Abstract
Additive manufacturing (AM) of biomaterials has evolved from a rapid prototyping tool into a viable approach for the manufacturing of patient-specific implants over the past decade. It can tailor to the unique physiological and anatomical criteria of the patient’s organs or bones through precise controlling of the structure during the 3D printing. Silicone elastomers, which is a major group of materials in many biomedical implants, have low viscosities and can be printed with a special AM platform, known as freeform 3D printing systems. The freeform 3D printing systems are composed of a supporting bath and a printing material. Current supporting matrices that are either commercially purchased or synthesized were usually disposed of after retrieval of the printed part. In this work, we proposed a new and improved supporting matrix comprises of synthesized calcium alginate microgels produced via encapsulation which can be recycled, reused, and recovered for multiple prints, hence minimizing wastage and cost of materials. The dehydration tolerance of the calcium alginate microgels was improved through physical means by the addition of glycerol and chemical means by developing new calcium alginate microgels encapsulated with glycerol. The recyclability of the heated calcium alginate microgels was also enhanced by a rehydration step with sodium chloride solution and a recovery step with calcium chloride solution via the ion exchange process. We envisaged that our reusable and recyclable biocompatible calcium alginate microgels can save material costs, time, and can be applied in various freeform 3D printing systems.
Keywords: Freeform 3D printing, Hydrogel-based supporting matrix, Calcium alginate microgels, Silicone elastomer
Introduction
Over the last decade, additive manufacturing (AM) in the biomedical field, also known as bioprinting, has developed from a rapid prototyping tool to a viable approach for the production of patient-specific implants. It enables precise three-dimensional regulation of structure and material properties adapted to the specific anatomical and physiological requirements of the patient’s organs or bones [1]. Particularly, silicone elastomers are one of the widely used biomaterials for surgical implants as well as many other biomedical devices such as prosthetic, transdermal therapeutic systems, and orthodontics [2]. However, the challenges associated with the printing of silicone elastomers in conventional AM systems are the difficulty of handling their low viscosity before curing, thus hindering the sculpting and shaping of the silicone after material extrusion. Therefore, silicone implants were still mostly manufactured by indirect molding techniques whereby the silicone would be poured into machined or 3D printed molds and subsequently left to cure. This conventional fabrication method for silicone is time-consuming and technically challenging when customizing implants with complex and hollow geometries [3, 4].
In this context, one of the promising AM systems that can offer an alternative platform for the fabrication of customizable silicone products is the freeform 3D printing systems where those soft and low viscosity materials are omnidirectionally printed within a viscous fluid matrix [5–11]. In particular, two major components are involved in freeform 3D printing systems: (1) a supporting bath that is made of shear-thinning viscous fluid, and supports printed materials so that they will not collapse or deform under their weight, and (2) the printing materials that are directly printed within a supporting bath. Both the supporting matrix and the printing materials should be shear-thinning with matching rheological properties (e.g., shear moduli and yield stresses) such that stable deposition and embedment of materials within the supporting matrices can be achieved [5]. This will ensure the structural integrity and print fidelity of the intended model throughout the printing and curing process. In general, the freeform 3D printing systems can be classified into positive 3D printing and negative 3D printing, depending on whether the final printed product is from the ink or the supporting matrix [12]. In this work, we focus on positive freeform 3D printing (Fig. 1) where the final product obtained is the cured deposited material that follows the geometry defined by the user. For instance, Bhattacharjee et al. [8, 9] printed hydrophobic polydimethylsiloxane (PDMS) within an oil-based granular gel made of silicone elastomer. The printed materials were cured within the supporting matrix before the removal of the printed part. This system was used to create 3D objects, shelled models, and branched tubular networks with silicones.
Fig. 1.
Schematics of the positive freeform 3D printing process
However, most silicone elastomers used for freeform 3D printing have been photo-curable or room temperature-curable, thus a freeform 3D printing system for more widely used heat-curable silicones should be developed. In order to successfully print heat-curable silicone rubber, the supporting matrix must be able to support printing and withstand high-temperature post-curing conditions as silicone resins need to be treated at temperature > 70 °C for a few hours [13]. Furthermore, it would be more beneficial if a supporting matrix is recyclable, eco-friendly, and biocompatible since many silicone products have been used for medical or epidermal electronic devices. As the larger volume of a supporting matrix is often required compared to the volume of its counterpart inks, the preparation of the supporting matrix for printing increases the time and waste of the whole printing process when the matrix has to be destroyed or disposed of after each print. Particularly, a supporting matrix which consists of non-degradable microbeads leads to another waste management issue.
Herein, considering these additional requirements of a supporting matrix besides its supportability for embedded inks, we have introduced alginate microgels as a material for a biocompatible and recyclable supporting matrix that can be used for freeform 3D printing with silicone rubber cured at high temperature. The alginate-based supporting matrix was introduced in our previous work [14] for the direct freeform 3D printing of intrinsically curved flexible membranes. Alginate was chosen for its biocompatibility that allows printing of biomaterials for biomedical engineering, heat stability which allows it to withstand high-temperature curing of silicone ink as well as degradability that prevents waste generation. Alginate-based supporting matrix was fabricated by alginate microgels dispersed in water, denoted as “alginate slurries”. The alginate microparticles were synthesized through the encapsulation process, carefully controlling the particle sizes and shapes of the microgels, to create a slurry with different flow properties. In encapsulation technologies, surfactants are essential components to be added to the gelation bath to overcome the high surface tension force during the impact when spherical alginate droplets penetrate the gelation bath [15, 16]. Typical surfactants used are Tween 20, Tween 80, cetyltrimethylammonium bromide (CTAB), or dodecyl trimethyl ammonium bromide (DTAB) [16–18]. To avoid microparticle agglomeration through particle–particle interaction, Tween 20 was first used as a dispersant during the encapsulation process in this work. However, printed silicone in the alginate-based supporting matrix would undergo thermal curing where significant dehydration of the supporting matrix is often observed. Dried alginate hydrogels shrank in size and have more cracks appearing on the surface of the hydrogels after drying the beads at 70 °C for 24 h [13]. Extensive dehydration will destroy the hydrogel-based supporting matrix, resulting in poor print fidelity, resolution, and wastage of materials. Thus, we also introduced glycerol as a humectant to alginate slurry systems which can be recycled, reused, and recovered for multiple prints, hence minimizing wastage and cost of materials. Glycerol is commonly used as a humectant for food, cosmetics, pharmaceutical products, due to its hygroscopic nature, biocompatibility, and solubility in water that helps to reduce water loss and retain moisture [19]. A systematic investigation was conducted to (1) improve the intrinsic dehydration tolerance of the alginate-based supporting matrix through the incorporation of glycerol, and (2) to improve the recyclability of the dehydrated alginate slurries through the ion exchange process. Finally, we proposed a recycling process of the alginate supporting matrix for large-scale freeform 3D printing systems. We envisaged that our reusable and recyclable biocompatible alginate slurries can save material costs, time, and can be applied in various freeform 3D printing systems, giving more options for users depending on their target system.
Materials and methods
Chemicals and materials
Chemicals used for the preparation of supporting matrix included alginic acid sodium salt (Sigma Aldrich, USA), sodium chloride (Merck, USA), calcium chloride (Sigma Aldrich, USA), Tween 20 (Sigma Aldrich, USA), and glycerol (Sigma Aldrich, USA). Chemicals used for the preparation of printing materials included heat-curable silicone elastomer DOWSIL SE1700 White (DOW Corning, Japan), mineral oil (Sigma Aldrich, USA), and food coloring dye (Wilton, USA). A Milli-Q system (Millipore Singapore Pte Ltd) was used to supply ultrapure water for the dissolution of solutes.
Fabrication of alginate-based supporting matrices
Preparation of alginate slurry with tween 20 (Alg/T20)
Figure 2a illustrates the process of making alginate slurry with Tween 20 (Alg/T20) and the physical addition of glycerol to improve the dehydration tolerance of the slurry. Alg/T20 microgels were produced with Encapsulator B-390 (Buchi, Switzerland) whereby 1 wt% alginate solution was pumped at a flow rate of 3 mL/min through a 120 µm nozzle into a gelation bath. The gelation bath is a mixture of 0.5 wt% Tween 20 and 0.05 M calcium chloride (CaCl2) solution with an alginate-CaCl2 volume composition of 3:4, stirred at 200 rpm at a temperature of 40 °C. Tween 20 is a common surfactant used in encapsulation to overcome the high surface tension force during the impact and prevent deformation of the alginate microgels as the alginate droplets penetrate through the gelation bath [16–18]. The vibration unit in Encapsulator B-390 provided electric oscillations at a frequency of 6000 Hz to break up the alginate droplets. The electrode of 1700 V voltage helped to electrostatically disperse the alginate droplets before reaching the gelation mixture to prevent agglomeration. These cross-linked Alg/T20 calcium alginate microgels were subsequently filtered by a nylon mesh filter of 500 µm pore size together with a coffee filter after encapsulation. The microgels were first rinsed and washed with 50 mL of 0.45 wt% or 0.9 wt% NaCl solution thrice, stirred in the same solution at a microgels-NaCl solution mass composition of 1:7 for at least 24 h at 200 rpm, and washed thrice again before being filtered dry for use. Subsequently, Alg/T20 microgel-based slurry systems were prepared by dispersing microgels in 0.90 wt% NaCl solution with a weight ratio of microgels to NaCl solution = 1:2 or 1:7, varying the concentrations of glycerol from 5 to 15 wt%. A see-saw rocker SSL4 (Stuart equipment, USA) was used to provide continuous shaking and mixing for 90 min.
Fig. 2.
Schematics of the preparation of the slurry from the encapsulation of calcium alginate microgels to their post-treatment. a Preparation of Alg/T20 calcium alginate microgels with physical addition of glycerol, and b Preparation of Alg/Gly calcium alginate microgels
Preparation of alginate slurry with glycerol (Alg/Gly)
Alginate slurry with glycerol (Alg/Gly) was produced in similar steps as Alg/T20 slurry except that a different gelation bath was used (see Fig. 2b). Alg/Gly microgels were produced with Encapsulator B-390 whereby 1 wt% alginate solution was pumped at a flow rate of 3 mL/min through a 120 µm nozzle into a gelation bath with an oscillatory frequency of 6000 Hz and the electrode of 1700 V voltage. The gelation bath was a mixture of 0.05 M calcium chloride solution and glycerol at various concentrations from 0.5 to 3.0 wt%. Alg/Gly microgels were subsequently filtered, followed by rinsing/washing with 50 mL of 0.45 wt% NaCl solution thrice, stirred in the same solution at a microgels-NaCl solution mass composition of 1:7 for at least 24 h at 200 rpm, and washed thrice again before being filtered dry for use. Subsequently, Alg/T20 microgel-based slurry systems were prepared by dispersing microgels in 0.90 wt% NaCl solution with a weight ratio of microgels to NaCl solution = 1:2 or 1:7, varying the concentrations of glycerol from 5 to 15 wt%. A see-saw rocker SSL4 (Stuart equipment, USA) was used to provide continuous shaking and mixing for 90 min.
Dehydration of alginate microgels during heat-curing of ink
Alginate slurries were filtered dry and packed into small Petri dishes of diameter 30 mm with 8 g of microgels each. They were then placed in a 75 °C oven for 24 h to stimulate the heat-curing of ink after printing. The extent of dehydration was calculated by the percentage of weight loss of the microgels after 24 h of heating.
Rheological testing of alginate microgels
The alginate slurries were loaded onto a rheometer (TA Instrument AR2000 Ex, USA) with a 20 mm, 1° conical plate. Oscillatory stress sweep tests were conducted from 0.1 to 100 Pa at a gap of 1500 µm and a temperature of 25 °C.
Improving recyclability of alginate-based supporting matrix
Post-encapsulation treatment for small and large scale production of Alg/Gly slurry
To minimize wastage of resources, the post-encapsulation treatment approaches for small scale production (i.e., below 50 mL of alginate solution) and large scale production (i.e., at least 360 mL of alginate solution) were different. For small scale production of Alg/T20 or Alg/Gly slurry, the microgels were subsequently filtered by a nylon mesh filter of 500 µm pore size together with a coffee filter after encapsulation. The microgels were first rinsed and washed with 50 mL of 0.45 wt% or 0.9 wt% NaCl solution thrice, stirred in the same solution at a microgels-NaCl solution mass composition of ~ 1:7 for at least 24 h at 200 rpm, and washed thrice again before being filtered dry for use.
For large scale production of microgels, the microgels were filtered by two stacked metal sieves of 500 µm and 90 µm pore sizes after encapsulation. The collated sieve of microgels was first rinsed and washed in a container filled with 2 L of 0.45 wt% NaCl (i.e., microgels-NaCl mass composition of ~ 1:7) with manual stirring using a plastic scraper for 1 min before being lifted and drained repeatedly for 6 cycles. Next, another 3 similar cycles were repeated in a new container of 0.45 wt% NaCl solution. Lastly, the microgels were soaked and stirred in 500 mL of fresh 0.45 wt% NaCl for 24 h. Before usage, the microgels were sieved and compressed with a customized thick flat plate printed by a polyjet Objet350 Connex3 (Stratasys, USA) to drain off excess NaCl solution.
Optical transparency of alginate microgels
Alg/T20 and Alg/Gly microgels were separately dispersed in 0.90 wt% NaCl solution with mass compositions of microgels-NaCl solution at ratios of 1:2 and 1:7. A see-saw rocker SSL4 (Stuart equipment, USA) was used to provide continuous shaking and mixing for 90 min. After the shaking stopped, the microgels were soaked for 24 h and filtered to dry state before being measured for absorbance via a UV–Vis spectrophotometer (SpectraMax M5, Molecular Devices, USA) at wavelengths ranging from 400 to 700 nm. The transmittance percentages were calculated from the measured absorbance values.
Rehydration and recovery of alginate microgels
Heated alginate microgels were rehydrated by sieving and rinsing in 0.45 wt% or 0.9 wt% NaCl solution as shown in Fig. 3a. This is the rehydration process that has to be conducted after every heating cycle to prevent the drying of microgels. After several printing and heating cycles, alginate microgels softened and became unsuitable for printing. Softened alginate microgels would undergo the recovery process where the microgels were sieved and rinsed in 10 mM CaCl2 solution for 10 s with manual stirring using a plastic scraper as illustrated in Fig. 3b. The sieve of microgels will be lifted after 10 s and compressed to drain off the CaCl2 solution from the microgels. Next, the sieve of microgels was rinsed in 0.45 wt% NaCl solution with manual stirring for 1 min and drained repeatedly for 3 cycles before being compressed to remove the excess NaCl solution from the microgels.
Fig. 3.
Schematics of the processes for improving the recyclability of calcium alginate microgels: a Rehydration of heated calcium alginate microgels by rinsing in NaCl solution. Rehydration needs to be conducted after every printing-heating cycle. b Recovery of softened calcium alginate microgels by rinsing in CaCl2 solution. Recovery to be performed only when rehydrated microgels softened beyond printing capability
The 3D printing process
All 3D printing in this work followed the positive freeform 3D printing process illustrated in Fig. 1. Printing was conducted via an extrusion-based 3D Bio-printer (RegenHu 3D Discovery, Switzerland). The printing materials (i.e., the ink) comprised silicone elastomer base DOWSIL SE1700 White (DOW Corning, Japan), its catalyst, and mineral oil at a mass composition of 10:1:1.5. Orange food dye was used for visualization purposes. The supporting matrix was composed of calcium alginate microgels produced via encapsulation with Encapsulator B-390 (Buchi, Switzerland). The printhead moved in three dimensions across the building platform and extruded the ink within the supporting matrix according to the printing path. The printing path was controlled via G-codes that could be customized accordingly to the desired geometry. G-codes of the contours of a hemisphere with a 20 mm diameter and a 3D scanned elbow surface with a dimension of 70 mm by 70 mm were generated and used for printing in this work. Small Petri dishes with a diameter of 30 mm were used as the containers for alginate slurry to print the hemisphere whereas a large printing dish, which was 82 mm × 82 mm x 33 mm in size was used as the container for the elbow contour printing. After the prints were completed, the embedded parts were cured in the oven at 75 °C overnight. The cured parts were retrieved by washing away the alginate slurry.
Results and discussion
Recyclability of alginate slurries with Tween 20 (Alg/T20) and glycerol
The dehydration tolerance of the calcium alginate microgels of alginate slurries with the addition of glycerol was first investigated in terms of weight loss and rheological behavior after heating the slurries at 75 °C. 5 wt%, 10 wt%, and 15 wt% glycerol were added to alginate/Tween 20 (Alg/T20) slurries to examine the optimal concentration of glycerol that could prevent extensive dehydration of the microgels during heating with 25–30% relative humidity. Figure 4a shows the image of the dehydrated Alg/T20 slurry with different concentrations of glycerol compared with Alg/T20 slurry without any glycerol addition after 24 h of heating. Water loss was significantly minimized after the addition of glycerol as compared to Alg/T20 slurry without glycerol after heating. The percentage loss in weight of the slurry decreased when the concentration of glycerol added increased. 5 wt% glycerol addition had the highest weight loss percentage at ~ 31%, 10 wt% glycerol addition had ~ 24% weight loss and 15 wt% glycerol addition had the lowest weight loss percentage at ~ 18%. This was also observable from the decrease in voidage of slurry inside the Petri dishes when the microgels dried up (demarcated by the red boundary in Fig. 4a) when the concentration of glycerol added increased. Besides, without glycerol addition, the calcium alginate network in Alg/T20 slurry was destroyed and the microgels shriveled into thin rigid solid pieces after 24 h of direct heating in the oven with 25–30% relative humidity. Due to the high heating rate, the calcium alginate microgels changed from a predominantly amorphous structure to form crystalline structures in the calcite phase and calcium oxides [20]. These results showed that adding glycerol to the microgels had significantly reduced water loss, keeping the microgels in a moist state after heating. Without the addition of glycerol, the microgels would be entirely dried up and would not be reusable for subsequent printings.
Fig. 4.
Alginate slurry comprising of Alg/T20 microgels with glycerol addition. a Alg/T20 microgels added with 0 wt% (no glycerol), 5 wt%, 10 wt%, and 15 wt% glycerol that underwent 24 h of heating. Red boundaries indicated voids created when dehydration occurred during heating. b Oscillatory stress sweep test of Alg/T20 microgels rinsed and washed with 0.90 wt% NaCl before heating and Alg/T20 microgels added with 5 wt%, 10 wt%, and 15 wt% glycerol after 24 h of heating. c Oscillatory stress sweep test of Alg/T20 microgels rinsed and washed with 0.45 wt% NaCl before heating and Alg/T20 microgels added with 7.5 wt% and 12.5 wt% glycerol after 24 h of heating. d G-code profile of hemispherical structure (left) Printed structure in Alg/T20 slurry with 10 wt% glycerol (right). All scale bars: 1 cm
To quantitatively evaluate whether glycerol addition had helped to retain the original rheological properties of Alg/T20 slurry after 24 h of direct heating, oscillatory stress sweep tests were conducted. Alg/T20 slurries with 5–15 wt% glycerol addition were tested after heating and compared to the fresh Alg/T20 slurry before heating which acts as the control. It would be favorable for the slurry with glycerol addition to preserve its viscoplastic behavior whereby the printing nozzle can move through the slurry and deposit the ink without resistance and without disrupting the surrounding matrix. In the oscillatory stress sweep test, the shear modulus G’/G’’ cross over point is defined as the yield stress of the slurry which signifies the transition of the slurry from elastic (solid-like) to viscous (liquid-like) behavior [21]. As seen in Fig. 4b, all the Alg/T20 slurries prepared with 0.90 wt% NaCl solution and with glycerol addition did not produce any cross over points after heating except the fresh Alg/T20 slurry. Furthermore, different concentrations of NaCl could affect the yield stresses and shear moduli of the slurry. The overall yield stresses of slurries were greatly reduced when another set of Alg/T20 slurries was rinsed and sieved with 0.45 wt% NaCl solution, separately mixed with 0 wt% (no glycerol), 7.5 wt%, 12.5 wt% of glycerol, and subjected to the same heating process (Fig. 4c). Nevertheless, the G’/G’’ cross over points were still not obtainable from all the heated Alg/T20 slurries with glycerol. The yield stresses and shear moduli G’ were summarized in Table 1. For both NaCl concentrations, the heated microgels were stiff and remained solid-like at shear stresses beyond 100 Pa after 24 h of direct heating despite being able to retain moisture with the help of glycerol that was added to the samples.
Table 1.
Yield stress and shear elastic modulus of Alg/T20 slurry with or without glycerol addition, before and after heating
| Alg/T20 slurry | Yield stress (Pa) | G’ (Pa) |
|---|---|---|
| Prepared with 0.90 wt% NaCl solution | ||
| Before heating | 93.4 ± 5.1 | 249.5 ± 42.4 |
| 5.0 wt% glycerol addition after heating | No crossover point (yield stress > 100 Pa) | |
| 100 wt% glycerol addition after heating | ||
| 15.0 wt% glycerol addition after heating | ||
| Prepared with 0.45 wt% NaCl solution | ||
| Before heating | 18.1 ± 0.7 | 160.8 ± 1.0 |
| 7.5 wt% glycerol addition after heating | No crossover point (yield stress > 100 Pa) | |
| 12.5 wt% glycerol addition after heating | ||
These findings affirmed that the introduction of glycerol to the alginate microgels through physical addition would not be able to retain the original rheological properties of the slurry before heating although it would help in avoiding mass loss and extreme dehydration of the alginate microgels. The significant improvement in the reduction of water loss in the samples had definitely shown glycerol’s potential as a humectant in this work. Although glycerol was able to keep the microgels in a good state after printing, it is also important to examine if the addition of glycerol to Alg/T20 slurry would compromise the print fidelity and resolution of parts printed in the slurry. Therefore, a hemispherical structure of 20 mm diameter was 3D printed in Alg/T20 slurry with a 10 wt% glycerol addition as it had a better condition (i.e., less watery) than the Alg/T20 slurry with 15 wt% glycerol addition. The G-code profile of the hemispherical structure and the printed structure in the slurry were shown in Fig. 4d. Despite being printable in the slurry using the optimized printing parameters at a pressure of 4.5 bar and feed rate of 3 mm/min, the printed structure had breakages at different parts of the print. This might be due to the inhomogeneity of the slurry when glycerol was physically added and manually mixed with the microgels. Without proper control of the homogeneity of the slurry, there would be an undesirable impact on the print fidelity and resolution of printed parts in the freeform 3D printing system.
Fabrication of alginate slurries with glycerol (Alg/Gly) as an alternative
As the addition of glycerol through physical means had shown potential in reducing water loss yet could not preserve the original rheological properties of the alginate microgels, another method of introducing glycerol to alginate microgels through chemical means was examined (see Fig. 2b). To ensure that glycerol could be incorporated into the alginate microgels, glycerol was used as a substitute for Tween 20 in the gelation bath during encapsulation. Different concentrations of glycerol from 1.0 to 3.0 wt% were used in the preparation of the gelation bath during encapsulation instead of Tween 20. Both Alg/T20 and Alg/Gly microgels produced from 1.0 to 2.0 wt% had spherical shapes whereas Alg/Gly microgels produced from 2.5 and 3.0 wt% began to show some irregularity and deformation as indicated by the black arrows in Fig. 5a. The average particle size of Alg/T20 beads is 230 ± 40 µm whereas all Alg/Gly beads produced are in the size range of ~ 190 µm to ~ 220 µm as shown in Fig. 5b. When the concentration of glycerol increased beyond 2.0 wt%, the sphericity of the microgels was affected. No significant trend between glycerol concentration and particle size was observed. With increasing concentration of glycerol, the viscosity of the gelation bath increases. The alginate droplets would flatten in the impact direction upon hitting the gelation bath with increasing viscosity of the gelation bath. Therefore, the Alg/Gly microgels produced from higher glycerol concentration experienced some deformation resulting in lower sphericity. This was in agreement with Dohnal et al.’s work [22] that demonstrated the particle aspect ratio was sigmoidally affected by the viscosity of the gelation bath, but there was no systematic dependency between the particle size and the viscosity of the gelation bath. The shape and size of the microgels would affect the rheological behavior of the slurry and also surface roughness of the printed parts. Spherical microgels with a homogenous size distribution between 200 µm to 300 µm would be desirable in a supporting matrix such that the printing resolution would be consistent.
Fig. 5.
Characterization of alginate slurry comprising of Alg/Gly microgels. a Microscopic images of Alg/T20 microgels (control) and Alg/Gly microgels produced from encapsulation with 1.0 wt% glycerol, 2.0 wt% glycerol, and 3.0 wt% glycerol. Black arrows indicated irregularities in the sphericity of beads. Scale bar: 200 µm, inset:100 µm. b Particle sizes of Alg/Gly microgels produced from the different concentrations of glycerol encapsulated from 1.0 to 3.0 wt%. Data was measured from an average of 90 beads taken across different microscopic images. c Transmittance of light (indicating transparency of calcium alginate slurry) for Alg/T20 microgels and Alg/Gly microgels with a mass composition of 1:2 or 1:7 (microgels-0.90 wt% NaCl solution) with and without continuous mixing for 90 min. Insets show images of microgels for the transmittance test. **p < 0.01
Microgels are usually washed with deionized water or salt solution such as NaCl solution or potassium chloride solution [23, 24]. Therefore, it is important to study the influence of the washing/rehydration step on the alginate microgels. To compare the differences among the microgels obtained with different washing steps, two mass compositions of Alg/T20 or Alg/Gly microgels to 0.9 wt% NaCl solution at ratios of 1:2 and 1:7 (microgels-NaCl solution) were subjected to a simulated soaking and washing step with and without continuous mixing for 90 min using a see-saw rocker SSL4 (Stuart equipment, USA). Apparent changes in the clarity of the microgels were observed and shown in Fig. 5c. Microgels-NaCl solution ratios of 1:7 had higher transmittances of light in the range of 60–70% at 700 nm wavelength than microgels-NaCl solution ratios of 1:2 with transmittance in the range of 30–35%. This showed that the mass compositions of the microgels to the soaking salt solution greatly affected the transparency of the microgels at 1% significance level. During the soaking and washing, the calcium ions in the calcium alginate microgels were released into the surrounding salt solution through the ion exchange process and swelling of the alginate network would occur [24]. Soaking the microgels in a larger volume of the salt solution allowed a better dispersion of microgels and ensured that every bead would have access to surrounding sodium ions for ion exchange to take place. As such, a higher microgels-NaCl solution ratio would result in clearer microgels with higher transmittance. Also, at 1% significance level, Alg/Gly microgels exhibited a statistically higher transmittance of ~ 70% compared to Alg/T20 with lower transmittance of ~ 60% for microgels-NaCl solution ratios of 1:7. The inset images in Fig. 5c had also reflected these transmittance values in the visual appearance of the microgels. The rehydration rates of the microgels were highly dependent on the ionic strength and type of salt solution. Increasing the salt concentration of the washing medium would increase the rehydration rates of microgels [24]. The maximum transmittance achievable for Alg/T20 microgels was ~ 60% and could be reached with 0.45 wt% NaCl solution or 0.90 wt% NaCl solution slurry with microgels-NaCl solution ratio of 1:7. No significant difference was observed for microgels with or without continuous shaking and mixing for 90 min at 1% significance level. The high transmittance of slurry is an additional advantage for ease of monitoring the ink deposition within the transparent and clear slurry during the printing process. To minimize the usage of resources, the post-encapsulation treatment for the production of high transmittant or clear Alg/Gly slurry was conducted by washing, filtering, and stirring in 0.45 wt% NaCl solution with microgels-NaCl solution ratio of 1:7.
Recyclability of alginate slurries with glycerol (Alg/Gly)
Thermal stability of Alg/Gly slurries
The thermal stability of Alg/Gly slurries is an important aspect to consider especially when high-temperature printing or curing is involved in the freeform 3D printing process. To examine whether incorporation of glycerol in alginate microgels through encapsulation would be effective in improving the dehydration tolerance of alginate microgels during the heat-curing process, weight loss analysis after 24 h of direct heating in the oven was also conducted for the Alg/Gly microgels produced from the various glycerol concentration. As seen in Fig. 6a, Alg/T20 microgels had the highest weight loss at ~ 60%. Alg/Gly microgels produced from 1.0 wt% glycerol had the next greatest weight loss at ~ 42% while Alg/Gly microgels produced from 2.0 wt% glycerol had the lowest weight loss at ~ 18%. When glycerol concentration increased from 1.0 to 2.0 wt% glycerol, the weight loss after heating decreased due to the ability of glycerol to absorb moisture from surrounding thus minimizing dehydration. However, beyond 2.0 wt% glycerol, weight loss began to increase from ~ 19% for 2.5 wt% glycerol to 32% for 3.0 wt% glycerol. These may be attributable to the deformation of the beads for Alg/Gly microgels produced from 2.5 and 3.0 wt% glycerol as discussed in the previous section. The effectiveness of glycerol in retaining moisture was diminished by the deformation of the microgels. As such, Alg/Gly microgels produced from 2.0 wt% glycerol with an average particle diameter of 212 ± 28 µm was eventually chosen for the production of slurry as it was the maximum allowable glycerol concentration that can be incorporated into the microgels during encapsulation without compromising their sphericity. It was also the most effective in improving the dehydration tolerance of Alg/Gly slurry in the heating test.
Fig. 6.
Thermal stability of Alg/Gly microgels. a Weight loss of Alg/Gly microgels produced from encapsulation with 1.0 wt% to 3.0 wt% glycerol after 24 h of heating. b Oscillatory stress sweep test of Alg/Gly microgels produced from 2.0 wt% glycerol before heating, after heating, and after rehydrating with 0.45 wt% NaCl solution. c Alg/Gly microgels processed with 0.90wt % NaCl solution at the mass composition of 1:2 (microgels-NaCl solution) and Alg/Gly microgels processed with 0.90 wt% NaCl solution at the mass composition of 1:2 (microgels-NaCl solution) before and after the usage for silicone printing and curing after 24 h of heating in a 75 °C oven. Zoomed-in microscopic images of the unclear and clear beads after undergoing the heat-curing process. Scale bars: 500 µm
Furthermore, the rheological behavior of the Alg/Gly slurry produced from 2.0 wt% glycerol before, after heating, and after rehydrating was also assessed by oscillatory stress sweep tests and compared in Fig. 6b. The yield stress and shear elastic modulus G’ were compared in Table 2. The yield stress of the slurry before and after heating was successfully preserved by the introduction of glycerol into the microgels through encapsulation. Alg/Gly slurry had shown substantial improvement in withstanding the dehydration during heating as compared to the results obtained from Alg/T20 slurry with glycerol addition (see Fig. 4b, c). Dehydration during heating caused the microgels to become stiffer and hence the increase in shear modulus G’. During the dehydration process, the concentration of calcium ions increased, resulting in the formation of egg-box multimer structures in the absence of salt solution [24–26]. Therefore, rehydration was performed by sieving and rinsing the heated microgels in 0.45 wt% NaCl solution (see Fig. 3a). In the presence of salt solution, the multimer structures broke up during ion exchange, and dehydrated microgels gradually became rehydrated, swelled, and softened when calcium ions were released from the alginate microgels into the surrounding solution [24–26]. Rehydration allowed the Alg/Gly slurry to go back to its original state and regain its rheological properties such that consistency in printing could be achieved. However, from Fig. 6b, the yield stress of the rehydrated microgels became slightly lower than the original microgels before heating and after one cycle of heating and rehydrating. This implied that the Alg/Gly slurry might become degraded after multiple cycles of heating and rehydrating, affecting the consistency of the printing and the recyclability of the microgels.
Table 2.
Yield stress and shear elastic modulus of Alg/Gly slurries produced from 2.0 wt% glycerol before and after heating, and after rehydrating
| Alg/Gly slurry | Yield stress (Pa) | G’ (Pa) |
|---|---|---|
| Before heating | 34.4 ± 3.75 | 72.5 ± 9.95 |
| After heating | 34.3 ± 2.25 | 128.1 ± 1.48 |
| After rehydrating | 25.3 ± 2.85 | 84.1 ± 1.89 |
Other than the rheological properties of slurries, it is also critical to determine the print fidelity and recyclability of Alg/Gly slurries after heating to evaluate the thermal stability of the slurry. In Fig. 6c, both clear (microgels-NaCl solution ratio of 1:7) and unclear (microgels-NaCl solution ratio of 1:2) Alg/Gly slurries were packed into Petri dishes for the printing of the hemispherical structures. The clarity of microgels was apparent when comparing the visibility of the words shown in the background. After 24 h of heat-curing in 75 °C oven, the cured structure that was printed in the unclear microgels had several breakages and had poorer print fidelity as compared to that of the clear microgels. Besides, the unclear microgels became clumpy and were in a much drier state after heating whereas the clear microgels remained moist and soft after heating. From the zoomed-in microscopic images of the heated microgels taken, the clear microgels remained spherical as before while the unclear microgels became deformed and lost its sphericity. The rupture strength of calcium alginate microgels increased and their size decreased when heating temperature raised [13, 27]. Moreover, the unclear microgels were unable to regain its sphericity despite the attempt of rehydrating them with 0.45% NaCl solution. On the other hand, the clear microgels could be stored aside to be reused for subsequent printing after rehydration. Therefore, it is important to optimize the post-encapsulation treatment or the washing steps such that clear calcium alginate microgels could be obtained. These findings further affirmed that clear Alg/Gly microgels produced with 2.0 wt% glycerol are desirable for visualization, printing, recycling, and overall thermal stability.
Rheological behavior of rehydrated and recovered Alg/Gly slurries
The sustainability of calcium alginate microgels is another important aspect to consider for a good supporting matrix. After multiple printing and heating cycles, the microgels showed signs of softening. To prevent degradation of the microgels after several repeating cycles, a reversal process involving the recovery of the softened microgels with calcium chloride (CaCl2) solution was investigated. The concept of the CaCl2 recovery method was considered for the reversal of the ion exchange process that took place during the washing and rehydration of microgels. CaCl2 solution is one of the major components of the gelation bath during the encapsulation process. During gelation, calcium ions bound to the guluronate units of the alginate chains and formed junctions with adjacent guluronate units of polymer chains, creating an egg-box model of cross-linking. During dehydration, the increase in calcium concentration formed egg box multimers but these calcium ions were released into the surrounding NaCl solution via ion exchange process between sodium ions and calcium ions during the rehydration [24–26]. As dehydration and rehydration is a reversible process, the CaCl2 recovery method allows the reverse ion exchange between sodium ions and calcium ions to regain a stiffer polymer chain [28, 29].
To investigate the effectiveness of CaCl2 solution in improving the yield stress of the rehydrated Alg/Gly slurry, a preliminary test of rinsing the rehydrated microgels with 100 mM CaCl2 solution followed by 0.45 wt% NaCl solution was conducted. The rheological test results of the oscillatory stress sweep tests demonstrated the feasibility of using CaCl2 solution to recover the original gel strength of the softened Alg/Gly microgels (Fig. 7a). However, the CaCl2 recovery procedure had to be carefully controlled to prevent the ‘over-hardening’ of the Alg/Gly microgels as exhibited in this preliminary test. The concentration of CaCl2 solution and the duration of the rinsing were deemed to be critical in affecting the yield stresses and shear moduli of the recovered Alg/Gly microgels, thus the CaCl2 recovery procedure was optimized by varying those key parameters for large scale Alg/Gly slurry production. Two continuous cycles of heating-rehydration-recovery of the Alg/Gly slurry were conducted and analyzed for their rheological behavior. As shown in Fig. 7b, c, the shear moduli for all the Alg/Gly slurry before heating, after rehydrating and recovery for two continuous cycles were similar in the range of ~ 130–154 Pa. The yield stresses were comparable for all at ~ 49–55 Pa except for the first recovery that reached ~ 106 Pa. This anomaly might be due to (1) a delay in human reaction time when rinsing during the recovery procedure and hence causing more calcium ions to enter the alginate network or (2) the variation in the kinetics for the ion exchange reactions due to indeterminate concentration of sodium or calcium ions brought over by the rehydrated microgels. This further affirms the significance of the concentration of calcium ions and the rinsing duration in this CaCl2 recovery method. However, the calcium concentration present in the solution could not be accurately monitored in situ due to a lack of suitable equipment. Nevertheless, these findings showed that CaCl2 recovery is an effective method to recover the initial yield stresses and shear moduli of the microgels by reversing the ion exchange between sodium ions and calcium ions under precise control. In fact, we recommend performing the CaCl2 recovery after several heating-rehydration cycles when Alg/Gly slurry became degraded beyond the printable range.
Fig. 7.
Rheological behavior of rehydrated and recovered Alg/Gly microgels. a Oscillatory stress sweep test of Alg/Gly microgels after rehydrating with 0.45 wt% NaCl solution and after recovery with 100 mM CaCl2. b Oscillatory stress sweep test of Alg/Gly microgels before heating, after the first cycle of rehydration, recovery, and after the second cycle of rehydration and recovery. c Yield stress and shear modulus results obtained from b on Alg/Gly microgels before heating, after the first cycle of rehydration, recovery, and after the second cycle of rehydration and recovery. Rehydration of b and c are conducted by rinsing with 0.45 wt% NaCl solution and recovery was conducted by rinsing with 10 mM CaCl2
Printability of recycled Alg/Gly slurries
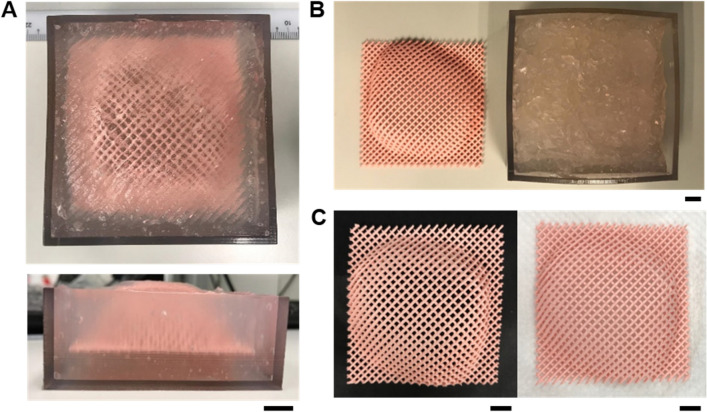
To demonstrate the overall thermal stability and sustainability of the Alg/Gly slurries, the printability of the recycled Alg/Gly slurries was investigated by printing large structures in the fresh Alg/Gly slurry and its recycled Alg/Gly slurry. An elbow mesh structure created from customized G-codes derived from a 3D scanned human elbow was printed in the Alg/Gly slurry as shown in Fig. 8a. The elbow structures were dual-layered meshes with each layer placed perpendicularly on top of each other and had printed filament diameters of approximately 1 mm. The overall dimensions of the elbow meshes were 70 mm x 70 mm. The 3D elbow mesh was successfully printed in the fresh and recycled Alg/Gly slurry via freeform 3D printing and cured after 24 h of heating in a 75 °C oven. Figure 8b shows the printed and cured elbow mesh from the fresh Alg/Gly slurry. Moreover, the Alg/Gly slurry remained moist and soft after 24 h of direct heating without a water bath, enabling the microgels to be reused for multiple printing after rehydration. Figure 8c shows the elbow meshes printed from Alg/Gly slurry that underwent one cycle of recycling (Fig. 8c left) and two cycles of recycling (Fig. 8c right). There were no breakages and the dual-layer meshes were well connected at all points for all three meshes. This proves that our Alg/Gly slurry can be reusable for printing after recycling.
Fig. 8.
Printability of recycled Alg/Gly slurries. a Images of silicone elbow mesh printed within the fresh Alg/Gly slurry with freeform 3D printing. b Image of printed silicone elbow mesh in fresh Alg/Gly slurry and Alg/Gly slurry condition immediately after 24 h of heat-curing in 75 °C oven (right). c Printed silicone elbow meshed in Alg/Gly slurry after 1st recycling (left) and 2nd recycling (right). All scale bars: 1 cm
Scalability of alginate-based supporting matrix for large scale manufacturing
The scaling up of the Alg/Gly slurry production was necessary for the printing of large objects and large scale manufacturing. Herein, we recommend an optimal process cycle for large scale manufacturing with alginate-based supporting matrices, as illustrated in Fig. 9. Beginning with slurry formation, alginate microgels could be consistently produced with a yield rate of 3 mL/min based on our experimental conditions for encapsulation. However, considering losses from scattering during electrostatic dispersion, transferring, and filtering, the effective yield rate could be decreased by approximately 40–50%. The optimized post-encapsulation treatment method for the production of clear alginate microgels by washing, filtering, and stirring in 0.45 wt% NaCl solution with microgels-NaCl solution ratio of 1:7 was applicable for both small scale (less than 50 mL alginate) and large scale (more than 300 mL alginate) production. However, the filtering procedure needed to be slightly modified to ensure that high transmittance could be obtained for an extensive number of microgels in the large scale production with time and resource efficiency. After slurry formation, the fresh alginate-based slurry undergoes printing followed by heat-curing for thermally cross-linked inks e.g. silicone elastomer ink. Upon heat-curing, the alginate slurry has to undergo a rehydration process whereby the microgels will be rehydrated with NaCl solution and revert to the fresh alginate slurry condition. In instances where the rehydrated alginate slurry becomes degraded, softened, and no longer supports consistent printing, the slurry will need to undergo a recovery process whereby the microgels will be “hardened” with CaCl2 solution to revert to the fresh alginate slurry condition. The exact number of cycles, n, before a recovery process has to be performed is dependent on the condition of the slurry. Based on our experimental tests, we can determine that n > 3 given adherence to our proposed methodology. We have also included an economic analysis involving only the material costs for the entire slurry preparation process with and without recycling. Considering 10 printing cycles where each cycle uses 360 mL of alginate solution, the material cost for a process without recycling (all 10 batches of fresh alginate slurry) is ~ USD75 whereas the material cost for a process with recycling (1 fresh batch + 9 batches of recycled slurry with 1 recovery performed after every 3 cycles) is USD14. We believed that the cost efficiency of our recyclable and reusable alginate-based supporting matrix is a great advantage for freeform 3D printing systems.
Fig. 9.
Recommended optimal process cycle for large scale manufacturing of recyclable and reusable alginate-based supporting matrices for multiple freeform 3D printing cycles
Conclusion
We have developed a new and improved supporting matrix that is biocompatible, reusable, and recyclable for freeform 3D printing systems which are one of the latest bioprinting techniques for 3D printing of low viscosity inks such as hydrogels, cells, elastomers, etc. This supporting matrix is made up of synthesized calcium alginate microgels produced via encapsulation with an average particle diameter of 212 ± 28 µm. Although alginate has good heat stability, under high heating rate and prolonged heating, the calcium alginate microgels are prone to dehydration that leads to deformation and destruction of the alginate network. In this work, we investigated several strategies to improve the dehydration tolerance of calcium alginate microgels by incorporating glycerol as a humectant through physical means of external addition to the microgels and chemical means of incorporating the glycerol together with the alginate microgels during the encapsulation process. Calcium alginates microgels that were produced with 2.0 wt% glycerol (Alg/Gly) had proven to be more effective in withstanding prolonged high-temperature heating of 75 °C for 24 h at 25–30% relative humidity than calcium alginates microgels (Alg/T20) with physically added glycerol. Among which, Alg/Gly slurry with higher clarity or transparency could remain in the moist state after heating whereas Alg/Gly slurry of lower clarity or transparency would lose its sphericity and become deformed. We also explored the significance of the post-treatment methods in affecting the thermal stability, recyclability, and print fidelity of the Alg/Gly slurry. The recyclability of the microgels was compared via rheological testing of microgels before, after heating, and after rehydration. Rehydration of heated or dehydrated microgels with sodium chloride solution allowed the microgels to regain its original rheology behavior. Numerous cycles of heating and rehydration caused microgels to degrade from its original state. In such instances, we had proposed a recovery step with a calcium chloride solution to recover the original state of the degraded microgels such that consistency in printing can be maintained. The feasibility of recovering the original rheological properties of the microgels with our calcium chloride recovery procedure was successfully demonstrated in this work. However, one limitation is the uncertainty of the calcium ions and sodium ions concentrations in the recovery procedure due to a lack of suitable testing equipment. This impedes the finetuning of the calcium chloride recovery procedure to achieve better and more consistent results. Nevertheless, the calcium alginate microgels produced with glycerol (Alg/Gly) is a promising addition to the currently available supporting matrices for both positive and negative freeform 3D printing systems. This new supporting matrix is biocompatible, highly heat resistant, recyclable, reusable, and recoverable for multiple prints. Lastly, we also proposed an optimal process cycle for large scale manufacturing with alginate-based supporting matrices and conducted a cost analysis. We envisaged that our alginate slurries can save material costs, time, and can be applied in various d freeform 3D printing systems and a wide range of markets including food, drug delivery, and tissue engineering.
Acknowledgements
The authors thank Ms. Oh Jhing Ruong for her help in gathering experiment data. Ms. Turlapati Sri Harsha, Juhi Gurnani for their help in 3D scanning and Muhammad Azhar and Muhammad Aidil for his support in the G-code generation. Mr. Han Win Tun for his support in the material formulation. This research was supported by Nanyang Technological University.
Funding
This research was supported by National Additive Manufacturing—Innovation Cluster (NAMIC) Singapore (NTU@NAMIC 2018197).
Compliance with ethical standards
Conflict of interest
T.W.S, M.A.B.J, S.J are co-inventors of Singapore provisional patent application number 10202003037W. The remaining authors declare no competing interests.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Touri M, Kabirian F, Saadati M, Ramakrishna S, Mozafari M. Additive manufacturing of biomaterials—the evolution of rapid prototyping. Adv Eng Mater. 2019;21(2):1800511. doi: 10.1002/adem.201800511. [DOI] [Google Scholar]
- 2.Yoda R. Elastomers for biomedical applications. J Biomater Sci Polym Ed. 1998;9(6):561–626. doi: 10.1163/156856298X00046. [DOI] [PubMed] [Google Scholar]
- 3.Luis E, et al. Silicone 3D printing: process optimization, product biocompatibility, and reliability of silicone meniscus implants. 3D Print Addit Manuf. 2019;6(6):319–332. doi: 10.1089/3dp.2018.0226. [DOI] [Google Scholar]
- 4.Luis E, Pan MH, Sing LS, Bajpai R, Song J, Yeong YW. 3D direct printing of silicone meniscus implant using a novel heat-cured extrusion-based printer. Polymers. 2020;12(5):1031. doi: 10.3390/polym12051031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosskopf AK, Truby RL, Kim H, Perazzo A, Lewis JA, Stone HA. Viscoplastic matrix materials for embedded 3D printing. ACS Appl Mater Interfaces. 2018;10(27):23353–23361. doi: 10.1021/acsami.7b19818. [DOI] [PubMed] [Google Scholar]
- 6.Hinton TJ, Hudson A, Pusch K, Lee A, Feinberg AW. 3D printing PDMS elastomer in a hydrophilic support bath via freeform reversible embedding. ACS Biomater Sci Eng. 2016;2(10):1781–1786. doi: 10.1021/acsbiomaterials.6b00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinton TJ, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9):e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharjee T, et al. Writing in the granular gel medium. Sci Adv. 2015;1(8):e1500655. doi: 10.1126/sciadv.1500655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Bryan CS, et al. Self-assembled micro-organogels for 3D printing silicone structures. Sci Adv. 2017;3(5):e1602800. doi: 10.1126/sciadv.1602800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez MJ, Dixon TA, Cohen E, Huang W, Omenetto FG, Kaplan DL. 3D freeform printing of silk fibroin. Acta Biomater. 2018;71:379–387. doi: 10.1016/j.actbio.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willie W, Adam D, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv Mater. 2011;23(24):H178–H183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Tan WS, Shi Q, Cheng XS, Chan WL, Song J. Freeform 3D printing of soft matters: recent advances in technology for biomedical engineering. Biomed Eng Lett. 2020. [DOI] [PMC free article] [PubMed]
- 13.Abubakr N, Lin SX, Chen XD. Effects of drying methods on the release kinetics of vitamin B12 in calcium alginate beads. Dry Technol. 2009;27(11):1258–1265. doi: 10.1080/07373930903267732. [DOI] [Google Scholar]
- 14.Tan WS, Juhari MAB, Shi Q, Chen S, Campolo D, Song J. Development of a new additive manufacturing platform for direct freeform 3D printing of intrinsically curved flexible membranes. Addit Manuf. 2020;36-101563.
- 15.Chan E-S, Lee B-B, Ravindra P, Poncelet D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J Colloid Interface Sci. 2009;338(1):63–72. doi: 10.1016/j.jcis.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Lee B-B, Ravindra P, Chan E-S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem Eng Technol. 2013;36(10):1627–1642. [Google Scholar]
- 17.Buitelaar R, Bucke C, Tramper J, Wijffels R. Immobilized cells: basics and applications. Amsterdam: Elsevier; 1996. [Google Scholar]
- 18.Seifert DB, Phillips JA. Production of small, monodispersed alginate beads for cell immobilization. Biotechnol Progress. 1997;13(5):562–568. doi: 10.1021/bp9700723. [DOI] [Google Scholar]
- 19.Pagliaro M, Rossi M. The future of glycerol. 2008;1-127
- 20.dos Santos Araújo P, Belini GB, Mambrini GP, Yamaji FM, Waldman WR. Thermal degradation of calcium and sodium alginate: a greener synthesis towards calcium oxide micro/nanoparticles. Int J Biol Macromol. 2019;140:749–760. doi: 10.1016/j.ijbiomac.2019.08.103. [DOI] [PubMed] [Google Scholar]
- 21.Cheng DC-H. Yield stress: a time-dependent property and how to measure it. Rheol Acta J. 1986;25(5):542–554. doi: 10.1007/BF01774406. [DOI] [Google Scholar]
- 22.Dohnal J, Štěpánek F. Inkjet fabrication and characterization of calcium alginate microcapsules. Powder Technol. 2010;200(3):254–259. doi: 10.1016/j.powtec.2010.02.032. [DOI] [Google Scholar]
- 23.Segale L, Giovannelli L, Mannina P, Pattarino F. Calcium alginate and calcium alginate-chitosan beads containing celecoxib solubilized in a self-emulsifying phase. Scientifica. 2016;2016:5062706 [DOI] [PMC free article] [PubMed]
- 24.Vreeker R, Li L, Fang Y, Appelqvist I, Mendes E. Drying and rehydration of calcium alginate gels. Food Biophys J. 2008;3(4):361–369. doi: 10.1007/s11483-008-9087-2. [DOI] [Google Scholar]
- 25.Li L, Fang Y, Vreeker R, Appelqvist I, Mendes E. Reexamining the egg-box model in calcium − alginate gels with X-ray diffraction. Biomacromol. 2007;8(2):464–468. doi: 10.1021/bm060550a. [DOI] [PubMed] [Google Scholar]
- 26.Lyn ME, Ying D. Drying model for calcium alginate beads. Ind Eng Chem Res. 2010;49(4):1986–1990. doi: 10.1021/ie901451m. [DOI] [Google Scholar]
- 27.Kim S, Jeong C, Cho S, Kim S-B. Effects of thermal treatment on the physical properties of edible calcium alginate gel beads: response surface methodological approach. Foods. 2019;8(11):578. doi: 10.3390/foods8110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermansson E, Schuster E, Lindgren L, Altskär A, Ström A. Impact of solvent quality on the network strength and structure of alginate gels. Carbohydr Polym. 2016;144:289–296. doi: 10.1016/j.carbpol.2016.02.069. [DOI] [PubMed] [Google Scholar]
- 29.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Progress Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]