Abstract
Microbial and plant assisted bioremediation is an emerging way for the remediation of soils polluted with heavy metals. To screen the cadmium tolerant bacteria, soil samples were collected from Nanjing mining area, China. The average cadmium content of the mine soil reached 45.71 mg/kg, which was indicating serious pollution and potential ecological risk. From the mine soil, six cadmium tolerant plant growth-promoting rhizobacteria (PGPR) were isolated. The isolated bacterial strain “SY-2” showed maximum cadmium tolerance and it was selected for further experimentation. This strain was identified as Stenotrophomonas maltophilia by 16S rRNA gene sequencing (GenBank accession number MG597057). SY-2 was found to tolerate maximum cadmium at 1.0 mM concentration. This strain also exhibited good adsorption capacity (up to 35.7%) of heavy metal at 0.5 mM concentration. The results of this study exhibited organic phosphorus solubilization (37.08 mg/L) and IAA biosynthesis (15.11 mg/L) ability of isolated S. maltophilia. Scanning electron microscopy (SEM) revealed cell shrinkage and the cell wall of S. maltophilia was very rough. Moreover, the energy dispersive X-ray (EDX) analysis endorsed the adsorption of Cd ions on the surface of biomass. FT-IR study described the presence of functional groups and the nature of chemical bonds, before and after cadmium stress. At 0.25 mM cadmium concentration, S. maltophilia treated seeds of Capsicum annuum L. developed 1.46 times longer roots than untreated seeds. The results of this study helped us to conclude that SY-2 strain of S. maltophilia possesses significant metal tolerance and bioremediation potential against cadmium. In the future, this strain can be used as a microbial remediation agent to detoxify heavy metals in contaminated soils.
Electronic Supplementary Material
The online version of this article (10.1007/s13205-020-02524-7s) contains supplementary material, which is available to authorized users.
Keywords: Stenotrophomonas maltophilia, Heavy metal, PGPR, Cadmium, SEM
Introduction
Mining activity is one of the major sources of heavy metals, released into the ecosystem. Soil, air, and water are the key environmental commodities, contaminated by heavy metals (Afzal et al. 2017). Metals cannot be degraded and with increasing industrial and mining activities, large volumes of heavy metals have been deposited in the soil (Li et al. 2015). The presence and accumulation of heavy metals in the rhizosphere persists and accumulates in the food chain and triggers severe human health issues (Chibuike and Obiora 2014). Soil heavy metal pollution could be described as the deposition of heavy metals like lead, mercury, cadmium, and chromium, etc. Cadmium (Cd) is described as a very toxic and nonessential metal that causes diseases in humans and animals (Cuypers et al. 2010). Cd is harmful, even at low concentrations (Alkorta et al. 2004). At high concentrations, Cd adversely affects plant growth (Wani et al. 2007). Cd accumulates in plants and causes various physiological and biochemical alterations (Feng et al. 2010). It affects mineral uptake and photosynthetic processes (Hossain et al. 2010). Due to Cd contamination, the crop yield is reduced, severely (di Toppi and Gabbrielli 1999).
Few studies have described the remediation of polluted sites with landfilling, physio-chemical extraction, and excavation but these techniques are very costly and require high energy and chemicals (Jeyasingh and Philip 2005). In the recent era, ‘‘bioremediation’’ approach has gained extreme attention to clean up polluted soils. This technique is cost-effective and ecofriendly (Wu et al. 2006). For the detoxification of heavy metals, different fungi, bacteria, algae, and plants have been extensively used (Nevita et al. 2013). In microbial remediation, microorganisms do immobilization or transformation to cope up with the heavy metal stress (Ma et al. 2011). Through biosorption and bioaccumulation processes, the living and dead biomass of microbes remove metal ions (Joutey et al. 2015). Biosorption is an energy-independent process (Velásquez and Dussan 2009), with low operational cost and better performance (Göksungur et al. 2005).
Bio-fertilizers are composed of living organisms (particularly microorganisms) and these are considered as one of the best alternatives of synthetic chemical agents (Ashraf et al. 2011). Among these microorganisms, plant growth-promoting rhizobacteria (PGPR) are considered the most promising group (Haiyambo et al. 2015). PGPRs, initially described by Kloepper and Schroth (1978), are mainly defined as a heterogeneous group of bacteria that promotes the growth of plants by directly or indirectly affecting the soil environment (Wang et al. 2014). The beneficial effects of PGPRs are generally observed by the increase in seed germination, shoot and root growth, crop yield, total leaf area, total chlorophyll, and nitrogen content, and delay in leaf senescence (Ashraf et al. 2011). PGPRs including Agrobacterium spp., Acinetobacter spp., Bacillus spp., Xanthomonas spp., Pseudomonas spp., Azotobacter spp., Erwinia spp., Enterobacter spp., Flavobacterium spp. and Serratia spp., etc. have been reported to be useful in soil remediation (Ullah et al. 2015). Due to these valuable properties, isolation, characterization, and application of plant growth-promoting indigenous bacterial strains have become a key concept to develop novel bio-fertilizers (Karadayi et al. 2016).
The current investigation was designed for the isolation, morphological observation, and phylogenetic identification of cadmium tolerant PGPRs, from the mining fields of Nanjing, China. This study also focused on phosphate solubilization, siderophore secretion, and IAA producing properties of isolated bacteria.
Materials and methods
Soil collection and analysis
To isolate heavy metal tolerant bacteria, soil samples were collected from the mine (latitude 118˚57′2" E, longitude 32˚9′23" N), located in Nanjing, the northern part of Jiangsu Province, China. The whole site was divided into three plots and the samples were taken according to its topographical features. From each plot, three samples were taken at 0–20 cm depth and transferred aseptically to the research laboratory, within 1 h (Naseem and Bano 2014). To determine the content of manganese (Mn), nickel (Ni), chromium (Cr), arsenic (As), cadmium (Cd), and copper (Cu), the air-dried soil samples were ground and digested (Sinha and Paul 2014). For metal analysis, conditions described in AOAC (1990) were followed and atomic absorption spectrophotometer (AAS) was used, with a graphite furnace (Hitachi Z-8100, Japan).
Isolation of cadmium tolerant bacteria
For this purpose, 3 g of soil samples were aseptically suspended in 100 mL sterilized water and placed in an incubator shaker at 35 °C and 150 rpm, for 30 min. The serial dilution was performed to isolate bacterial strains. The solution (1 mL) was further diluted in 9 mL sterile water and the dilution series was prepared between 10–1 and 10–7. These dilutions were aseptically spread on plates containing Luria Bertini (LB) agar media, supplemented with peptone (10 g/L), yeast extract (5 g/L), NaCl (10 g/L), agar (15 g/L) and CdCl2 (0.89 mM). The petri plates were incubated for 48 h at 35 °C to grow distinct bacterial colonies on LB media. Well grown colonies were considered as Cd resistant bacteria and the plates were further stored at − 80 °C.
Molecular characterization and phylogenetic analysis
For the identification of isolated bacterial strain, 16S rRNA gene was amplified and sequenced. DNA from bacterial culture was isolated using a standard protocol (Wright et al. 2017). The isolated DNA was visualized on 0.8% agarose gel and its quality was evaluated by using NanoDrop ND-1000 (NanoDrop Technologies Inc., Wilmington, DE, USA). For the amplification of isolated DNA, universal forward and reverse primers (27F, 1492R) were used in the C-1000 thermal cycler (Bio-Rad, Hercules, USA). For PCR, 20–30 ng genomic DNA and 10 mM of each forward and reverse primers were mixed in the master mix. The cycling parameters were 94 °C for 4 min, followed by 34 cycles of 94 °C for 40 s, 55 °C for 50 s, 72 °C for 45 s, and a final extension step of 72 °C for 4 min. The amplified PCR product was purified and sequenced. BLASTn search program (https://www.ncbi.nlm.nih.gov) was used to determine the homology of 16S rRNA gene sequence. Sixteen-related sequences were used to construct a phylogenetic tree, using neighbor-joining method. The obtained sequence was submitted in GenBank NCBI database (accession number MG597057).
Effect of cadmium on bacterial growth
The influence of Cd on the growth of the bacteria was studied by conducting a time course growth analysis of the bacteria, in the presence of Cd. For this purpose, LB broth media supplemented with 0.5 mM CdCl2 and without CdCl2 (control) were prepared. These medium tubes were inoculated with 20 μL inoculums of isolated strains and incubated on a shaker at 35 °C, at 150 rpm. After every 2 h, tubes were removed and cell density was measured with a spectrophotometer. The growth curve was plotted by the readings, obtained from the experiment (Shakoori et al. 2010).
Maximum tolerable concentration (MTC)
To determine the MTC of Cd, the methodology of Vashishth and Khanna (2015) was followed. The Cd stock solution was filter-sterilized and added into autoclaved LB medium to the final concentration of 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, and 1.75 mM for testing bacterial MTCs. Maximum concentration that showed visible bacterial growth was selected after 18 h of incubation.
Biosorption potential evaluation
The biosorption potential of the selected bacterial strain (SY-2) was evaluated against Cd. For this purpose, the initial solution of Cd (0.5 mM) was autoclaved and 2 mL of 18–20 h old bacterial culture (10–8 CFU/mL) having 0.5 McFarland turbidity was inoculated in 200 mL sterilized LB broth. Bacterial culture bottles were placed in an incubator shaker for 24 h at 35 °C and 150 rpm. The culture bottles were then centrifuged for 5 min at 14,000 rpm. Pellets were discarded and the supernatants were kept at − 20 °C. Inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 8000, Perkin Elmer, USA) was used to measure the residual concentrations of Cd in supernatants (Ramyakrishna and Sudhamani 2016). The experiment was performed in three replicates. Adsorption rate percentage was calculated by using the following equation:
wherein, C0 is the initial concentration of Cd (mM) and C is a residual concentration of Cd (mM).
Plant growth-promoting characteristics of bacterial strain
Phosphorus solubilizing capacity
For this analysis, SY-2 strain was plated onto Pikovskaya’s agar plates and incubated at 35 °C. To see halo formation, plates were observed for up to 5–7 days (Ramachandran et al. 2007). To measure soluble phosphorus in culture media, Pikovskaya’s broth containing SY-2 strain (100 mL) was placed at 35 °C for 72 h, in a shaking incubator. After specific intervals (24, 48, and 72 h), each culture was centrifuged for 10 min and the supernatants were collected to observe the soluble phosphorus content, using the Molybdate blue (Mo-blue) method (Murphy and Riley 1962).
Indole-3-acetic acid-producing ability
To determine the amounts of IAA synthesized by the bacterial strain, Salkowski's method was used (Khamna et al. 2010). The strain was inoculated in Yeast malt dextrose (YMD) broth supplemented with 0, 250, 500 and 750 mg/L tryptophan, respectively, and incubated on a shaker at 35 °C and 150 rpm for 3 days. The broth was centrifuged for 10 min at 12,000×g after incubation and 1 mL of supernatant was mixed with 2 mL of reagent (2% 0.5 M FeCl3 in 35% HClO4 solution). This mixture was kept in the dark for 30 min, and its optical density (OD) was recorded at 530 nm.
Siderophore producing ability
Siderophore production capability of the isolate was measured by chrome azurol-S (CAS) analytical method (Goswami et al. 2013). In this procedure, the isolate was inoculated in Fiss minimal medium (containing 5.03 g/L KH2PO4, 40 mg/L MgSO4, 100 µg/L MnSO4, 5 g/L glucose, 500 µg/L ZnCl2 and 5.03 g/L asparagine). Fiss minimal medium was amended with 0.25 mM and 0.5 mM Cd and the bacterial isolate was grown for 48 h at 30 °C. After incubation, 10 ml of the sample was collected and centrifuged for 15 min. The supernatant (0.5 ml) was taken, mixed with CAS assay solution (0.5 ml), and allowed to stand for 20 min. Finally, the siderophore production was done at 630 nm.
Seed germination assay
The SY-2 strain was cultured for 24 h at 35 ℃ in LB medium and the culture was diluted to 108 CFU/mL concentration. Seeds of Capsicum annuum L. were surface sterilized with 1% sodium hypochlorite solution (for 3 min) and 70% ethanol (for 2 min) and rinsed with distilled water. Sterilized seeds were soaked in bacterial suspension (108 CFU/mL) for 4 h. Seeds soaked in distilled water were used as control. In individual sterile petri plates, 25 treated and control seeds were placed, separately and the plates were supplemented with 3 ml of solutions containing 0, 0.25, and 0.5 mM Cd, respectively. Seeds were germinated at 25 °C in an incubator (HP1000GS, Ruihua, Wuhan, China). Seeds germination rate was recorded every 24 h and root length was measured. This experiment was performed in three replicates (Saleemi et al. 2017).
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) analysis
Effect of Cd on surface features of SY-2 strain was observed using SEM, coupled with EDX. For this purpose, strain SY-2 was grown for 24 h in LB broth, supplemented with 0.5 mM CdCl2 and without Cd (control). The broth was centrifuged for 15 min at 8000 rpm, and the pellet was washed thrice with phosphate buffer (pH 7.0). At 4 °C, the samples were fixed with 10% glutaraldehyde for 100–120 min. The biomass was centrifuged for 15 min at 8000 rpm and dehydrated with 50% and 100% ethanol. The samples were dried at 60 °C (Moharrer et al. 2012). The dried samples were then coated with carbon tapes and sputtered thrice with gold particles to make them electrically conducting. The topological and morphological characteristics of samples were determined using SEM (JOEL JED 230 instrument) and EDX was used to observe their chemical characteristics (Hartmann et al. 2010).
Fourier transform infrared spectroscopy (FT‑IR) analysis of SY-2 strain biomass
For FT-IR analysis of the control and tested samples, 2 mg of freeze-dried biomass was ground with 200 mg of dry potassium bromide powder in 1:100 ratios. To get translucent sample discs of the mixture, the pressure bench press was used. PerkinElmer Spectrum Version 10.4.3 was used to perform FT-IR analysis and over the range of 450–4000/cm, the spectral data were collected (Ramyakrishna and Sudhamani 2016).
Statistical analysis
All the experiments were performed in triplicate. To get mean and standard deviation, SPSS and MS-Excel 2007 software (Microsoft Inc.) were used. Post hoc Fisher Least Significant Difference (LSD) test was used to compare treatment means at P < 0.05 level. Pollution index (Pi) and Nemerow pollution index (Pcom) were calculated, following the methods of Guan et al. (2014) and Kowalska et al. (2016). Potential Ecological Risk (RI) was determined, following Hakanson (1980) and Kowalska et al. (2016).
Results
Soil analysis
The results of atomic absorption spectroscopy of nine samples revealed the presence of six different heavy metals (Table 1). Contamination levels of six individual elements were found to be in descending order of Cd > As > Mn > Cu > Cr > Ni. The comprehensive analysis revealed serious heavy metal pollution of the plot, in which Cd was the most dominating element. On the basis of these results, Cd was selected for further study.
Table 1.
Heavy metal content, pollution index and the potential ecological risk index of collected soil
| Elements | ||||||
|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Ni | |
| Content (mg/kg) | 503.41 ± 501.45 | 45.71 ± 32.35 | 92. ± 30.73 | 216.46 ± 84.42 | 14,015.31 ± 8581.75 | 41.13 ± 49.54 |
| Pi | 12.59 ± 12.54 | 45.71 ± 32.35 | 0.31 ± 0.10 | 0.54 ± 0.21 | 11.68 ± 7.15 | 0.21 ± 0.25 |
| Eri | 474.91 ± 473.07 | 7217.05 ± 5107.89 | 3.15 ± 1.04 | 33.61 ± 3.11 | 27.43 ± 16.79 | 5.88 ± 7.08 |
Pi pollution index, Eri potential ecological risk index
Identification of bacterial isolate
In six out of nine soil samples, Cd tolerant bacteria were present. About 3–6 colonies were grown on each plate and 26 total colonies were observed and isolated in LB broth. Among these, one bacterial strain, showing the most significant growth was purified and referred to as Strain SY-2. This strain was used for further characterization. SY-2 colonies appeared in pale yellow color on LB media plate and showed violet color by Gram staining. The cells were in rod shape with polar flagella.
Molecular characterization and phylogenetic analysis
Molecular characterization confirmed 99% similarity of SY-2 with Stenotrophomonas maltophilia strain IAM 12423 (NR041577). The phylogenetic tree also helped us to identify this isolate (Supplementary 1). The strain SY-2 sequence was submitted to GenBank NCBI database (accession number MG 597057).
Effect of cadmium on bacterial growth
The growth curve experiment helped us to observe the effect of Cd on bacterial growth at different time intervals (Fig. 1) and concentrations (Fig. 2). At 0, 0.25, and 0.50 mM concentration of Cd, the bacterial strain showed strong resistance to Cd, while at 0.75 and 1.0 mM concentration, SY-2 strain did grow normally and showed Cd tolerance. A higher concentration of Cd (1.25 and 1.50 mM) completely inhibited the growth of the bacterial strain. These findings declared 1 mM as the MTC of Cd for strain SY-2.
Fig. 1.

Growth curve of strain SY-2 in control (CK) and at 0.5 mM Cd concentration, at different time intervals
Fig. 2.
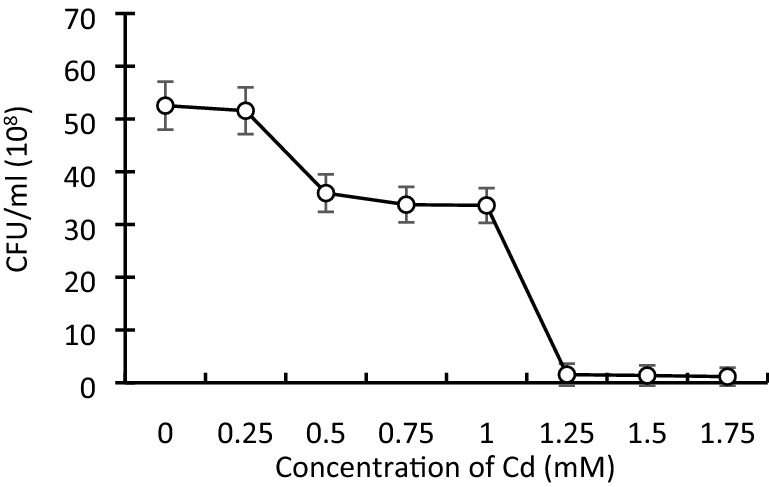
Effect of various Cd concentrations on the growth of SY-2 strain
Evaluation of biosorption potential
Results showed that the growth of bacteria and Cd biosorption rate increased rapidly with the culture time extending at the early stage (Table 2). After 24 h, Cd biosorption rate was the maximum (35.7%). Subsequently, the growth and adsorption rate decreased, simultaneously.
Table 2.
Growth of SY-2 strain and the biosorption rate of Cd at different incubation intervals
| Time (h) | 6 | 12 | 18 | 24 | 30 | 36 |
|---|---|---|---|---|---|---|
| CFU (108/mL) | 4.98 | 8.23 | 12.71 | 19.11 | 10.63 | 8.56 |
| Biosorption rate | 3.64% | 19.71% | 25.20% | 35.72% | 31.07% | 30.50% |
Plant growth-promoting characteristics of bacterial strain
The formation of a clear zone around the colony of SY-2 strain indicated its organic phosphorus solubilizing ability (Supplementary 2). The results indicated that the concentration of available phosphorus in the media increased from 1 to 7 days (Fig. 3). The strain was found to produce phytohormone indole acetic acid (IAA). It was observed that by the addition of l-tryptophan, the IAA producing ability of strain was enhanced (Fig. 4). Strain SY-2 also showed the ability to produce siderophore. The absorbance of sample (A) and absorbance of reference (Ar) were used to calculate A/Ar value which represented relative capability to produce siderophore. It was evident from our results that the SY-2 did not have a strong ability to produce siderophore (Fig. 5).
Fig. 3.
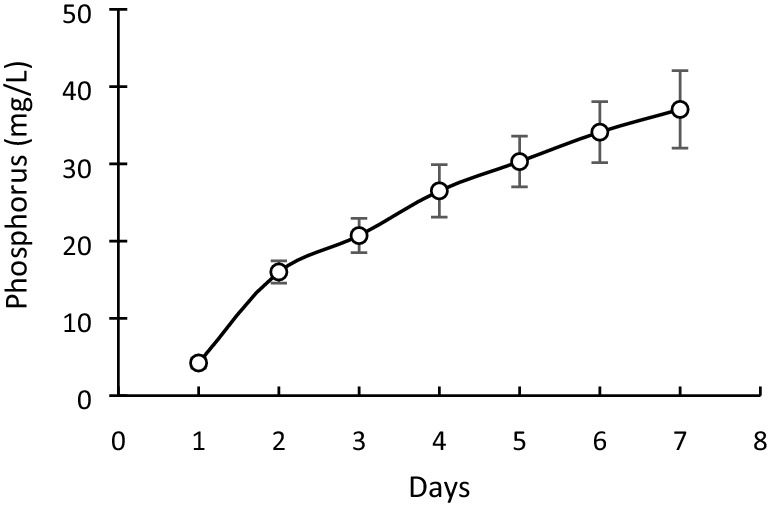
Estimation of available P in SY-2 strain, at different time intervals. The bars indicate standard deviations
Fig. 4.

IAA concentrations in SY-2 strain cultures, supplemented with various dosages of tryptophan. The bars indicate standard deviations
Fig. 5.

Siderophore production ability of SY-2 strain. The bars indicate standard deviations
Seed germination assay
Increasing concentration of Cd negatively affected the germination (Fig. 6a) and root growth of C. annuum seeds (Fig. 6b). The germination rate of seeds treated with SY-2 was higher than that of the seeds treated with water at 0.25 and 0.5 mM concentration of Cd (P > 0.05). Water treated seeds of C. annuum exhibited shorter root length than SY-2 strain treated seeds. SY-2 strain treatment significantly enhanced root growth at 0 mM Cd concentration. At 0.25 mM Cd concentration, the root growth of SY-2 treated plants was decreased but it was still better than water treated seeds. At 0.5 mM Cd concentration, root growth of all plants was inhibited.
Fig. 6.
Germination rate (a) and root length (b) of Capsicum annuum seeds soaked in water (Control = CK) and SY-2 strain culture at different concentrations of Cd
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) analysis
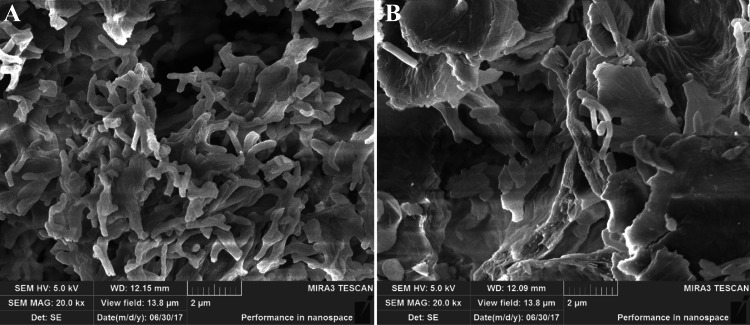
Variable changes in the surface of the bacteria treated by Cd were observed with SEM. In Cd free culture, the bacterial cell number was significantly higher, and the bacterial cell surface was smooth. The cells were closely connected to each other, providing a large surface area for biosorption. After the adsorption of Cd, the surface of the bacterial cell became rough and the cell shrinkage was observed (Fig. 7).
Fig. 7.
SEM images of SY-2 strain before (a) and after (b) cadmium uptake
X-ray spectrum analyzer (EDX) efficiently measured mass changes and atoms’ number of each element. The results indicated significant changes in the cell surface due to Cd adsorption (Supplementary 3). In the control, the main elements of the cell surface were carbon, oxygen, and phosphorus in a weight percentage (Wt%) of 72.00%, 23.54% and 1.76%, respectively, and the number percentages of these atoms (At %) were 79.08%, 19.41% and 0.75%, respectively. There no Cd was detected in control cells. On the surface of bacteria cells treated with 0.5 mM Cd for 24 h, carbon dramatically decreased to 49.27% in the weight percentage, and decreased to 70.01% in the number percentage of atoms, while phosphorus increased to 2.33% (Wt%) and 1.29% (At %). Moreover, Cd increased to 17.46% (Wt%) and 2.65% (At %), respectively (Table 3).
Table 3.
EDX analysis of SY-2 strain before and after cadmium uptake
| Elements | Before Cd uptake | After Cd uptake | ||
|---|---|---|---|---|
| Weight (%) | Atoms (%) | Weight (%) | Atoms (%) | |
| C | 72.00 | 79.08 | 49.27 | 70.01 |
| O | 23.54 | 19.41 | 20.91 | 22.30 |
| P | 1.76 | 0.75 | 2.33 | 1.29 |
| S | 0.39 | 0.16 | 5.90 | 3.14 |
| Ca | 0.40 | 0.13 | 0.76 | 0.32 |
| Cd | – | – | 17.46 | 2.65 |
FTIR analysis
In Cd free culture conditions, FTIR analysis showed peaks in 3500–1000/cm range. FTIR spectra depicted peaks at 3302, 2927, 1652, 1452, and 1397/cm, which are attributed to the hydroxyl group, carboxyl group, amino group, and hydrocarbon group, respectively. After the adsorption of Cd, FTIR spectra showed peaks at 3417, 2922, 1650, and 1402/cm. Characteristic peaks changed without changing the structure of the original chemical group. The results indicated that after the adsorption of Cd, many functional groups like hydroxyl group, hydrocarbon group, carboxyl group, and the amino group were still attached to the cell surface (Supplementary 4, Table 4).
Table 4.
FTIR analysis of SY-2 strain, before and after cadmium uptake
| Peak | Chemically/functional group | |
|---|---|---|
| Before biosorption | After biosorption | |
| 3302 | 3417 | O–H stretch/hydroxyl and carboxyl groups (carbohydrate and protein) N–H stretch/amide (protein) |
| 2927 | 2922 | C–H stretch/alkanes (lipid) |
| 1652 | 1650 | C = O stretch/proteinamide band I |
| 1452 | – | C–N bend/proteinamide band III |
| 1397 | 1402 | C–H bend/alkyl (protein) |
Discussion
Generally, high levels of toxic heavy metals impose effective pressure on the local biodiversity and microorganisms (Burges et al. 2016). Metal-tolerant bacteria can easily survive in these habitats and can be used for the bioremediation of polluted sites (Piotrowska-Seget et al. 2005). In this regard, plant growth-promoting bacteria are considered as promising candidates to remediate chemically affected soils and meet the agricultural demands of the world population (Mahmoud et al. 2014). During the last few decades, many studies have focused on the isolation and characterization of diverse bacterial strains with plant growth-promoting potential.
The current study described the isolation, phylogenetic identification, and biosorptive potential evaluation of cadmium tolerant bacteria from the lead zinc mining area of Nanjing, China. SY-2 strain was observed to be the Cd tolerant strain and it was identified as Stenotrophomonas maltophilia. In biosorption potential evaluation after 24 h, the percentage reduction in metal concentration was assessed through ICP-OES. The findings of our study are similar to Siñeriz et al. (2009), who observed Cd biosorption by Streptomyces sp. from a uranium mine. Some studies showed that S. maltophilia strain can tolerate Cd, Pb, Co, Zn, Hg, Cu and other heavy metals (Wierzba 2015). Gao et al. (2013) found that S. maltophilia C6 phenanthrene may be utilized as the sole carbon source and it can effectively decompose organic pollutants like phenanthrene. Growing Bacillus cereus M116 has been found to be efficient in the sorption of Cd from aqueous solutions (Ganguly et al. 2011). Raja et al. (2006) found good biosorption potential of Pseudomonas aeruginosa against Ni, Cr, Cd, and Pb. Enterobacter cloacae and Klebsiella species have been described to be resistant against Cd, Cr, and Pb (Haq et al. 1999). Similar findings were described by Afzal et al. (2017) who isolated Klebsiella variicola from industrial effluents and observed a negative impact of bacterial isolates on nickel and cobalt concentrations. Selvi et al. (2012) isolated and characterized metal tolerant bacteria from tannery effluents and described their tolerance to Pb, Zn, Cu, Hg, and Cr.
Our results indicated the organic phosphate solubilizing ability of SY-2 strain. The P-solubilizing activity is actually the microbial biochemical ability to release organic acids (Chen et al. 2006). SY-2 strain also showed a direct relationship of IAA production with L-tryptophan, the precursor of IAA biosynthesis. IAA can promote plant growth by promoting plant lateral and adventitious root development, and increase the tolerance of plants to heavy metals. Pan et al. (2017) have also screened IAA producing Cd resistant Bacillus megaterum (SaN1) strain. Similar findings have been described in copper resistance (He et al. 2010). Ahmad et al. (2008) reported copper resistant Azotobacter, Pseudomonas, and Bacillus. IAA production by Kocuria rosea (Godinho et al. 2010), Kocuria sp. (Vicene et al. 2012) and Kocuria turfanensis (Goswami et al. 2014) has been described earlier. SY-2 strain showed a positive result for siderophore production. Siderophore producing capability of PGPR and relieve heavy metal stress, promote plant growth, and provide iron to the plants (Imsande 1998). The presence of more than one plant growth-promoting traits in bacterial isolates can facilitate plant growth (Luo et al. 2011). The seeds of C. annuum pre-treated with SY-2 strain showed better seed germination and root growth. Strain SY-2 could produce auxin which promotes the growth of young roots. The root length of SY-2 treated seedlings was 1.46 times more than that of control, which is consistent with the previous findings (Tan et al. 2015).
Similar to previous findings, cell morphology of SY-2 strain changed after Cd adsorption; resulting in shrinking of the bacterial cell and the surface became rough (Chakravarty and Banerjee 2008). Further, the EDX analysis confirmed the adsorption of Cd ions on the surface of biomass. Without adsorption of Cd, the bacteria did not show the characteristic signal of Cd. After Cd treatment, a clear signal of Cd peak was observed, which are similar to the findings of Arivalagan et al. (2014). FT-IR study depicted the complex nature of the biomass. The presence of carboxyl amide and phosphate group favors Cd binding to the cell surface. Similar findings have also been described by Park et al. (2005).
Conclusion
On the basis of the above findings, it can be concluded that S. maltophilia, isolated from mining soil of Nanjing, China has remarkable Cd tolerance ability and biosorption potential under varying concentrations of Cd. Isolated SY-2 strain of S. maltophilia has the potential to bio-remediate Cd contaminated soil, in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are highly thankful to the Instrumental Analysis Center of Shanghai Jiao Tong University for analyzing the heavy metal concentration, SEM-EDX and FT-IR.
Author contributions
LQL designed the experiments and supervised the research work. LJK executed the experiments. FL analyzed the data and wrote the manuscript. MFHM supervised manuscript write up and submission. UH collected samples, SA did data compilation and CSQ supervised the research work. All authors read and approved the final manuscript.
Funding
Supported by research fund of Shanghai Landscape Architecture Construction Co., Ltd.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Accession number
MG597057 (Stenotrophomonas maltophilia).
References
- Afzal A, Rasool M, Waseem MH, Aslam MB. Assessment of heavy metal tolerance and biosorptive potential of Klebsiella variicola isolated from industrial effluents. AMB Express. 2017;7(1):184. doi: 10.1186/s13568-017-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163(2):173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alkorta I, Hernandez-Allica J, Becerril JM, Amezaga I, Albizu I, Garbisu I. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Rev Environ Sci Biotechnol. 2004;3:71–90. [Google Scholar]
- Arivalagan P, Singaraj D, Haridass V, Kaliannan T. Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol Eng. 2014;71:728–735. [Google Scholar]
- Ashraf MA, Rasool M, Mirza MS. Nitrogen fixation and indole acetic acid production potential of bacteria isolated from rhizosphere of sugarcane (Saccharum officinarum L.) Adv Biol Res. 2011;6:348–355. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) Official methods of analysis: changes in official methods of analysis made at the annual meeting. Arlington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Burges A, Epelde L, Benito G, Artetxe U, Becerril JM, Garbisu C. Enhancement of ecosystem services during endophyte-assisted aided phytostabilization of metal contaminated mine soil. Sci Total Environ. 2016;562:480–492. doi: 10.1016/j.scitotenv.2016.04.080. [DOI] [PubMed] [Google Scholar]
- Chakravarty R, Banerjee PC. Morphological changes in an acidophilic bacterium induced by heavy metals. Extremophiles. 2008;12(2):279–284. doi: 10.1007/s00792-007-0128-4. [DOI] [PubMed] [Google Scholar]
- Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol. 2006;34(1):33–41. [Google Scholar]
- Chibuike GU, Obiora SC. Heavy metal polluted soils: effect on plants and bioremediation methods. Appl and Environ Soil Sci. 2014;2014:1–12. [Google Scholar]
- Cuypers A, Plusquin M, Remans T, Jozefczak M, Smeets K. Cadmium stress : an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- di Toppi LS, Gabbrielli R. Response to cadmium in higher plants. Environ Exp Bot. 1999;41:105–130. [Google Scholar]
- Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumissativus L. Sci Hortic. 2010;123:521–530. [Google Scholar]
- Ganguly A, Guha AK, Ray L. Adsorption behaviour of cadmium by Bacillus cereus M116: some physical and biochemical studies. Chem Spec Bioavailab. 2011;23(3):175–182. [Google Scholar]
- Gao S, Seo JS, Wang J, Keum YS, Li J, Li QX. Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6[J] Int Biodeter Biodegr. 2013;79(2):98–104. doi: 10.1016/j.ibiod.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho A, Ramesh R, Bhosle S. Bacteria from sand dunes of Goa promoting growth in eggplant. World J Agric Sci. 2010;6:555–564. [Google Scholar]
- Göksungur Y, Üren S, Güvenc U. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol. 2005;96:103–109. doi: 10.1016/j.biortech.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Goswami D, Vaghela H, Parmar S, Dhandhukia C, Thakker JN. Whole genome sequencing and analysis of plant growth promoting potentials of Pseudomonas spp. strain OG isolated from marine water. J Plant Interact. 2013;4:281–290. [Google Scholar]
- Goswami D, Dhandhukia P, Patel P, Thakker JN. Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res. 2014;169(1):66–75. doi: 10.1016/j.micres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Guan Y, Shao C, Ju M. Heavy metal contamination assessment and partition for industrial and mining gathering areas. Int J Env Res Pub He. 2014;11(7):286–7303. doi: 10.3390/ijerph110707286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiyambo DH, Chimwamurombe PM, ReinholdHurek B. Isolation and screening of rhizosphere bacteria from grasses in East Kavango region of Namibia for plant growth-promoting characteristics. Curr Microbiol. 2015;71:566–571. doi: 10.1007/s00284-015-0886-7. [DOI] [PubMed] [Google Scholar]
- Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980;14(8):975–1001. [Google Scholar]
- Haq R, Zaidi SK, Shakoori AR. Cadmium resistant Enterobacter cloacae and Klebsiella sp isolated from industrial effluents and their possible role in cadmium detoxification. World J Microbiol Biotechnol. 1999;15(2):283–290. [Google Scholar]
- He LY, Zhang YF, Ma HY, Chen ZJ, Wang QY, Qian M, Sheng XF. Characterization of copper-resistant bacteria and assessment of bacterial communities in rhizosphere soils of copper-tolerant plants. Appl Soil Ecol. 2010;44(1):49–55. [Google Scholar]
- Hossain MA, Hasanuzzaman M, Fujita M. Upregulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants. 2010;16:259–272. doi: 10.1007/s12298-010-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. Iron, sulfur, and chlorophyll deficiencies: a need for an integrative approach in plant physiology. Physiol Plant. 1998;103(1):139–144. [Google Scholar]
- Jeyasingh J, Philip L. Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. J Hazard Mater. 2005;118(1):113–120. doi: 10.1016/j.jhazmat.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Joutey NT, Sayel H, Bahafid W, El Ghachtouli N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev Environ Contamin Toxicol. 2015;233:45–69. doi: 10.1007/978-3-319-10479-9_2. [DOI] [PubMed] [Google Scholar]
- Karadayi M, Gulluce M, Dogan S, Gulluce E. Isolation and molecular characterization of bacteria with plant growth-promoting characteristics from magnesite mining fields in Kütahya-Turkey. J Appl Biol Sci. 2016;10(3):27–32. [Google Scholar]
- Khamna S, Yokota A, Peberdy JF, Lumyong S. Indole-3-acetic acid production by Streptomyces spp. isolated from some Thai medicinal plant rhizosphere soils. Eurasia J Biosci. 2010;4:23–32. [Google Scholar]
- Kloepper JW, Schroth MN. Association of in vitro antibiosis with inducibility of increased plant growth by Pseudomonas spp. (Abstr) Phytopathol. 1978;12:136. [Google Scholar]
- Kowalska J, Mazurek R, Gąsiorek M, Setlak M, Zaleski T, Waroszewski J. Soil pollution indices conditioned by medieval metallurgical activity—a case study from Krakow (Poland) Environ Pollut. 2016;218:1023–1036. doi: 10.1016/j.envpol.2016.08.053. [DOI] [PubMed] [Google Scholar]
- Li J, Yu H, Luan Y. Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metal-polluted water. Int J Environ Res Public Health. 2015;12:14958–14973. doi: 10.3390/ijerph121214959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SL, Chen L, Chen JL, Xiao X, Xu TY, Wan Y, Zeng GM. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere. 2011;85(7):1130–1138. doi: 10.1016/j.chemosphere.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Ma Y, Prasad MNV, Rajkumar M, Freitas H. Plant growth-promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv. 2011;29(2):248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Mahmoud E, Gizawy EE, Geries L. Effect of compost extract, N2-fixing bacteria and nitrogen levels applications on soil properties and onion crop. Arch Agron Soil Sci. 2014;61:185–201. [Google Scholar]
- Moharrer S, Mohammadi B, Gharamohammadi RA, Yargoli M. Biological synthesis of silver nanoparticles by Aspergillus flavus, isolated from soil of ahar copper mine. Indian J Sci Technol. 2012;5:2443–2444. [Google Scholar]
- Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- Naseem H, Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact. 2014;9:689–701. [Google Scholar]
- Nevita T, Pandey P, Maheshwari DK, Sood A. bacteria in agrobiology: crop productivity. Berlin, Heidelberg: Springer; 2013. Interactions in rhizosphere for bioremediation of heavy metals; pp. 439–461. [Google Scholar]
- Pan F, Meng Q, Luo S, Shen J, Chen B, Khan KY, Japenga J, Ma X, Yang X, Feng Y. Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth-promoting bacteria isolated from Cd hyperaccumulator Sedum Hance. Int J Phytorem. 2017;19(3):281–289. doi: 10.1080/15226514.2016.1225280. [DOI] [PubMed] [Google Scholar]
- Park D, Yun YS, Park JM. Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere. 2005;60(10):1356–1364. doi: 10.1016/j.chemosphere.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Seget Z, Cycoń M, Kozdroj J. Metal-tolerant bacteria occurring in heavily polluted soil and mine spoil. Appl Soil Ecol. 2005;28(3):237–246. [Google Scholar]
- Raja CE, Anbazhagan K, Selvam GS. Isolation and characterization of a metal-resistant Pseudomonas aeruginosa strain. World J Microbiol Biotechnol. 2006;22:577–585. [Google Scholar]
- Ramachandran K, Srinivasan V, Hamza S, Anandaraj M (2007) Phosphate solubilizing bacteria isolated from the rhizosphere soil and its growth promotion on black pepper (Piper nigrum L.) cuttings. In: First international meeting on microbial phosphate solubilization. Springer, Dordrecht, pp 325–331
- Ramyakrishna K, Sudhamani M. The metal binding potential of a dairy isolate. J Water Reuse Desalin. 2016;7(4):429–441. [Google Scholar]
- Saleemi M, Kiani MZ, Sultan T, Khalid A, Mahmood S. Integrated effect of plant growth-promoting rhizobacteria and phosphate-solubilizing microorganisms on growth of wheat (Triticum aestivum L.) under rainfed condition. Agric Food Secur. 2017;6(1):1–8. [Google Scholar]
- Selvi AT, Anjugam E, Devi RA, Madhan B, Kannappan S, Chandrasekaran B. Isolation and characterization of bacteria from tannery effluent treatment plant and their tolerance to heavy metals and antibiotics. Asian J Exp Biol Sci. 2012;3:34–41. [Google Scholar]
- Siñeriz ML, Kothe E, Abate CM. Cadmium biosorption by Streptomyces sp. F4 isolated from former uranium mine. J Basic Microbiol. 2009;49(S1):S55–S62. doi: 10.1002/jobm.200700376. [DOI] [PubMed] [Google Scholar]
- Sinha SN, Paul D. Heavy metal tolerance and accumulation by bacterial strains isolated from wastewater. J Chem Biol Phy Sci. 2014;4:812–817. [Google Scholar]
- Tan S, Yi Y, Wang H, Zhou X, Tan Z, Liu Q. Screening, identification and growth-promoting effects of ramie-promoting bacteria. Bull Microbiol. 2015;42(3):525–533. [Google Scholar]
- Ullah A, Heng S, Munis MFH, Fahad S. Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. J Environ Exp Bot. 2015;117:28–40. [Google Scholar]
- Vashishth A, Khanna S. Toxic heavy metals tolerance in bacterial isolates based on their inducible mechanism. Int J Novel Res Life Sci. 2015;2:34–41. [Google Scholar]
- Velásquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater. 2009;167:713–716. doi: 10.1016/j.jhazmat.2009.01.044. [DOI] [PubMed] [Google Scholar]
- Vicene CS, Nascimento F, Espada M, Barbosa P, Mota M, Glick BR, Oliveira S. Characterization of bacteria associated with pinewood nematode Bursaphelenchus xylophilus. PLoS ONE. 2012;7:e46661. doi: 10.1371/journal.pone.0046661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ouyang L, Ju X, Zhang L, Zhang Q, Li Y. Survey of plant drought-resistance promoting bacteria from Populus euphratica tree living in arid area. Indian J Microbiol. 2014;54:419–426. doi: 10.1007/s12088-014-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani PA, Khan MS, Zaidi A. Cadmium, chromium and copper in green gram plants. Agron Sustain Deve. 2007;27:145–153. [Google Scholar]
- Wierzba S. Biosorption of lead (II), zinc (II) and nickel (II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis. Pol J Chem Technol. 2015;17(1):79–87. [Google Scholar]
- Wright MH, Adelskov J, Greene AC. Bacterial DNA extraction using individual enzymes and phenol/chloroform separation. J Microbiol Boil Educ. 2017;18(2):18. doi: 10.1128/jmbe.v18i2.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Wood TK, Mulchandani A, Chen W. Engineering plant–microbe symbiosis for rhizoremediation of heavy metals. Appl Environ Microbiol. 2006;72(2):1129–1134. doi: 10.1128/AEM.72.2.1129-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.