Abstract
Cancer remains the second leading cause of mortality worldwide. In the course of this multistage and multifactorial disease, a set of alterations takes place, with genetic and environmental factors modulating tumorigenesis and disease progression. Metabolic alterations of tumors are well-recognized and are considered as one of the hallmarks of cancer. Cancer cells adapt their metabolic competences in order to efficiently supply their novel demands of energy to sustain cell proliferation and metastasis. At present, there is a growing interest in understanding the metabolic switch that occurs during tumorigenesis. Together with the Warburg effect and the increased glutaminolysis, lipid metabolism has emerged as essential for tumor development and progression. Indeed, several investigations have demonstrated the consequences of lipid metabolism alterations in cell migration, invasion, and angiogenesis, three basic steps occurring during metastasis. In addition, obesity and associated metabolic alterations have been shown to augment the risk of cancer and to worsen its prognosis. Consequently, an extensive collection of tumorigenic steps has been shown to be modulated by lipid metabolism, not only affecting the growth of primary tumors, but also mediating progression and metastasis. Besides, key enzymes involved in lipid-metabolic pathways have been associated with cancer survival and have been proposed as prognosis biomarkers of cancer. In this review, we will analyze the impact of obesity and related tumor microenviroment alterations as modifiable risk factors in cancer, focusing on the lipid alterations co-occurring during tumorigenesis. The value of precision technologies and its application to target lipid metabolism in cancer will also be discussed. The degree to which lipid alterations, together with current therapies and intake of specific dietary components, affect risk of cancer is now under investigation, and innovative therapeutic or preventive applications must be explored.
Keywords: lipid metabolism, cancer prognosis, tumor microenviroment (TME), obesity, cancer risk, precision medicine, precision nutrition
Introduction
Cancer is a significant public health problem and is the second leading cause of death globally (1). The World Health Organization (WHO) has indicated that lung, prostate, colorectal (CRC), stomach, and liver cancers are among the most frequent types of cancer in men, whereas breast, CRC, lung, cervical, and thyroid cancers are the most frequent among women. Together with the genetic alterations, environmental factors orchestrate the multifactorial and multistage characteristics of cancer, modulating the expression of both tumor suppressor genes and oncogenes.
One of the hallmarks of cancer is the abnormal regulation of cellular metabolism (2). Tumor cells exhibit high rates of aerobic glycolysis and an increased anabolism to support growth, proliferation, and survival. Consequently, metabolism-related pathways have acquired enormous relevance in cancer research. Together with the Warburg effect and the increased glutaminolysis, lipid metabolism plays a key role in cancer metabolic reprogramming (3). Lipids, a highly diverse class of biological molecules, exert three main functions in the cells. First, they are employed for energy storage, principally as triacylglycerol esters and steryl esters, in lipid droplets (LDs). In addition, lipids are structural components of cellular membranes, and they also operate as metabolic signaling messengers (4). The sterol regulatory element-binding proteins (SREBPs) are transcription factors that coordinate and regulate the synthesis of lipids. They act in response to upstream signaling networks and to the intracellular nutrient status, to regulate the expression of enzymes involved in cholesterol and fatty acid (FA) synthesis and uptake (5).
Together with genetic alterations mediating the metabolic reprogramming in a cell autonomous manner, cancer progression and dissemination also depend on the availability of nutrients and oxygen at the tumor microenvironment. Tumors communicate with the surrounding microenvironment, which includes fibroblasts, adipocytes, immune cells, endothelial cells, and components of the extracellular matrix—to support cancer proliferation and dissemination (6).
Furthermore, key lipid metabolism genes have been proposed as prognostic biomarkers in several types of cancer associated with tumor recurrence and/or survival (7, 8). Indeed, the role of lipid metabolism alterations in tumor cell migration, invasion, and angiogenesis has been clearly demonstrated (9–11).
The technical improvement and development of “omics” approaches, together with the availability of large public accessible databases, have redefined current strategies of cancer research (12) allowing to reanalyze, recapitulate, and update our knowledge of the relevance of lipid metabolism–related genes in cancer. Genomics and transcriptomics are being applied for precision medicine purposes in cancer. The design, validation, and use of polygenetic scores open a window of new opportunities to integrate “omics” technologies into clinical advice. Moreover, proteomics, metabolomics, lipidomics, and metagenomics will complete the full scenario (13). Additionally, clinical trials combining current chemotherapies with natural bioactive compounds toward altered lipid metabolism represent a promising strategy to improve cancer treatment (14).
In this review, we will discuss about the role of lipid metabolism alterations in cancer. We will explore their mechanism of action and their oncologic implications. Moreover, we will analyze current reports and knowledge of lipid metabolism biomarkers in the most frequent types of cancer. Finally, we will investigate their emergent use in precision medicine and precision nutrition strategies.
Impact of Obesity in Cancer
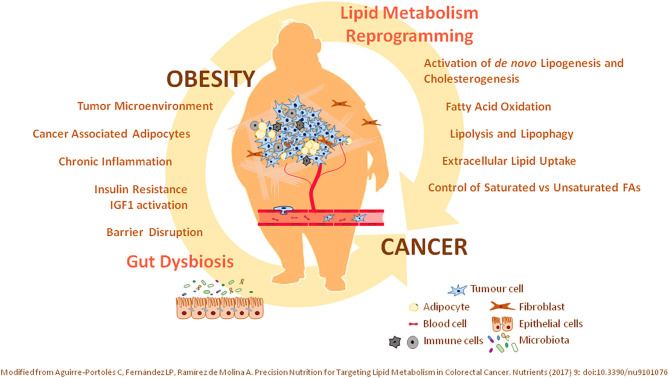
In recent years, it has demonstrated that cancer malignancy not only relays on the genetic factors—oncogenic and tumor suppressor alterations—from patients, but also on environmental factors associated with lifestyle (15). In this regard, it has been shown that up to one-third of cancer deaths could be prevented by modifying environmental factors related to lifestyle such as physical activity and diet, alcohol consumption, and smoking. Unhealthy diets—high consumption of saturated FAs or high-glucose-content beverages—are also associated with the development of systemic metabolic alterations including obesity, insulin resistance, and metabolic syndrome, among others. Obesity, which is defined as a high body weight with excessive adipose tissue accumulation, can be considered as a chronic, multifactorial, and proinflammatory disease (6, 16). Obesity is a risk factor for several chronic diseases including type 2 diabetes mellitus, cardiovascular diseases, hepatic steatosis, and cancer initiation and progression (17, 18). In fact, the overall risk of cancer death is around 1.5- to 1.6-fold in individuals with a body mass index higher than 40 kg/m2 (19). The main types of cancer where obesity has been found associated with are prostate cancer (20), postmenstrual endometrial (21), breast cancer (22), ovary (23), bladder (24), liver (25), colon (26), and pancreas (22). During obesity, adipocytes accumulate in locations not classically associated with adipose tissue. Fat accumulation in ectopic sites is classified as central adipose tissue with systemic effects and locally accumulated adipose tissue supporting tumor microenvironment. The central adipose tissue leads to alterations in the levels of steroidal sex hormones, decreased insulin sensitivity, and low-grade inflammation (27), and it has been associated mainly with CRC (27) and breast cancer (6, 28). In addition, visceral depots of adipose tissue may provoke alterations in the cellular composition of cells surrounding the tumor microenvironment contributing to tumor cell proliferation and dissemination such as in the case of tumors located close to adipose tissues, such as breast, ovary, or colon tumors (6, 29).
The effects of tumor cells at the tumor microenvironment has been also found to associate with drug resistance (30). Cancer-associated adipocytes present metabolic features that sustain tumor progression and dissemination, because of the release of FAs and proinflammatory mediators, which contribute to support the surrounding tumor microenvironment (6). Thus, ovarian cancer partially relies on lipids provided by adipocytes at the tumor microenvironment (29, 31). Moreover, the hyperplasia and hypertrophy of adipose tissue diminish the levels of oxygen available, promoting angiogenesis, which may contribute to tumor dissemination (32). In this regard, breast, gastric, and colon cancers preferentially grow in adipocyte-enriched environments. In addition, excess of adipose tissue induces low chronic inflammation augmenting the circulating levels of proinflammatory interleukins (IL-6 and IL-8), tumor necrosis factor α, vascular endothelial growth factor (VEGF), and prostaglandins and leukotrienes, which have protumorigenic effects. Arachidonic acid (AA) is the main precursor of proinflammatory lipid mediators, such as prostaglandins, thromboxanes, and leukotrienes, which promote proliferation, cell survival, and dissemination of cancer cells. Inflammatory prostaglandins, such as prostaglandin E2 produced by COX2 (cyclooxygenase 2), activate epidermal growth factor receptor cell signaling to promote angiogenesis and the expression of matrix metalloproteases in colon cancer (33). Prostaglandins have been shown to inhibit the antitumor immune response by diminishing the activation of cytotoxic CD8+ T lymphocytes and the infiltration of natural killer cells and dendritic cells to the tumor (34). In this regard, COX2 inhibitors have been demonstrated to augment the response to immune checkpoint inhibitors in melanomas (35, 36).
In addition, it has been described that obese individuals present an altered gut microbiota and disrupted intestinal epithelium barrier. Dysbiosis is associated with microbial diversity together with an increase in proinflammatory species. Intestinal dysbiosis has been associated with gastric, CRC, and esophageal cancers (37, 38). Thus, the design of microbiota-targeting therapies is now considered as a feasible strategy in the clinic.
Because of the important metabolic link between obesity and the tumorigenic process (Figure 1), effective control of the nutritional and metabolic status of individuals (control of glucose, lipid levels, blood pressure, and chronic inflammation) might represent a specific and mechanistic approach to prevent and/or ameliorate cancer progression. In this scenario, precision nutrition has emerged as a complementary therapeutic tool in the management of metabolic alterations associated with cancer prognosis. Personalized nutrition compiles nutrigenetics (genetic variants and epigenetic signatures), deep phenotyping, and a wide spectrum of data concerning metabolic personalization through omics technologies—transcriptomics, metabolomics, lipidomics, and metagenomics. Importantly, nutritional interventions based on the knowledge of how nutrients and bioactive dietary compounds interact with the genome, metabolism, microbiome, etc., at the molecular level, represent an effective tool to fight against metabolic alterations.
Figure 1.
Relevance of lipid metabolism alterations in cancer. Illustrated is the crucial role of (i) oncogenic mutations supporting the lipid metabolism reprogramming in cancer, together with (ii) systemic lipid metabolic alterations associated with obesity—as an environmental modifiable risk factor. Precision interventions should include therapeutic clinical drugs targeting identified lipid metabolism molecular targets together with nutritional interventions—bioactive compounds, diet-derived ingredients—considering the nutritional and metabolic status of patients. T2DM, type 2 diabetes mellitus; IR, Insulin Resistance; TME, tumor microenviroment; CAAs, cancer-associated adipocytes; FAO, fatty acid oxidation; FA, fatty acid.
Lipid Metabolic Reprogramming of Oncogenic Pathways in Cancer
Cancer cells present metabolic alterations to provide the additional requirements of energy and metabolites for cancer cell proliferation and dissemination (2). Enormeous diversity exists between the different types of cancer, and even within the same tumor. Moreover, cancer cells are characterized by the continuous capacity to adapt to changes in the levels of nutrients and oxygen at the tumor microenvironment (6). The altered tumor metabolism depends not only on the cell autonomous genetic alterations, but also on additional factors including diet, food behavior, exercise, and microbiome. All these factors together will determine the biology of the developing tumor (39) (Figure 1).
One of the most frequent metabolic alterations observed in cancer is the increased of the glycolytic pathway, independently of the oxygen levels (Warburg effect) (40). Aerobic glycolysis in cancer is coupled to increase glutamine metabolism for the anaplerosis of intermediated of the tricarboxylic acid (TCA) cycle (41). In addition, different studies including in vitro, preclinical, and clinical trials have demonstrated the relevance of lipid metabolism to sustain cancer initiation and progression (6). The inhibition of lipid metabolic enzymes has been shown to induce tumor regression, to inhibit the metastatic spread, and/or to avoid drug resistance. Lipids not only are structural components of biological membranes, but also provide energy by means of β-FA oxidation (β-FAO), control the redox homeostasis, and act as signaling molecules affecting a plethora of crucial processes in cancer including proliferation, migration, invasion, transformation, tumor microenvironment reshaping, and/or modulation of inflammation (42). Cholesterol is a key component of the cell membranes affecting its fluidity, stabilizing specific areas (lipid rafts) to transduce intracellular cell signaling pathways (43), and being precursor of steroidal hormones (44). In addition, lipids are also signaling molecules such as proinflammatory prostaglandins or tromboxanes—synthesized from omega-6 AA (45), or anti-inflammatory omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid, which availability depends on lipids provided from diet.
Herein, we describe potential strategies to target the altered lipid metabolism in cancer. In addition, as the uptake of high levels of saturated FAs from diet is a risk factor in several types of cancers, strategies to diminish lipolysis and promotion of healthy diets should also be considered.
Activation of de novo Lipogenesis and Cholesterogenesis
Lipid metabolism alterations affect not only tumor cell proliferation, but also dissemination and resistance to chemotherapeutic drugs (46). Most of adult tissues obtain FAs, cholesterol, and lipids from diet; meanwhile, de novo synthesis of FAs and cholesterol is restricted to the liver and adipocytes. Tumors frequently present the capability to activate the de novo synthesis of cholesterol and FAs (47) making them more independent from externally provided lipids (48, 49). Importantly, targeting enzymes associated with de novo lipogenesis and/or the mevalonate pathway has been shown to inhibit tumor growth (6, 50).
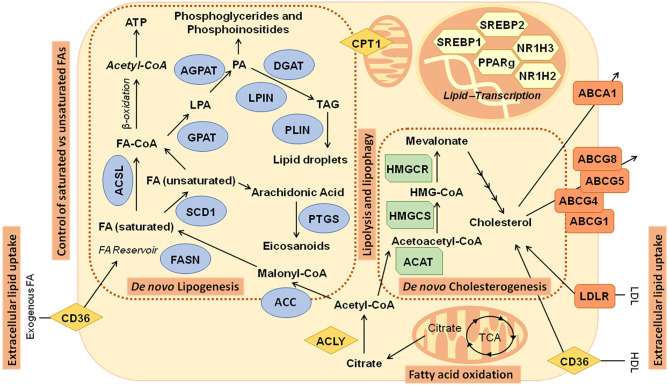
FAs are synthesized from cytoplasmic acetyl-CoA (AcCoA), generated from citrate produced from glucose, glutamine, or acetate (48). ATP-citrate lyase (ACLY) generates AcCoA and oxaloacetate (OAA) from citrate (48, 51). AcCoA carboxylases (ACC1/2) carboxylase AcCoA to form malonyl-CoA. Subsequent condensation steps, catalyzed by FA synthase (FASN), forms the 16-carbon saturated FA palmitate. Palmitate is then elongated by FA elongases (ELOVL) and desaturated by stearoyl-CoA desaturase (SCD1) or FA desaturases (FADS) to form other nonessential FAs, such as the 18-carbon monounsaturated FA (MUFA) oleate (C18:1) (Figure 2).
Figure 2.
Main metabolic pathways related to lipid metabolism in cancer: Illustration of pathways and genes implicated in de novo lipogenesis—fatty acids and cholesterol biosynthesis. ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette subfamily G member 1; ABCG4, ATP-binding cassette subfamily G member 4; ABCG5, ATP-binding cassette subfamily G member 5; ABCG8, ATP-binding cassette subfamily G member 8; ACAT, acetyl-CoA acetyltransferase; ACC, acetyl- CoA carboxylase; ACLY, ATP citrate lyase; ACSL, acyl-CoA synthetase long chain; AGPAT, 1-acylglycerol-3-phosphate O-acyltransferase; CD36, CD36 molecule; CPT1, carnitine palmitoyltransferase; DGAT, diacylglycerol O-acyltransferase; FA, Fatty acids; FASN, fatty acid synthase; GPAT, glycerol-3-phosphate acyltransferase; HDL, high-density lipoprotein; HMGCR: 3-hydroxy-3-methylglutaryl-CoA reductase; HMGCS, 3-hydroxy-3-methylglutaryl-CoA synthase; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPIN, Lipin; NR1H2, nuclear receptor subfamily 1 group H member 2; NR1H3, nuclear receptor subfamily 1 group H member 3; PLIN, perilipin; PPARγ, peroxisome proliferator-activated receptor γ; PTGS, prostaglandin-endoperoxide synthase; SCD1, stearoyl-CoA desaturase; SREBP1, Sterol regulatory element binding transcription factor 1; SREBP2, sterol regulatory element binding transcription factor 2; TCA, tricarboxylic acid cycle.
Many enzymes implicated in de novo synthesis of FAs and cholesterol have been proposed as biomarkers for prognosis in specific types of cancer. FASN is found upregulated in prostate and breast cancer (47, 52), and ACLY has been shown to support tumor formation and transformation (51). Inhibition of several enzymes of de novo lipogenesis, such as FASN, and ACC1 and ACC2, has been tested in different cancer models showing their relevance on tumor growth inhibition (53).
Similarly, inhibition of hydroxymethylglutaryl-CoA (HMGCoA) reductase (HMGCR), by statins, leads to inhibition of cell proliferation of breast cancer cells (54) and tumor regression in several preclinical mouse models, and it is being tested in clinical trials (43). The overexpression of enzymes of the mevalonate pathway has been proposed as biomarkers of poor prognosis in breast cancer (55). Cholesterol is generated by the mevalonate pathway, by condensation of two AcCoA molecules to form 3-HMGCoA, which is then reduced to form mevalonate, and then isoprenoid farnesyl-pyrophosphate. Several studies have shown that targeting the synthesis of cholesterol inhibits cancer cell proliferation and transformation (56).
De novo synthesis of FAs and cholesterogenesis are transcriptionally regulated by SREBPs, which are downstream oncogenic pathways including PI3K/Akt (57) and c-Myc (47) (Figure 2).
The SREBP family includes three transcription factors: SREBP1a and SREBP1c, which are derived from SREBF1 gene by alternative splicing (58), and SREBP2, which is encoded by SREBF2 gene. SREBPs are bound to the endoplasmic reticulum (ER) as inactive precursors (59). When the intracellular levels of cholesterol are high, insulin-induced genes interact with SREBP-cleavage–activating proteins (SCAPs) to retain SREBP inactive precursors attached to the ER. When cholesterol levels are low, SCAPs facilitate the translocation SREBPs to the Golgi apparatus to be further processed releasing the active forms (56). SREBP1 promotes the expression of lipogenic genes; meanwhile, SREBP2 regulates the expression of genes involved in the synthesis, uptake, and efflux of cholesterol. Nevertheless, SREBP1 and SREBP2 have overlapping activities. Both SREBP1 and SREBP2 are found overexpressed in several cancers. Regulation of the intracellular content of cholesterol has also been shown crucial for cancer cell survival. The ATP-binding cassette transporter (ABCA1) controls the efflux of cholesterol to ApoA-coated lipoproteins (57). Recently, it has been demonstrated that activation of p53 increases the retrograde transport of cholesterol from the plasma membrane to the ER, to prevent SREBP2 maturation (60). In addition, cholesterol levels are fine tune regulated by microRNA33—encoded by an intron within the SREBF2 gene (51)—which targets ABCA1. In addition, the esterification of cholesterol for storage in LDs, by sterol O-acyltransferase 1 (ACAT1), has been shown to augment the survival in prostate cancer (61).
Fatty Acid Oxidation in Cancer
In addition to de novo synthesis of FAs and cholesterol, the mobilization of intracellular FAs for FAO at mitochondria is crucial for cancer survival and dissemination. It is well-known that tumor cells present higher levels of reactive oxygen species (ROS) than not tumor cells, which allow them to activate prosurvival and epithelial-to-mesenchymal transition programs to support cancer progression and dissemination. Nevertheless, excessive ROS may promote apoptotic cell death. It has been demonstrated that enzymes implicated in the mobilization of intracellular neutral lipids provide metabolic flexibility to increase the levels of FAs for oxidation at mitochondria. In the FAO pathway, acyl-CoAs are cyclically dehydrogenated, hydrated, and decarboxylated, resulting in the progressive shortening of the FA, together with the production of NADH and FADH2 and AcCoA. NADH and FADH2 will be used for ATP production in the electron transport chain, and AcCoA can enter the Krebs cycle. AcCoA together with OAA gives rise to citrate, which after being exported to cytoplasm, can enter two metabolic pathways to produce cytosolic NADPH (62).
Enhanced mitochondrial β-oxidation of FAs has been described in pancreatic cancer (63, 64) and in metastatic breast cancer (65). FAO not only provides energy when glucose becomes limiting, but it also contributes to a better control of the oxidative stress, by augmenting the intracellular levels of NADPH (66). Increased FAO augments survival in leukemia and gliomas by counteracting the metabolic oxidative stress. Moreover, FAO has been shown crucial for the survival of cells from solid tumors when undergoing loss of attachment, which triggers anoikis or cell death due to oxidative stress (67, 68).
In addition, FAO is also influenced by the tumor microenvironment such as in the case of ovarian cancers, which preferentially metastasizes to the omentum enriched in adipocytes, which provides lipids for ATP and NADPH production to control metabolic stress during metastasis.
Regulation of FA Storage and Intracellular FA Mobilization (Lipolysis and Lipophagy)
De novo synthesis of FAs in cancer cells is coupled to additional processes to accommodate the increase in the intracellular lipid content, to preserve the homeostasis between lipid storage and lipid mobilization (69). FAs from de novo lipogenesis are accumulated into neutral lipids (stored in LDs) and phospholipids (in membranes). LDs are complex and dynamic organelles consisting of a neutral lipid core surrounded by a phospholipid monolayer and a complex proteome associated. LDs itself have been proposed as novel diagnostic biomarkers for glioblastoma. It has been demonstrated that while they are not detectable in low-grade gliomas or normal brain tissues, they are common in glioblastoma, the most lethal brain tumor (70). Among the LD-associated proteins, there are enzymes of the sterol biosynthetic pathway, the acyl-CoA metabolism (ACSLs), and triacylglycerol (TAG) biosynthesis. Structural proteins, such as perilipins (PLINs) or caveolins, are critical for the integrity of LDs to avoid collapse and to protect them from lipolysis (Figure 2). Cancer cells present higher amounts of LDs than normal cells (71). Increased expression of PLIN2 has been shown to favor the accumulation of LDs (72), contributing to a better control of the ER stress, to increase the protection against ROS, and to augment the resistance to therapeutic drugs in cancer cells. On the contrary, PLIN2 depletion significantly attenuated the proliferation of colon cancer cells (73), supporting the LD-associated proteins as potential druggable targets for cancer treatment (11).
The increase in de novo synthesis of FAs in cancer cells requires efficient and complementary lipolytic mechanisms to accommodate the intracellular lipid content. Thus, lipolysis allows the stored lipids to be available for the synthesis of phospholipids and lipid signaling mediators and/or to increase the levels of ATP or NADPH when required. Several enzymes involved in lipolysis—adipose TAG lipase (ATGL), hormone-sensitive lipase (HSL), monoacylglycerol lipase (MAGL)—have been described to promote tumorigenesis (74). In this sense, ATGL knockdown in HCT116 CRC cells reduced cell proliferation (75). Increased levels of MAGL are associated with aggressive cancer types such as melanoma and ovarian and breast cancer (74), and inhibition of MAGL suppresses cancer cell migration, invasion, and survival (76). Recently, it has been demonstrated that glioblastomas, which acquire large amounts of free FAs, upregulate diacylglycerol-acyltransferase 1 (DGAT1) to store the excess FAs into triglycerides and LDs (77). Inhibition of DGAT1 disrupted lipid homeostasis, resulting in increased levels of ROS leading to apoptotic cell death.
In addition, a specific function of autophagy associated with the regulation of the intracellular lipid content—lipophagy—has been described to augment resistance to cell death in cancer (78).
Extracellular Lipid Uptake
In addition, similar to normal cells, cancer cells can uptake exogenous lipids when de novo lipogenesis is inhibited. Upregulation of cell surface receptors, such as cluster of differentiation 36 (CD36) (Figure 2), has been found to augment metastasis (79, 80). CD36 inhibition diminished tumor growth and metastasis in preclinical models of prostate cancer (80). Moreover, the expression of low-density lipoprotein receptor (LDLR) for the internalization of low-density lipoproteins (LDLs) has been found upregulated in renal cell carcinoma (RCC) cells (81). FA-binding proteins (FABPs) contribute to augment the lipid uptake, as well as the intracellular lipid trafficking in cancer cells (82). In breast cancer and glioblastoma cell lines, it has been shown that the uptake of extracellular FAs during hypoxia is sustained by the upregulation of FABP3 and FABP7; meanwhile, FABP5 increases cell proliferation and growth in prostate cancer (83).
Control of Saturated vs. Unsaturated FAs
Depending on the source of FAs, de novo lipogenesis or extracellular lipid uptake, the levels of saturated FAs incorporated in the phospholipids of cell membranes are different. The lipogenic pathway increases the saturation level of cell membranes with saturated and MUFAs (84), which are less sensitive to suffer lipid peroxidation compared to polyunsaturated acyl chains (PUFAs) mainly obtained from diet. This way, de novo lipogenesis contributes to augment the resistance to oxidative stress and chemotherapy in cancer cells (85).
Nevertheless, excessive accumulation of saturated FAs in the cell membranes can lead to lipotoxicity. In this regard, SCD1 inhibition induces ER stress and apoptosis in cancer cells and diminishes the tumor growth in xenografts models of colon and lung cancers (86). During tumor growth, inner parts of the tumors are faced to hypoxia and reduced nutrient availability. Tumors have developed different strategies to balance the levels of saturated vs. unsaturated FAs. Thus, tumors anticipate lipotoxicity by augmenting the uptake of MUFAs/PUFAs from plasma, which are further stored into LDs or incorporated into phospholipids at the cell membranes. As SCD1 activity requires oxygen, during hypoxia some tumors rely on the activity of DGATs to incorporate MUFAs into TG, which are further accumulated into LDs (Figure 2). In addition, tumors balance, via the Lands cycle, the levels of saturated vs. unsaturated FAs in the phospholipids at the cell membranes. Recently, a process known as ferroptosis has been described associated with high levels of MUFA/PUFAs in the phospholipids of cell membranes, which induce cell death by means of their oxidation through the Fenton pathway. Long-chain FA acyl CoA synthetases (ACSLs)—implicated in the long chain FA activation—may control ferroptosis, as distinct isoforms use distinct substrates. Meanwhile, ACSL4 has PUFAS as main substrates such as AA, ACSL3 can activate both MUFAs and PUFAs, allowing a better control of the excessive accumulation of PUFAs in phospholipids (87). In addition, ACSL3 allows a better control of FA distribution between LD storage or β-FAO, providing a better control of the oxidative stress (42).
Lipid Metabolism Alterations and Cancer Prognosis
Alterations of lipid metabolism genes are found in many tumor types, predominantly, but not exclusively, because lipid metabolism can modulate different cellular processes that go from plasmatic and organelle membrane organization and plasticity (88, 89), substrate supply for ATP synthesis, (62) and intracellular cell signaling activation (90). Cancer tissues display abnormal activation of de novo lipogenesis and cholesterogenesis (91). Extremely proliferative cancer cells exhibit an intense lipid and cholesterol avidity, which they satisfy by increasing the uptake of dietary or exogenous lipids and lipoproteins or activating lipogenesis or cholesterol synthesis (3). Importantly, this aberrant lipid metabolism does not only influence the primary tumor, but the exogenous lipids produced by tumor microenvironment can also influence malignancy (14, 92–95). Besides, three basic steps during metastasis: migration (96), invasion (9, 10) and angiogenesis (97, 98), are affected by lipid metabolism regulation (11).
Nowadays, there are increasing evidences of the role of lipid metabolism alterations as biomarkers of cancer prognosis and survival. Here, we are going to review previous knowledge on the prognostic value of lipid-related genes that belong to FAs and cholesterol pathways (Figure 2) in the most frequent types of cancer according to the WHO: lung, CRC, breast, and prostate.
Furthermore, “omics” data publicly available in huge searchable databases facilitate addressing specific medical issues in thousands of patients. Remarkably, The Cancer Genome Atlas (TCGA) gene expression dataset (https://www.cancer.gov/tcga) and The Human Protein Atlas website together with The Pathology Atlas online tool (https://www.proteinatlas.org/humanproteome/pathology), which contains mRNA data from TCGA study and protein expression data from different forms of human cancer (99–101), allowing us to obtain a global view of the putative implications of lipid metabolism–related genes in cancer prognosis. Data from TCGA visualized using The Pathology Atlas online tool, are summarized in Table 1.
Table 1.
Prognostic value of lipid metabolism–related genes.
| Literature | The Cancer Genome Atlas (TCGA)-The Protein Atlas | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostic value | LC | CRC | BC | PC | LC | CRC | BC | PC | CC | EC | G | HNC | LC | M | OC | PC | RC | SC | TC | ThC | UC | ||||
| Fatty acid–related pathways | |||||||||||||||||||||||||
| Fatty acid synthesis | |||||||||||||||||||||||||
| ACLY | (61, 102, 103) | (104) | |||||||||||||||||||||||
| pACC | (105) | (39) | |||||||||||||||||||||||
| ACACA | |||||||||||||||||||||||||
| ACACB | (106) | ||||||||||||||||||||||||
| FASN | (107) | (36, 108–112) | (113–115) | (116–119) | |||||||||||||||||||||
| ACSL1 | (7–9, 120, 121) | ||||||||||||||||||||||||
| ACSL3 | (+) | ||||||||||||||||||||||||
| ACSL4 | (7–9, 120, 121) | (122) | |||||||||||||||||||||||
| ACSL5 | (121, 123) | ||||||||||||||||||||||||
| ACSL6 | |||||||||||||||||||||||||
| SCD1 | (124) | (7, 125) | (126) | ||||||||||||||||||||||
| FADS1 | (127) | ||||||||||||||||||||||||
| FADS2 | |||||||||||||||||||||||||
| FADS3 | |||||||||||||||||||||||||
| FADS4 | |||||||||||||||||||||||||
| FADS6 | |||||||||||||||||||||||||
| FADS7 | |||||||||||||||||||||||||
| FADS8 | |||||||||||||||||||||||||
| PTGS1 | |||||||||||||||||||||||||
| PTGS2 | (128–130) | (131) | (132) | (133) | |||||||||||||||||||||
| GPAT1 | |||||||||||||||||||||||||
| GPAT2 | |||||||||||||||||||||||||
| GPAT3 | |||||||||||||||||||||||||
| GPAT4 | |||||||||||||||||||||||||
| AGPAT1 | (7, 8) | ||||||||||||||||||||||||
| AGPAT2 | |||||||||||||||||||||||||
| AGPAT3 | |||||||||||||||||||||||||
| AGPAT4 | |||||||||||||||||||||||||
| AGPAT5 | |||||||||||||||||||||||||
| LPIN1 | (134) | (135) | (136) | ||||||||||||||||||||||
| LPIN2 | |||||||||||||||||||||||||
| LPIN3 | |||||||||||||||||||||||||
| PLIN1 | (137) | (138) | |||||||||||||||||||||||
| PLIN2 | (139) | ||||||||||||||||||||||||
| PLIN3 | |||||||||||||||||||||||||
| PLIN4 | |||||||||||||||||||||||||
| PLIN5 | |||||||||||||||||||||||||
| DGAT1 | |||||||||||||||||||||||||
| DGAT2 | (140) | ||||||||||||||||||||||||
| Fatty acids–related transportation | |||||||||||||||||||||||||
| CD36 | (141) | (141) | (141) | ||||||||||||||||||||||
| CPT1A | (142, 143) | ||||||||||||||||||||||||
| CPT1B | |||||||||||||||||||||||||
| CPT1C | |||||||||||||||||||||||||
| Cholesterol-related pathways | |||||||||||||||||||||||||
| Cholesterol synthesis | |||||||||||||||||||||||||
| ACAT1 | (144, 145) | ||||||||||||||||||||||||
| ACAT2 | |||||||||||||||||||||||||
| HMGCS1 | |||||||||||||||||||||||||
| HMGCS2 | (146) | (147) | |||||||||||||||||||||||
| HMGCR | (148) | (149, 150) | |||||||||||||||||||||||
| Cholesterol-related transportation | |||||||||||||||||||||||||
| ABCA1 | (7, 8, 151) | ||||||||||||||||||||||||
| ABCG1 | |||||||||||||||||||||||||
| ABCG4 | (152) | ||||||||||||||||||||||||
| ABCG5 | (153) | ||||||||||||||||||||||||
| ABCG8 | |||||||||||||||||||||||||
| LDLR | (154) | ||||||||||||||||||||||||
| Lipid transcription | |||||||||||||||||||||||||
| Transcription factors | |||||||||||||||||||||||||
| SREBP1 | (155) | ||||||||||||||||||||||||
| SREBP2 | (156) | (157) | |||||||||||||||||||||||
| PPARγ | (158, 159) | (160) | (161, 162) | (163) | |||||||||||||||||||||
| NR1H2 | (156) | ||||||||||||||||||||||||
| NR1H3 | (164) | (156) | |||||||||||||||||||||||
Favorable

Unfavorable

Gene prognostic value reported in the literature in most frequent types of cancer according to the World Health Organization (WHO) together with gene prognostic value using data from The Cancer Genome Atlas (TCGA) and visualized using The Pathology Atlas online tool. Abbreviations: LC, lung cancer; CRC, colorectal cancer; BC, breast cancer; PC, prostate cancer; CC, cervical cancer; EC, endometrial cancer; G, glioma; HNC, head and neck cancer; LC, liver cancer; M, melanoma; OC, ovarian cancer; PC, pancreatic cancer; RN, renal cancer; SC, stomach cancer; TC, testis cancer; ThC, thyroid cancer; UC, urothelial cancer; ACLY, ATP citrate lyase; pACC, phospo acetyl-CoA carboxylase; ACACA, acetyl-CoA carboxylase A; ACACB, acetyl-CoA carboxylase B; FASN, fatty acid synthase; ACSL1, acyl-CoA synthetase long chain family member 1; ACSL3, acyl-CoA synthetase long chain family member 3; ACSL4, acyl-CoA synthetase long chain family member 4; ACSL5, acyl-CoA synthetase long chain family member 5; ACSL6, acyl-CoA synthetase long chain family member 6; SCD1, stearoyl-CoA desaturase1; FADS1, fatty acid desaturase 1; FADS2, fatty acid desaturase 2; FADS3, fatty acid desaturase 3; FADS4, fatty acid desaturase 4; FADS6, fatty acid desaturase 6; FADS7, fatty acid desaturase 7; FADS8, fatty acid desaturase 8; PTGS1, prostaglandin-endoperoxide synthase 1; PTGS2, prostaglandin-endoperoxide synthase 2; GPAT1, glycerol-3-phosphate acyltransferase 1; GPAT2, glycerol-3-phosphate acyltransferase 2; GPAT3, glycerol-3-phosphate acyltransferase 3; GPAT4, glycerol-3-phosphate acyltransferase 4; AGPAT1, 1-acylglycerol-3-phosphate O-acyltransferase 1; AGPAT2, 1-acylglycerol-3-phosphate O-acyltransferase 2; AGPAT3, 1-acylglycerol-3-phosphate O-acyltransferase 3; AGPAT4, 1-acylglycerol-3-phosphate O-acyltransferase 4; AGPAT5, 1-acylglycerol-3-phosphate O-acyltransferase 5; LPIN1, lipin 1; LPIN2, lipin 2; LPIN3, lipin 3; PLIN1, perilipin 1; PLIN2, perilipin 2; PLIN3, perilipin 3; PLIN4, perilipin 4; PLIN5, perilipin 5; DGAT1, diacylglycerol O-acyltransferase 1; DGAT2, diacylglycerol O-acyltransferase 2; CD36, CD36 molecule; CPT1A, carnitine palmitoyltransferase 1A; CPT1B, carnitine palmitoyltransferase 1B; CPT1C, carnitine palmitoyltransferase 1C; ACAT1, acetyl-CoA acetyltransferase 1; ACAT2, acetyl-CoA acetyltransferase 2; HMGCS1, 3-hydroxy-3-methylglutaryl-CoA synthase 1; HMGCS2, 3-hydroxy-3-methylglutaryl-CoA synthase 2; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette subfamily G member 1; ABCG4, ATP-binding cassette subfamily G member 4; ABCG5, ATP-binding cassette subfamily G member 5; ABCG8, ATP-binding cassette subfamily G member 8; LDLR, low-density lipoprotein receptor; SREBP1, Sterol regulatory element binding transcription factor 1; SREBP2, Sterol regulatory element binding transcription factor 2; PPARγ, peroxisome proliferator-activated receptor γ; NR1H2, nuclear receptor subfamily 1 group H member 2; NR1H3, nuclear receptor subfamily 1 group H member 3. (+) Unpublished results.
Fatty Acid–Related Alterations as Biomarkers of Cancer Prognosis and Survival
De novo FA biosynthesis occurs in the cellular cytoplasm. FAs originate from acetyl-coenzyme A, which is mostly provided by citrate produced by the TCA cycle. Switch of citrate into AcCoA is catalyzed by ATP citrate lyase (ACLY) (Figure 2). Consequently, ACLY is a key enzyme connecting carbohydrate to lipid metabolism by producing AcCoA from citrate for both FA and cholesterol synthesis (61). Several studies have associated ACLY expression in tumor tissues with worse prognosis. ACLY overexpression correlated with stage, differentiation grade, and a poorer prognosis in non–small cell lung cancer (NSCLC) (61). Besides, in combination with the glucose transporter GLUT1, ACLY was also an independent prognostic factor for overall survival (OS) in node-negative patients with NSCLC (102). However, one study reports that young NSCLC patients overexpressing ACLY had longer OS, in contrast to older patients where overexpression of ACLY appears to predict the opposite prognosis (103). ACLY also facilitates colon cancer cell metastasis, and high expression levels of ACLY and Catenin β1 (CTNNB1) protein were positively correlated with metastasis of colon cancer (104). Data from TCGA showed ACLY as a putative unfavorable marker of cervical and liver cancer (Table 1).
At the genomic level, single nucleotide polymorphisms (SNPs) in ACLY gene have been described as independent cancer prognostic markers in Asiatic populations. SNP rs9912300 in ACLY gene was significantly associated with OS in lung cancer patients (165). rs9912300 and rs2304497, both functional ACLY SNPs, exhibited a significant association with risks of death and recurrence in patients with advanced stages of colon cancer (166).
The following step of FA biosynthesis involves the activation of AcCoA to malonyl-CoA, which is catalyzed by AcCoA carboxylase (ACC) (Figure 2). ACC is a complex multifunctional enzyme system. There are two ACC forms, α (ACACA) and β (ACACB), encoded by two different genes. High phospho-acetylCoA carboxylase (pACC) was an independent marker for prediction of better survival in lung adenocarcinoma patients (105), and low pACC levels detected by immunohistochemistry were associated both with worse OS and progression-free survival in advanced stage CRC (167). In the same line, gene expression analysis reported that patients with upregulation of six of these hub genes (genes with high correlation in candidate modules) (ACACB, acyl-CoA dehydrogenase medium chain, adiponectin, C1Q and collagen domain containing, acyl-CoA synthetase short-chain family member 2, phosphoenolpyruvate carboxykinase 1 and PLIN1) displayed improved breast cancer prognosis (106). In TCGA dataset, ACACA gene expression is an unfavorable risk factor for liver cancer, whereas ACACB is a favorable prognostic factor for both renal and pancreas tumors (Table 1). Finally, it has been described in prostate cancer that genetic alterations of ACACA, FASN, and SREBF1 predicted worse overall patient survival (168).
Malonyl-CoA is coupled to the multifunctional enzyme FASN. Repeated cycles of acetyl group's condensation produce the primary FA palmitate that can suffer separate elongation and/or unsaturation cycles to yield other FA molecules (169) (Figure 2). FASN is the key enzyme necessary for the de novo synthesis of long-chain FAs. FASN has been found overexpressed in nearly all of cancer tissues, and its expression is associated with a poorer prognosis.
One study reported that FASN gene expression was higher in the adjacent non-cancer tissue than in the NSCLC tissue, but authors concluded that it was a weaker predictor of shorter patient survival (170). However, a correlation analysis between expression levels of CD276 (B7-H3) and FASN exhibited a positive correlation with poor prognosis in clinical lung cancer tissues (107).
FASN levels were clearly upregulated in CRC tissues with high expression of FASN significantly associated with lymph node metastasis (108), liver metastasis (109), TNM (tumor, node, metastasis) stage, and poor prognosis (36). Moreover, a significant association was shown between FASN and VEGF expression, suggesting the involvement of FAS in tumor angiogenesis (110). Interestingly, one study reported that, among non-obese patients with colon cancer, tumoral FASN overexpression is associated with better survival, while among moderately overweight or obese patients, FASN overexpression may predict a poorer outcome (111). Furthermore, a panel of five genes including FASN (ACOT8/ACSL5/FASN/HMGBCS2/SCD1) has been reported to display a improved prognostic performance than validated clinical risk scales, and it is applicable for early discovery of CRC and tumor recurrence (112). Finally, FASN levels in serum were also examined in CRC patients, where it was associated with tumor stage (171), and high FASN levels are considered as a promising independent predictor of CRC with advanced phases, late clinical stages, and shorter survival (172).
FASN is associated with poor prognosis in breast and prostate cancer, and its inhibition is selectively cytotoxic to human cancer cells (113). FASN was found overexpressed in most of the triple-negative breast cancer (TNBC) patients but not always correlated with OS or disease-free survival. High FASN was significantly associated with positive node status (114). A greater part of clinically HER2-positive tumors was achieved as FASN overexpressors. Reclassification of HER2-positive breast tumors based on FASN gene expression predicted a significantly inferior relapse-free and distant metastasis-free survival in HER2+/FASN+ patients (115).
A substantial subset of prostatic cancers displays clearly elevated expression of immunohistochemically detectable FASN, a feature that has been associated with poorer prognosis (116–119). Furthermore, high expression level of FASN resulted in a significantly poor prognosis of pancreatic cancer (173), and data from TCGA study suggest that FASN expression could be a marker of bad outcome in cervical and renal cancer (Table 1).
In addition, several genetic changes in FASN gene have been associated with cancer prognosis. Two SNPs rs4246444 and rs4485435 were significantly associated with the recurrence of NSCLC (165). Finally, as it has been previously mentioned in prostate cancer that genetic alterations of FASN together with ACACA and SREBF1 predicted worse prognosis (168).
Then, FAs are activated with CoA by fatty acyl-CoA synthetases (ACSLs) (Figure 2), which is essential for phospholipid and triglyceride synthesis and lipid modification of proteins in addition to for FA β-oxidation (169).
Family of long-chain acyl-CoA synthetases has been extensively proposed as putative prognostic biomarkers of cancer. ACSL3 is up-regulated in lung cancer compared to the healthy lung tissue (174), and recently, an association with ACSL3 expression, NSCLC prognosis, and the efficacy of statins treatment has been discovered (L. P. Fernandez et al., unpublished results). ACSL3 was also found to be overexpressed in estrogen receptor–negative breast cancer (175) and prostate cancer (176). ACSL1 and ACSL4 overexpression was associated with a poor clinical outcome in stage II CRC patients (7–9, 120, 121). In addition, ACSL4 is considered a biomarker for liver and breast cancers (122, 177). By contrast, downregulation of ACSL5 in breast cancer was associated with a poorer prognosis (121, 123). There have not been reported associations between ACSL6 and cancer survival (178).
An in silico study (121) also suggested that high ACSL1 expression was associated with worse outcome in lung cancer patients, and ACSL3 overexpression was associated with worse survival in patients with melanoma. In contrast, high ACSL3 expression predicted a better prognosis in ovarian cancer. In the same study, ACSL4 overexpression predicted bad prognosis in CRC, but good prognosis in breast, brain, and lung cancers. High expression of ACSL5 predicted good prognosis in breast, ovarian, and lung cancers. Finally, low ACSL6 predicted a worse prognosis in acute myeloid leukemia. In silico analysis of TCGA data (Table 1) suggested that ACSL1, ACSL4, and ACSL5 are associated with favorable outcome in renal, urothelial, and endometrial cancers, respectively, whereas ACSL3 expression predicts poor survival in lung and liver tumors.
Genetically, a 3′-UTR polymorphism in ACSL1 is associated with ACSL1 expression levels and poor clinical outcome in CRC patients (14, 120). Patients carrying the ACSL1 rs8086 T/T genotype had significantly reduced disease-free survival compared with patients carrying the C/T or C/C genotype, with 3-fold higher risk of recurrences. T/T genotype for rs8086 is correlated with worse clinical outcome and simultaneously associates with high ACSL1 mRNA levels (14, 120).
Stearoyl CoA desaturase 1 (SCD1) catalyzes the rate-limiting step in the synthesis of MUFAs that are the main components of tissue lipids. SCD1 has been associated with tumor development, late stage, and reduced survival in lung adenocarcinoma (124). Together with other three lipid metabolism–related genes (ABCA1, ACSL1, and AGPAT1), SCD1 expression separated stage II colon cancer patients with a 5-fold higher risk of relapse (7). Moreover, positive associations between SCD1 expression and CRC patient clinical status and the expression of cancer stem cell–related genes (WNT and NOTCH signaling) were found based on TCGA data analysis (125). In the same line, high SCD1 expression is associated with shorter survival in breast cancer patients (126). Table 1 shows that SCD1 is an unfavorable marker of survival in renal and urothelial cancer in TCGA tumors. Other desaturases have also been analyzed as prognostic markers, and, for example, reduced expression of FADS1 suggests pessimistic prognosis for NSCLC patients (127).
Glycerol-3-phosphate acyltransferase (GPAT) catalyzes the first step in the production of almost all membrane phospholipids. GPAT transfers an acyl group from acyl-CoA or acyl-ACP at the sn-1 or-2 position of glycerol 3-phosphate originating lysophosphatidic acids (LPAs) (179). LPA is a substrate for synthesis of numerous important glycerolipid intermediates, such as storage lipids, extracellular lipid polyesters, and membrane lipids (Figure 2). Four GPATs have been discovered; nevertheless, only GPAT1 (GPAM) has been related to cancer outcome. High GPAT1 expression has been associated with reduced OS in ovarian cancer (180). Data from TCGA suggested that GPAT1 could be a favorable prognostic marker in renal cancer, while GPAT3 is a putative biomarker of good prognosis in renal cancer in contrast to urothelial cancer. Finally, GPAT4 expression could have a risk effect in ovarian and endometrial cancers, and a protective one in prostate and urothelial cancer (Table 1).
LPA is further metabolized to phosphatidic acid (PA) by AGPATs (1-acylglycerol-3-phosphate O-acyltransferases) (Figure 2). AGPAT1 belongs to previously mentioned transcriptional signature where combined analysis of four genes, ABCA1, ACSL1, AGPAT1, and SCD1, is associated with higher risk of relapse in stage II CRC patients (7). Furthermore, individuals with upregulation of AGPAT1 expression have an increased risk of CRC recurrence, independently of tumor stage (8). Expression of AGPAT2 was significantly related to decreased OS as well as to shorter progression-free survival in ovarian cancer patients younger than 60 years (181). When we consider tumors from TCGA study, several associations were found (Table 1). AGPAT3 is a marker of good prognosis in renal cancer and predicts bad outcome in cervical cancer. High expression levels of AGPAT4 may be associated with poor prognosis in cervical and renal cancers, whereas AGPAT5 is an unfavorable prognostic marker in liver cancer and a favorable one in CRC.
Then PA is converted to diacylglycerol (DAG) by LPIN, a PA phosphatase. Three LPIN isoforms have been described. LPIN1 is upregulated in lung adenocarcinoma tumor tissues, and high LPIN1 expression was correlated with poor prognosis of patients with lung adenocarcinoma (134). In breast cancer, previous results seem to indicate that the high LPIN expression is related to a good prognosis (135). However, in basal-like TNBC, high LPIN1 expression correlates with the poor prognosis of these patients (136). In TCGA dataset analysis, LPIN2 appears as a favorable prognostic marker in head and neck cancers, while LPIN3 could be an unfavorable biomarker of endometrial, ovarian, and renal tumors (Table 1).
The final step in triacylglycerols synthesis is catalyzed by DGAT, which esterifies the DAG with a FA. Two human DGAT isoforms have been described (182). The expression of DGAT2 in HER2-positive breast cancer was decreased and was closely related to patient prognosis (140). However, data from TCGA reported DGAT2 as an unfavorable prognostic factor for endometrial cancer (Table 1).
Subsequently, TAGs could be stored in LDs, and PLINs, an LD surface family of proteins, are necessary for optimal lipid storage and FA release. There are multiple PLIN proteins encoded by mRNA splice variants of a single PLIN gene. PLIN1 expression in lung adenocarcinoma is associated with apocrine-like features and poor clinical prognosis (137). In contrast, PLIN1 mRNA expression is significantly downregulated in human breast cancer. The reduced expression of PLIN1 is an independent predictor of OS in estrogen receptor–positive and luminal A-subtype breast cancer patients (138). Also in breast cancer, low expression of PLIN2 was associated with favorable prognosis (139). The prognostic effects of PLINs in several types of cancer from TCGA analysis are multiple and very diverse (Table 1).
Eicosanoids are biologically active metabolites of AA and are produced by cyclooxygenases 1 and 2 (COX1 and COX2) [also known as prostaglandin-endoperoxide synthase 1 and 2 (PTGS1 and PTGS2)]. They are overexpressed in a variety of malignant tumors. It has been reported that the mRNA levels of COX-1 and COX-2 in lung cancer patients were significantly higher than in normal patients (183). However, another study reports that in tumor cells COX-2 rather than COX-1 expression may account for the variable prostanoid production seen in NSCLC (128). It is clear that multivariate analysis showed that tumoral COX-2 mRNA expression and lymph node status were the most important independent prognostic predictors for NSCLC survival and disease relapse (129). Elevated COX-2 expression in tumors was significantly associated with lower survival in NSCLC and might be useful in identifying patients who would benefit from additional therapies for managing their disease (130).
The same tendency was observed in CRC, where elevated COX-2 expression, but not that of COX-1, was significantly associated with reduced survival and recognized as an independent prognostic factor (131). However, it has been reported that COX-1 and COX-2 expression is highly variable in Dukes' C tumors, and changes in COX-1 expression may be of importance in CRC (184).
COX-2 expression level and its prognostic value are also a matter of debate in breast cancer (185). Nevertheless, at least eight immunohistochemical reports have explored expression of COX-2 in a total of 2,392 primary breast carcinomas, of which 40% were found to be COX-2 positive (132). At least, four studies have detected that overexpression of COX-2 is linked to poor prognosis in breast cancer. These studies provide the basis for further estimation of a possible therapeutic effect of COX inhibitors in therapy of breast cancer.
In prostate cancer, a subset of Chinese patients with high-COX-2 expression showed minor disease-free and OS rates than those with low COX-2 expression. In this work, univariate and multivariate analyses suggested that the status of COX-2 protein expression was an independent prognostic factor for patients' survival (133).
Data from TCGA showed COX-1 and COX-2 as unfavorable markers of renal cancer, whereas only COX-1 was a risk biomarker of urothelial cancer (Table 1).
Chronic inflammation is a recognized risk factor for CRC, and polymorphisms in genes regulating inflammatory processes appear to modify the risk of neoplasia and the efficacy of non-steroidal anti-inflammatory drugs in CRC chemoprevention. COX-1 polymorphism G213G was significantly associated with an increased CRC (186). Finally, another study reports four COX-1 variants that were associated with CRC survival. rs1213266 was associated with approximately 50% lower CRC mortality. Three other variants, including L237M, resulted in significantly elevated CRC mortality risk (187).
Proteins related to FAs transportation are also relevant as cancer biomarkers. Carnitine palmitoyltransferase, CPT1A, is a protein that is responsible for the translocation of FAs from the cytosol to the mitochondrial matrix, where FA oxidation occurs. Associations of shorter disease-free survival with CPT1A positivity in invasive lobular carcinoma of the breast have been found (142).
Another study recognized a gene expression signature composed of 19 genes associated with FAO that was significantly associated with breast cancer patient survival. These 19 genes are referred to as the “fatty acid oxidation (FAO)” signature. Included in this signature were genes that have previously been identified as the core components of the FA β-oxidation pathway, such as CPT1A. Moreover, the expression of CPT1A was elevated in estrogen receptor–positive, compared to estrogen receptor–negative tumors and cell lines (143). Data from TCGA clearly confirm a CPT1A association with poor prognosis in breast cancer, whereas CPT1A is a marker of good prognosis in renal cancer and CPT1C in pancreas (Table 1).
Other relevant FA transporter is CD36. CD36, a scavenger receptor expressed in multiple cell types, mediates lipid uptake, immunological recognition, inflammation, molecular adhesion, and apoptosis. CD36 has been continually proposed as a prognostic marker in diverse cancers, mostly of epithelial origin (breast, prostate, ovary, and colon) and also for hepatic carcinoma and gliomas (141). Through systematic analysis of the multiple omics data from TCGA, it has been found that the most widely altered lipid metabolism pathways in pan-cancer are FA metabolism, AA metabolism, cholesterol metabolism, and peroxisome proliferator-activated receptor (PPAR) signaling. Genes related to lipid metabolism and immune response that were associated with poor prognosis were discovered including CD36 (188).
Cholesterol-Related Alterations as Biomarkers of Cancer Prognosis and Survival
First step of cholesterol or mevalonate pathway is catalyzed by acetyl-coenzyme A ACAT1 (Figure 2). ACAT1 is a mitochondrial enzyme that catalyzes the reversible formation of acetoacetyl-CoA from two molecules of AcCoA. An increased expression of ACAT1 in intratumor cholesteryl ester–rich breast tumors was reported (189). Also it has been proposed that ACAT1 expression could serve as a potential prognostic marker in prostate cancer, specifically in differentiating indolent and aggressive forms of cancer (144, 145). Data from TCGA suggest that ACAT1 is a marker of good prognosis in liver and renal tumors. Interestingly, isoform 2 (ACAT2) is a marker of good prognosis in CRCs, whereas in endometrial and renal tumors, ACAT2 has the opposite effect (Table 1).
Next step in cholesterol synthesis is mediated by 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS). This enzyme, with two isoforms, condenses AcCoA with acetoacetyl-CoA to form HMG-CoA, which is the substrate for HMG-CoA reductase. HMGCS2 expression is associated with reduced clinical prognosis and outcomes in patients with CRC and oral squamous cell carcinoma. It has been suggested that HMGCS2 may act as a helpful prognostic marker and essential target for potential therapeutic strategies against advanced cancer (146). Also, it has been described that HMGCS2 works as a tumor suppressor and has a prognostic impact in prostate cancer, capable of predicting the risk of biochemical recurrence (147). However, in TCGA population, both isoforms are favorable makers of renal cancer. Besides, HMGCS2 determines good prognosis in ovarian and liver cancer (Table 1).
HMGCR is the rate-limiting enzyme of the mevalonate pathway (Figure 2). HMG-CoA reductase expression in CRC and breast cancer correlates with favorable clinicopathological characteristics and an improved clinical outcome (148–150). Besides, HMCGR expression is a predictor of response to tamoxifen in breast cancer (190) and also may predict patient response to radiotherapy in ductal carcinoma in situ (191). In TCGA subset, HMGCR also is a good prognosis marker of renal tumors (Table 1). Statins, lipid-lowering compounds commonly used in cardiovascular disease, are competitive inhibitors of HMGCR. The value of HMGCR as a predictor of response to neoadjuvant or adjuvant statin treatment in cancer was also studied (192).
Once that cholesterol is synthesized, there are several cholesterol transporter proteins that play key roles in cholesterol and phospholipids homeostasis. The ATP-binding cassette transporter ABCA1 is a transmembrane protein responsible for the reverse cholesterol transport from the inner cell to circulatory system. ABCA1 is significantly overexpressed in patients of all stages of CRC, and its overexpression gives proliferative advantages together with caveolin-1–dependent increased migratory and invasive capacities (151). Individuals with upregulation of ABCA1 expression have an improved risk of CRC recurrence and OS independently of tumor stage (8). ABCA1 also forms part of the metabolic-signature ColoLipidGene able to precisely stratify stage II CRC with 5-fold higher risk of relapse (7). Moreover, the presence of tumoral genetic variants located in ABCA1 coding region seems to be associated with CRC risk of death (8). In other tumor types, ABCA1 expression was related to positive lymph nodes, but not significantly associated with tumor recurrence or breast cancer–specific survival (193).
Together with ABCA1, ATP-binding cassette G1 (ABCG1) also initiates and propagates cellular cholesterol efflux. Several genetic variants in ABCG1 have been associated with survival of NSCLC patients (194). Moreover, ABCG1 expression seems to be a favorable prognostic marker of renal cancer in data from TCGA (Table 1).
Other members of the family are the ATP-binding cassettes G4, G5, and G8. High ABCG4 expression has been associated with poor prognosis in NSCLC patients treated with cisplatin-based chemotherapy (152). ABCG5 positivity in tumor buds have been proposed as an indicator of poor prognosis in node-negative CRC patients (153), whereas in TCGA tumors, ABCG5 seems to have a favorable effect in liver prognosis (Table 1).
While cellular cholesterol efflux is mainly performed via ABCA1, cholesterol uptake is principally executed via the LDLR. The prognostic value of LDLR expression was analyzed in CRC where authors found that the absence of LDLR predicts a shorter survival (154). In the same line, lower LDLR expression was an independent prognostic factor associated with longer survival in patients with small cell lung cancer (195). By contrast, TCGA data suggest that LDLR could be a bad prognostic marker of pancreatic, renal, and urothelial cancers (Table 1).
Lipid-Related Transcription Factor Alterations as Biomarkers of Cancer Prognosis and Survival
Five are the main transcription factors that regulate the expression of mediators of lipid metabolism: SREBP1, SREBP2, PPARγ, NR1H3, and NR1H2. Sterol regulatory element-binding protein 1 (SREBP1) is a known transcription factor of lipogenic genes, which plays important roles in regulating de novo lipogenesis. SREBP1 is overexpressed and strongly associated with worse clinical outcomes in breast cancer (155). Moreover, SREBP1 also seems to have an essential role in pancreatic cancer, regulating tumorigenesis and being associated with bad prognosis (196). However, data from TCGA propose SREBP1 as a favorable prognostic marker in pancreatic and endometrial cancers (Table 1).
The combined expression of sterol regulatory element-binding protein 2 (SREBP2) together with HMGCR, NR1H3, and NR1H2 genes was associated with poor CRC clinical outcome independent of lymph node metastasis, distant metastasis, and advanced stage (156). Besides, expression of SREBP-2 was elevated in advanced pathologic grade and metastatic prostate cancer and significantly associated with poor clinical outcomes (157).
The PPARγ is a nuclear receptor that controls expression of mediators of lipid metabolism but also the inflammatory response. Additionally, it has been demonstrated that PPAR b/d and a isotypes also have important roles in FAO, FA storage, and cholesterogenesis (197).
Decreased expression of PPARγ has been observed in many tumor types. In this sense, reduced PPARγ expression within the tumor is associated with poor prognosis in lung cancer patients (158, 159). In the same line, tumor expression of PPARγ is independently associated with increased survival of CRC patients (160). Also in patients with breast and prostate cancer, PPARγ is a marker of better prognosis and is associated with better survival (161–163). Importantly, one study reports that cytoplasmic PPARγ expression appeared as an independent marker of poor prognosis in primary breast cancers (198). TCGA analysis proposed PPARγ as a favorable prognostic marker for renal and urothelial cancers (Table 1).
Finally, several studies have also evaluated the association between PPARγ genetic variants and the risk of CRC (199). In patients with stages II/III CRC, polymorphism rs1801282 in PPARγ was significantly associated with tumor recurrence (200).
NR1H3 and NR1H2 encode for liver X receptor (LXR) α and LXR β, respectively. They are intimately related nuclear receptors that react to elevated levels of intracellular cholesterol by enhancing transcription of genes that control cholesterol efflux and FA biosynthesis. NR1H3 expression was significantly correlated to better survival in completely resected stages II and III NSCLC patients (164). Moreover, one study reports that NR1H3 and NR1H2 belong to a transcription signature associated with poor CRC clinical outcome independent of lymph node metastasis, distant metastasis, and advanced stage (156). This result is validated in TCGA dataset (Table 1) where NR1H2 was also associated with CRC poor prognosis.
Targeting the Altered Lipid Metabolism in Cancer
Because of the essential role of FAs for cancer cell proliferation and progression, drugs to target lipogenic enzymes and/or transcription factors regulating the intracellular lipid homeostasis are considering as promising therapeutic strategies against cancer.
Different drugs have been already evaluated to target (i) lipogenic enzymes (FASN, ACLY, ACC); (ii) the exogenous lipid uptake (LXR, CD36, FABP4/5); (iii) inflammatory signaling pathways (PTGS2); (iv) regulation of intracellular lipid homeostasis (PPARγ, CPT1a, lipin2, HSL, MAGAT, DAGAT…); and/or (v) saturated vs. unsaturated FAs. Their efficacy has been demonstrated in numerous models of cancer, including in vitro preclinical and clinical studies.
In Table 2, we summarize main drugs evaluated in preclinical and clinical studies. Nevertheless, although the results of these studies are encouraging, side effects due to the many different regulatory mechanisms of lipid metabolism are still a big challenge.
Table 2.
Preclinical and clinical studies with main drugs evaluated to target the altered lipid metabolism in cancer.
| Target | Drug | Type of cancer | Preclinical/clinical trial | |
|---|---|---|---|---|
| FASN | Cerulenin | Breast Cancer | (48) | |
| Ovarian Cancer | (201) | |||
| C75 | Renal Cancer | (59) | ||
| Breast Cancer | (53) | |||
| Lung Cancer | (43) | |||
| Orlistat | Melanoma | (57, 202) | ||
| Prostate Cancer | (86) | |||
| Fasnall | Breast Cancer | (87) | ||
| C93 | NSCLC | (42, 43) | ||
| C247 | Breast Cancer | (44) | ||
| TV3166 | CRC | (45) | ||
| TVB-2640 | NSCLC | NCT03808558 | (56) | |
| TNBC | NCT03179904 | (56) | ||
| HG Astrocytoma | NCT03032484 | (203) | ||
| Ovarian, Breast Cancer | NCT02223247 | (204) | ||
| Triclosan | Breast | (58, 60) | ||
| ACLY | SB-204990 | NSCLC, Prostate, Ovarian | (51) | |
| NSCLC | (61) | |||
| ACC1/2 | ND-630 (GS-0976) | NASH | (71) | |
| TOFA | HNSCC | (205) | ||
| Ovarian | (33) | |||
| ND-654 | HCC | (34) | ||
| GS-0976 | NASH | (36) | ||
| NCT02856555 | (35) | |||
| ND-646 | NSCL | (206) | ||
| SCD1 | CVT-12 | HCC | (207) | |
| SSI-4 | HCC | (208) | ||
| Betulinic acid | CRC | (209) | ||
| GBC | (210) | |||
| MF-438 | NSCLC | (211) | ||
| A939572 | NSCLC | (212) | ||
| ccRCC | (213) | |||
| Prostate | (213) | |||
| CPT1A | Etomoxir | Leukemia | (214) | |
| Ranolazine | Prostate Cancer | (215) | ||
| Glioblastoma | (216) | |||
| Etomoxir, Ranolazine, Perhexiline | Prostate Cancer | (217) | ||
| Perhexiline | CLL | (218) | ||
| Breast Cancer | (219, 220) | |||
| SREBP | Betulin | HCC | (221) | |
| Melanoma | (222) | |||
| Fatostatin | Prostate | (223, 224) | ||
| Glioma | (225) | |||
| HCC | (226) | |||
| LXR | T0901317/GW3965 | BPDCN | (227) | |
| LXR623 and GW3965 | Colon/Glioblastoma | (228) | ||
| GW3965 | Glioma | (229) | ||
| ACAT1 | Avasimive | Prostate/Colon Cancer | (230) | |
| GBM | (231) | |||
| CML | (232) | |||
| CD36 | FA6.152 | Oral Cancer | (80) | |
| Prostate Cancer | (233) | |||
| HMGCR | Fluvastatin | Prostate | NCT01992042 | (234) |
| NCT00608595 | ||||
| Simvastatin | CRC | NCT00994903 | (235) | |
| NSCLC | NCT00452244 | (236) | ||
| MAGL | URB602 | Colon | (237) | |
| PTGS2 | Celecoxib | Lung Cancer | (238) | |
| Ovarian Cancer (HFD) | (239) | |||
| NSCLC | NCT00046839 | (+) | ||
| PDAC | NCT01111591 | (240) | ||
| Prostate cancer | NCT00073970 | (+) | ||
| Early CRC | NCT00608595 | (+) | ||
| PPARG | VSP-17 | Breast Cancer | (241) | |
| FABP4 | BMS309403 | HCC | (242) | |
| Prostate Cancer | (243) | |||
| FABP5 | SBFI26 | CRPC | (244) | |
(+) Unpublished results.
Recently, there is growing interest on complementary approaches by means of dietary interventions for cancer treatment. The success of such interventions requires a deep knowledge of the metabolic requirements of tumors, considering the nutritional status of the individuals—obesity, metabolic syndrome and/or insulin resistance, among others—and the genetic susceptibilities to metabolic alterations. Moreover, the knowledge of the molecular targets and mechanism of action of dietary ingredients will be crucial to apply these approaches with the conventional chemotherapy in order to improve the responses to the clinical treatments and the well-being of patients.
Precision nutrition should be considered at three levels: (1) nutritional guidelines based on age, gender, and other sociocultural factors; (2) individualized recommendations after refined phenotyping; and a (3) genetic-nutrition based on genetic variants with high penetrance and on the response to nutritional interventions (6).
The improvement of the “omics” sciences, including transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, provides a more complete scenario for personalized nutritional interventions (13, 245). The main challenge is to define tumor heterogeneities, which can be originated by genomic, epigenomic, transcriptomic, and immune variability. This will lead to patients' stratification for personalized treatments in the clinics (246).
Nutrigenetics aims to study the effect of genetic variants on the dietary response and the risk of several diseases. For example, SNPs in the CD36 gene associate with dyslipidemia when high amounts of fats are consumed (247). In addition, dietary ingredients affect cancer risk and progression affecting gene expression. Nutrigenomics considers the effect of diet-derived ingredients on gene expression and, consequently, on the proteome and metabolome.
Dietary ingredients and nutrients from natural sources, such as epigallocatechin-3-gallate, curcumin, sulforaphane, and genistein, have been shown to have anticancer properties regulating the expression of genes related to cancer. Polyphenols contribute to the prevention of obesity through the modulation of genes implicated in adipogenesis, lipolysis, and FAO (248–251).
Importantly, in the frame of precision nutrition, dietary interventions might also provide systemic responses affecting the antitumoral response of the immune system, as well as the reduction of low-grade chronic inflammation, dyslipidemia, insulin resistance, and/or obesity.
The direct association of diet with obesity and dysbiosis requires further research to understand the impact of diet on cancer prognosis. High intake of saturated FAs increases the expression of genes related to inflammation, insulin resistance, and/or hepatic steatosis. In contrast, Mediterranean diet downregulates the expression of genes related to oxidative stress, inflammation, and/or insulin signaling (252, 253). Importantly, high levels of triglycerides and LDLs have been associated with CRC prognosis and distant metastasis. Cholesterol in high-fat diets associates with colorectal tumorigenesis (254). Ceramide sphingolipids have been shown to be antitumoral in combination with tamoxifen (255). Phosphatidylcholine is increased in CRC cells. Increased intake of MUFAs is associated with reduce inflammation in CRC cancer (256). Energy-restricted diets supplemented with EPA and α-lipoic acid increase the expression of FAO genes, diminishing the expression of genes related to de novo lipogenesis and inflammation (257) (Table 3).
Table 3.
Preclinical and clinical studies with bioactive compounds from natural sources to target the altered lipid metabolism and/or associated risk factors (mainly obesity and T2DM) in cancer.
| Family | Bioactive compounds | Molecular targets, metabolic effects | Preclinical/clinical trials | References |
|---|---|---|---|---|
| Polyphenols | ||||
| Flavonoids | Gallic acid and its derivatives EGCG, gallate, ethyl gallate, gallocatechin gallate, methyl gallate, propyl gallate, theaflavin-3-gallate | ↑AMPK, FAO, thermogenesis | (258) | |
| ↓antiobesity | (259) | |||
| ↓Cholesterol, LDL | NCT02147041 | (260) | ||
| ↓lipogenesis, ↓PPARG, LXR, ↑AMPK | (261, 262) | |||
| ↑AMPK, SIRT, PGC1a, FAO, UCP1, CYp7a1 | (263) | |||
| ↓dyslipidemia | (264) | |||
| ↓dyslipidemia | NCT02627898 | (265) | ||
| ↑FAO, ↓antiobesity | NCT02381145 | (266) | ||
| ↓HOMAIR, T2DM | Human study | (267) | ||
| Citrus flavonoids | ||||
| Nobilettin | ↓HSL, ACC, ↑AMPK, CPT1a, ACOX1, FAO | (268) | ||
| Naringenin | ↑PPARα, CPT-1, UCP-2, FAO, ↓SREBP1c, 3HMGCR, hepatic steatosis | (269–272) | ||
| Tangeretin | ↑PPARα, FAO | (273) | ||
| Hesperetin | ↑PPARα, PPARγ, AMPK, FAO, ↓lipogenesis | (274) | ||
| Baicalin | ↓SREBP-1c, FASN, ACC | (275) | ||
| Hispidulin | ↑PPARα, CPT1α ↑Acat1, Acad1, HMGCS2 | (276, 277) | ||
| Mangiferin | ↓inflammation, T2DM, steatosis, ACC, DGAT2, ↑ FAO(CPT1a) | |||
| Dihydromyricetin | ↓hepatic steatosis | ChiCTRTRC12002377 | (278) | |
| Berberin | ↓hepatic steatosis, TG and cholesterol levels | NCT00633282 | (279) | |
| Luteolin | ↑FAO, ↓lipogenesis, cholesterogenesis, HMGCS1 | NCT00633282 | (280) | |
| Quercetin | ↓ CYP2E1, inflammation, obesity, T2DM | (281, 282) | ||
| Stilbenos | Resveratrol | ↓ steatosis, adipogenesis, SREBP1c, lipin1, ACC, ↑AMPK, SIRT1, FAO | (283–285) | |
| Curcuminoids | Curcumin | ↓steatosis, adipogenesis, SREBP1c, FASN, SCD1, GPAT-1, ↑1AMPK, FAO | (286, 287) | |
| Phenolic acids | Ellagic acid | ↓steatosis, Insulin resistance | (288) | |
| Terpenoids | ||||
| Carnosol | ↓hyperglycemia, inflammation, lipogenesis, anticancer | (289, 290) | ||
| Betulinic acid | ↓SCD, steatosis, lipogenesis | (209) | ||
| Ursolic acid | ↑AMPK, FAO, ↓lipogenesis | (291) | ||
| Ginsenoside | ↑AMPK, perilipin, FAO | (292–294) | ||
| Licopene | ↓inflammation | ISRCTN99660610 | (295) |
Importantly, the efficacy of fasting cycles or cycles of fasting mimicking diets in dampening tumor development has already been established (296), and the implementation of other dietary approaches for cancer therapy is likely to take a similar approach.
Concluding Remarks
Metabolic alterations of tumors have been well-recognized as one of the hallmarks of cancer. At present, several investigations have demonstrated the consequences of lipid metabolism deregulation in cancer not only sustain tumor growth but also promote cell migration, invasion, and angiogenesis. In this review, we have discussed about the main lipid metabolism alterations found in cancer by describing their mechanism of action and their oncologic implications. Importantly, we emphasize the crucial role of the aberrant lipid metabolism not only affecting the primary tumors but also shaping the tumor microenvironment to promote malignancy and dissemination. Moreover, we have explored the available public data bases containing mRNA data (TCGA) and protein expression data (The Human Protein Atlas) to obtain a global view of the putative implications of lipid metabolism–related genes in cancer prognosis of the most frequent types of cancer according to the WHO: lung, CRC, breast, and prostate cancers.
We also highlight the relevance of “omics” technologies, including genomic and transcriptomic data, considering the phenotypic metabolic status (mainly obesity) to define lipid metabolic scores to be integrated into the clinical advice. Thus, the use of this knowledge will allow a better stratification of patients, which will be translated into improvements on the OS and well-being of the patients. In the frame of precision medicine, new clinical trials integrating classical chemotherapies with precision nutrition–based strategies—bioactive products and diet derived nutrients—will provide an unquestionable line of research in cancer treatment.
Author Contributions
LPF and MGC wrote the paper. AR performed the critical revision of the article. All authors conceptually designed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Plan Nacional I + D + i PID2019-110183RB-C21; Regional Government of Community of Madrid P2018/BAA-4343-ALIBIRD2020-CM; Ramón Areces Foundation; EU Structural Funds and COST Action CA17118.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Cancer J Clin. (2020) 70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. (2016) 5:e189. 10.1038/oncsis.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. (2008) 9:112–24. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peck B, Schulze A. Lipid metabolism at the nexus of diet and tumor microenvironment. Trends in Cancer. (2019) 5:693–703. 10.1016/j.trecan.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Gómez de Cedrón M, Ramírez de Molina A. Chapter 28 - Precision nutrition to target lipid metabolism alterations in cancer. In: Faintuch J, Faintuch S. editors. Precision Medicine for Investigators, Practitioners Providers. Academic Press (2019). p. 291–9. 10.1016/B978-0-12-819178-1.00028-9 [DOI] [Google Scholar]
- 7.Vargas T, Moreno-Rubio J, Herranz J, Cejas P, Molina S, González-Vallinas M, et al. ColoLipidGene: signature of lipid metabolism-related genes to predict prognosis in stage-II colon cancer patients. Oncotarget. (2015) 6:7348–63. 10.18632/oncotarget.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández LP, Ramos-Ruiz R, Herranz J, Martín-Hernández R, Vargas T, Mendiola M, et al. The transcriptional and mutational landscapes of lipid metabolism-related genes in colon cancer. Oncotarget. (2017) 9:5919–30. 10.18632/oncotarget.23592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Martínez R, Cruz-Gil S, Gómez de Cedrón M, Álvarez-Fernández M, Vargas T, Molina S, et al. A link between lipid metabolism and epithelial-mesenchymal transition provides a target for colon cancer therapy. Oncotarget. (2015) 6:38719–36. 10.18632/oncotarget.5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling. Mol Cancer. (2006) 5:69. 10.1186/1476-4598-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. (2017) 16:76. 10.1186/s12943-017-0646-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rung J, Brazma A. Reuse of public genome-wide gene expression data. Nat Rev Genet. (2013) 14:89–99. 10.1038/nrg3394 [DOI] [PubMed] [Google Scholar]
- 13.Ferguson LR, De Caterina R, Görman U, Allayee H, Kohlmeier M, Prasad C, et al. Guide and position of the international society of nutrigenetics/nutrigenomics on personalised nutrition: part 1 - fields of precision nutrition. J Nutrigenet Nutrigenomics. (2016) 9:12–27. 10.1159/000445350 [DOI] [PubMed] [Google Scholar]
- 14.Aguirre-Portolés C, Fernández LP, Ramírez de Molina A. precision nutrition for targeting lipid metabolism in colorectal cancer. Nutrients. (2017) 9:1076. 10.3390/nu9101076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonds NI, Ghazarian AA, Pimentel CB, Schully SD, Ellison GL, Gillanders EM, et al. Review of the gene-environment interaction literature in cancer: what do we know?. Genet Epidemiol. (2016) 40:356–365. 10.1002/gepi.21967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. (2017) 3:17034 10.1038/nrdp.2017.34 [DOI] [PubMed] [Google Scholar]
- 17.Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. (2014) 5:444–70. 10.4239/wjd.v5.i4.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalitin S, Battelino T, Moreno LA. Obesity, metabolic syndrome and nutrition. World Rev Nutr Diet. (2016) 114:21–49. 10.1159/000441810 [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348:1625–38. 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhou G, Sun B, Zhao G, Liu D, Sun J, et al. Impact of obesity upon prostate cancer-associated mortality: a meta-analysis of 17 cohort studies. Oncol Lett. (2015) 9:1307–12. 10.3892/ol.2014.2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers. (2014) 29:e21–9. 10.5301/jbm.5000047 [DOI] [PubMed] [Google Scholar]
- 22.Dobbins M, Decorby K, Choi BC. The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med. (2013) 2013:680536. 10.5402/2013/680536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Zhang TT, Zhao JJ, Qi SF, Du P, Liu DW, et al. The association between overweight, obesity and ovarian cancer: a meta-analysis. Jpn J Clin Oncol. (2015) 45:1107–15. 10.1093/jjco/hyv150 [DOI] [PubMed] [Google Scholar]
- 24.Qin Q, Xu X, Wang X, Zheng XY. Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. (2013) 14:3117–21. 10.7314/apjcp.2013.14.5.3117 [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. (2007) 97:1005–8. 10.1038/sj.bjc.6603932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. (2007) 16:2533–47. 10.1158/1055-9965.EPI-07-0708 [DOI] [PubMed] [Google Scholar]
- 27.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. (2007) 17:319–26. 10.1016/j.numecd.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 28.Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM. Visceral obesity and breast cancer risk. Cancer. (1994) 74:632–9. [DOI] [PubMed] [Google Scholar]
- 29.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. (2011) 17:1498–503. 10.1038/nm.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. (2012) 21:309–22. 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Outschoorn UE, Pestell RG, Howell A, Tykocinski ML, Nagajyothi F, Machado FS, et al. Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and achilles' heel of tumor cells. Cell Cycle. (2011) 10:4208–16. 10.4161/cc.10.24.18487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Cho SY, Lee SB, Son H, Jeong H. Obesity is associated with higher risk of prostate cancer detection in a biopsy population in Korea. BJU Int. (2014) 114:891–5. 10.1111/bju.12600 [DOI] [PubMed] [Google Scholar]
- 33.Li S, Qiu L, Wu B, Shen H, Zhu J, Zhou L, et al. TOFA suppresses ovarian cancer cell growth in vitro and in vivo. Mol Med Rep. (2013) 8:373–8. 10.3892/mmr.2013.1505 [DOI] [PubMed] [Google Scholar]
- 34.Lally JSV, Ghoshal S, DePeralta DK, Moaven O, Wei L, Masia R, et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. (2019) 29:174–82.e5. 10.1016/j.cmet.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loomba R, Kayali Z, Noureddin M, Ruane P, Lawitz EJ, Bennett M, et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. (2018) 155:1463–73.e6. 10.1053/j.gastro.2018.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu T, Sun L, Wang Z, Zhang Y, He Z, Xu C. Fatty acid synthase enhances colorectal cancer cell proliferation and metastasis via regulating AMPK/mTOR pathway. Onco Targets Ther. (2019) 12:3339–47. 10.2147/OTT.S199369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. (2014) 12:661–72. 10.1038/nrmicro3344 [DOI] [PubMed] [Google Scholar]
- 38.Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. (2014) 16:406. 10.1007/s11912-014-0406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta. (2012) 1826:370–84. 10.1016/j.bbcan.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 40.Warburg O. On the origin of cancer cells. Science. (1956) 123:309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 41.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. (2008) 7:11–20. 10.1016/j.cmet.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 42.Orita H, Coulter J, Lemmon C, Tully E, Vadlamudi A, Medghalchi SM, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. (2007) 13:7139–45. 10.1158/1078-0432.CCR-07-1186 [DOI] [PubMed] [Google Scholar]
- 43.Orita H, Coulter J, Tully E, Kuhajda FP, Gabrielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clin Cancer Res. (2008) 14:2458–64. 10.1158/1078-0432.CCR-07-4177 [DOI] [PubMed] [Google Scholar]
- 44.Alli PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. (2005) 24:39–46. 10.1038/sj.onc.1208174 [DOI] [PubMed] [Google Scholar]
- 45.Zaytseva YY, Rychahou PG, Le AT, Scott TL, Flight RM, Kim JT, et al. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget. (2018) 9:24787–800. 10.18632/oncotarget.25361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashima T, Sato S, Okabe S, Miyata S, Matsuura M, Sugimoto Y, et al. Acyl-CoA synthetase as a cancer survival factor: its inhibition enhances the efficacy of etoposide. Cancer Sci. (2009) 100:1556–62. 10.1111/j.1349-7006.2009.01203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menendez JA, Vellon L, Espinoza I, Lupu R. The metastasis inducer CCN1 (CYR61) activates the fatty acid synthase (FASN)-driven lipogenic phenotype in breast cancer cells. Oncoscience. (2016) 3:242–57. 10.18632/oncoscience.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. (1996) 56:2745–7 [PubMed] [Google Scholar]
- 49.Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. (2011) 9:1551–61. 10.1158/1541-7786.MCR-11-0126 [DOI] [PubMed] [Google Scholar]
- 50.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. (2012) 279:2610–3. 10.1111/j.1742-4658.2012.08644.x [DOI] [PubMed] [Google Scholar]
- 51.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. (2005) 8:311–21. 10.1016/j.ccr.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 52.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. (2007) 7:763–77. 10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- 53.Menendez JA, Vellon L, Colomer R, Lupu R. Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances taxol (paclitaxel)-induced cytotoxicity. Int J Cancer. (2005) 115:19–35. 10.1002/ijc.20754 [DOI] [PubMed] [Google Scholar]
- 54.Rao S, Lowe M, Herliczek TW, Keyomarsi K. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene. (1998) 17:2393–402. 10.1038/sj.onc.1202322 [DOI] [PubMed] [Google Scholar]
- 55.Clendening JW, Pandyra A, Boutros PC, El Ghamrasni S, Khosravi F, Trentin GA, et al. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci USA. (2010) 107:15051–6. 10.1073/pnas.0910258107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Che L, Paliogiannis P, Cigliano A, Pilo MG, Chen X, Calvisi DF. Pathogenetic, prognostic, and therapeutic role of fatty acid synthase in human hepatocellular carcinoma. Front Oncol. (2019) 9:1412. 10.3389/fonc.2019.01412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seguin F, Carvalho MA, Bastos DC, Agostini M, Zecchin KG, Alvarez-Flores MP, et al. The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br J Cancer. (2012) 107:977–87. 10.1038/bjc.2012.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadowski MC, Pouwer RH, Gunter JH, Lubik AA, Quinn RJ, Nelson CC. The fatty acid synthase inhibitor triclosan: repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget. (2014) 5:9362–81. 10.18632/oncotarget.2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horiguchi A, Asano T, Ito K, Sumitomo M, Hayakawa M. Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J Urol. (2008) 180:729–36. 10.1016/j.juro.2008.03.186 [DOI] [PubMed] [Google Scholar]
- 60.Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol. (2014) 27:834–42. 10.1021/tx5000156 [DOI] [PubMed] [Google Scholar]
- 61.Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. (2008) 68:8547–54. 10.1158/0008-5472.CAN-08-1235 [DOI] [PubMed] [Google Scholar]
- 62.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. (2013) 13:227–32. 10.1038/nrc3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khasawneh J, Schulz MD, Walch A, Rozman J, Hrabe de Angelis M, Klingenspor M, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci USA. (2009) 106:3354–9. 10.1073/pnas.0802864106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. (2013) 14:1045–53. 10.1038/ni.2704 [DOI] [PubMed] [Google Scholar]
- 65.Li S, Zhou T, Li C, Dai Z, Che D, Yao Y, et al. High metastaticgastric and breast cancer cells consume oleic acid in an AMPK dependent manner. PLoS ONE. (2014) 9:e97330. 10.1371/journal.pone.0097330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ludtmann MH, Angelova PR, Zhang Y, Abramov AY, Dinkova-Kostova AT. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem J. (2014) 457:415–24. 10.1042/BJ20130863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. (2011) 1807:726–34. 10.1016/j.bbabio.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 68.Carracedo A, Weiss D, Leliaert AK, Bhasin M, de Boer VCJ, Laurent G, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. (2012) 122:3088–100. 10.1172/JCI62129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Przybytkowski E, Joly E, Nolan CJ, Hardy S, Francoeur AM, Langelier Y, et al. Upregulation of cellular triacylglycerol - free fatty acid cycling by oleate is associated with long-term serum-free survival of human breast cancer cells. Biochem Cell Biol. (2007) 85:301–10. 10.1139/o07-001 [DOI] [PubMed] [Google Scholar]
- 70.Geng F, Guo D. Lipid droplets, potential biomarker and metabolic target in glioblastoma. Intern Med Rev. (2017) 3. 10.18103/imr.v3i5.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harriman G, Greenwood J, Bhat S, Huang X, Wang R, Paul D, et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci USA. (2016) 113:E1796–805. 10.1073/pnas.1520686113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Togashi A, Katagiri T, Ashida S, Fujioka T, Maruyama O, Wakumoto Y, et al. Hypoxia-inducible protein 2 (HIG2), a novel diagnostic marker for renal cell carcinoma and potential target for molecular therapy. Cancer Res. (2005) 65:4817–26. 10.1158/0008-5472.CAN-05-0120 [DOI] [PubMed] [Google Scholar]
- 73.Penrose H, Heller S, Cable C, Makboul R, Chadalawada G, Chen Y, et al. Epidermal growth factor receptor mediated proliferation depends on increased lipid droplet density regulated via a negative regulatory loop with FOXO3/Sirtuin6. Biochem Biophys Res Commun. (2016) 469:370–6. 10.1016/j.bbrc.2015.11.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. (2010) 140:49–61. 10.1016/j.cell.2009.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ou J, Miao H, Ma Y, Guo F, Deng J, Wei X, et al. Loss of abhd5 promotes colorectal tumor development and progression by inducing aerobic glycolysis and epithelial-mesenchymal transition. Cell Rep. (2018) 24:2795–97. 10.1016/j.celrep.2018.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nomura DK, Lombardi DP, Chang JW, Niessen S, Ward AM, Long JZ, et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. (2011) 18:846–56. 10.1016/j.chembiol.2011.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng X, Geng F, Pan M, Wu X, Zhong Y, Wang C, et al. Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metabolism. (2020) 32:229–42.e8. 10.1016/j.cmet.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. (2011) 5:159–66. 10.1586/egh.11.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. (2011) 10:427–36. 10.1158/1535-7163.MCT-10-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. (2017) 541:41–5. 10.1038/nature20791 [DOI] [PubMed] [Google Scholar]
- 81.Sundelin JP, Stahlman M, Lundqvist A, Levin M, Parini P, Johansson ME, et al. Increased expression of the very low-density lipoprotein receptor mediates lipid accumulation in clear-cell renal cell carcinoma. PLoS ONE. (2012) 7:e48694. 10.1371/journal.pone.0048694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koizume S, Miyagi Y. Lipid droplets: a key cellular organelle associated with cancer cell survival under normoxia and hypoxia. Int J Mol Sci. (2016) 17:1430. 10.3390/ijms17091430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levi L, Wang Z, Doud MK, Hazen SL, Noy N. Saturated fatty acids regulate retinoic acid signalling and suppress tumorigenesis by targeting fatty acid-binding protein 5. Nat Commun. (2015) 6:8794. 10.1038/ncomms9794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. (2014) 19:393–406. 10.1016/j.cmet.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. (2010) 70:8117–26. 10.1158/0008-5472.CAN-09-3871 [DOI] [PubMed] [Google Scholar]
- 86.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. (2004) 64:2070–5. 10.1158/0008-5472.can-03-3645 [DOI] [PubMed] [Google Scholar]
- 87.Alwarawrah Y, Hughes P, Loiselle D, Carlson DA, Darr DB, Jordan JL, et al. Fasnall, a selective FASN inhibitor, shows potent anti-tumor activity in the MMTV-Neu model of HER2(+) Breast cancer. Cell Chem Biol. (2016) 23:678–88. 10.1016/j.chembiol.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. (2009) 1788:2022–31. 10.1016/j.bbamem.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 89.Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L, et al. Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Res. (2016) 76:2037–49. 10.1158/0008-5472.CAN-15-1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. (2016) 16:732–49. 10.1038/nrc.2016.89 [DOI] [PubMed] [Google Scholar]
- 91.Zhu Z, Zhao X, Zhao L, Yang H, Liu L, Li J, et al. p54 nrb /NONO regulates lipid metabolism and breast cancer growth through SREBP-1A. Oncogene. (2016) 35:1399–410. 10.1038/onc.2015.197 [DOI] [PubMed] [Google Scholar]
- 92.Carito V, Bonuccelli G, Martinez-Outschoorn UE, Whitaker-Menezes D, Caroleo MC, Cione E, et al. Metabolic remodeling of the tumor microenvironment: migration stimulating factor (MSF) reprograms myofibroblasts toward lactate production, fueling anabolic tumor growth. Cell Cycle. (2012) 11:3403–14. 10.4161/cc.21701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guaita-Esteruelas S, Gumà J, Masana L, Borràs J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol Cell Endocrinol. (2017) 462:107–18. 10.1016/j.mce.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 94.Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. (2017) 7:68. 10.3389/fonc.2017.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ventura R, Mordec K, Waszczuk J, Wang Z, Lai J, Fridlib M, et al. Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine. (2015) 2:808–24. 10.1016/j.ebiom.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang L, Xiao L, Sugiura H, Huang X, Ali A, Kuro-o M, et al. Metabolic reprogramming during TGFβ1-induced epithelial-to-mesenchymal transition. Oncogene. (2015) 34:3908–16. 10.1038/onc.2014.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.English D, Brindley DN, Spiegel S, Garcia JGN. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim Biophys Acta. (2002) 1582:228–39. 10.1016/s1388-1981(02)00176-2 [DOI] [PubMed] [Google Scholar]
- 98.Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, et al. The role of fatty acid β-oxidation in lymphangiogenesis. Nature. (2017) 542:49–54. 10.1038/nature21028 [DOI] [PubMed] [Google Scholar]
- 99.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. (2015) 347:1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 100.Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. (2017) 357:eaan2507. 10.1126/science.aan2507 [DOI] [PubMed] [Google Scholar]
- 101.Uhlén M, Björling E, Agaton C, Szigyarto CA-K, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. (2005) 4:1920–32. 10.1074/mcp.M500279-MCP200 [DOI] [PubMed] [Google Scholar]
- 102.Osugi J, Yamaura T, Muto S, Okabe N, Matsumura Y, Hoshino M, et al. Prognostic impact of the combination of glucose transporter 1 and ATP citrate lyase in node-negative patients with non-small lung cancer. Lung Cancer. (2015) 88:310–8. 10.1016/j.lungcan.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 103.Csanadi A, Kayser C, Donauer M, Gumpp V, Aumann K, Rawluk J, et al. Prognostic value of malic enzyme and ATP-citrate lyase in non-small cell lung cancer of the young and the elderly. PLoS ONE. (2015) 10:e0126357. 10.1371/journal.pone.0126357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wen J, Min X, Shen M, Hua Q, Han Y, Zhao L, et al. ACLY facilitates colon cancer cell metastasis by CTNNB1. J Exp Clin Cancer Res. (2019) 38:401. 10.1186/s13046-019-1391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conde E, Suarez-Gauthier A, García-García E, Lopez-Rios F, Lopez-Encuentra A, García-Lujan R, et al. Specific pattern of LKB1 and phospho-acetyl-CoA carboxylase protein immunostaining in human normal tissues and lung carcinomas. Hum Pathol. (2007) 38:1351–60. 10.1016/j.humpath.2007.01.022 [DOI] [PubMed] [Google Scholar]
- 106.Bai J, Zhang X, Kang X, Jin L, Wang P, Wang Z. Screening of core genes and pathways in breast cancer development via comprehensive analysis of multi gene expression datasets. Oncol Lett. (2019) 18:5821–30. 10.3892/ol.2019.10979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo D, Xiao H, Dong J, Li Y, Feng G, Cui M, et al. B7-H3 regulates lipid metabolism of lung cancer through SREBP1-mediated expression of FASN. Biochem Biophys Res Commun. (2017) 482:1246–51. 10.1016/j.bbrc.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 108.Wang H, Xi Q, Wu G. Fatty acid synthase regulates invasion and metastasis of colorectal cancer via Wnt signaling pathway. Cancer Med. (2016) 5:1599–606. 10.1002/cam4.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O'Connor K, et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. (2012) 72:1504–17. 10.1158/0008-5472.CAN-11-4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohamed AH, Said NM. Immunohistochemical expression of fatty acid synthase and vascular endothelial growth factor in primary colorectal cancer: a clinicopathological study. J Gastrointest Cancer. (2019) 50:485–92. 10.1007/s12029-018-0104-5 [DOI] [PubMed] [Google Scholar]
- 111.Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. (2008) 26:5713–20. 10.1200/JCO.2008.18.2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gharib E, Nasrinasrabadi P, Zali MR. Development and validation of a lipogenic genes panel for diagnosis and recurrence of colorectal cancer. PLoS ONE. (2020) 15:e0229864. 10.1371/journal.pone.0229864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puig T, Porta R, Colomer R. Fatty acid synthase: a new anti-tumor target. Med Clin. (2009) 132:359–63. 10.1016/j.medcli.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 114.Giró-Perafita A, Sarrats A, Pérez-Bueno F, Oliveras G, Buxó M, Brunet J, et al. Fatty acid synthase expression and its association with clinico-histopathological features in triple-negative breast cancer. Oncotarget. (2017) 8:74391–405. 10.18632/oncotarget.20152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Corominas-Faja B, Vellon L, Cuyàs E, Buxó M, Martin-Castillo B, Serra D, et al. Clinical and therapeutic relevance of the metabolic oncogene fatty acid synthase in HER2+ breast cancer. Histol Histopathol. (2017) 32:687–98. 10.14670/HH-11-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kristiansen G. Immunohistochemical algorithms in prostate diagnostics: what's new? Pathologe. (2009) 30:146–53. 10.1007/s00292-009-1230-4 [DOI] [PubMed] [Google Scholar]
- 117.Shah US, Dhir R, Gollin SM, Chandran UR, Lewis D, Acquafondata M, et al. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum Pathol. (2006) 37:401–9. 10.1016/j.humpath.2005.11.022 [DOI] [PubMed] [Google Scholar]
- 118.Myers JS, von Lersner AK, Sang Q-XA. Proteomic upregulation of fatty acid synthase and fatty acid binding protein 5 and identification of cancer- and race-specific pathway Associations in Human Prostate Cancer Tissues. J Cancer. (2016) 7:1452–64. 10.7150/jca.15860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. (2000) 88:176–9. [DOI] [PubMed] [Google Scholar]
- 120.Vargas T, Moreno-Rubio J, Herranz J, Cejas P, Molina S, Mendiola M, et al. 3'UTR polymorphism in ACSL1 gene correlates with expression levels and poor clinical outcome in colon cancer patients. PLoS ONE. (2016) 11:e0168423. 10.1371/journal.pone.0168423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W-C, Wang C-Y, Hung Y-H, Weng T-Y, Yen M-C, Lai M-D. Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-coenzyme a synthetase family in cancer. PLoS ONE. (2016) 11:e0155660. 10.1371/journal.pone.0155660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu X, Li Y, Wang J, Wen X, Marcus MT, Daniels G, et al. Long chain fatty Acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PLoS ONE. (2013) 8:e77060. 10.1371/journal.pone.0077060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yen M-C, Kan J-Y, Hsieh C-J, Kuo P-L, Hou M-F, Hsu Y-L. Association of long-chain acyl-coenzyme a synthetase 5 expression in human breast cancer by estrogen receptor status and its clinical significance. Oncol Rep. (2017) 37:3253–60. 10.3892/or.2017.5610 [DOI] [PubMed] [Google Scholar]
- 124.Huang J, Fan X-X, He J, Pan H, Li R-Z, Huang L, et al. SCD1 is associated with tumor promotion, late stage and poor survival in lung adenocarcinoma. Oncotarget. (2016) 7:39970–9. 10.18632/oncotarget.9461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi S, Yoo YJ, Kim H, Lee H, Chung H, Nam M-H, et al. Clinical and biochemical relevance of monounsaturated fatty acid metabolism targeting strategy for cancer stem cell elimination in colon cancer. Biochem Biophys Res Commun. (2019) 519:100–5. 10.1016/j.bbrc.2019.08.137 [DOI] [PubMed] [Google Scholar]
- 126.Holder AM, Gonzalez-Angulo AM, Chen H, Akcakanat A, Do K-A, Fraser Symmans W, et al. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat. (2013) 137:319–27. 10.1007/s10549-012-2354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang D, Lin Y, Gao B, Yan S, Wu H, Li Y, et al. Reduced expression of fads1 predicts worse prognosis in non-small-cell lung cancer. J Cancer. (2016) 7:1226–32. 10.7150/jca.15403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Watkins DN, Lenzo JC, Segal A, Garlepp MJ, Thompson PJ. Expression and localization of cyclo-oxygenase isoforms in non-small cell lung cancer. Eur Respir J. (1999) 14:412–8. 10.1034/j.1399-3003.1999.14b28.x [DOI] [PubMed] [Google Scholar]
- 129.Yuan A, Yu C-J, Shun C-T, Luh K-T, Kuo S-H, Lee Y-C, et al. Total cyclooxygenase-2 mRNA levels correlate with vascular endothelial growth factor mRNA levels, tumor angiogenesis and prognosis in non-small cell lung cancer patients. Int J Cancer. (2005) 115:545–55. 10.1002/ijc.20898 [DOI] [PubMed] [Google Scholar]
- 130.Brabender J, Park J, Metzger R, Schneider PM, Lord RV, Hölscher AH, et al. Prognostic significance of cyclooxygenase 2 mRNA expression in non-small cell lung cancer. Ann Surg. (2002) 235:440–3. 10.1097/00000658-200203000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. (2004) 10:8465–71. 10.1158/1078-0432.CCR-04-0653 [DOI] [PubMed] [Google Scholar]
- 132.Denkert C, Winzer K-J, Hauptmann S. Prognostic impact of cyclooxygenase-2 in breast cancer. Clin Breast Cancer. (2004) 4:428–33. 10.3816/cbc.2004.n.006 [DOI] [PubMed] [Google Scholar]
- 133.Bin W, He W, Feng Z, Xiangdong L, Yong C, Lele K, et al. Prognostic relevance of cyclooxygenase-2 (COX-2) expression in Chinese patients with prostate cancer. Acta Histochem. (2011) 113:131–6. 10.1016/j.acthis.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 134.Fan X, Weng Y, Bai Y, Wang Z, Wang S, Zhu J, et al. Lipin-1 determines lung cancer cell survival and chemotherapy sensitivity by regulation of endoplasmic reticulum homeostasis and autophagy. Cancer Med. (2018) 7:2541–54. 10.1002/cam4.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dinarvand N, Khanahmad H, Hakimian SM, Sheikhi A, Rashidi B, Bakhtiari H, et al. Expression and clinicopathological significance of lipin-1 in human breast cancer and its association with p53 tumor suppressor gene. J Cell Physiol. (2020) 235:5835–46. 10.1002/jcp.29523 [DOI] [PubMed] [Google Scholar]
- 136.He J, Zhang F, Tay LWR, Boroda S, Nian W, Levental KR, et al. Lipin-1 regulation of phospholipid synthesis maintains endoplasmic reticulum homeostasis and is critical for triple-negative breast cancer cell survival. FASEB J. (2017) 31:2893–904. 10.1096/fj.201601353R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fujimoto M, Yoshizawa A, Sumiyoshi S, Sonobe M, Menju T, Hirata M, et al. Adipophilin expression in lung adenocarcinoma is associated with apocrine-like features and poor clinical prognosis: an immunohistochemical study of 328 cases. Histopathology. (2017) 70:232–41. 10.1111/his.13048 [DOI] [PubMed] [Google Scholar]
- 138.Zhou C, Wang M, Zhou L, Zhang Y, Liu W, Qin W, et al. Prognostic significance of PLIN1 expression in human breast cancer. Oncotarget. (2016) 7:54488–502. 10.18632/oncotarget.10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lucenay KS, Doostan I, Karakas C, Bui T, Ding Z, Mills GB, et al. Cyclin E associates with the lipogenic enzyme ATP-citrate lyase to enable malignant growth of breast cancer cells. Cancer Res. (2016) 76:2406–18. 10.1158/0008-5472.CAN-15-1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao C, Zhuang J, Li H, Liu C, Zhou C, Liu L, et al. Development of a risk scoring system for evaluating the prognosis of patients with Her2-positive breast cancer. Cancer Cell Int. (2020) 20:121. 10.1186/s12935-020-01175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enciu A-M, Radu E, Popescu ID, Hinescu ME, Ceafalan LC. Targeting CD36 as biomarker for metastasis prognostic: how far from translation into clinical practice? Biomed Res Int. (2018) 2018:7801202. 10.1155/2018/7801202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cha YJ, Kim HM, Koo JS. Expression of lipid metabolism-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Int J Mol Sci. (2017) 18:232. 10.3390/ijms18010232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aiderus A, Black MA, Dunbier AK. Fatty acid oxidation is associated with proliferation and prognosis in breast and other cancers. BMC Cancer. (2018) 18:805. 10.1186/s12885-018-4626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saraon P, Trudel D, Kron K, Dmitromanolakis A, Trachtenberg J, Bapat B, et al. Evaluation and prognostic significance of ACAT1 as a marker of prostate cancer progression. Prostate. (2014) 74:372–80. 10.1002/pros.22758 [DOI] [PubMed] [Google Scholar]
- 145.Saraon P, Cretu D, Musrap N, Karagiannis GS, Batruch I, Drabovich AP, et al. Quantitative proteomics reveals that enzymes of the ketogenic pathway are associated with prostate cancer progression. Mol Cell Proteomics. (2013) 12:1589–601. 10.1074/mcp.M112.023887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen S-W, Chou C-T, Chang C-C, Li Y-J, Chen S-T, Lin I-C, et al. HMGCS2 enhances invasion and metastasis via direct interaction with PPARα to activate Src signaling in colorectal cancer and oral cancer. Oncotarget. (2017) 8:22460–76. 10.18632/oncotarget.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wan S, Xi M, Zhao H-B, Hua W, Liu Y-L, Zhou Y-L, et al. HMGCS2 functions as a tumor suppressor and has a prognostic impact in prostate cancer. Pathol Res Pract. (2019) 215:152464. 10.1016/j.prp.2019.152464 [DOI] [PubMed] [Google Scholar]
- 148.Bengtsson E, Nerjovaj P, Wangefjord S, Nodin B, Eberhard J, Uhlén M, et al. HMG-CoA reductase expression in primary colorectal cancer correlates with favourable clinicopathological characteristics and an improved clinical outcome. Diagn Pathol. (2014) 9:78. 10.1186/1746-1596-9-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim H, Seol YM, Choi YJ, Shin H-J, Chung JS, Shin N, et al. HMG CoA reductase expression as a prognostic factor in korean patients with breast cancer: a retrospective study. Medicine. (2019) 98:e14968. 10.1097/MD.0000000000014968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Borgquist S, Jögi A, Pontén F, Rydén L, Brennan DJ, Jirström K. Prognostic impact of tumour-specific HMG-CoA reductase expression in primary breast cancer. Breast Cancer Res. (2008) 10:R79. 10.1186/bcr2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aguirre-Portolés C, Feliu J, Reglero G, Ramírez de Molina A. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BET inhibitor apabetalone. Mol Oncol. (2018) 12:1735–52. 10.1002/1878-0261.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang G, Wang X-J, Huang L-J, Zhou Y-A, Tian F, Zhao J-B, et al. High ABCG4 expression is associated with poor prognosis in non-small-cell lung cancer patients treated with cisplatin-based chemotherapy. PLoS ONE. (2015) 10:e0135576. 10.1371/journal.pone.0135576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hostettler L, Zlobec I, Terracciano L, Lugli A. ABCG5-positivity in tumor buds is an indicator of poor prognosis in node-negative colorectal cancer patients. World J Gastroenterol. (2010) 16:732–9. 10.3748/wjg.v16.i6.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Caruso MG, Osella AR, Notarnicola M, Berloco P, Leo S, Bonfiglio C, et al. Prognostic value of low density lipoprotein receptor expression in colorectal carcinoma. Oncol Rep. (1998) 5:927–30. 10.3892/or.5.4.927 [DOI] [PubMed] [Google Scholar]
- 155.Zhang N, Zhang H, Liu Y, Su P, Zhang J, Wang X, et al. SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with snail and HDAC1/2. Cell Death Differ. (2019) 26:843–59. 10.1038/s41418-018-0158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharma B, Gupta V, Dahiya D, Kumar H, Vaiphei K, Agnihotri N. Clinical relevance of cholesterol homeostasis genes in colorectal cancer. Biochim Biophys Acta Mol Cell Biol Lipids. (2019) 1864:1314–27. 10.1016/j.bbalip.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 157.Li X, Wu JB, Li Q, Shigemura K, Chung LWK, Huang W-C. SREBP-2 promotes stem cell-like properties and metastasis by transcriptional activation of c-Myc in prostate cancer. Oncotarget. (2016) 7:12869–84. 10.18632/oncotarget.7331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hazra S, Peebles KA, Sharma S, Mao JT, Dubinett SM. The role of ppargamma in the cyclooxygenase pathway in lung cancer. PPAR Res. (2008) 2008:790568. 10.1155/2008/790568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sasaki H, Tanahashi M, Yukiue H, Moiriyama S, Kobayashi Y, Nakashima Y, et al. Decreased perioxisome proliferator-activated receptor gamma gene expression was correlated with poor prognosis in patients with lung cancer. Lung Cancer. (2002) 36:71–6. 10.1016/s0169-5002(01)00449-4 [DOI] [PubMed] [Google Scholar]
- 160.Ogino S, Shima K, Baba Y, Nosho K, Irahara N, Kure S, et al. Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology. (2009) 136:1242–50. 10.1053/j.gastro.2008.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abduljabbar R, Al-Kaabi MM, Negm OH, Jerjees D, Muftah AA, Mukherjee A, et al. Prognostic and biological significance of peroxisome proliferator-activated receptor-gamma in luminal breast cancer. Breast Cancer Res Treat. (2015) 150:511–22. 10.1007/s10549-015-3348-9 [DOI] [PubMed] [Google Scholar]
- 162.Jiang Y, Zou L, Zhang C, He S, Cheng C, Xu J, et al. PPARgamma and Wnt/beta-Catenin pathway in human breast cancer: expression pattern, molecular interaction and clinical/prognostic correlations. J Cancer Res Clin Oncol. (2009) 135:1551–9. 10.1007/s00432-009-0602-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nakamura Y, Suzuki T, Sugawara A, Arai Y, Sasano H. Peroxisome proliferator-activated receptor gamma in human prostate carcinoma. Pathol Int. (2009) 59:288–93. 10.1111/j.1440-1827.2009.02367.x [DOI] [PubMed] [Google Scholar]
- 164.Melloni G, Muriana P, Bandiera A, Fontana R, Maggioni D, Russo V, et al. Prognostic role of liver X receptor-alpha in resected stage II and III non-small-cell lung cancer. Clin Respir J. (2018) 12:241–6. 10.1111/crj.12522 [DOI] [PubMed] [Google Scholar]
- 165.Jin X, Zhang K-J, Guo X, Myers R, Ye Z, Zhang Z-P, et al. Fatty acid synthesis pathway genetic variants and clinical outcome of non-small cell lung cancer patients after surgery. Asian Pac J Cancer Prev. (2014) 15:7097–103. 10.7314/apjcp.2014.15.17.7097 [DOI] [PubMed] [Google Scholar]
- 166.Xie S, Zhou F, Wang J, Cao H, Chen Y, Liu X, et al. Functional polymorphisms of ATP citrate lyase gene predicts clinical outcome of patients with advanced colorectal cancer. World J Surg Oncol. (2015) 13:42. 10.1186/s12957-015-0440-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zulato E, Bergamo F, De Paoli A, Griguolo G, Esposito G, De Salvo GL, et al. Prognostic significance of AMPK activation in advanced stage colorectal cancer treated with chemotherapy plus bevacizumab. Br J Cancer. (2014) 111:25–32. 10.1038/bjc.2014.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O'Malley J, Kumar R, Kuzmin AN, Pliss A, Yadav N, Balachandar S, et al. Lipid quantification by Raman microspectroscopy as a potential biomarker in prostate cancer. Cancer Lett. (2017) 397:52–60. 10.1016/j.canlet.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Merino Salvador M, Gómez de Cedrón M, Moreno Rubio J, Falagán Martínez S, Sánchez Martínez R, Casado E, et al. Lipid metabolism and lung cancer. Crit Rev Oncol Hematol. (2017) 112:31–40. 10.1016/j.critrevonc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 170.Cerne D, Zitnik IP, Sok M. Increased fatty acid synthase activity in non-small cell lung cancer tissue is a weaker predictor of shorter patient survival than increased lipoprotein lipase activity. Arch Med Res. (2010) 41:405–9. 10.1016/j.arcmed.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 171.Notarnicola M, Tutino V, Calvani M, Lorusso D, Guerra V, Caruso MG. Serum levels of fatty acid synthase in colorectal cancer patients are associated with tumor stage. J Gastrointest Cancer. (2012) 43:508–511. 10.1007/s12029-011-9300-2 [DOI] [PubMed] [Google Scholar]
- 172.Long Q, Yi Y, Qiu J, Xu C, Huang P. Fatty acid synthase (FASN) levels in serum of colorectal cancer patients: correlation with clinical outcomes. Tumour Biol. (2014) 35:3855–9. 10.1007/s13277-013-1510-8 [DOI] [PubMed] [Google Scholar]
- 173.Bian Y, Yu Y, Wang S, Li L. Up-regulation of fatty acid synthase induced by EGFR/ERK activation promotes tumor growth in pancreatic cancer. Biochem Biophys Res Commun. (2015) 463:612–7. 10.1016/j.bbrc.2015.05.108 [DOI] [PubMed] [Google Scholar]
- 174.Padanad MS, Konstantinidou G, Venkateswaran N, Melegari M, Rindhe S, Mitsche M, et al. Fatty acid oxidation mediated by Acyl-CoA synthetase long chain 3 is required for mutant kras lung tumorigenesis. Cell Rep. (2016) 16:1614–28. 10.1016/j.celrep.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang J, Scholtens D, Holko M, Ivancic D, Lee O, Hu H, et al. Lipid metabolism genes in contralateral unaffected breast and estrogen receptor status of breast cancer. Cancer Prev Res. (2013) 6:321–30. 10.1158/1940-6207.CAPR-12-0304 [DOI] [PubMed] [Google Scholar]
- 176.Obinata D, Takayama K, Fujiwara K, Suzuki T, Tsutsumi S, Fukuda N, et al. Targeting Oct1 genomic function inhibits androgen receptor signaling and castration-resistant prostate cancer growth. Oncogene. (2016) 35:6350–8. 10.1038/onc.2016.171 [DOI] [PubMed] [Google Scholar]
- 177.Xia H, Lee KW, Chen J, Kong SN, Sekar K, Deivasigamani A, et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov. (2017) 3:17058. 10.1038/cddiscovery.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tang Y, Zhou J, Hooi SC, Jiang Y-M, Lu G-D. Fatty acid activation in carcinogenesis and cancer development: essential roles of long-chain acyl-CoA synthetases. Oncol Lett. (2018) 16:1390–6. 10.3892/ol.2018.8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. (2009) 296:E1195–209. 10.1152/ajpendo.90958.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marchan R, Büttner B, Lambert J, Edlund K, Glaeser I, Blaszkewicz M, et al. Glycerol-3-phosphate acyltransferase 1 promotes tumor cell migration and poor survival in ovarian carcinoma. Cancer Res. (2017) 77:4589–601. 10.1158/0008-5472.CAN-16-2065 [DOI] [PubMed] [Google Scholar]
- 181.Niesporek S, Denkert C, Weichert W, Köbel M, Noske A, Sehouli J, et al. Expression of lysophosphatidic acid acyltransferase beta (LPAAT-beta) in ovarian carcinoma: correlation with tumour grading and prognosis. Br J Cancer. (2005) 92:1729–36. 10.1038/sj.bjc.6602528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Roe ND, Handzlik MK, Li T, Tian R. The role of diacylglycerol acyltransferase (DGAT) 1 and 2 in cardiac metabolism and function. Sci Rep. (2018) 8:4983. 10.1038/s41598-018-23223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xin C, Chu L, Zhang L, Geng D, Wang Y, Sun D, et al. Expression of cytosolic phospholipase A2 (cPLA2)-Arachidonic acid (AA)-Cyclooxygenase-2 (COX-2) pathway factors in lung cancer patients and its implication in lung cancer early detection and prognosis. Med Sci Monit. (2019) 25:5543–51. 10.12659/MSM.915314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Church RD, Yu J, Fleshman JW, Shannon WD, Govindan R, McLeod HL. RNA profiling of cyclooxygenases 1 and 2 in colorectal cancer. Br J Cancer. (2004) 91:1015–8. 10.1038/sj.bjc.6602119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tury S, Becette V, Assayag F, Vacher S, Benoist C, Kamal M, et al. Combination of COX-2 expression and PIK3CA mutation as prognostic and predictive markers for celecoxib treatment in breast cancer. Oncotarget. (2016) 7:85124–41. 10.18632/oncotarget.13200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Frank B, Hoffmeister M, Klopp N, Illig T, Chang-Claude J, Brenner H. Polymorphisms in inflammatory pathway genes and their association with colorectal cancer risk. Int J Cancer. (2010) 127:2822–30. 10.1002/ijc.25299 [DOI] [PubMed] [Google Scholar]
- 187.Coghill AE, Newcomb PA, Poole EM, Hutter CM, Makar KW, Duggan D, et al. Genetic variation in inflammatory pathways is related to colorectal cancer survival. Clin Cancer Res. (2011) 17:7139–47. 10.1158/1078-0432.CCR-11-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hao Y, Li D, Xu Y, Ouyang J, Wang Y, Zhang Y, et al. Investigation of lipid metabolism dysregulation and the effects on immune microenvironments in pan-cancer using multiple omics data. BMC Bioinform. (2019) 20:195. 10.1186/s12859-019-2734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.de Gonzalo-Calvo D, López-Vilaró L, Nasarre L, Perez-Olabarria M, Vázquez T, Escuin D, et al. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. (2015) 15:460. 10.1186/s12885-015-1469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brennan DJ, Laursen H, O'Connor DP, Borgquist S, Uhlen M, Gallagher WM, et al. Tumor-specific HMG-CoA reductase expression in primary premenopausal breast cancer predicts response to tamoxifen. Breast Cancer Res. (2011) 13:R12. 10.1186/bcr2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Butt S, Butt T, Jirström K, Hartman L, Amini R-M, Zhou W, et al. The target for statins, HMG-CoA reductase, is expressed in ductal carcinoma-in situ and may predict patient response to radiotherapy. Ann Surg Oncol. (2014) 21:2911–9. 10.1245/s10434-014-3708-4 [DOI] [PubMed] [Google Scholar]
- 192.Bjarnadottir O, Romero Q, Bendahl P-O, Jirström K, Rydén L, Loman N, et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat. (2013) 138:499–508. 10.1007/s10549-013-2473-6 [DOI] [PubMed] [Google Scholar]
- 193.Schimanski S, Wild PJ, Treeck O, Horn F, Sigruener A, Rudolph C, et al. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm Metab Res. (2010) 42:102–9. 10.1055/s-0029-1241859 [DOI] [PubMed] [Google Scholar]
- 194.Wang Y, Liu H, Ready NE, Su L, Wei Y, Christiani DC, et al. Genetic variants in ABCG1 are associated with survival of nonsmall-cell lung cancer patients. Int J Cancer. (2016) 138:2592–601. 10.1002/ijc.29991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhou T, Zhan J, Fang W, Zhao Y, Yang Y, Hou X, et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC). BMC Cancer. (2017) 17:269. 10.1186/s12885-017-3239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, et al. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. (2015) 36:4133–41. 10.1007/s13277-015-3047-5 [DOI] [PubMed] [Google Scholar]
- 197.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications – a review. Nutr J. (2014) 13:17. 10.1186/1475-2891-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shao W, Kuhn C, Mayr D, Ditsch N, Kailuwait M, Wolf V, et al. Cytoplasmic PPARγ is a marker of poor prognosis in patients with Cox-1 negative primary breast cancers. J Transl Med. (2020) 18:94. 10.1186/s12967-020-02271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liang X, Fan X, Tan K, Zhang L, Jian L, Yu L. Peroxisome proliferators-activated receptor gamma polymorphisms and colorectal cancer risk. J Cancer Res Ther. (2018) 14:S306–10. 10.4103/0973-1482.235346 [DOI] [PubMed] [Google Scholar]
- 200.Sebio A, Gerger A, Matsusaka S, Yang D, Zhang W, Stremitzer S, et al. Genetic variants within obesity-related genes are associated with tumor recurrence in patients with stages II/III colon cancer. Pharmacogenet Genomics. (2015) 25:30–7. 10.1097/FPC.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bauerschlag DO, Maass N, Leonhardt P, Verburg FA, Pecks U, Zeppernick F, et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J Transl Med. (2015) 13:146. 10.1186/s12967-015-0511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Malvi P, Chaube B, Pandey V, Vijayakumar MV, Boreddy PR, Mohammad N, et al. Obesity induced rapid melanoma progression is reversed by orlistat treatment and dietary intervention: role of adipokines. Mol Oncol. (2015) 9:689–703. 10.1016/j.molonc.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Konkel B, Caflisch L, Diaz Duque AE, Brenner A. Updated results from a prospective, randomized phase 2 study in patients with first relapse of high-grade astrocytoma using TVB-2640 in combination with avastin versus avastin alone. NeuroOncology. (2018) 20:vi16 10.1093/NEUONC/NOY148.058 [DOI] [Google Scholar]
- 204.Dean EJ, Falchook GS, Patel MR, Brenner AJ, Infante JR, Arkenau HT, et al. Preliminary activity in the first in human study of the first-in-class fatty acid synthase (FASN) inhibitor, TVB-2640. J Clin Oncol. (2017) 34:2512 10.1200/JCO.2016.34.15_suppl.2512 [DOI] [Google Scholar]
- 205.Luo J, Hong Y, Lu Y, Qiu S, Chaganty BK, Zhang L, et al. Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the warburg effect by cetuximab. Cancer Lett. (2017) 384:39–49. 10.1016/j.canlet.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. (2016) 22:1108–19. 10.1038/nm.4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Koltun DO, Zilbershtein TM, Migulin VA, Vasilevich NI, Parkhill EQ, Glushkov AI, et al. Potent, orally bioavailable, liver-selective stearoyl-CoA desaturase (SCD) inhibitors. Bioorg Med Chem Lett. (2009) 19:4070–4. 10.1016/j.bmcl.2009.06.017 [DOI] [PubMed] [Google Scholar]
- 208.Ma MKF, Lau EYT, Leung DHW, Lo J, Ho NPY, Cheng LKW, et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J Hepatol. (2017) 67:979–90. 10.1016/j.jhep.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 209.Potze L, di Franco S, Kessler JH, Stassi G, Medema JP. Betulinic acid kills colon cancer stem cells. Curr Stem Cell Res Ther. (2016) 11:427–33. 10.1038/onc.2015.102 [DOI] [PubMed] [Google Scholar]
- 210.Wang H, Dong F, Wang Y, Wang X, Hong D, Liu Y, et al. Betulinic acid induces apoptosis of gallbladder cancer cells via repressing SCD1. Acta Biochim Biophys Sin. (2020) 52:200–6. 10.1093/abbs/gmz148 [DOI] [PubMed] [Google Scholar]
- 211.Pisanu ME, Noto A, De Vitis C, Morrone S, Scognamiglio G, Botti G, et al. Blockade of stearoyl-CoA-desaturase 1 activity reverts resistance to cisplatin in lung cancer stem cells. Cancer Lett. (2017) 406:93–104. 10.1016/j.canlet.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 212.Noto A, Raffa S, De Vitis C, Roscilli G, Malpicci D, Coluccia P, et al. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. (2013) 4:e947. 10.1038/cddis.2013.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, et al. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. (2013) 19:2368–80. 10.1158/1078-0432.CCR-12-3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. (2010) 120:142–56. 10.1172/JCI38942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schlaepfer IR, Rider L, Rodrigues LU, Gijon MA, Pac CT, Romero L, et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. (2014) 13:2361–71. 10.1158/1535-7163.MCT-14-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lin H, Patel S, Affleck VS, Wilson I, Turnbull DM, Joshi AR, et al. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro Oncol. (2017) 19:43–54. 10.1093/neuonc/now128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Flaig TW, Salzmann-Sullivan M, Su LJ, Zhang Z, Joshi M, Gijon MA, et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget. (2017) 8:56051–65. 10.18632/oncotarget.17359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu PP, Liu J, Jiang WQ, Carew JS, Ogasawara MA, Pelicano H, et al. Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline. Oncogene. (2016) 35:5663–73. 10.1038/onc.2016.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ren XR, Wang J, Osada T, Mook RA, Jr, Morse MA, Barak LS, et al. Perhexiline promotes HER3 ablation through receptor internalization and inhibits tumor growth. Breast Cancer Res. (2015) 17:20. 10.1186/s13058-015-0528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. (2016) 22:427–32. 10.1038/nm.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li N, Zhou ZS, Shen Y, Xu J, Miao HH, Xiong Y, et al. Inhibition of the sterol regulatory element-binding protein pathway suppresses hepatocellular carcinoma by repressing inflammation in mice. Hepatology. (2017) 65:1936–47. 10.1002/hep.29018 [DOI] [PubMed] [Google Scholar]
- 222.Soica C, Dehelean C, Danciu C, Wang HM, Wenz G, Ambrus R, et al. Betulin complex in gamma-cyclodextrin derivatives: properties and antineoplasic activities in in vitro and in vivo tumor models. Int J Mol Sci. (2012) 13:14992–5011. 10.3390/ijms131114992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li X, Wu JB, Chung LW, Huang WC. Anti-cancer efficacy of SREBP inhibitor, alone or in combination with docetaxel, in prostate cancer harboring p53 mutations. Oncotarget. (2015) 6:41018–32. 10.18632/oncotarget.5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. (2014) 13:855–66. 10.1158/1535-7163.MCT-13-0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Williams KJ, Argus JP, Zhu Y, Wilks MQ, Marbois BN, York AG, et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res. (2013) 73:2850–62. 10.1158/0008-5472.CAN-13-0382-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y, et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol. (2009) 16:882–92. 10.1016/j.chembiol.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 227.Ceroi A, Masson D, Roggy A, Roumier C, Chague C, Gauthier T, et al. LXR agonist treatment of blastic plasmacytoid dendritic cell neoplasm restores cholesterol efflux and triggers apoptosis. Blood. (2016) 128:2694–707. 10.1182/blood-2016-06-724807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nguyen TTT, Ishida CT, Shang E, Shu C, Torrini C, Zhang Y, et al. Activation of LXRbeta inhibits tumor respiration and is synthetically lethal with Bcl-xL inhibition. EMBO Mol Med. (2019) 11:e10769. 10.15252/emmm.201910769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. (2011) 1:442–56. 10.1158/2159-8290.CD-11-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee SS, Li J, Tai JN, Ratliff TL, Park K, Cheng JX. Avasimibe encapsulated in human serum albumin blocks cholesterol esterification for selective cancer treatment. ACS Nano. (2015) 9:2420–32. 10.1021/nn504025a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liu JY, Fu WQ, Zheng XJ, Li W, Ren LW, Wang JH, et al. Avasimibe exerts anticancer effects on human glioblastoma cells via inducing cell apoptosis and cell cycle arrest. Acta Pharmacol Sin. (2020). 10.1038/s41401-020-0404-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bandyopadhyay S, Li J, Traer E, Tyner JW, Zhou A, Oh ST, et al. Cholesterol esterification inhibition and imatinib treatment synergistically inhibit growth of BCR-ABL mutation-independent resistant chronic myelogenous leukemia. PLoS ONE. (2017) 12:e0179558. 10.1371/journal.pone.0179558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Watt MJ, Clark AK, Selth LA, Haynes VR, Lister N, Rebello R, et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Transl Med. (2019) 11:eaau5758. 10.1126/scitranslmed.aau5758 [DOI] [PubMed] [Google Scholar]
- 234.Longo J, Hamilton RJ, Masoomian M, Khurram N, Branchard E, Mullen PJ, et al. A pilot window-of-opportunity study of preoperative fluvastatin in localized prostate cancer. Prostate Cancer Prostatic Dis. (2020). 10.1038/s41391-020-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Singh PP, Lemanu DP, Soop M, Bissett IP, Harrison J, Hill AG. Perioperative simvastatin therapy in major colorectal surgery: a prospective, double-blind randomized controlled trial. J Am Coll Surg. (2016) 223:308–20 e1. 10.1016/j.jamcollsurg.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 236.Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T, et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. (2011) 17:1553–60. 10.1158/1078-0432.CCR-10-2525 [DOI] [PubMed] [Google Scholar]
- 237.Pagano E, Borrelli F, Orlando P, Romano B, Monti M, Morbidelli L, et al. Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol Res. (2017) 119:227–36. 10.1016/j.phrs.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 238.Liu R, Zheng H, Li W, Guo Q, He S, Hirasaki Y, et al. Anti-tumor enhancement of Fei-Liu-Ping ointment in combination with celecoxib via cyclooxygenase-2-mediated lung metastatic inflammatory microenvironment in Lewis lung carcinoma xenograft mouse model. J Transl Med. (2015) 13:366. 10.1186/s12967-015-0728-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Suri A, Sheng X, Schuler KM, Zhong Y, Han X, Jones HM, et al. The effect of celecoxib on tumor growth in ovarian cancer cells and a genetically engineered mouse model of serous ovarian cancer. Oncotarget. (2016) 7:39582–94. 10.18632/oncotarget.8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Marks EI, Yee NS. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J Gastroenterol. (2016) 22:1335–47. 10.3748/wjg.v22.i4.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang Y, Zhu M, Yuan B, Zhang K, Zhong M, Yi W, et al. VSP-17, a new ppargamma agonist, suppresses the metastasis of triple-negative breast cancer via upregulating the expression of E-Cadherin. Molecules. (2018) 23:121. 10.3390/molecules23010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Laouirem S, Sannier A, Norkowski E, Cauchy F, Doblas S, Rautou PE, et al. Endothelial fatty liver binding protein 4: a new targetable mediator in hepatocellular carcinoma related to metabolic syndrome. Oncogene. (2019) 38:3033–3046. 10.1038/s41388-018-0597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Huang M, Narita S, Inoue T, Koizumi A, Saito M, Tsuruta H, et al. Fatty acid binding protein 4 enhances prostate cancer progression by upregulating matrix metalloproteinases and stromal cell cytokine production. Oncotarget. (2017) 8:111780–94. 10.18632/oncotarget.22908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Al-Jameel W, Gou X, Forootan SS, Al Fayi MS, Rudland PS, Forootan FS, et al. Inhibitor SBFI26 suppresses the malignant progression of castration-resistant PC3-M cells by competitively binding to oncogenic FABP5. Oncotarget. (2017) 8:31041–56. 10.18632/oncotarget.16055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kohlmeier M, De Caterina R, Ferguson LR, Görman U, Allayee H, Prasad C, et al. Guide and position of the International Society of Nutrigenetics/Nutrigenomics on personalized nutrition: part 2 - ethics, challenges and endeavors of precision nutrition. J Nutrigenet Nutrigenomics. (2016) 9:28–46. 10.1159/000446347 [DOI] [PubMed] [Google Scholar]
- 246.Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. (2017) 17:79–92. 10.1038/nrc.2016.126 [DOI] [PubMed] [Google Scholar]
- 247.Ramos-Lopez O, Roman S, Martinez-Lopez E, Fierro NA, Gonzalez-Aldaco K, Jose-Abrego A, et al. CD36 genetic variation, fat intake and liver fibrosis in chronic hepatitis C virus infection. World J Hepatol. (2016) 8:1067–74. 10.4254/wjh.v8.i25.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Carpene C, Gomez-Zorita S, Deleruyelle S, Carpene MA. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr Med Chem. (2015) 22:150–64. 10.2174/0929867321666140815124052 [DOI] [PubMed] [Google Scholar]
- 249.Farhat G, Drummond S, Al-Dujaili EAS. Polyphenols and their role in obesity management: a systematic review of randomized clinical trials. Phytother Res. (2017) 31:1005–18. 10.1002/ptr.5830 [DOI] [PubMed] [Google Scholar]
- 250.Novotny JA, Chen T-Y, Terekhov AI, Gebauer SK, Baer DJ, Ho L, et al. The effect of obesity and repeated exposure on pharmacokinetic response to grape polyphenols in humans. Mol Nutr Food Res. (2017) 61:11. 10.1002/mnfr.201700043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jiao X, Wang Y, Lin Y, Lang Y, Li E, Zhang X, et al. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem. (2019) 64:88–100. 10.1016/j.jnutbio.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 252.Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. (2012) 67:3–10. 10.1093/gerona/glr167 [DOI] [PubMed] [Google Scholar]
- 253.Lopez-Moreno J, Quintana-Navarro GM, Delgado-Lista J, Garcia-Rios A, Alcala-Diaz JF, Gomez-Delgado F, et al. mediterranean diet supplemented with coenzyme q10 modulates the postprandial metabolism of advanced glycation end products in elderly men and women. J Gerontol A Biol Sci Med Sci. (2018) 73:340–6. 10.1093/gerona/glw214 [DOI] [PubMed] [Google Scholar]
- 254.Bayerdörffer E, Mannes GA, Richter WO, Ochsenkühn T, Seeholzer G, Köpcke W, et al. Decreased high-density lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. Ann Intern Med. (1993) 118:481–7. 10.7326/0003-4819-118-7-199304010-00001 [DOI] [PubMed] [Google Scholar]
- 255.Morad SAF, Madigan JP, Levin JC, Abdelmageed N, Karimi R, Rosenberg DW, et al. Tamoxifen magnifies therapeutic impact of ceramide in human colorectal cancer cells independent of p53. Biochem Pharmacol. (2013) 85:1057–65. 10.1016/j.bcp.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Varela LM, Ortega-Gomez A, Lopez S, Abia R, Muriana FJG, Bermudez B. The effects of dietary fatty acids on the postprandial triglyceride-rich lipoprotein/apoB48 receptor axis in human monocyte/macrophage cells. J Nutr Biochem. (2013) 24:2031–9. 10.1016/j.jnutbio.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 257.Huerta AE, Prieto-Hontoria PL, Fernández-Galilea M, Escoté X, Martínez JA, Moreno-Aliaga MJ. Effects of dietary supplementation with EPA and/or α-lipoic acid on adipose tissue transcriptomic profile of healthy overweight/obese women following a hypocaloric diet. Biofactors. (2017) 43:117–31. 10.1002/biof.1317 [DOI] [PubMed] [Google Scholar]
- 258.Boschmann M, Thielecke F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. J Am Coll Nutr. (2007) 26:389S–95S. 10.1080/07315724.2007.10719627 [DOI] [PubMed] [Google Scholar]
- 259.Cardoso GA, Salgado JM, Cesar Mde C, Donado-Pestana CM. The effects of green tea consumption and resistance training on body composition and resting metabolic rate in overweight or obese women. J Med Food. (2013) 16:120–7. 10.1089/jmf.2012.0062 [DOI] [PubMed] [Google Scholar]
- 260.Chen IJ, Liu CY, Chiu JP, Hsu CH. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. (2016) 35:592–9. 10.1016/j.clnu.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 261.Santamarina AB, Carvalho-Silva M, Gomes LM, Okuda MH, Santana AA, Streck EL, et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J Nutr Biochem. (2015) 26:1348–56. 10.1016/j.jnutbio.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 262.Huang J, Feng S, Liu A, Dai Z, Wang H, Reuhl K, et al. Green tea polyphenol EGCG alleviates metabolic abnormality and fatty liver by decreasing bile acid and lipid absorption in mice. Mol Nutr Food Res. (2018) 62 10.1002/mnfr.201700696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Doan KV, Ko CM, Kinyua AW, Yang DJ, Choi YH, Oh IY, et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology. (2015) 156:157–68. 10.1210/en.2014-1354 [DOI] [PubMed] [Google Scholar]
- 264.Huang DW, Chang WC, Yang HJ, Wu JS, Shen SC. Gallic acid alleviates hypertriglyceridemia and fat accumulation via modulating glycolysis and lipolysis pathways in perirenal adipose tissues of rats fed a high-fructose diet. Int J Mol Sci. (2018) 19:254. 10.3390/ijms19010254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Quezada-Fernandez P, Trujillo-Quiros J, Pascoe-Gonzalez S, Trujillo-Rangel WA, Cardona-Muller D, Ramos-Becerra CG, et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled trial. Int J Food Sci Nutr. (2019) 70:977–85. 10.1080/09637486.2019.1589430 [DOI] [PubMed] [Google Scholar]
- 266.Most J, Timmers S, Warnke I, Jocken JW, van Boekschoten M, de Groot P, et al. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. Am J Clin Nutr. (2016) 104:215–27. 10.3945/ajcn.115.122937 [DOI] [PubMed] [Google Scholar]
- 267.Mellor DD, Sathyapalan T, Kilpatrick ES, Beckett S, Atkin SL. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet Med. (2010) 27:1318–21. 10.1111/j.1464-5491.2010.03108.x [DOI] [PubMed] [Google Scholar]
- 268.Lee YS, Cha BY, Choi SS, Choi BK, Yonezawa T, Teruya T, et al. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J Nutr Biochem. (2013) 24:156–62. 10.1016/j.jnutbio.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 269.Huong DT, Takahashi Y, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition. (2006) 22:546–52. 10.1016/j.nut.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 270.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, et al. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. (2009) 58:2198–210. 10.2337/db09-0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cho KW, Kim YO, Andrade JE, Burgess JR, Kim YC. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor alpha protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr. (2011) 50:81–8. 10.1007/s00394-010-0117-8 [DOI] [PubMed] [Google Scholar]
- 272.Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ, et al. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. (2011) 55:963–71. 10.1016/j.jhep.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ren B, Qin W, Wu F, Wang S, Pan C, Wang L, et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol. (2016) 773:13–23. 10.1016/j.ejphar.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 274.Yang MY, Chan KC, Lee YJ, Chang XZ, Wu CH, Wang CJ. Sechium edule shoot extracts and active components improve obesity and a fatty liver that involved reducing hepatic lipogenesis and adipogenesis in high-fat-diet-fed rats. J Agric Food Chem. (2015) 63:4587–96. 10.1021/acs.jafc.5b00346 [DOI] [PubMed] [Google Scholar]
- 275.Zhang J, Zhang H, Deng X, Zhang N, Liu B, Xin S, et al. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice. Life Sci. (2018) 192:46–54. 10.1016/j.lfs.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 276.Guo HX, Liu DH, Ma Y, Liu JF, Wang Y, Du ZY, et al. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. (2009) 30:1505–12. 10.1038/aps.2009.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Han M, Gao H, Ju P, Gao MQ, Yuan YP, Chen XH, et al. Hispidulin inhibits hepatocellular carcinoma growth and metastasis through AMPK and ERK signaling mediated activation of PPARgamma. Biomed Pharmacother. (2018) 103:272–83. 10.1016/j.biopha.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 278.Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang X, et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: a randomized controlled trial. Pharmacol Res. (2015) 99:74–81. 10.1016/j.phrs.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 279.Chang X, Wang Z, Zhang J, Yan H, Bian H, Xia M, et al. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. J Transl Med. (2016) 14:266. 10.1186/s12967-016-0982-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kasala ER, Bodduluru LN, Barua CC, Gogoi R. Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed Pharmacother. (2016) 82:568–77. 10.1016/j.biopha.2016.05.042 [DOI] [PubMed] [Google Scholar]
- 281.Chen S, Jiang H, Wu X, Fang J. therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators Inflamm. (2016) 2016:9340637. 10.1155/2016/9340637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Porras D, Nistal E, Martinez-Florez S, Pisonero-Vaquero S, Olcoz JL, Jover R, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. (2017) 102:188–202. 10.1016/j.freeradbiomed.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 283.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. (2008) 29:698–706. 10.1111/j.1745-7254.2008.00807.x [DOI] [PubMed] [Google Scholar]
- 284.Aguirre L, Portillo MP, Hijona E, Bujanda L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J Gastroenterol. (2014) 20:7366–80. 10.3748/wjg.v20.i23.7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Andrade JM, Paraiso AF, de Oliveira MV, Martins AM, Neto JF, Guimaraes AL, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. (2014) 30:915–9. 10.1016/j.nut.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 286.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. (2009) 139:919–25. 10.3945/jn.108.100966 [DOI] [PubMed] [Google Scholar]
- 287.Colacino JA, McDermott SP, Sartor MA, Wicha MS, Rozek LS. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res Treat. (2016) 158:29–41. 10.1007/s10549-016-3854-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Polce SA, Burke C, Franca LM, Kramer B, de Andrade Paes AM, Carrillo-Sepulveda MA. Ellagic acid alleviates hepatic oxidative stress and insulin resistance in diabetic female rats. Nutrients. (2018) 10:531. 10.3390/nu10050531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Moore J, Yousef M, Tsiani E. Anticancer effects of rosemary (rosmarinus officinalis l.) extract and rosemary extract polyphenols. Nutrients. (2016) 8:731. 10.3390/nu8110731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Naimi M, Vlavcheski F, Shamshoum H, Tsiani E. Rosemary extract as a potential anti-hyperglycemic agent: current evidence and future perspectives. Nutrients. (2017) 9:968. 10.3390/nu9090968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lodi A, Saha A, Lu X, Wang B, Sentandreu E, Collins M, et al. Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ Precis Oncol. (2017) 1:1–12. 10.1038/s41698-017-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Park KH, Shin HJ, Song YB, Hyun HC, Cho HJ, Ham HS, et al. Possible role of ginsenoside Rb1 on regulation of rat liver triglycerides. Biol Pharm Bull. (2002) 25:457–60. 10.1248/bpb.25.457 [DOI] [PubMed] [Google Scholar]
- 293.Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, et al. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. (2013) 54:1430–8. 10.1194/jlr.M035907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Qiao L, Zhang X, Liu M, Liu X, Dong M, Cheng J, et al. Ginsenoside Rb1 enhances atherosclerotic plaque stability by improving autophagy and lipid metabolism in macrophage foam cells. Front Pharmacol. (2017) 8:727 10.3389/fphar.2017.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Colman-Martinez M, Martinez-Huelamo M, Valderas-Martinez P, Arranz-Martinez S, Almanza-Aguilera E, Corella D, et al. Trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: an intervention trial. Mol Nutr Food Res. (2017) 61. 10.1002/mnfr.201600993 [DOI] [PubMed] [Google Scholar]
- 296.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. (2015) 22:86–99. 10.1016/j.cmet.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]