBF-LB/M and PBF-EB/M of metallic materials have great advantages over other AM of metallic materials with better quality of the finished products in terms of resolution and dimensional accuracy thus have shown promising results in orthopedic applications [42, 76, 77]. PBF-EB/M has been used with femoral knee implants with Co-29Cr-6Mo [78]. Complex structures produced by PBF-EB/M [79, 80], corrosion resistance [81], and biocompatibility [82, 83] include some of recent research topics with PBF-EB/M-fabricated materials.
Directed energy deposition (DED)
DED technology is an additive manufacturing technique first developed by Sandia National Laboratory in the 1990s [84]. The technology is one of the widely studied technology which uses injected powder, instead of a powder bed, along with laser to melt and build on substrate as the schematic shows in Fig. 4. Its advantages include capability to produce complex shapes with near net-shape parts, capability to produce parts with gradients of pores or different metals (such as Ti alloys, stainless steel, and shape memory alloys) and also produce parts with a mixture of different metal powders to create alloys [85]. Moreover, the big differences from powder bed fusion techniques is that it has better fabrication efficiency and cooling effect and re-fabricating capability [86]. The applications of the technology not only include making near net-shaped parts but also used in repairing, modifying the surface, and adding features to parts [43]. The components of DED technology include laser beam, powder nozzle, and a build platform. The DED AM process starts with the delivery of powder (or mixture of powders) with argon-pressurized nozzles to the point on which the laser beam is focused on. Then melting occurs and forms a melt pool while the platform is moving according to the designed CAD model [86, 87]. The melted regions solidifies and forms a layer and the successive layer is repeated after raising the laser and the powder nozzle. This all takes place in an enclosed and oxygen-controlled (< 10 ppm) environment to minimize oxidation of the fabricated parts [88]. Significant amount of recent research trend of DED technology is geared toward biomedical load-bearing applications [89, 90]. Functionally structured materials fabricated with DED technology were reported such as porous Ti-based implant designed with the mechanical properties to mimic the human bone more closely compared to dense Ti material [91]. Moreover, the microstructural and corrosion-related effects were studied in terms of the processing parameters of NiTi alloys using DED technology for bone implants [89]. Some recent applications of the technology include developing implants that are antimicrobial and biocompatible with Tanium-silver alloys [92], studying the surface properties [93, 94], and load-bearing properties [91, 95] of Ti–6Al–4V alloys.
Fig. 4.
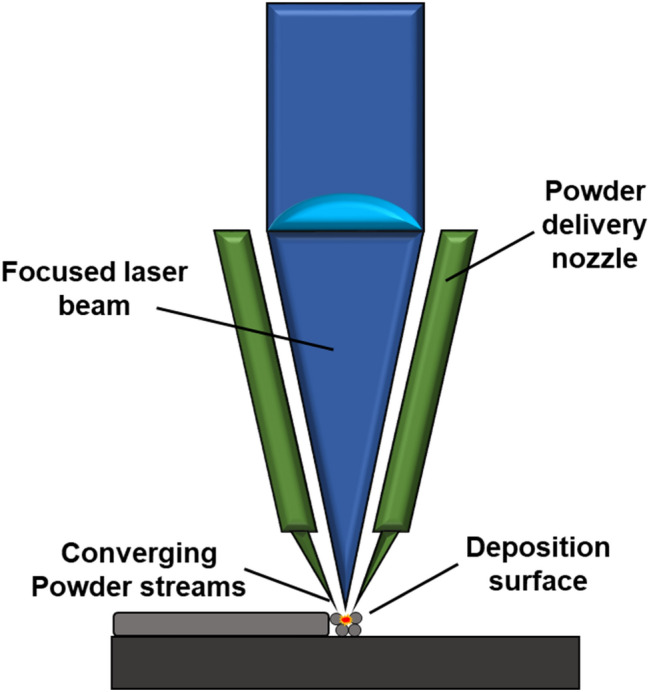
Schematic diagram of the directed energy deposition (DED)
Binder jetting (BJ)
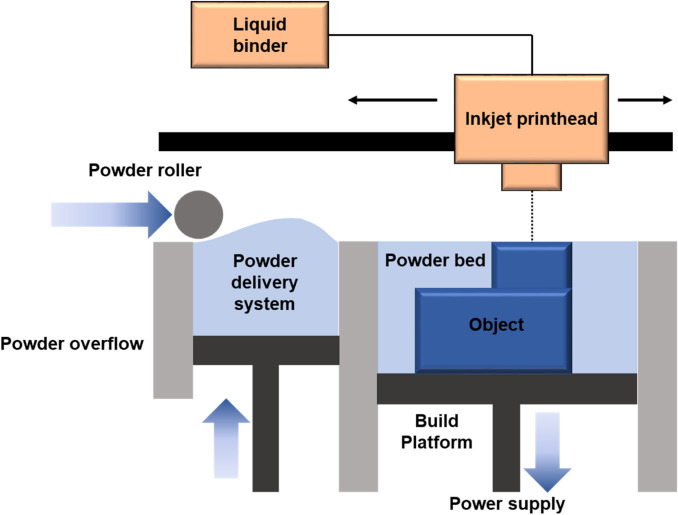
Binder jetting, also known as 3D printing (3DP), is an additive manufacturing technology which uses powder and liquid binding agent and it was developed in the early 1990s at Massachusetts Institute of Technology [68, 96, 97]. Figure 5 shows the components that make up binder jetting include powder spreader, print-head, and build platform [98]. The binding agent is selectively deposited onto a bed of metallic powder followed by lowering of the powder bed and repeating the process layer-buy-layer to obtain a “green” body using a predesigned CAD model. The fabricated green body is cured to enhance mechanical strength and is sintered. The sintering process (1100 °C, 24–36 h) burns off binding agent and sinters the green body. Additionally, to 1iquid state infiltrates the porous scaffold through capillary action as the driving force [68]. The advantages of the manufacturing technique are that it is cost-effective with high efficiency and that no residual stress is involved whereas the disadvantages include shrinkage during sintering, poor quality and low mechanical properties [68].
Fig. 5.
Schematic diagram of the binder jetting (BJ)
Powder production processes
Gas atomization (GA)
With a great demand of spherical Ti powder for AM technologies, many studies have focused on developing alternative methods to reduce the cost of traditional wrought method and improve the quality of powder. Powder metallurgy technology which allows near-net-shape manufacturing, includes processes such as powder production, compaction, sintering, and post-sintering processing [99]. The quality of the parts created using AM will depend heavily on the powder deposition and the manufacturing process of powder which determines the powder properties such as morphology, size, size distribution, tap density, and flowability. The Hausner ratio is the ratio of the tap to apparent densities and serves as a simple way to describe the flowability of powders.
Commonly used methods to fabricate spherical Ti powder today include gas atomization, plasma atomization, and plasma rotating electrode process [99, 100]. Gas atomization is a method that was introduced 1980s by Crucible Materials Corporation [100–102]. As shown in the simplified schematic of GA in Fig. 6, starting material (raw or pre-alloyed materials) in the shape of ingots or bars are induction skull melted in vacuum into a homogeneously melted state which is contained in a thin skull of the same composition which forms in the inner wall of the crucible. When the melt is ready for atomization, a localized melting occurs with a smaller induction coil at the bottom of the skull and it is poured into an induction heated nozzle and atomized with high pressure gas. The droplets then undergoes cooling while in free fall state which is called free-fall gas atomization (FFGA) [100, 103]. The particles generally have a wide size range less than 500 μm [103].
Fig. 6.
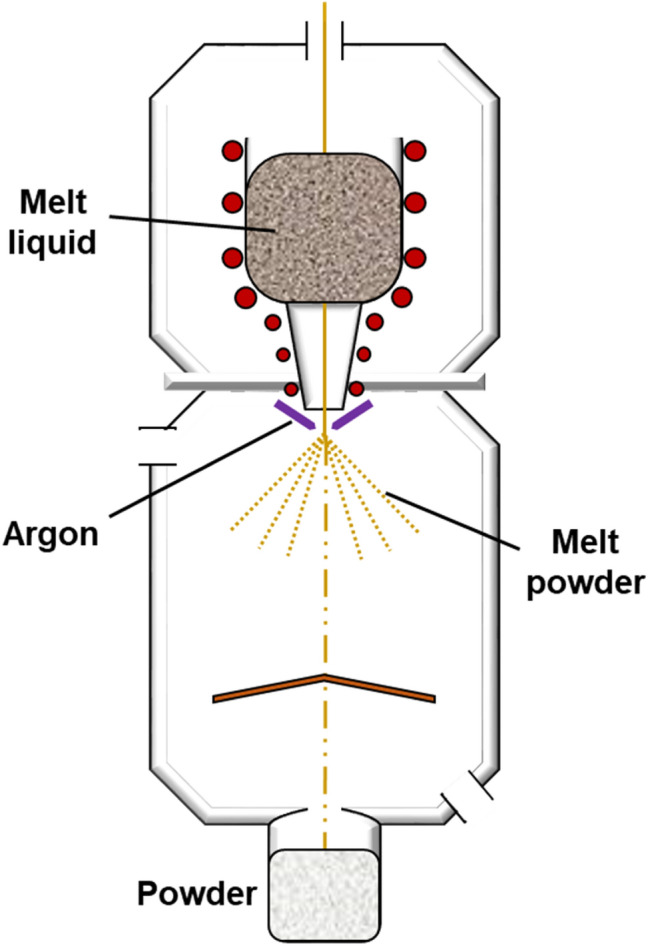
Schematic diagram of the GA process for AM
There are several issues which are addressed with the GA technology. First, particles produced with GA tends to have satellite particles which play a negative role on the free-flow of particles. The reason for production of satellite particles is that fine particle interact with droplets. Second, ceramic powder contamination from erosion of the nozzle by the melted liquid is possible. Lastly, argon gas may be trapped within the melted state resulting in porous powder particles which is not removable even with hot isostatic pressing and have detrimental results on the mechanical properties [99, 100].
A critical factor in determining the particle size distribution of GA powder is the gas-to-metal flow ratio [104]. A higher ratio would result in a higher yield of fine particles which is approximately 15% for FFGA. Close coupled gas atomization (CCGA) is the further developed technique which is used to target the fine particles with a size of less than 45 μm and to increase the production yield and to enhance the atomization efficiency [105, 106]. Y2O3-W-YSZ composite tubes were used to Ti powder with CCGA. However, to knowledge no studies have yet reported the powder yield of fine Ti powder [99].
In addition, with high reactivity of titanium with ceramics and metals, electrode induction gas atomization (EIGA) technology was developed to make ceramic contamination-free powder by ensuring that the melt does not come into contact with any metal or ceramic parts [107]. A rod is melted in cone-shaped induction coil while in slow rotation which is later atomized into small droplets. The diameter of the rod can be increased from the initial 25–70 mm up to 90–120 mm to increase productivity [100]. The atomization apparatus coated with Ti in the inner walls and other walls of the flow path was developed to minimize contamination during atomization [108].
Plasma atomization (PA)
Plasma atomization was first developed in 1990s to make fine, spherical powder [109, 110]. As shown in Fig. 7, it uses pre-alloyed wire as a feed material which is melted using high velocity plasma torches and broken into droplets simultaneously. It is capable of producing high purity powder since the melted droplets do not come into contact with any metal or other materials prior to solidifying unlike the gas atomization process. The cooling rate is typically around 100–1000 °C s−1 with the particle size range of 25–250 μm [99]. Generally, compared to the powder produced with gas atomization, the yield of fine powder and purity are higher [99, 111].
Fig. 7.
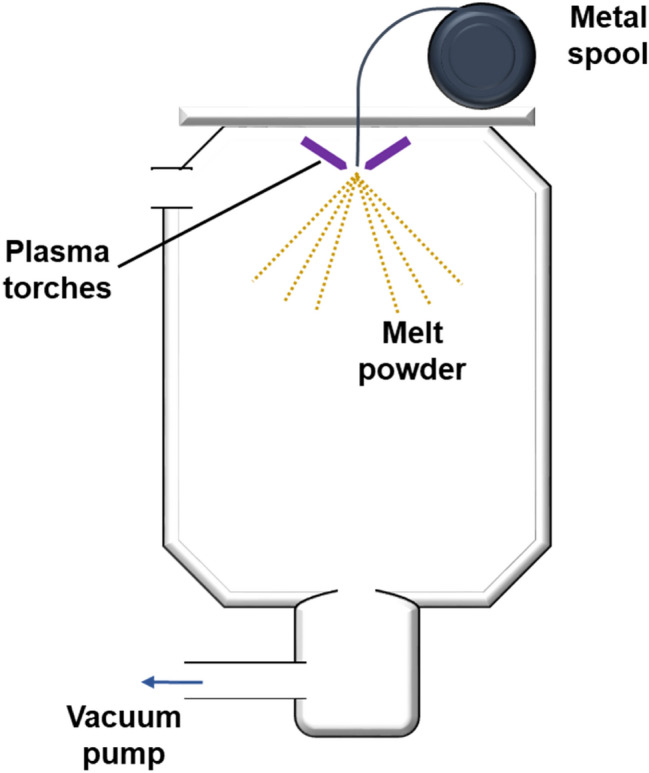
Schematic diagram of the PA process for AM
The morphology of plasma atomized particles are high quality spheres, and they tend to have fewer satellite particles than gas atomized particles. However, the issues of potential residual gas pores within the produced particle and existence of satellites are still of concern. The restriction in the type of feedstock usable for the process pose limitations to plasma atomization studies. It increases the manufacturing cost since wire forms are generally more expensive and also limits the experimentation of materials that do not exist in wire form such as Ti3Al [100]. Factors that can adjusted to alter the yield of fine particles and the capacity include the following: diameter of wire, feed rate of wire, gas pressure, and the angle and distance between the wire and plasma outlet [100, 111]. A study also reported that an induction coil added plasma apparatus can improve the production rate [111].
Plasma rotating electrode process (PREP)
The plasma rotating electrode process was developed by modifying the rotating electrode process (REP) developed in the 1960s by Nuclear Metals/Starmet [63, 111] by replacing the heat source with a transferred arc plasma torch to avoid tungsten contamination. The PREP is carried out in stainless steel chamber. A rapidly rotating metal electrode rod is melted in helium environment with helium plasma torch and with the centrifugal force from the rotation. Helium is chosen for its heat transfer and electric arc properties [111]. The rotation speed is generally between 3000 and 15,000 rpm. The melted liquid spins off and forms droplets which solidifies in flight as shown in Fig. 8 [77, 103]. The particle size of the produced Ti–6Al–4V powder is approximately 100–300 μm [103].
Fig. 8.
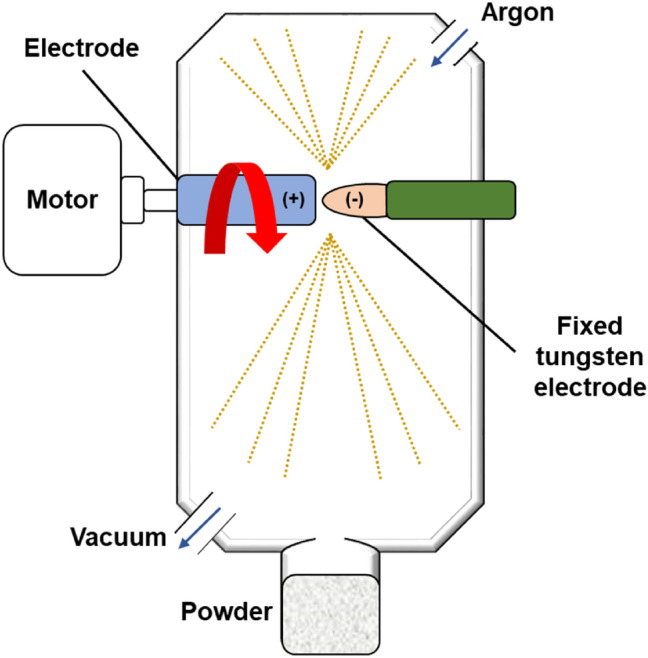
Schematic diagram of the PREP process for AM
The process is one of the common methods to make spherical Ti alloy powders which possesses good packing and flow properties. The apparatus design allows for the final product to have high purity from avoiding contact with other materials prior to solidifying. In addition, interstitial impurities such as oxygen and nitrogen is minimal during the manufacturing process due to its relatively large size and low specific surface area [111]. Since the process does not involve high-pressure gas, there close to no gas pores in the powder particles. In addition, the droplets formed fly away from the melted surface without back flow of fine particles. Therefore, the possibility of fine particles from colliding with other particles is very low, consequently lowering the possibility of having satellite particles [112]. Moreover, the yield of finer powder can be increased by increasing the rotation speed and the diameter of the electrode [113]. Yet, one of the drawbacks for PREP is that the size of the produced particles is suitable for powder metallurgy involving hot isostatic pressing but it is not suitable for powder-bed-based AM.
Conclusion
The development and progress in additive manufacturing techniques of metals have played an important role in manufacturing customized near-net-shape orthopedic and dental implants that are not possible through other fabrication techniques. In this review, commonly used powder-based additive manufacturing processes which include PBF-LB/M, PBF-EB/M, DED, and binder jetting are discussed. In addition, some of the common powder production techniques to make spherical titanium-based powder which are preferable for metal-AM are presented. These include GA, PA, and PREP.
Although significant progress has been made in the metal AM technology allowing fabrication of complex structures, each technique has its own advantages and disadvantages in terms of cost, accuracy, quality, and speed. In order for metal AM technology to be more widely used in medical applications, an optimized processing method that is capable of printing with high accuracy, high quality, high output, low defect, and low cost is needed. In addition, further study on the effects of processing parameters on the structure and mechanical stability is needed to predict and optimize manufacturing strategies. Moreover, the performance of the parts also depend on the quality of the starting powder in which spherical powder is preferred. The above mentioned methods which are used to make spherical powder all have advantages and disadvantages as well. There is still room for improvement to reduce the cost and to minimize possible pores, contamination, and satellite particles.
Acknowledgements
This work was supported by the Basic Science Research Program [Nos. 2018R1C1B6001003 and 2020R1F1A1072103] through the National Research Foundation of Korea funded by the Korea government (MSIT).
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tae-Sik Janga and DongEung Kim have contributed equally to this work.
Contributor Information
Chang-Bun Yoon, Email: cbyoon@kpu.ac.kr.
Hyun-Do Jung, Email: hdjung@catholic.ac.kr.
References
- 1.Bae BH, Lee JW, Cha JM, Kim I-W, Jung H-D, Yoon C-B. Preliminary characterization of glass/alumina composite using laser powder bed fusion (L-PBF) additive manufacturing. Materials. 2020;13(9):2156. doi: 10.3390/ma13092156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A. Additive manufacturing of biomaterials. Prog Mater Sci. 2018;93:45–111. doi: 10.1016/j.pmatsci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Lee H, Cheon K-H, Park C, Jang T-S, Kim H-E, Jung H-D. Fabrication of poly(lactic acid)/Ti composite scaffolds with enhanced mechanical properties and biocompatibility via fused filament fabrication (FFF)-based 3D printing. Addit Manuf. 2019;30:100883. [Google Scholar]
- 4.Frazier WE. Performance: metal additive manufacturing: a review. J Mater Eng Perform. 2014;23(6):1917–1928. [Google Scholar]
- 5.Jung H-D, Jang T-S, Lee JE, Park SJ, Son Y, Park S-H. Enhanced bioactivity of titanium-coated polyetheretherketone implants created by a high-temperature 3D printing process. Biofabrication. 2019;11(4):045014. doi: 10.1088/1758-5090/ab376b. [DOI] [PubMed] [Google Scholar]
- 6.Dutta B, Froes FHS. The additive manufacturing (AM) of titanium alloys. In: Titanium powder metallurgy. Elsevier; 2015. p. 447–68.
- 7.Gill S, Arora H, Sheth V. On the development of Antenna feed array for space applications by additive manufacturing technique. Addit Manuf. 2017;17:39–46. [Google Scholar]
- 8.Xiong J, Yin Z, Zhang WJT. Forming appearance control of arc striking and extinguishing area in multi-layer single-pass GMAW-based additive manufacturing. Int J Adv Manuf Technol. 2016;87(1–4):579–586. [Google Scholar]
- 9.Jang T-S, Jung H-D, Pan MH, Han WT, Chen S, Song J. 3D printing of hydrogel composite systems: recent advances in technology for tissue engineering. 2018;4(1):1–21. [DOI] [PMC free article] [PubMed]
- 10.Vaezi M, Yang S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys Prototyp. 2015;10(3):123–135. [Google Scholar]
- 11.Parthasarathy J, Starly B, Raman S. A design for the additive manufacture of functionally graded porous structures with tailored mechanical properties for biomedical applications. J Manuf Process. 2011;13(2):160–170. [Google Scholar]
- 12.Bose S, Robertson SF, Bandyopadhyay A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018;66:6–22. doi: 10.1016/j.actbio.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Ramakrishna S, Singh R. Material issues in additive manufacturing: a review. J Manuf Process. 2017;25:185–200. [Google Scholar]
- 14.Lee H, Jung H-D, Kang M-H, Song J, Kim H-E, Jang T-S. Design: effect of HF/HNO3-treatment on the porous structure and cell penetrability of titanium (Ti) scaffold. Mater Des. 2018;145:65–73. [Google Scholar]
- 15.Kang M-H, Lee H, Jang T-S, Seong Y-J, Kim H-E, Koh Y-H, Song J, Jung H-D. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019;84:453–467. doi: 10.1016/j.actbio.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 16.Moon B-M, Seo JH, Lee H-J, Jung KH, Park JH, Jung H-D. Compounds: method of recycling titanium scraps via the electromagnetic cold crucible technique coupled with calcium treatment. J Alloys Compd. 2017;727:931–939. [Google Scholar]
- 17.Kang M-H, Jang T-S, Kim SW, Park H-S, Song J, Kim H-E, Jung K-H, Jung H-D. MgF2-coated porous magnesium/alumina scaffolds with improved strength, corrosion resistance, and biological performance for biomedical applications. Mater Sci Eng, C. 2016;62:634–642. doi: 10.1016/j.msec.2016.01.085. [DOI] [PubMed] [Google Scholar]
- 18.Jung H-D, Jang T-S, Wang L, Kim H-E, Koh Y-H, Song J. Novel strategy for mechanically tunable and bioactive metal implants. Biomaterials. 2015;37:49–61. doi: 10.1016/j.biomaterials.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Jung HD, Yook SW, Han CM, Jang TS, Kim HE, Koh YH, Estrin Y. Highly aligned porous Ti scaffold coated with bone morphogenetic protein-loaded silica/chitosan hybrid for enhanced bone regeneration. J Biomed Mater Res Part B Appl Biomater. 2014;102(5):913–921. doi: 10.1002/jbm.b.33072. [DOI] [PubMed] [Google Scholar]
- 20.Jung H-D, Yook S-W, Kim H-E, Koh Y-H. Fabrication of titanium scaffolds with porosity and pore size gradients by sequential freeze casting. Mater Lett. 2009;17(63):1545–1547. [Google Scholar]
- 21.Long M, Rack HJ. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19(18):1621–1639. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 22.Niinomi M, Nakai M, Hieda J. Development of new metallic alloys for biomedical applications. Acta Bimater. 2012;8(11):3888–3903. doi: 10.1016/j.actbio.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Matassi F, Botti A, Sirleo L, Carulli C, Innocenti M. Porous metal for orthopedics implants. Clin Cases Miner Bone Metab. 2013;10(2):111. [PMC free article] [PubMed] [Google Scholar]
- 24.Jang T-S, Lee JH, Kim S, Park C, Song J, Jae HJ, Kim H-E, Chung JW, Jung H-D. Ta ion implanted nanoridge-platform for enhanced vascular responses. Biomaterials. 2019;223:119461. doi: 10.1016/j.biomaterials.2019.119461. [DOI] [PubMed] [Google Scholar]
- 25.Jang T-S, Cheon K-H, Ahn J-H, Song E-H, Kim H-E, Jung H-S. In-vitro blood and vascular compatibility of sirolimus-eluting organic/inorganic hybrid stent coatings. Colloids Surf B Biointerfaces. 2019;179:405–413. doi: 10.1016/j.colsurfb.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Shin YC. Additive manufacturing of Ti6Al4V alloy: a review. Mater Des. 2019;164:107552. [Google Scholar]
- 27.Qiu C, Ravi G, Attallah MM. Microstructural control during direct laser deposition of a β-titanium alloy. Mater Des. 2015;81:21–30. [Google Scholar]
- 28.Crupi V, Epasto G, Guglielmino E, Squillace A. Influence of microstructure [alpha + beta and beta] on very high cycle fatigue behaviour of Ti–6Al–4V alloy. Int J Fatigue. 2017;95:64–75. [Google Scholar]
- 29.Li Y, Ng HP, Jung H-D, Kim H-E, Estrin Y. Enhancement of mechanical properties of grade 4 titanium by equal channel angular pressing with billet encapsulation. Mater Lett. 2014;114:144–147. [Google Scholar]
- 30.Kim Y, Kim E-P, Song Y-B, Lee SH, Kwon Y-S. Compounds: microstructure and mechanical properties of hot isostatically pressed Ti–6Al–4V alloy. J Alloys Compd. 2014;603:207–212. [Google Scholar]
- 31.Cui C, Hu B, Zhao L, Liu S. Titanium alloy production technology, market prospects and industry development. Mater Des. 2011;32(3):1684–1691. [Google Scholar]
- 32.Chan C-W, Lee S, Smith G, Sarri G, Ng C-H, Sharba A, Man H-C. Enhancement of wear and corrosion resistance of beta titanium alloy by laser gas alloying with nitrogen. Appl Surf Sci. 2016;367:80–90. [Google Scholar]
- 33.Boyer RR. An overview on the use of titanium in the aerospace industry. Mater Sci Eng, A. 1996;213(1–2):103–114. [Google Scholar]
- 34.Wu B, Pan Z, Li S, Cuiuri D, Ding D, Li H. The anisotropic corrosion behaviour of wire arc additive manufactured Ti–6Al–4V alloy in 35% NaCl solution. Corros Sci. 2018;137:176–183. [Google Scholar]
- 35.Jiang J, Xu J, Liu Z, Deng L, Sun B, Liu S, Wang L, Liu H. Preparation, corrosion resistance and hemocompatibility of the superhydrophobic TiO2 coatings on biomedical Ti–6Al–4V alloys. Appl Surf Sci. 2015;347:591–595. [Google Scholar]
- 36.Jang T-S, Kim S, Jung H-D, Chung J-W, Kim H-E, Koh Y-H, Song J. Large-scale nanopatterning of metal surfaces by target-ion induced plasma sputtering (TIPS) RSC Adv. 2016;6(28):23702–23708. [Google Scholar]
- 37.Qi Y, Contreras KG, Jung H-D, Kim H-E, Lapovok R, Estrin Y. Ultrafine-grained porous titanium and porous titanium/magnesium composites fabricated by space holder-enabled severe plastic deformation. Mater Sci Eng, C. 2016;59:754–765. doi: 10.1016/j.msec.2015.10.070. [DOI] [PubMed] [Google Scholar]
- 38.Emelogu A, Marufuzzaman M, Thompson SM, Shamsaei N, Bian L. Additive manufacturing of biomedical implants: a feasibility assessment via supply-chain cost analysis. Addit Manuf. 2016;11:97–113. [Google Scholar]
- 39.Kumar AY, Bai Y, Eklund A, Williams CB. The effects of Hot Isostatic Pressing on parts fabricated by binder jetting additive manufacturing. Addit Manuf. 2018;24:115–124. [Google Scholar]
- 40.Calignano F, Manfredi D, Ambrosio EP, Biamino S, Lombardi M, Atzeni E, Salmi A, Minetola P, Iuliano L, Fino P. Overview on additive manufacturing technologies. Proc IEEE. 2017;105(4):593–612. [Google Scholar]
- 41.Harun W, Kamariah M, Muhamad N, Ghani S, Ahmad F, Mohamed Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018;327:128–151. [Google Scholar]
- 42.Wang X, Xu S, Zhou S, Xu W, Leary M, Choong P, Qian M, Brandt M, Xie YM. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials. 2016;83:127–141. doi: 10.1016/j.biomaterials.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Ni J, Ling H, Zhang S, Wang Z, Peng Z, Benyshek C, Zan R, Miri AK, Li Z, Zhang X. Three-dimensional printing of metals for biomedical applications. Mater Today Bio. 2019;3:100024. doi: 10.1016/j.mtbio.2019.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H, Qian M, Liu N, Zhang X, Yang G, Wang J. Effect of powder reuse times on additive manufacturing of Ti–6Al–4V by selective electron beam melting. JOM. 2015;67(3):555–563. [Google Scholar]
- 45.Sun P, Fang ZZ, Xia Y, Zhang Y, Zhou C. A novel method for production of spherical Ti–6Al–4V powder for additive manufacturing. Powder Technol. 2016;301:331–335. [Google Scholar]
- 46.Sun Y, Aindow M, Hebert RJ. Comparison of virgin Ti–6Al–4V powders for additive manufacturing. Addit Manuf. 2018;21:544–555. [Google Scholar]
- 47.Carroll BE, Palmer TA, Beese AM. Anisotropic tensile behavior of Ti–6Al–4V components fabricated with directed energy deposition additive manufacturing. Acta Mater. 2015;87:309–320. [Google Scholar]
- 48.Chen G, Zhao S, Tan P, Wang J, Xiang C, Tang H. A comparative study of Ti–6Al–4V powders for additive manufacturing by gas atomization, plasma rotating electrode process and plasma atomization. Powder Technol. 2018;333:38–46. [Google Scholar]
- 49.McCracken CG, Motchenbacher C, Barbis DP. Review of titanium-powder-production methods. Int J Powder Metall. 2010;46(5):19–26. [Google Scholar]
- 50.Kruth J-P, Wang X, Laoui T, Froyen L. Lasers and materials in selective laser sintering. Assem Autom. 2003;23:357–371. [Google Scholar]
- 51.Beaman JJ, Deckard CR. Selective laser sintering with assisted powder handling. In: Google Patents; 1990.
- 52.Xie F, He X, Cao S, Mei M, Qu X. Influence of pore characteristics on microstructure, mechanical properties and corrosion resistance of selective laser sintered porous Ti–Mo alloys for biomedical applications. Electrochim Acta. 2013;105:121–129. [Google Scholar]
- 53.Xie F, He X, Lv Y, Wu M, He X, Qu X. Selective laser sintered porous Ti–(4–10) Mo alloys for biomedical applications: structural characteristics, mechanical properties and corrosion behaviour. Corros Sci. 2015;95:117–124. [Google Scholar]
- 54.Guden M, Celik E, Cetiner S, Aydin A. Metals foams for biomedical applications: processing and mechanical properties. In: Biomaterials. Springer; 2004. p. 257–66. [DOI] [PubMed]
- 55.Zhou B, Zhou J, Li H, Lin F. A study of the microstructures and mechanical properties of Ti6Al4V fabricated by SLM under vacuum. Mater Sci Eng, A. 2018;724:1–10. [Google Scholar]
- 56.Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Kadri NA, Osman NAA. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater. 2015;16(3):033502. doi: 10.1088/1468-6996/16/3/033502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kruth J-P, Vandenbroucke B, Van Vaerenbergh J, Naert I. Rapid manufacturing of dental prostheses by means of selective laser sintering/melting. Proc AFPR S. 2005;4:176–186. [Google Scholar]
- 58.Ataee A, Li Y, Brandt M, Wen C. Ultrahigh-strength titanium gyroid scaffolds manufactured by selective laser melting (SLM) for bone implant applications. Acta Mater. 2018;158:354–368. [Google Scholar]
- 59.Yan C, Hao L, Hussein A, Wei Q, Shi Y. Microstructural and surface modifications and hydroxyapatite coating of Ti–6Al–4V triply periodic minimal surface lattices fabricated by selective laser melting. Mater Sci Eng, C. 2017;75:1515–1524. doi: 10.1016/j.msec.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 60.Jamshidi P, Aristizabal M, Kong W, Villapun V, Cox SC, Grover LM, Attallah MM. Selective laser melting of Ti–6Al–4V: the impact of post-processing on the tensile, fatigue and biological properties for medical implant applications. Materials. 2020;13(12):2813. doi: 10.3390/ma13122813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cox SC, Jamshidi P, Eisenstein NM, Webber MA, Hassanin H, Attallah MM, Shepherd DE, Addison O, Grover LM. Adding functionality with additive manufacturing: fabrication of titanium-based antibiotic eluting implants. Mater Sci Eng, C. 2016;64:407–415. doi: 10.1016/j.msec.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Hassanin H, Finet L, Cox SC, Jamshidi P, Grover LM, Shepherd DE, Addison O, Attallah MM. Tailoring selective laser melting process for titanium drug-delivering implants with releasing micro-channels. Addit Manuf. 2018;20:144–155. [Google Scholar]
- 63.Vaithilingam J, Kilsby S, Goodridge RD, Christie SD, Edmondson S, Hague RJ. Functionalisation of Ti6Al4V components fabricated using selective laser melting with a bioactive compound. Mater Sci Eng, C. 2015;46:52–61. doi: 10.1016/j.msec.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 64.Attar H, Calin M, Zhang L, Scudino S, Eckert J. Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mater Sci Eng, A. 2014;593:170–177. [Google Scholar]
- 65.Parthasarathy J, Starly B, Raman S, Christensen A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM) J Mech Behav Biomed Mater. 2010;3(3):249–259. doi: 10.1016/j.jmbbm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Koike M, Martinez K, Guo L, Chahine G, Kovacevic R, Okabe T. Evaluation of titanium alloy fabricated using electron beam melting system for dental applications. J Mater Process Technol. 2011;211(8):1400–1408. [Google Scholar]
- 67.Heinl P, Körner C, Singer RF. Selective electron beam melting of cellular titanium: mechanical properties. Adv Eng Mater. 2008;10(9):882–888. [Google Scholar]
- 68.Gao C, Wang C, Jin H, Wang Z, Li Z, Shi C, Leng Y, Yang F, Liu H, Wang J. Additive manufacturing technique-designed metallic porous implants for clinical application in orthopedics. RSC Adv. 2018;8(44):25210–25227. doi: 10.1039/c8ra04815k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sing SL, An J, Yeong WY, Wiria FE. Laser and electron-beam powder-bed additive manufacturing of metallic implants: a review on processes, materials and designs. J Orthop Res. 2016;34(3):369–385. doi: 10.1002/jor.23075. [DOI] [PubMed] [Google Scholar]
- 70.Herzog D, Seyda V, Wycisk E, Emmelmann C. Additive manufacturing of metals. Acta Mater. 2016;117:371–392. [Google Scholar]
- 71.Sames WJ, List F, Pannala S, Dehoff RR, Babu SS. The metallurgy and processing science of metal additive manufacturing. Int Mater Rev. 2016;61(5):315–360. [Google Scholar]
- 72.Körner C. Additive manufacturing of metallic components by selective electron beam melting—a review. Int Mater Rev. 2016;61(5):361–377. [Google Scholar]
- 73.Milberg J, Sigl M. Electron beam sintering of metal powder. Prod Eng. 2008;2(2):117–122. [Google Scholar]
- 74.Cheng X, Li S, Murr L, Zhang Z, Hao Y, Yang R, Medina F, Wicker R. Compression deformation behavior of Ti–6Al–4V alloy with cellular structures fabricated by electron beam melting. J Mech Behav Biomed Mater. 2012;16:153–162. doi: 10.1016/j.jmbbm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Mumtaz K, Hopkinson N. Selective laser melting of thin wall parts using pulse shaping. J Mater Process Technol. 2010;210(2):279–287. [Google Scholar]
- 76.Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng, C. 2017;78:1246–1262. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 77.Yan C, Hao L, Hussein A, Young P. Ti–6Al–4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J Mech Behav Biomed Mater. 2015;51:61–73. doi: 10.1016/j.jmbbm.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 78.Murr LE, Gaytan SM, Martinez E, Medina F, Wicker RB. Next generation orthopaedic implants by additive manufacturing using electron beam melting. Int J Biomater. 2012;2012:245727. [DOI] [PMC free article] [PubMed]
- 79.Yan R, Luo D, Huang H, Li R, Yu N, Liu C, Hu M, Rong Q. Electron beam melting in the fabrication of three-dimensional mesh titanium mandibular prosthesis scaffold. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-017-15564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yánez A, Herrera A, Martel O, Monopoli D, Afonso H. Compressive behaviour of gyroid lattice structures for human cancellous bone implant applications. Mater Sci Eng, C. 2016;68:445–448. doi: 10.1016/j.msec.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 81.Acquesta A, Monetta T. As-built EBM and DMLS Ti–6Al–4V parts: topography-corrosion resistance relationship in a simulated body fluid. Metals. 2020;10(8):1015. [Google Scholar]
- 82.Chudinova EA, Surmeneva MA, Timin AS, Karpov TE, Wittmar A, Ulbricht M, Ivanova A, Loza K, Prymak O, Koptyug A. Adhesion, proliferation, and osteogenic differentiation of human mesenchymal stem cells on additively manufactured Ti6Al4V alloy scaffolds modified with calcium phosphate nanoparticles. Colloids Surf B Biointerfaces. 2019;176:130–139. doi: 10.1016/j.colsurfb.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 83.Ataee A, Li Y, Wen C. A comparative study on the nanoindentation behavior, wear resistance and in vitro biocompatibility of SLM manufactured CP–Ti and EBM manufactured Ti64 gyroid scaffolds. Acta Biomater. 2019;97:587–596. doi: 10.1016/j.actbio.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Selcuk C. Laser metal deposition for powder metallurgy parts. Powder Metall. 2011;54(2):94–99. [Google Scholar]
- 85.Dinda G, Song L, Mazumder J. Fabrication of Ti–6Al–4V scaffolds by direct metal deposition. Metall Mater Trans A. 2008;39(12):2914–2922. [Google Scholar]
- 86.Li Y, Hu Y, Cong W, Zhi L, Guo Z. Additive manufacturing of alumina using laser engineered net shaping: effects of deposition variables. Ceram In. 2017;43(10):7768–7775. [Google Scholar]
- 87.Sahasrabudhe H, Bandyopadhyay A. Additive manufacturing of reactive in situ Zr based ultra-high temperature ceramic composites. JOM. 2016;68(3):822–830. [Google Scholar]
- 88.España FA, Balla VK, Bose S, Bandyopadhyay A. Design and fabrication of CoCrMo alloy based novel structures for load bearing implants using laser engineered net shaping. Mater Sci Eng, C. 2010;30(1):50–57. [Google Scholar]
- 89.Marattukalam JJ, Singh AK, Datta S, Das M, Balla VK, Bontha S, Kalpathy SK. Microstructure and corrosion behavior of laser processed NiTi alloy. Mater Sci Eng, C. 2015;57:309–313. doi: 10.1016/j.msec.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 90.Balla VK, Bodhak S, Bose S, Bandyopadhyay A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010;6(8):3349–3359. doi: 10.1016/j.actbio.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue W, Krishna BV, Bandyopadhyay A, Bose S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007;3(6):1007–1018. doi: 10.1016/j.actbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 92.Maharubin S, Hu Y, Sooriyaarachchi D, Cong W, Tan GZ. Laser engineered net shaping of antimicrobial and biocompatible titanium-silver alloys. Mater Sci Eng, C. 2019;105:110059. doi: 10.1016/j.msec.2019.110059. [DOI] [PubMed] [Google Scholar]
- 93.Revathi A, Mitun D, Balla VK, Dwaipayan S, Devika D, Manivasagam G. Surface properties and cytocompatibility of Ti–6Al–4V fabricated using Laser Engineered Net Shaping. Mater Sci Eng, C. 2019;100:104–116. doi: 10.1016/j.msec.2019.02.099. [DOI] [PubMed] [Google Scholar]
- 94.Revathi A, Das M, Balla VK, Devika D, Sen D, Manivasagam G. Surface engineering of LENS-Ti–6Al–4V to obtain nano-and micro-surface topography for orthopedic application. Nanomed Nanotechnol Biol Med. 2019;18:157–168. doi: 10.1016/j.nano.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Bandyopadhyay A, Espana F, Balla VK, Bose S, Ohgami Y, Davies NM. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010;6(4):1640–1648. doi: 10.1016/j.actbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meteyer S, Xu X, Perry N, Zhao YF. Energy and material flow analysis of binder-jetting additive manufacturing processes. Procedia CIRP. 2014;15:19–25. [Google Scholar]
- 97.Sachs E, Cima M, Williams P, Brancazio D, Cornie J. Three dimensional printing: rapid tooling and prototypes directly from a CAD model. J Ind Eng. 1992;114:481–488. [Google Scholar]
- 98.Ziaee M, Crane NB. Binder jetting: a review of process, materials, and methods. Addit Manuf. 2019;28:781–801. [Google Scholar]
- 99.Fang ZZ, Paramore JD, Sun P, Chandran KR, Zhang Y, Xia Y, Cao F, Koopman M, Free M. Powder metallurgy of titanium—past, present, and future. Int Mater Sci. 2018;63(7):407–459. [Google Scholar]
- 100.Sun P, Fang ZZ, Zhang Y, Xia Y. Review of the methods for production of spherical Ti and Ti alloy powder. JOM. 2017;69(10):1853–1860. [Google Scholar]
- 101.Yolton CF. Method for producing titanium particles. In: Google Patents; 1992.
- 102.Yolton CF. Gas atomized titanium and titanium aluminide alloys. In: P/M in aerospace and defense technologies; 1990. p. 123–31.
- 103.Yolton C, Froes FHS. Conventional titanium powder production. In: Titanium powder metallurgy. Elsevier; 2015. p. 21–32.
- 104.Suzuki RO, Ono K. OS process—a new calciothermic reduction of TiO2 in the molten CaCl2. In: Proceedings of the 18th Annual ITA Conference; 2002. p. 6–8.
- 105.Heidloff A, Rieken J, Anderson I, Byrd D, Sears J, Glynn M, Ward R. Advanced gas atomization processing for Ti and Ti alloy powder manufacturing. JOM. 2010;62(5):35–41. [Google Scholar]
- 106.Heidloff A, Rieken J, Anderson I, Byrd D. Advancements in Ti alloy powder production by close-coupled gas atomization. In: Ames Laboratory (AMES), Ames, IA (United States); 2011.
- 107.Hohman M, Ludwig N. System for the production of powders from metals. In: Google Patents; 1994.
- 108.Hanusiak WM, McBride DR. Titanium powder production apparatus and method. In: Google Patents; 2018.
- 109.Entezarian M, Allaire F, Tsantrizos P, Drew R. Plasma atomization: a new process for the production of fine, spherical powders. JOM. 1996;48(6):53–55. [Google Scholar]
- 110.Tsantrizos PG, Allaire F, Entezarian M. Method of production of metal and ceramic powders by plasma atomization. In: Google Patents; 1998.
- 111.Dion CAD, Kreklewetz W, Carabin P. Plasma apparatus for the production of high quality spherical powders at high capacity. In: Google Patents; 2018.
- 112.Miller S, Roberts P. ASM handbook volume 7, powder metal technologies and applications. Materials Park: ASM International; 1990. [Google Scholar]
- 113.Dai Y, Li L. Preparation of airborne 3D-printed metallic powders by plasma rotational atomization. Adv Mater Ind. 2016;16(8):57–63. [Google Scholar]