Abstract
Mucuna pruriens (Mp) is an annual and perennial legume which belongs to the family Fabaceae having different types of therapeutic activity. Anti-oxidative, anti-inflammatory, anti-epileptic, anti-microbial, etc. are the example of some most common activities of Mp. It is widely utilized as a potent aphrodisiac. The anti-Parkinsonian activity of Mp was explored since the nineteenth century. The neuroprotective activity of Mp was shown by several researchers. Levodopa (L-DOPA) is the important constituents responsible for the anti-Parkinsonian activity of Mp. Apart from L-DOPA, several other important bioactive components like Ursolic acid (UA) and Betulinic acid (BA) also exhibit a similar neuroprotective activity. Parkinson’s disease (PD) is mainly sporadic. A very small proportion shows the genetic nature of PD. The anti-Parkinsonian activity of Mp was explored in different toxin-induced PD models as like MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), Rotenone, Paraquat, 6-hydroxydopamine (6-OHDA) as suggested by several pieces of literature. Various parts of Mp’s like seed, leaf, and stem exhibit potent neuroprotective attributes. Among different parts, seeds are widely utilized as anti-PD agents because of the higher percentage of L-DOPA. Besides anti-PD activity, Mp’s neuroprotective potential was also explored in the ischemic model of stroke that also shows positive results. Recently, several clinical trials have been performed on the anti-PD activity of Mp on PD patients that show convincing results. Although, a small population-based study needs to be further validated in the broader population. Apart from anti-PD activity, Mp also shows its therapeutic activity in some other diseases like cancer, diabetes, skin infection, anemia, antihypertensive, etc. that are summarized in Table 1. In this review, we have discussed the anti-PD potential of Mp in the sporadic and genetic model along with some clinical trials that have performed on PD patients. Some other activity of Mp is also summarized in this review. There is a strong need to test the efficacy of Mp in some other neurodegenerative diseases along with PD. Following this, this review emphasizes the role of Mp in PD systematically through literature analysis available to date.
Table 1.
The generalized activities of Mp in some other diseases published in recent years
| Authors and Year | Type of study | Article Major Findings |
|---|---|---|
| Ashidi et al. (2019) | In vivo study using rat model. |
The reproductive function of male albino rat was enhanced at the dose of 0.75 g of Mp. Dose higher than 0.75 g might be toxic for the rat. |
| Ameh et al. (2019) | In vitro study. | For the environment which is prone toward snack bite, Mp and Mpu show potent anti-venom activity which is very beneficial for them. |
| Anosike et al. (2018) | Clinical Study |
Methanolic extract of Mp shows erythrocyte stabilizing ability. Mp shows this ability by its anti-oxidant potential and might be utilized for the treatment of sickle cell anemia. |
| Pinto et al. (2019) | In vivo study using animal model. |
In the ethanol-induced gastropathy model, lectin from Mp exhibits gastroprotective along with its anti-oxidant activities Mp exhibits this activity through alpha-2 adrenoceptors and prostaglandins without any toxicity sign. |
| Ulu et al. (2018) | In vivo study using rat model. |
In high-fructose diet-fed rats, Mp alleviates the adverse effect of the same. Mp modulates the expression of nuclear transcription factors present in kidney to remove the adverse effect of high-fructose diet. |
| Saiyad Musthafa et al. (2018) | In vivo animal model. |
As a feed additive, Mp seed stimulates immunity along with disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Thus, Mp seed can be commercially utilized as a food additive for the economic production of freshwater fish, O. mossambicus. |
| Seppan et al. (2018) | In vivo rat model. |
This interesting study has explored the impact of Mp on reproductive potential of male albino rats. The authors have shown that Mp prolonged the time of erectness of penis which dorsal nerve was getting damaged due to aging. |
| Sinha et al. (2018) | In vitro cell line study. |
Mp shows the anti-cancer potential against human breast cancer cells through JAK2/STAT5A signaling. Thus, Mp might be recommended as a supplement in diet |
| Khan and Kumar (2017) | In vitro and vivo study. | Through inhibiting activity on angiotensin I-converting enzyme (ACE), seed extract of Mp shows potent antihypertensive activity in vitro and in vivo |
Keywords: Parkinson's disease, Mucuna pruriens, Levodopa, Ursolic acid, Sporadic model, Genetic model
Introduction
Parkinson’s disease (PD) is the result of a complex interaction between genetic, environmental, and pathological factors that affect the general population all over the world with intense medical and financial burdens (Surathi et al. 2016; Ball et al. 2019). From the past few decades, researchers have tried and succeeded to develop a preclinical model that has helped in the early-stage diagnosis of PD. Researchers have found only limited success in the correct diagnosis of PD because of the complexity of this disease. Iron metabolism was significantly impaired in PD and responsible for its progression as suggested by molecular-level study (Chi et al. 2020). In general, this disease affects all age groups, most commonly affecting the large percentage of the elderly population that include patients, caregivers, and consequently progressively increases the financial load of the country (DeMaagd and Philip 2015). For the possible treatment of this disease, researchers have faced a tremendous amount of challenges. The medication available so far for this disease has only provided symptomatic relief to the patients and also caused severe side effects later in their lives (Goldenberg 2008). Ayurveda opens an effective and alternative way of treating this disease with minimal side effects. Some medicinal plants like Mucuna pruriens (Mp), Withania somnifera (Ws), Tinospora cordifolia (Tc) exhibits beneficial property for the treatment of neurodegenerative disease (Rai et al. 2017a, b; Prakash et al. 2014; Birla et al. 2019a, b). In the case of mutation-induced neurodegenerative disease, gene editing shows potential impact using the CRISPR/Cas9 system with some disadvantages (Shin and Lee 2017; Im et al. 2016). Day to day, researchers develop new surgical tools that effectively targets particularly the affected area and provides targeted treatment of PD (Lee et al. 2018). Medicinal plants have numerous bioactive components like Mp contain Ursolic acid (UA) as one of the powerful bioactive components that has shown potent anti-oxidative and anti-inflammatory activity in toxin-induced PD model (Rai et al. 2016, 2019c). Like UA, Chlorogenic acid (CA) found in Ws shows potent anti-Parkinsonian activity in toxin-induced Parkinsonian mouse model (Singh et al. 2018). The nanoformulation and targeted drug delivery based on this bioactive component have enhanced the neuroprotective efficacy in a manifold. The major hurdle in the development of the treatment for PD is the absence of accurate diagnostic system which could not possible yet due to the complex nature of this disease. In the last few decades, researchers have used several chemical-induced models for PD which shows very positive findings along with some disadvantages. The cellular model, genetic model, and transgenetic model have also been used since the nineties that helped in the rapid development of a neuroprotective drugs for the PD. Recently, 3D organoid model showed a prominent response as it recapitulates most of the conditions found for in vivo condition. These 3D models have been utilized worldwide by the researchers for the development of the PD (Kaushik et al. 2018). Nowadays, researchers have focused on the causation of the PD. The gut microbiome axis has been widely utilized by the researchers to know the exact pathophysiology of the PD (Fitzgerald et al. 2019; Yang et al. 2019). In addition, PI3K/Akt and quality control pathway can also be utilized for the effect of medicinal plant and effect of some other chemical compounds in PD treatment (Rai et al. 2019a, b). Medicinal plants like Mp might solve some of the major puzzles responsible for the progression of PD.
Mucuna pruriens (Mp) commonly known as ‘‘velvet’’ bean; and ‘‘Atmagupta’’ in India is a climbing legume prevalent in India and other parts of the tropics including Central and South America. It has been used by the ancient time since 1500 BC for the treatment of medical disorders. Kampavata has been described by Ayurveda as a nervous difficulty bearing similarities to PD and Mucuna seed preparations are in contemporary use for the treatment of PD in India (Manyam 1990; Manyam & Sánchez-Ramos, 1999). Mp is a mysterious and efficiently very vital plant. Moreover, it is a potential source for food because it is very rich in crude protein, essential fatty acids, starch, along with essential amino acids. Mp also has a variety of anti-nutritional factors like inhibitors for protease, total phenolics, oligosaccharides, and few cyclitols having anti-diabetic activity. In general, almost all parts of the Mp plant exhibit potent medicinal ability. L-DOPA (5%) is the main phenolic compound found in Mp. Mohapatra have found about 3.02–4.72% L-DOPA in Mp by HPTLC method (Mohapatra et al. 2019). The synthesis of this L-DOPA was regulated by polyphenol oxidase and not by CYP 450/tyrosine hydroxylase in Mp (Saranya et al. 2020). Seeds of Mp also have anti-venom activity (Pathania et al. 2020; Ameh et al. 2020). Additionally, methanolic extracts of Mp leaves show anti-microbial and anti-oxidant activities (Lampariello et al. 2012). L-DOPA was first isolated from the seeds of Mp in 1937 when the worth of L-DOPA for the treatment of PD became identified; scientific interest in plants rich in L-DOPA was invigorated. Between 18 and 60 patients were involved in three open-label studies in which mean dosages of 45 g/day of Mucuna seed powder extract (containing about 1500 mg L-DOPA) were used and have reported significant improvements in PD in about 12–20 weeks (Damodaran and Ramaswamy 1937). One study suggested that tolerability might be better with Mp than with standard L-DOPA preparations in PD patients (Cilia et al. 2017). The role of Mp in the sporadic and genetic model along with some relevant and informative clinical trials has been performed in the last few decades based on PD patients that are discussed below in sequence manner. Recently, a review article has been published that shows the medicinal and nutritive properties of Mp (Pathania et al. 2020). Mp offers therapeutic activity not only in developed countries, but also in low-income countries (Fothergill-Misbah et al. 2020). A dietary supplement that included Mp, CoenzymeQ 10, Multivitamins, Melatonin and N-acetylcysteine has been included for PD patients (Ferguson et al. 2019). Therefore, there should be an effective discussion between PD patients and healthcare practitioner about their dietary lifestyle. Figure 1 shows the generalized activities of Mp.
Fig. 1.
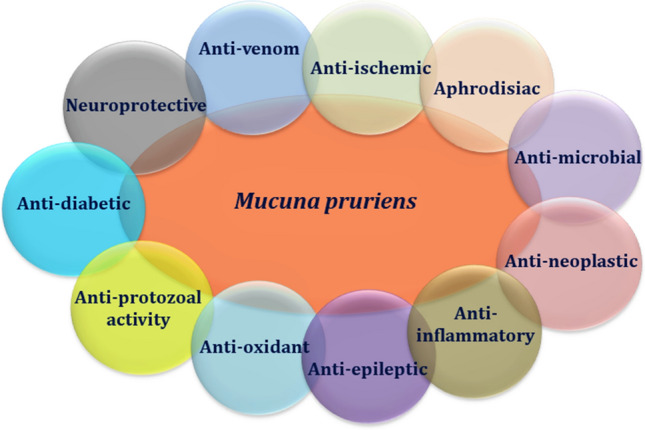
The generalized therapeutic activities of Mucuna pruriens
Anti-PD potential of Mucuna pruriens (Mp) in sporadic model
All parts of Mp possess valuable medicinal properties. Reportedly, they possess analgesic, anti-inflammatory, anti-neoplastic, anti-epileptic, and anti-microbial activities (Adepoju and Odubena 2009). In India, seeds of Mp are used as a tonic and aphrodisiac for male virility. Manyam et al. have shown the neuroprotective role of Mp in the 6-hydroxydopamine (6-OHDA)-induced Parkinsonian rat model. They have also found that Mp shows the more significant neuroprotective activity as compared to L-DOPA. They have also performed an in vitro experiment and have shown that cotyledon powder of Mp significantly enhanced the mitochondrial complex-I activity, while the monoamine oxidase activity remains unaffected. They have successfully demonstrated that the neurorestorative potential of Mp cotyledon powder was due to increased complex-I activity along with the presence of NADH and coenzyme Q-10 in Parkinsonian rats (Manyam et al. 2004). Thus, Mp has even been said to be a rejuvenator drug having neuroprotective property (Manyam et al. 2004). They should also compare the potential of different extract of Mp like methanolic, ethanolic, and aqueous in toxin-induced Parkinsonian mouse model that might surely help to validate their findings.
A double-blind clinical and pharmacological study on PD by Mp was effectively conducted by Katzenschlager et al. (2004). They have shown that for the long-term management of PD, Mp shows better efficacy as compared to conventional L-DOPA treatment which causes severe L-DOPA-induced dyskinesia upon prolonged treatment. Since the sample size was very small (8 PD patients), further study is needed in a large sample to validate the same (Katzenschlager et al. 2004). Age group selection is the major limitations in his study as PD is more common among old age person. In addition, they should also have compared the similar activities in female PD patients, though the prevalence of PD is less common in female as compared to that in male but still this might show some additional findings which help to draw a useful conclusion.
Mp also shows its activity at the genomic level, Mucuna pruriens cotyledon powder (MPCP) effectively protected DNA of both plasmid and genomic against L-DOPA along with copper-induced DNA damage (Tharakan et al. 2007). Along with cotyledon powder, leaf extract and some other vital part of Mp should also be studied in this report.
The antioxidant and metal chelating activity of Mp was demonstrated by Dhanasekaran et al. (2008). Mp was found to scavenge the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals and reactive oxygen species (ROS) and is also capable of inhibiting the oxidation of lipids and deoxyribose sugar. Mp shows divalent iron chelating activity and has no any genotoxic effect on DNA. Mp shows its neuroprotective activity which is independent of the symptomatic effect (Dhanasekaran et al. 2008). This is a very interesting study as it demonstrates the effect of Mp on the redox potential of the cell and also shows the focus on the metal homeostasis which plays a very significant role in the progression of PD. In any toxin-induced PD model, metal homeostasis is seriously disturbed (Chi et al. 2020); plant or any herbal medicine that balances the metal homeostasis inside dopaminergic neurons might protect the dopaminergic neurons from neurodegeneration.
Short-term treatment (a day) with soluble phenolic and antioxidant-rich Mp sprouts shows an improved concentration of L-DOPA which exhibits potent anti-Parkinsonian activity (Randhir et al. 2009). The extended form of this study should be needed to test the efficacy of this plant along with its bioactive component. Lieu et al. (2010) have suggested the anti-Parkinsonian activity of water extract of Mp (MPE) in the hemiparkinsonian rat model of PD. They have compared the activity of MPE alone and with an additive like peripheral dopa-decarboxylase inhibitor (DDCI) benserazide (BZ), and L-DOPA alone without BZ in the hemiparkinsonian rat model of PD at the behavioral level. Mp contains water soluble ingredients that show either intrinsic DDCI-like activity or alleviate the requirement of DDCI to improve Parkinsonism (Lieu et al. 2010). As toxin-induced model of PD shows significant variability between rats and mice, similar study should also be conducted in mice to find any definite conclusion that might help in the clinical trial regarding the same.
In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-(MPTP)-intoxicated nonhuman primate, the anti-Parkinsonian activity of Mp shows a reduced risk of dyskinesia which have been suggested by Lieu et al. (2012). This novel finding shows that Mp exhibits a strong ability to improve Parkinsonism without causing dyskinesia which is very different from that of L-DOPA treatment. Mp also alleviates the behavioral abnormalities in MPTP-intoxicated primate model. At the equivalent dose of both Mp and L-DOPA, electrophysiological studies show the better response of Mp as compared to L-DOPA via a unique mechanism. Thus, it is clear that Mp has some additional component along with L-DOPA that shows vital neuroprotective activity in basal ganglia. Researchers have identified more than 50 natural bioactive components in Mp and still many more might be present with neuroprotective potential. Thus, Lieu et al. have concluded that Mp has a vital bioactive component which is responsible for its anti-PD and anti-dyskinetic activity. Mp might be very beneficial for PD patients who have taken a regular dose of L-DOPA that caused dyskinesia. Further studies will be needed to identify every individual component in Mp that have anti-PD or anti-dyskinetic activity. Mp might offer a vital drug for PD patients (Lieu et al. 2012). This primate study surely adds additional steps towards the clinical trial of Mp for the PD patients.
Yadav et al. (2013) has shown the neuroprotective effect of Mp seed extract against Paraquat (PQ)-induced neurotoxicity in a mouse model of PD. The outcome of this study suggested that the aqueous extract of Mp seeds has a strong antioxidant property, which helps to reduce the oxidative stress generated in PD mice. The treatment of PQ-intoxicated mice with Mp seed extract improved motor behavior, due to a reduction in oxidative stress and also improved the expression of Tyrosine hydroxylase (TH) in substantia nigra (SN) and striatum of the brain. PQ enters the dopaminergic neurons through neutral amino acid transporter (NAT) and it also shows some affinity towards dopamine transporter (DAT). After entering the dopaminergic neurons, PQ impaired the redox potential of nigrostriatal pathways that is improved by the treatment with Mp. Therefore, this study strongly suggested that Mp, a valuable herbal plant, could be used for developing the drug for the treatment of PD (Yadav et al. 2013).
The neuroprotective role against the MPTP-induced Parkinsonian mouse model has been shown by the ethanolic extract of Mp seed. It shows a strong antioxidant potential against ROS generated in the MPTP model. Also, Mp treatment improves the motor behavior of Parkinsonian mice. Mp induced the level of catecholamines and stimulated antioxidant potential in the nigrostriatal region. Moreover, the expression of TH in the SN and striatal regions was improved by Mp treatment and normal expression levels of inducible nitric oxide synthase (iNOS) and glial fibrillary acidic protein (GFAP) in MPTP-treated animals were recovered. This demonstrates Mp’s tendency to aid in the recovery from neuronal injury and oxidative stress (Yadav et al. 2014). This study also compared the efficacy of Mp to estrogen that shows Mp have potent anti-Parkinsonian activities and having minimal side effects as compared to estrogen.
Yadav et al. (2016) has shown that Mp exhibits anti-apoptotic activity as the enhancement of Bcl2 expression and a decline in the level of Bax was seen. Thus, the results published by Yadav et al. elucidated the mechanism of Mp plant extract as a potent neuroprotectant. Yadav et al. also demonstrated HPTLC data of Mp and found two main constituents of its seed extracts, namely L-DOPA and UA (Yadav et al. 2016). As compared to L-DOPA, estrogen shows better neuroprotective activity in MPTP-intoxicated mouse model (Rai and Singh 2016). Additional pathways like Akt/Erk should also be discussed in this paper to find supportive information that might improve the findings of this study.
Mp shows anti-inflammatory activity by inhibiting the iNOS expression and ultimately protects the death of dopaminergic neurons in the SN in the PQ-intoxicated PD mouse model. MPTP intoxication causes the enhancement of the expression of iNOS in both SN and in the striatum, which is responsible for the reduction of the expression of TH. Mp improves the TH expression in SN and striatum via inhibiting the expression of iNOS. The mRNA expression of iNOS was also reduced due to the MPTP exposure along with the enhancement of the expression of a biochemical marker like nitrite and lipid peroxidation. Mp considerably reduces the biochemical markers along with the mRNA expression of iNOS which shows its neuroprotective activity (Yadav et al. 2017). The role of NF-κB should also be discussed in this paper as it is the vital inflammatory factor that activated the expression of proinflammatory cytokines which ultimately causes the progression of PD.
Recently, Rai et al. have elucidated the mechanism of action of Mp in MPTP-induced mouse model of PD. The authors have shown that Mp inhibits MPTP-induced neuroinflammation by inhibiting the nuclear translocation of NF-κB and also by inhibiting the expression of proinflammatory cytokines like Tumor necrosis factor alpha (TNF-α) and enzymes like iNOS (Rai et al. 2017b). DAT plays a vital role in the uptake of 1-methyl-4-phenylpyridinium (MPP+). During uptake of MPP+, DAT gets damaged which is reflected in the immunofluorescence staining of DAT. After entering the dopaminergic neurons, Mp causes the progressive degeneration of nigrostriatal neurons through inflammatory pathways. Following this, the expression of anti-apoptotic protein pAkt1 expression was reduced by MPTP intoxication. Mp shows its potent anti-inflammatory activity by improving the expression of DAT and pAkt1 along with the reduced expression of proinflammatory cytokines through inhibiting the nuclear translocation of NF-κB (Rai et al. 2017b). Thus, limitations in the Yadav et al. study have been performed by this study which ultimately suggests a possible mechanism of action behind Mp’s anti-inflammatory activity. Mp also shows immunomodulatory activities in various toxin-induced Parkinsonian mouse model (Rai et al. 2017a). Thus, Mp shows therapeutic potential by regulating the immunomodulatory pathways in toxin induced PD model.
Ursolic acid (UA) is the vital bioactive component in Mp seed extract as shown previously. UA also shows anti-oxidative and anti-inflammatory activity in different disease conditions. UA attenuates oxidative stress in nigrostriatal tissue and improves the neurobehavioral activity in the MPTP-induced Parkinsonian mouse model (Rai et al. 2016). UA also exhibits potent anti-inflammatory activity in MPTP-induced Parkinsonian mouse model. Similar to the anti-inflammatory activity of Mp, UA prevents the progression of PD via inhibiting the nuclear translocation of NF-κB into the nucleus, ultimately reducing the expression of proinflammatory cytokines. Thus, UA protects the death of dopaminergic neurons in basal ganglia through the NF-κB pathway (Rai et al. 2019c). Zahra et al. shows the anti-PD activity of UA in rotenone-induced PD mouse model (Zahra et al. 2020). Yadav et al. have shown in his study that along with UA, betulinic acid (BA) is also a bioactive component in Mp seed. Thus, the anti-Parkinsonian activity of BA should also be studied and compared with UA to find a logistic conclusion. Similar to UA, chlorogenic acid (CA) is also found in some medicinal plants like coffee beans and shows potent anti-inflammatory activity in neurodegenerative diseases. CA reduces the inflammatory loads generated by MPTP intoxication in a mouse via NF-κB pathways very similar to UA and Mp (Singh et al. 2018). CA also improves the mitochondrial abnormalities and maintains the apoptotic parameters in MPTP-intoxicated mouse model (Singh et al. 2020). Mp has reduced iNOS expression in the Parkinsonian mice model, and, thus, shows immunomodulatory activity in PD (Yadav et al. 2017). On another species of Mucuna called Mucuna sanjappae (Ms) also shows anti-Parkinsonian activity in MPTP-intoxicated mouse model. Ms also have L-DOPA similar to Mp but in lesser percentage but have potent anti-oxidative and anti-inflammatory activity. Ms significantly improves the behavioral and biochemical anomalies generated due to MPTP intoxication; it also improves the TH staining in the nigrostriatal region. Further studies are needed to explore various bioactive components found in Ms and behind its mechanism of action in PD models (Patil et al. 2016). The activity of Ms should also be tested in other toxin-induced PD models to suggest the neuroprotective activity of Ms. Also, the different extracts should also be prepared for Ms and tested and compared in the PD mouse model. Furthermore, other plant parts besides seed also should be tested to exhibit the anti-Parkinsonian activities of the whole plant of Ms.
Adi et al. have shown that n-Propanol extract of the boiled and fermented extract of Mp show prominent anti-Parkinsonian activity in PQ-induced PD rat model. PQ causes significant impairment in the nigrostriatal region at both biochemical and behavioral levels, while fermented extract of Mp significantly ameliorated these abnormalities and prevents the progression of PD (Adi et al. 2018). Bioactive component activities should also be checked in this study as this is the boiled and fermented extract which might alter the composition of Mp and might suggest a different mode of action as compared to the existing one.
Anjaneyulu et al. shows the effect of a combination of Ayurvedic nootropics in both C. elegans transgenic strains and MPTP-intoxicated model. In this combination, Mp is an important constituent. Apart from Mp, other vital Ayurvedic nootropics are Bacopa monnieri (BM), Withania somnifera (WS), Centella asiatica (CA), Sida cordifolia (SC), and Celastrus paniculatus (CP). Therefore, the efficacy of a combination of total 6 Ayurvedic nootropics was tested in both models of C. elegans. These combinations were very effective and show differential neuroprotective in this genetic and sporadic model. Among these 6 different Ayurvedic nootropics, BM exhibits the highest efficacy (~ 50%), while MP (~ 29%), and CP (~ 29%) shows similar potential in PD model. The major key player involved behind the neuroprotective activity of Mp was L-DOPA as suggested in this paper. L-DOPA significantly inhibited the MPTP-induced neurotoxicity. The other extract shows very minimal activity (Anjaneyulu and Godbole 2020). The combinatorial activity of these Ayurvedic nootropics should also be tested in other animal models to justify the findings. In addition, different toxin-induced PD model should also be tested for the same.
There will be a need to explore other herbal drugs having L-DOPA that might be useful for PD patients. Beans are generally included in the diet and its therapeutic activity was already discussed by researchers. Thus, similar to Mp, natural therapy of PD might be possible using beans in the diet (Rijntjes, 2019).
The anti-PD potential of Mp in genetic model
In the genetic model of PD, Poddighe et al. have suggested that Mp significantly ameliorates the motor abnormality, mitochondrial dysfunction, olfactory dysfunction along with synaptic impairment. In this study, they have shown that in Drosophila which is mutant for PINK1B9, Mp shows significant neuroprotective activity. They have used a methanolic extract of Mp to rescue the progression of PD in this genetic model of PD (Poddighe et al. 2014).
Solari et al. have demonstrated that muscle activity and the mitochondrial morphology of PD PINK1B9 Drosophila melanogaster (Dm) is significantly rescued by Mp. For studying the gastrointestinal symptoms in PD, PINK1B9 Dm offers a very useful translational model that can be utilized to confirm the anti-PD potential of Mp. At both larval and adult stages of Dm, Mp shows vital neuroprotective potential by improving the mitochondrial abnormalities. (Solari et al. 2018). Solari et al. provides evidence that the addition of Mp extract rescues the serotonergic pathway and improves the mitochondrial abnormalities in the PINK1B9 Dm model of PD. Moreover, further study will be needed to validate the same findings at the gut–microbiome axis (Solari et al. 2018).
Recently, Baroli et al. have compared the anti-oxidative potential of Mp and Ws in the genetic Dm model of PD. They have demonstrated 2 anti-oxidative enzymes, namely reduced glutathione (GSH) and superoxide dismutase (SOD) activity to compare the anti-PD potential of Mp and Ws. They have found that mutant PINK1B9 Dm shows reduced GSH and SOD activity along with an unexpected longer length of telomerase as compared to wild fly. Mp improved the GSH and SOD activity along with reducing the length of telomerase in the PINK1B9 mutant fly. On the other hand, Ws shows no significant impact on both SOD and GSH level and also has no any activity on the length of telomerase. Thus, Mp shows the better neuroprotective activity as compared to Ws in mutant fly model (Baroli et al. 2019).
The majority of the genetic study is conducted in Drosophila. To draw any possible and definite conclusion regarding the potential and mechanism of action of Mp’s anti-Parkinsonian activity, other animals should also be tested. In addition, role of Mp should also be studied at the CRISPER/CAS9 level to improve the existing finding regarding the Mp. Figure 2 shows the detailed mechanism of action behind Mp’s anti-Parkinsonian activity. Figure 3 exhibits different model system utilized for the generation and treatment of PD using Mp.
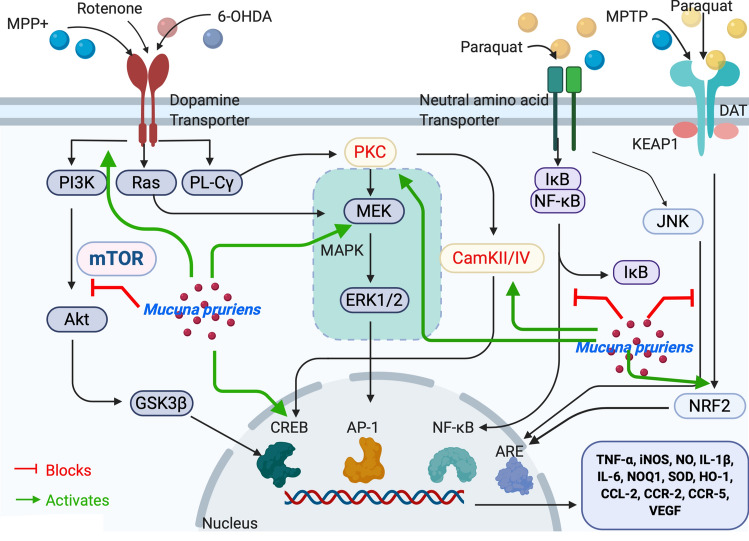
Fig. 2.
Schematic representation of Mucuna pruriens involved in neuroprotection against Parkinson’s disease. External stimulus as like MPP + and other toxins like 6-OHDA, Rotenone binds to Dopamine transporter (DAT) receptor present on dopaminergic neurons activating the PI3K/AKT, Ras/MAPK, and PL-C pathways. MPP + , 6-OHDA, Rotenone binds to DAT and finally activating NF-κB and JNK signaling. These stimuli also release Nrf2 from Nrf2-KEAP-1 complex and activates ARE. PQ also binds to neutral amino acid transporter and follows the similar route. PI3K, phosphatidylinsoitol-3-kinase; MAPK mitogen-activated protein kinase, mTOR mammalian target of rapamycin, ERK extracellular signal-regulated kinases, Nrf2 nuclear factor e2-related factor 2, KEAP-1 Kelch-like ECH-associated protein-1, MEK mitogen-activated protein kinase, PL-C phospholipase C, NF-κB nuclear factor-kappa B, PKC protein kinase C, JNK c-Jun N-terminal kinase, ARE Antioxidant response element, CREB cyclic adenosine monophosphate response element-binding protein, IκB inhibitory kappa B, CaMKII/IV Ca2 + –calmodulin kinase II/IV, GSK3β glucose synthase kinase-3β, Mp Mucuna pruriens
Fig. 3.
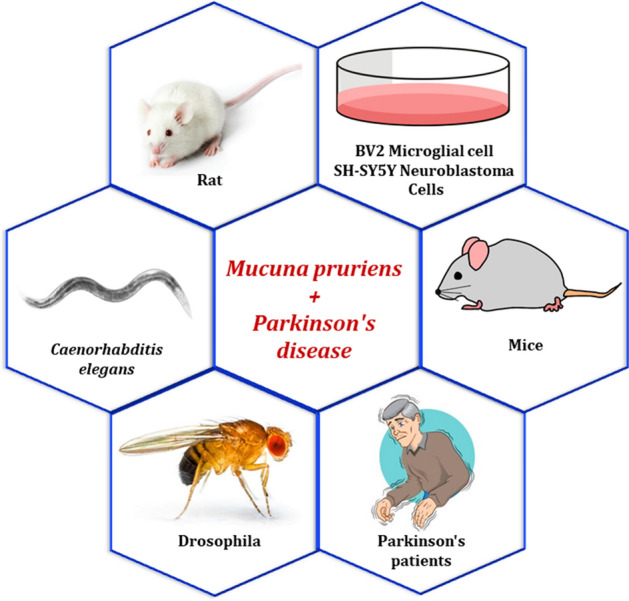
Different model system utilized for the generation and treatment of Parkinson’s disease using Mucuna pruriens. (Source-Google Images)
Clinical trial on Mp in PD patients
Recently, Cilia et al. (2017) have performed the clinical trial of Mp in PD patients. They have suggested that Mp might provide an excellent option for PD patients who belong to low-income countries in the place of marketed standard L-DOPA as it can be tolerated for the long term without causing severe dyskinesia. As compared to dispersible levodopa/benserazide treatment, a single dose of Mp from roasted seeds shows potent anti-Parkinsonian activity in 18 advanced PD patients. A large sample size is needed to validate the findings regarding the anti-PD potential of Mp (Cilia et al. 2017).
Another study done by Johnson et al. has suggested that Mp extract with reduced L-DOPA exhibits prominent neuroprotective activity in the PD model of Murine Microglia, Human Neuroblastoma Cells, Caenorhabditis elegans, and Drosophila melanogaster. Thus, L-DOPA is not only the main bioactive component in Mp which is responsible for its neuroprotective activity; but there might be some other key components that contribute to the activity in a synergistic way as well. Thus, Mp extract which contains reduced L-DOPA prevents the death of dopaminergic neurons in four different model system and clinical trial for the same is needed to justify this interesting finding (Johnson et al. 2018).
Cilia et al. have further performed the clinical trial in 2018 to show the effect of Mp in PD patients and have shown that its daily intake for 16 days has significantly more beneficial effects with minimal side effects. In this study, quality of life along with the motor and non-motor symptoms was compared between standard L-DOPA and Mp treatment for 16 weeks in 14 PD patients. Similar to previous clinical trials performed by Cilia et al., this clinical trial also has a very low number of PD patients, and a large sample size needed to again justify the same (Cilia et al. 2018).
Recently, a short communication demonstrated the combined potential of Mp and carbidopa in PD. This finding has shown that by adding a DDCI in Mp might result in a strong personalized alternative for PD patients who are unwilling to start L-DOPA treatment (Radder et al. 2019).
Major limitations in the above-discussed clinical trial regarding the anti-Parkinsonian activity of Mp are the sample size which was very small to apply for the general population. PD patients affected in different geographical region should be checked to prove the efficacy of Mp. Furthermore, the efficacy of Mp should also be compared in different large-sized populations.
Role of Mp in stroke and ischemia
Apart from PD, Mp also shows its therapeutic activity in some other brain diseases like in the ischemia model. An interesting finding published by Nayak et al. suggested that Mp might be utilized as an important agent to manage stroke-induced ischemia that is responsible for causing widespread brain injury. They have shown the anti-ischemic activity of Mp in the rat model of ischemia. They have generated the ischemia model of Wistar rat by bilateral carotid artery occlusion. The occlusion time for both the artery was 30 min. For the induction of global cerebral ischemia, after 30 min, the rat was reperfused. The global ischemic rat model was then treated with a methanolic extract of Mp for 10 days. The brain of the ischemia model shows multiple lesions that are not treated by Mp as shown by histopathological and biochemical parameters. Mp offers significant protection to these lesions in the rat model as compared to the negative control. The spatial memory of rats was significantly improved by Mp. Minimal neuronal damage in the CA1 region of the rat was observed in the Mp group as compared to ischemic rats. Furthermore, the ROS level was also decreased by Mp treatment as compared to ischemic rats. Thus, we can say that Mp offers potent anti-ischemic activity and there is a need to test the clinical efficacy of the same (Nayak et al. 2017).
Other vital activities of Mucuna pruriens: Along with anti-Parkinsonian activity, Mp also possesses some other vital activities which are very helpful in the area of medicines and pharmaceutical industries. Table No. 1 shows the role of Mp in some other diseases published in recent times.
Conclusion and future prospective
In conclusion, we can say that Mp possesses strong anti-PD potential in toxin-induced PD models and also shows significant improvement in the clinical trial. Mp shows this potent anti-PD activity due to the high percentage of L-DOPA. Different parts of the Mp have been explored in different diseased conditions with significant outcomes. The major question toward the efficacy of different parts of Mp is the pharmacokinetic effects on different organs. Another advancement that is required is the complete identification of various bioactive components in different vital parts of Mp. A large sample size will be needed to justify and validate the anti-PD potential of Mp in clinical trials. Different community-wise studies are needed to compare the efficacy of different parts of Mp in PD along with in some other diseases. In addition, the anti-PD potential of various bioactive components of Mp’s also needed to demonstrate any mechanism of action behind it. Furthermore, the potential of Mp should be explored in various brain regions to see any side effects associated with the plant. Strong in vitro activity in different cell lines should be required to compare Mp’s potential with in vivo models. Nanomaterials should be made of different bioactive components of Mp and should be tested in various disease conditions. The half-life of different parts of Mp should be checked in the brain to demonstrate its efficacy and sustainability. Finally, Mp might be a novel and potential drug in the near future when the above-mentioned necessary formalities are conducted in different disease conditions and compared with positive controls and find any positive outcomes in long-term clinical trials with a large sample size. There is also a need to compare the neuroprotective activity of different extract of Mp as aqueous, ethanolic, methanolic, etc. in PD and some other neurodegenerative diseases. Also, the neuroprotective activity of Mp should also be compared to another herbal plant extracts like Ws and Bm, etc. This might surely help in the development of the novel drug from Mp. Besides, Mp also shows vital medicinal properties in some other diseases like ischemia, diabetes, cancers and also shows potent aphrodisiac activity. Thus, Mp might be a potent and magical compound which might be used in the future to treat multiple disease condition. There will be a strong need to explore the neuroprotective potential of Mp in other neurodegenerative diseases like Alzheimer’s and Huntington’s disease. Mp might offer strong herbal drug that can be used for the treatment of neurodegenerative diseases.
Acknowledgments
Authors would like to acknowledge UGC Dr. D.S. Kothari Postdoctoral scheme for awarding the fellowship to Dr. Sachchida Nand Rai (Ref. No-F.4-2/2006 (BSR)/BL/19-20/0032).
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Sachchida Nand Rai, Email: raibiochem@gmail.com.
Vivek K. Chaturvedi, Email: vivekchaturvedi2013@gmail.com
Payal Singh, Email: payalsingh200012@gmail.com.
Brijesh Kumar Singh, Email: bks2139@cumc.columbia.edu.
M. P. Singh, Email: mpsingh.16@gmail.com
References
- Adepoju GKA, Odubena OO. Effect of Mucuna pruriens on some haematological and biochemical parameters. J Med Plant Res. 2009;3:073–076. [Google Scholar]
- Adi YK, Widayanti R, Pangestiningsih TW. n-Propanol extract of boiled and fermented koro benguk (Mucuna pruriens seed) shows a neuroprotective effect in paraquat dichloride-induced Parkinson’s disease rat model. Vet World. 2018;11(9):1250–1254. doi: 10.14202/vetworld.2018.1250-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameh MP, Mohammed M, Ofemile YP, Mohammed MG, Gabriel A, Isaac AO. Detoxifying action of aqueous extracts of Mucuna pruriens seed and Mimosa pudica root against venoms of Naja nigricollis and Bitis arietans. Recent Pat Biotechnol. 2020;14(2):134–144. doi: 10.2174/1872208313666191025110019. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu JRV, Godbole A. Differential effect of ayurvedic nootropics on Caenorhabditis elegans models of Parkinson’s disease. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anosike CA, Igboegwu ON, Nwodo OFC. Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. J Tradit Complement Med. 2018;9(4):278–284. doi: 10.1016/j.jtcme.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashidi JS, Owagboriaye FO, Yaya FB, Payne DE, Lawal OI, Owa SO. Assessment of reproductive function in male albino rat fed dietary meal supplemented with Mucuna pruriens seed powder. Heliyon. 2019;5(10):e02716. doi: 10.1016/j.heliyon.2019.e02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball N, Teo WP, Chandra S, Chapman J. Parkinson’s disease and the environment. Front Neurol. 2019;10:218. doi: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroli B, Loi E, Solari P, Kasture A, Moi L, Muroni P, Kasture S, Setzu MD, Liscia A, Zavattari P. Evaluation of oxidative stress mechanisms and the effects of phytotherapic extracts on Parkinson’s disease Drosophila PINK1B9 model. FASEB J. 2019;33(10):11028–11034. doi: 10.1096/fj.201901010. [DOI] [PubMed] [Google Scholar]
- Birla H, Keswani C, Rai SN, Singh SS, Zahra W, Dilnashin H, Rathore AS, Singh SP. Neuroprotective effects of Withania somnifera in BPA induced-cognitive dysfunction and oxidative stress in mice. Behav Brain Funct. 2019;15(1):9. doi: 10.1186/s12993-019-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birla H, Rai SN, Singh SS, Zahra W, Rawat A, Tiwari N, Singh RK, Pathak A, Singh SP. Tinospora cordifolia suppresses neuroinflammation in Parkinsonian mouse model. NeuroMol Med. 2019;21(1):42–53. doi: 10.1007/s12017-018-08521-7. [DOI] [PubMed] [Google Scholar]
- Chi H, Tang W, Bai Y. Molecular evidence of impaired iron metabolism and its association with Parkinson’s disease progression. 3 Biotech. 2020;10(4):173. doi: 10.1007/s13205-020-2162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia R, Laguna J, Cassani E, Cereda E, Pozzi NG, Isaias IU, Contin M, Barichella M, Pezzoli G. Mucuna pruriens in Parkinson disease A double-blind, randomized, controlled, crossover study. Neurology. 2017;89(5):432–438. doi: 10.1212/WNL.0000000000004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia R, Laguna J, Cassani E, Cereda E, Raspini B, Barichella M, Pezzoli G. Daily intake of Mucuna pruriens in advanced Parkinson’s disease: a 16-week, noninferiority, randomized, crossover, pilot study. Parkinsonism Relat Disord. 2018;49:60–66. doi: 10.1016/j.parkreldis.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Damodaran M, Ramaswamy R. Isolation of L-dopa from the seeds of Mucuna pruriens. Biochem J. 1937;31:2149–2451. doi: 10.1042/bj0312149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaagd G, Philip A. Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm Ther. 2015;40(8):504–532. [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran M, Tharakan B, Manyam BV. Antiparkinson drug—Mucunapruriensshows antioxidant and metal chelating activity. Phytother Res. 2008;22:6–11. doi: 10.1002/ptr.2109. [DOI] [PubMed] [Google Scholar]
- Ferguson CC, Knol LL, Halli-Tierney A, Ellis AC. Dietary supplement use is high among individuals with parkinson disease. South Med J. 2019;112(12):621–625. doi: 10.14423/SMJ.0000000000001041. [DOI] [PubMed] [Google Scholar]
- Fitzgerald E, Murphy S, Martinson HA. Alpha-synuclein pathology and the role of the microbiota in Parkinson’s Disease. Front Neurosci. 2019;13:369. doi: 10.3389/fnins.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill-Misbah N, Maroo H, Cham M, Pezzoli G, Walker R, Cilia R. Could Mucuna pruriens be the answer to Parkinson’s disease management in sub-saharan Africa and other low-income countries worldwide? Parkinsonism Relat Disord. 2020;73:3–7. doi: 10.1016/j.parkreldis.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Goldenberg MM. Medical management of Parkinson’s disease. Pharm Ther. 2008;33(10):590–606. [PMC free article] [PubMed] [Google Scholar]
- Im W, Moon J, Kim M. Applications of CRISPR/Cas9 for gene editing in hereditary movement disorders. J Movem Disord. 2016;9(3):136–143. doi: 10.14802/jmd.16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Park HY, DaSilva NA, Vattem DA, Ma H, Seeram NP. Levodopa-reduced Mucuna pruriens seed extract shows neuroprotective effects against Parkinson’s Disease in murine microglia and human neuroblastoma cells, Caenorhabditis elegans, and Drosophila melanogaster. Nutrients. 2018;10(9):1139. doi: 10.3390/nu10091139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, Timmermann L, Van der Giessen R, Lees AJ. Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. Neurol Neurosurg Psychiatry. 2004;75:1672–1677. doi: 10.1136/jnnp.2003.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik G, Ponnusamy MP, Batra SK. Concise review: current status of three-dimensional organoids as preclinical models. Stem Cells. 2018;36(9):1329–1340. doi: 10.1002/stem.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G. The magic velvet bean of mucuna pruriens. J Trad Complem Med. 2012;2(4):331–339. doi: 10.1016/s2225-4110(16)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Dallapiazza RF, De Vloo P, Lozano AM. Current surgical treatments for Parkinson’s disease and potential therapeutic targets. Neural Regener Res. 2018;13(8):1342–1345. doi: 10.4103/1673-5374.235220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu CA, Kunselman AR, Manyama BV, Venkiteswaran K, Subramanian T. A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. Parkinsonism Relat Disord. 2010;16(7):458–465. doi: 10.1016/j.parkreldis.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu CA, Venkiteswaran K, Gilmour TP, Rao AN, Petticoffer AC, Gilbert EV, Deogaonkar M, Manyam BV, Subramanian T. The antiparkinsonian and antidyskinetic mechanisms of Mucuna pruriens in the MPTP-treated nonhuman primate. Evid Based Complem Altern Med. 2012;840247:10. doi: 10.1155/2012/840247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyam B. Paralysis agitans and levodopa in ‘‘Ayurveda’’: ancient Indian medical treatise. Mov Disord. 1990;5:47–48. doi: 10.1002/mds.870050112. [DOI] [PubMed] [Google Scholar]
- Manyam BV, Sanchez-Ramos JR. Traditional and complementary therapies in Parkinson’s disease. Adv Neurol. 1999;80:565–574. [PubMed] [Google Scholar]
- Manyam BV, Dhanasekaran M, Hare T. Neuroprotective effects of antiparkinson drug Mucunapruriens. Phytother Res. 2004;18:706–712. doi: 10.1002/ptr.1514. [DOI] [PubMed] [Google Scholar]
- Mohapatra S, Ganguly P, Singh R, Katiyar CK. Estimation of levodopa in the unani drug Mucuna pruriens Bak and its marketed formulation by high-performance thin-layer chromatographic technique. J AOAC Int. 2019 doi: 10.5740/jaoacint.19-0288. [DOI] [PubMed] [Google Scholar]
- Nayak VS, Kumar N, D’Souza AS, Nayak SS, Cheruku SP, Pai KSR. The effects of Mucuna pruriens extract on histopathological and biochemical features in the rat model of ischemia. NeuroReport. 2017;28(18):1195–1201. doi: 10.1097/WNR.0000000000000888. [DOI] [PubMed] [Google Scholar]
- Pathania R, Chawla P, Khan H, Kaushik R, Khan MA. An assessment of potential nutritive and medicinal properties of Mucuna pruriens: a natural food legume. 3 Biotech. 2020;10(6):261. doi: 10.1007/s13205-020-02253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil RR, Rai SN, Jadhav JP, Singh SP. Mucuna sanjappae shows promising anti-Parkinson’s activity by reducing oxidative stress in mptp induced mouse model. EJPMR. 2016;3(11):452–463. [Google Scholar]
- Pinto IR, Chaves HV, Vasconcelos AS, de Sousa FCF, Santi-Gadelha T, de Lacerda JTJG, Ribeiro KA, Freitas RS, Maciel LM, Filho SMP, Viana AFSC, de Almeida Gadelha CA, Filho GC, de Paulo Teixeira Pinto V, Pereira KMA, Rodrigues E, Silva AA, Bezerra MM. Antiulcer and antioxidant activity of a lectin from Mucuna pruriens seeds on ethanol-induced gastropathy: involvement of alpha-2 adrenoceptors and prostaglandins. Curr Pharm Des. 2019;25(12):1430–1439. doi: 10.2174/1381612825666190524081433. [DOI] [PubMed] [Google Scholar]
- Poddighe S, De Rose F, Marotta R, Ruffilli R, Fanti M, Secci PP, Mostallino MC, Setzu MD, Zuncheddu MA, Collu I, Solla P, Marrosu F, Kasture S, Acquas E, Liscia A. Mucuna pruriens (Velvet bean) rescues motor, olfactory, mitochondrial and synaptic impairment in PINK1B9 Drosophila melanogaster genetic model of Parkinson’s disease. PLoS ONE. 2014;23:9. doi: 10.1371/journal.pone.0110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39:2527–2536. doi: 10.1007/s11064-014-1443-7. [DOI] [PubMed] [Google Scholar]
- Raddera DLM, Groenestege ATT, Boers E, Muilwijk EW, Bloem BR. Mucuna pruriens combined with carbidopa in Parkinson’s disease: a case report. J Parkinsons Dis. 2019;9(2):437–439. doi: 10.3233/JPD-181500. [DOI] [PubMed] [Google Scholar]
- Rai SN, Singh SP. Comparison of the neuroprotective potential of estrogen and levodopa in 1-methyl-4-pheny-l,2, 3,6-tetrahydropyridine (mptp)-induced cognitive deficit in parkinsonian mice model. EJPMR. 2016;3(9):317–328. [Google Scholar]
- Rai SN, Yadav SK, Singh D, Singh SP. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP- induced Parkinsonian mouse model. J Chem Neuroanat. 2016;71:41–49. doi: 10.1016/j.jchemneu.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Rai SN, Birla H, Singh SS, Zahra W, Patil RR, Jadhav JP, Gedda MR, Singh SP. Mucuna pruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-κB/Pakt signaling pathways. Front Aging Neurosci. 2017;9:421. doi: 10.3389/fnagi.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SN, Birla H, Zahra W, Singh SS, Singh SP. Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp) J Chem Neuroanat. 2017;85:27–35. doi: 10.1016/j.jchemneu.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Rai SN, Dilnashin H, Birla H, Singh SS, Zahra W, Rathore AS, Singh BK, Singh SP. The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res. 2019;35(3):775–795. doi: 10.1007/s12640-019-0003-y. [DOI] [PubMed] [Google Scholar]
- Rai SN, Singh BK, Rathore AS, Zahra W, Keswani C, Birla H, Singh SS, Dilnashin H, Singh SP. Quality control in huntington’s disease: a therapeutic target. Neurotox Res. 2019;36(3):612–626. doi: 10.1007/s12640-019-00087-x. [DOI] [PubMed] [Google Scholar]
- Rai SN, Zahra W, Singh SS, Birla H, Keswani C, Dilnashin H, Rathore AS, Singh R, Singh RK, Singh SP. Anti-inflammatory activity of ursolic acid in MPTP-induced Parkinsonian mouse model. Neurotox Res. 2019;36(3):452–462. doi: 10.1007/s12640-019-00038-6. [DOI] [PubMed] [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Improved health-relevant functionality in dark germinated Mucuna pruriens sprouts by elicitation with peptide and phytochemical elicitors. Biores Technol. 2009;100:4507–4514. doi: 10.1016/j.biortech.2009.01.078. [DOI] [PubMed] [Google Scholar]
- Rijntjes M. Knowing your beans in Parkinson’s disease: a critical assessment of current knowledge about different beans and their compounds in the treatment of Parkinson’s disease and in animal models. Parkinsons Dis. 2019;2019:1349509. doi: 10.1155/2019/1349509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyad Musthafa M, Asgari SM, Kurian A, Elumalai P, Jawahar Ali AR, Paray BA, Al-Sadoon MK. Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018;75:374–380. doi: 10.1016/j.fsi.2018.02.031. [DOI] [PubMed] [Google Scholar]
- Saranya G, Jiby MV, Jayakumar KS, Padmesh Pillai P, Jayabaskaran C. L-DOPA synthesis in Mucuna pruriens (L.) DC. is regulated by polyphenol oxidase and not CYP 450/tyrosine hydroxylase: an analysis of metabolic pathway using biochemical and molecular markers. Phytochemistry. 2020;178:112467. doi: 10.1016/j.phytochem.2020.112467. [DOI] [PubMed] [Google Scholar]
- Seppan P, Muhammed I, Mohanraj KG, Lakshmanan G, Premavathy D, Muthu SJ, Wungmarong Shimray K, Sathyanathan SB. Therapeutic potential of Mucuna pruriens (Linn.) on ageing induced damage in dorsal nerve of the penis and its implication on erectile function: an experimental study using albino rats. Aging Male. 2018;15:1–14. doi: 10.1080/13685538.2018.1439005. [DOI] [PubMed] [Google Scholar]
- Shin JW, Lee JM. The prospects of CRISPR-based genome engineering in the treatment of neurodegenerative disorders. Ther Adv Neurol Disord. 2017;11:1756285617741837. doi: 10.1177/1756285617741837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS, Rai SN, Birla H, Zahra W, Kumar G, Gedda MR, Tiwari N, Patnaik R, Singh RK, Singh SP. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front Pharmacol. 2018;9:757. doi: 10.3389/fphar.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Dilnashin H, Singh R, Singh SP. Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of DA neurons in a Parkinsonian mouse model. Oxid Med Cell Longev. 2020;27(2020):6571484. doi: 10.1155/2020/6571484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Sharma S, Vora J, Shah H, Srivastava A, Shrivastava N. Mucuna pruriens (L.) DC chemo sensitize human breast cancer cells via downregulation of prolactin-mediated JAK2/STAT5A signaling. J Ethnopharmacol. 2018;217:23–35. doi: 10.1016/j.jep.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Solari P, Maccioni R, Marotta R, Catelani T, Debellis D, Baroli B, Peddio S, Muroni P, Kasture S, Solla P, Stoffolano JG, Jr, Liscia A. The imbalance of serotonergic circuitry impairing the crop supercontractile muscle activity and the mitochondrial morphology of PD PINK1B9Drosophila melanogaster are rescued by Mucuna pruriens. J Insect Physiol. 2018;111:32–40. doi: 10.1016/j.jinsphys.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Surathi P, Jhunjhunwala K, Yadav R, Pal PK. Research in Parkinson’s disease in India: a review. Ann Indian Acad Neurol. 2016;19(1):9–20. doi: 10.4103/0972-2327.167713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharakan B, Dhanasekaran M, Mize-Berge J, Manyam BV. Anti-parkinson botanical Mucuna pruriens prevents levodopa induced plasmid and genomic DNA damage. Phytother Res. 2007;21:1124–1126. doi: 10.1002/ptr.2219. [DOI] [PubMed] [Google Scholar]
- Ulu R, Gozel N, Tuzcu M, Orhan C, Yiğit İP, Dogukan A, Telceken H, Üçer Ö, Kemeç Z, Kaman D, Juturu V, Sahin K. The effects of Mucuna pruriens on the renal oxidative stress and transcription factors in high-fructose-fed rats. Food Chem Toxicol. 2018;118:526–531. doi: 10.1016/j.fct.2018.05.061. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Prakash J, Chouhan S, Singh SP. Mucuna pruriens seed extract reduces oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in paraquat-induced Parkinsonian mouse model. Neurochem Int. 2013;62:1039–1047. doi: 10.1016/j.neuint.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Prakash J, Chouhan S, Westfall S, Verma M, Singh TD, Singh SP. Comparison of the neuroprotective potential of Mucuna pruriens seed extract with estrogen in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice model. Neurochem Int. 2014;65:1–13. doi: 10.1016/j.neuint.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Rai SN, Singh SP. Mucuna pruriens shows neuroprotective effect by inhibiting apoptotic pathways of dopaminergic neurons in the paraquat mouse model of parkinsonism. EJPMR. 2016;3(8):441–451. [Google Scholar]
- Yadav SK, Rai SN, Singh SP. Mp reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J Chem Neuroanat. 2017;80:1–10. doi: 10.1016/j.jchemneu.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhao D, Ali Shah SZ, Wu W, Lai M, Zhang X, Li J, Guan Z, Zhao H, Li W, Gao H, Zhou X, Yang L. The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol. 2019;10:1155. doi: 10.3389/fneur.2019.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahra W, Rai SN, Birla H, Singh SS, Rathore AS, Dilnashin H, Singh R, Keswani C, Singh RK, Singh SP. Neuroprotection of rotenone induced Parkinsonism by Ursolic acid in PD mouse model. CNS Neurol Disord. 2020 doi: 10.2174/1871527319666200812224457. [DOI] [PubMed] [Google Scholar]