Abstract
The most common cause of mortality due to malignant neoplasms in the general population around the world is lung cancer. In the last 10 years, there has been an enormous improvement in the treatment of this disease, mainly due to the immunotherapy that activates the immune system to fight cancer. Patients with metastatic non-small cell lung cancer are a special group of patients requiring not only cancer treatment but also considerable support in the treatment of cancer-related problems, as well as comorbidities. Early palliative care is important in this area. In addition, there is certain evidence that medicines most commonly administered in palliative care may lower the efficacy of immunotherapy. The present review article compares information on the prolonging of life after early hospice care, which has become the foundation of current standards of management in patients with metastatic lung cancer, and reports of decreased efficacy of the immunotherapy due to the administration of major palliative care medications.
Keywords: lung cancer, immunotherapy, early palliative care, opioids, glucorticosterois
1. Introduction
Lung cancer is the main cause of death due to cancer in men and women in the world (1,2). In 50–60% of the cases, the disease is diagnosed at the dissemination stage, and a 5-year survival rate is observed in only 6% of the patients (1). Characteristic for this group of patients is the presence of other tabacco-related diseases which are often the cause of numerous problems just like the cancer is. Frequently, it results in a more aggressive treatment at the end of life (3). Several articles have shown that the early palliative care (EPC) along with chemotherapy has beneficial effects not only on the quality of life of the patients, reducing the symptoms of depression, but also it reduces the number of intensive (not always necessary) medical procedures at the end of life. However, what is most important, EPC prolongs life (3–6). Palliative care consisting in the management of the cancer-related symptoms and psychological support is a key element in the treatment of patients with metastatic non-small cell lung cancer (NSCLC). Therefore, the palliative care physician becomes an important member of the multidisciplinary team deciding on the therapy management. The guidelines of international scientific societies emphasize the importance of the implementation of palliative care immediately after metastatic lung cancer was diagnosed (7,8).
The systemic treatment of metastatic lung cancer has changed significantly in the last five years. Presently, apart from chemotherapy and tyrosine kinase inhibitors, monoclonal antibodies directed against programmed cell death protein 1 (PD-1), nivolumab, pembrolizumab or its ligand (programmed cell death protein ligand 1, PDL-1) atezolizumab, which are immune-checkpoint inhibitors, are used (7,8). The immunotherapy has significantly improved prognosis of patients with metastatic lung cancer without epidermal growth factor receptor activating mutations and anaplastic lymphoma kinase translocation (9–26). The administration of these drugs in a group of patients in good general condition (performance status according to the Eastern Cooperative Oncology Group, ECOG 0–1) in the first- or second-line palliative systemic care is constantly increasing. It is also the group of patients that should be provided with EPC.
Studies on maintaining early palliative and palliative systemic care in parallel were undertaken in the last 10 years when the immunotherapy was not a standard practice. Despite changes in the guidelines, the recommendations concerning the implementation of EPC were maintained. However, is there any proof of evidence based medicine which confirms the justice concurrent use of the immunotherapy and EPC?
2. Chemotherapy and early palliative care
The turning point for the integration of EPC with cancer treatment in patients with metastatic lung cancer has become the study by Temel et al (3). The study randomized patients to either standard oncological care (exclusive cancer treatment) or the standard oncological care combined with early hospice care implemented eight weeks after the diagnosis of cancer at the latest. Palliative care included education of the patient and the family about the disease and prognosis and treatment of symptoms with a particular emphasis on pain, pulmonary symptoms (mainly cough and shortness of breath), fatigue, sleep disorders, mood changes (depression and anxiety), lack of appetite or nausea and vomiting, as well as patients participation in decisions concerning oncological treatment (27). The study showed that in week 12 of observation the patients randomized to EPC had a better quality of life than patients randomized to exclusive cancer treatment. The functional assessment of cancer therapy-lung which has the results from 0 to 136 showed a mean value of 98.0 compared to 91.5 in favor of EPC, and the difference was significant (P=0.03). The improvement in the quality of life observed in the study was similar to that observed in clinical trials which assessed the benefit of cisplatin-based chemotherapy. In patients undergoing EPC, the symptoms of depression were observed less frequently (16 vs. 38%, P=0.01). What is more, aggressive medical procedures at end of life were also less frequently used in this group (33 vs. 54%, P=0.05). Despite this, the median overall survival (OS) rate was significantly higher (11.6 vs. 8.9 months, P=0.02) (3). After the publication of that study, the American Society of Clinical Oncology (ASCO) established EPC as a standard in metastatic lung cancer (28).
Similar observations were obtained by other studies where the authors pointed to the benefit of implementing integrated oncological and palliative care (4–6) (Table I). Also, Temel et al (6) continued the study on EPC, extending their observations in other groups of patients. One study at week 24 of observation demonstrated an improvement in the quality of life and reduced percentage of depression among patients and, additionally, noted patients improved ability to cope with information about the prognosis. Another study also analyzed the ability of patients to speak with the clinicians about their end-of-life care (EOL-care) preferences (6). However, after week 12 a similar proportion of patients in both groups reported that the main goal of treatment was healing (28.7 vs. 34.5%, P=0.289), but patients in the early hospice intervention significantly more often declared that information on their prognosis was ‘very helpful’ or ‘extremely helpful’ in therapy decision-making (96.5 vs. 89.8%, P=0.043) and in coping with the disease (97.3 vs. 83.6%, P<0.001). After week 24, significantly more patients discussed their EOL-care requests with oncologists (30.2 vs. 14.5%, P=0.004). The results of the studies by Temel indicate that, apart from the management of cancer-related symptoms, other elements of EPC are equally important (6).
Table I.
Benefits of implementing integrated oncological and palliative care: Summary of the most important studies.
| First author, year | Type of study | Aim of study | Patients with lung cancer, n | Primary outcome measures | OS for the EPC group | Type of questionnaire to assess QoL | EPC group | QoL for the (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Temel, 2010 | Prospective, non-blinded, randomized, controlled trial | To compare two groups: Standard oncological treatment with palliative care given towards the end-of-life and standard oncological care integrated with EPC which is given soon after diagnosis | 151 | To assess the impact of early integration with palliative care on QoL in patients with advanced NSCL | Improved (P=0.02; HR 0.30) | FACT-L LCS, TOI | Improved (statistically significant) | (3) |
| Zimmermann, 2016 | Prospective, cluster-randomized controlled trial | To determine whether, compared with conventional cancer care, early involvement by a specialized symptom control and palliative care team in patients with advanced cancer will be associated with better quality of life, greater patient and caregiver satisfaction with care, better symptom control, improved communication with healthcare providers and improved caregiver quality of life | 101 | To assess Patient Heath Related Quality of Life | Improved (considering only patients with lung cancer) | FACIT-Sp, QUAL-E | Improved (statistically not significant) | (4) |
| King, 2016 | Retrospective | To compare outcomes from the EPC clinic with eligible patients treated by any other oncologist without the involvement of palliative care as the SC arm | 207 | Improved (P=0.032; HR 0.72) | Not used | Not measured | (5) | |
| Temel, 2017 | Prospective, non-blinded, randomized trial | EPC integrated with oncology care compared with usual oncology care | 191 | To assess changes in QoL | Not measured | FACT-G, PHQ-9, HADS | Improved (statistically improved) | (6) |
EPC, early palliative care; FACIT-Sp, The Functional Assessment of Chronic Illness; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-L, Functional Assessment of Cancer Therapy-Lung; LCS, lung-cancer subscale; QUAL-E, The Quality of Life at the End of Life; OS, overall survival; PHQ-9, Patient Health Questionnaire-9; SC, standard care; TOI, trial outcome index; HR, hazard ratio; HADS, Hospital Anxiety and Depression scale.
3. Mechanism of action of early palliative care
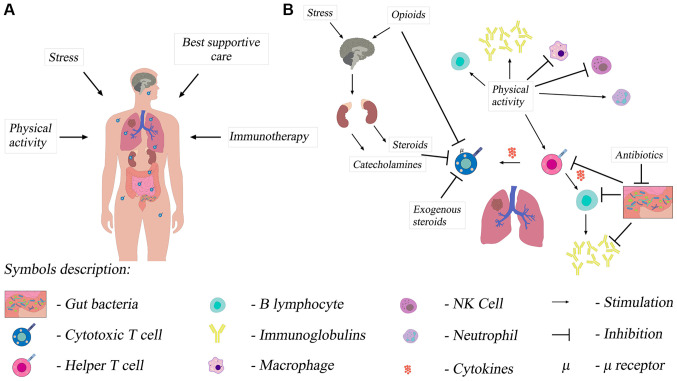
EPC is multidirectional and uses different mechanisms (29). The first mechanism is the biological mechanism, i.e. potentially beneficial anticancer action of the immune system (Fig. 1A and B). The improvement of the quality of life reduces chronic stress, which may improve the function of the immune system (Fig. 1A and B). High levels of chronic stress have been shown to be associated with higher baseline serum cortisol levels in the morning and reduced adaptive immune resistance. This results from the change in the T helper lymphocyte profile. T lymphocytes with the changed profile reduce the antitumor immunity. Patients with the improved quality of life showed the reduction of the cortisol level, which suggests a possible improvement in the function of the immune system (29).
Figure 1.
Influence of multiple factors on the immune system of patients with non-small-cell lung cancer. (A) The functioning of the immune system, including T lymphocytes, and the effectiveness of immunotherapy in a patient with non-small cell lung cancer depends on numerous factors. (B) The immunosuppressive effects of stress and opioids are based on a central mechanism of action. Opioids can also directly inhibit T lymphocytes. Additionally, antibiotic-induced dysbiosis adversely affects the immune system. Exercise slows down the inflammatory response of the immune system and increases the antitumor activity of immune cells. NK cell, natural killer cell.
Another important mechanism of action is the reduction of the symptoms of depression by early implementation of multidirectional palliative care. Patients with mild symptoms of depression are more likely to be physically active, which has a positive effect on general well-being (Fig. 1B). They also notice, more frequently and faster, the development of disturbing symptoms, both related to the disease and to cancer treatment, which may also be the variables that affect the survival rate. This additional information helps the clinician understand better the patient and undertake appropriate treatment (29).
Comprehensive EPC mechanisms may extend the survival rate through treatment of comorbidities (29). Understanding the prognosis by patients is also important. The Surveillance, Epidemiology, and End Results data indicates that chemotherapy undertaken in the last two weeks of life in patients over 65 years of age does not extend the survival rate, and the toxic effect of cytostatic agents may shorten the survival rate (30). If chemotherapy is stopped at the appropriate time, it may paradoxically prolong the patients life.
A palliative care practitioner can also implement an early and adequate treatment of adverse events (AE) of chemotherapy, which allows conducting chemotherapy in full doses and in the regular rhythm, and, thus, it can have a favorable effect on the patients survival rate (29).
It seems that all these elements have an additive effect, and their total effect is to extend the survival rate.
4. Immunotherapy in non-small cell lung cancer
The immunotherapy with the use of the immune-checkpoint inhibitors mentioned earlier, anti-PD1 and anti-PDL1 monoclonal antibodies, has become a breakthrough in the treatment of patients with advanced NSCLC, significantly changing the prognosis of such patients. It can now be used both in the first- and second-line therapy, after the failure of chemotherapy. Everyday clinical practice uses antibodies: pembrolizumab, nivolumab (anti-PD1 antibodies), atezolizumab (anti-PDL1 antibody) and ipilimumab [anti-T-lymphocyte-associated antigen 4 (CTLA4) antibody] (9–26).
In the first line of palliative systemic treatment of metastatic non-small cell lung cancer the immunotherapy can be used alone or combined with other drugs. Treatment options include pembrolizumab monotherapy if PDL-1 expression is strong (≥50%) (9,10), and regardless of PDL-1 expression, combination of nivolumab with ipilimumab in patients with high tumor mutation burden (TMB) (14,15), combination of chemotherapy with pembrolizumab (12) or combination of chemotherapy with atezolizumab and bevacizmumab [anti-vascular endothelial growth factor (VEGF) antibody] (17). All these schemes have been shown to affect OS and progression-free survival (PFS) (details in Table II). The overall response rate (ORR) evaluated by RECIST criteria were also higher in the group of patients receiving immunotherapy. The toxicity of the treatment clearly favors regimens with immunotherapy, in particular when monotherapy with pembrolizumab is used. The only exception is the combination of nivolumab and ipilimumab, where the toxicity of the treatment was slightly higher than in the case of nivolumab monotherapy (details in Table II). All the above regimens (except combination of chemotherapy with anti-PDL1 and anti-VEGF antibodies) also had a positive impact on the quality of life of patients, which is extremely important as it is one of the most important goals of palliative treatment (11,13,16,18).
Table II.
Summary of the crucial clinical trials which are the basis of the guidelines.
| Name of the trial | Expression of PD-L1 | Histology | Stage | Aim of the study | Median OS (IT vs. ChT) | Median PFS (IT vs. ChT) | ORR (IT vs. ChT) | AE (G3-G4) (IT vs. ChT) | QoL for IT | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|
| First-line therapy | ||||||||||
| KEYNOTE 024 | ≥50% | SCC and NSCC | IV | To compare pembrolizumab (at a fixed dose of 200 mg every 3 weeks) vs. the investigator's choice of platinum-based chemotherapy | 30 (95% CI, 18.3 months to NR) vs. 14.2 (95% CI, 9.8–19.0) months (HR = 0.63; 95% CI 0.47–0.86 months) | 10.3 (95% CI, 6.7 to not reached) vs. 6.0 months (95% CI, 4.2 to 6.2) | 45.5 vs. 29.8 % | G3-G5 31.2 vs. 53.3% | Improved (statistically significant) | (9–11) |
| KEYNOTE 189 | Benefit regardless of it | SCC and NSCC | IV | To compare pemetrexed and a platinum-based drug plus either 200 mg of pembrolizumab or placebo every 3 weeks for 4 cycles, followed by pembrolizumab or placebo for up to a total of 35 cycles plus pemetrexed maintenance therapy | 22.0 (19.5–25.2) vs. 10.7 (8.7–13.6) months (HR=0.56; 95% CI, 0.45–0.70) | 9.0 (8.1–9.9) vs. 4.9 (8.1–9.9) months (HR, 0.49; 95% CI, 0.40–0.59) | 48.0 vs. 19.4% | G3-G5 71.9 vs. 66.8% | Improved (statistically significant) | (12,13) |
| CheckMate 227 IT - ipi +nivo | Benefit regardless of it | SCC and NSCC | IV | To compare nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy alone | 17.1 (15.0–20.1) vs. 14.9 (12.7–16.7) months (HR=0.79; 97.72% CI, 0.65–0.96, P=0.007) | 7.2 (patients with high TMB) (95% CI, 5.5–13.2) vs. 5.5 months (95% CI, 4.4–5.8) (HR 0.58; 97.5% CI, 0.41–0.81; P<0.001) | 35.9 vs. 30.0% | 32.8 vs. 36.0% | Improved (statistically significant) | (14–16) |
| ImPower150 | Benefit regardless of it | NSCC | IV | To compare 3 treatments: atezolizumab+carboplatin+ paclitaxel (ACP), atezolizumab +carboplatin+paclitaxel +bevacizumab (ABCP) or carboplatin+paclitaxel +bevacizumab (BCP) | 19.2 for ABCP vs. 14.7 for BCP months (HR=0.78; 95% CI, 0.64–0.96; P=0.02) | 8.3 for ABCP vs. 6.8 for BCP months (HR 0.62; 95% CI, 0.52–0.74; P<0.001) | 63.5 in the ABCP group vs. 48.0 % in the BCP group | Treatment tolerability differed between induction and maintenance phases across treatment arms, more patients had grade 3/4 treatment-related AEs during the induction versus maintenance phase (ACP, 40.5 vs. 8.2%; ABCP, 48.6 vs. 21.2%; BCP, 44.7 vs. 11.1% | Similar across study's arms | (17,18) |
| Second-line therapy | ||||||||||
| CheckMate 017 | Benefit regardless of it | SCC | IIIB/IV | To compare nivolumab, at a dose of 3 mg per kilogram of body weight every 2 weeks, and docetaxel, at a dose of 75 mg per square meter of body-surface area every 3 weeks | 9.2 (95% CI, 7.3–13.3) vs. 6.0 (95% CI, 5.1–7.3) months (HR=0.59; 95% CI, 0.44–0.79; P<0.001) | 3.5 vs. 2.8 months (HR 0.62; 95% CI, 0.47–0.81; P<0.001) | 20 vs. 9% | 7 vs. 55% | Improved (statistically significant) | (19,20) |
| CheckMate 057 | Benefit regardless of it | NSCC | IIIB/IV | To compare nivolumab at a dose of 3 mg per kilogram of body weight every 2 weeks to docetaxel at a dose of 75 mg per square meter of body-surface area every 3 weeks | 12.2 (95% CI, 9.7 to 15.0) vs. 9.4 (95% CI, 8.1–10.7) months (HR=0.73; 96% CI, 0.59–0.89; P=0.002) | No difference | 19 vs. 12% | 10 vs. 54% | Improved (statistically significant) | (21,22) |
| KEYNOTE 010 | >1% | SCC, NSCC | IV | To compare two doses of pembrolizumab (2 and 10 mg/kg) vs. docetaxel 75 mg per square meter of body-surface area every 3 weeks | 10.4 (pembro 2 mg/kg), 12.7 (pembro 10 mg/kg) vs. 8·5 months pembro 2 mg/kg vs docetaxel (HR=0.71, 95% CI 0.58–0.88; p=0·0008) pembro 10 mg/kg vs. docetaxel (HR 0.61, 0.49–0·75; P<0·0001) | No significant difference between groups | 30% (pembro 2mg/kg), 29% pembro 10 mg/kg) vs. 8% | 13% (pembro 2mg/kg), 16% pembro 10 mg/kg vs. 35% | Improved (statistically significant) | (23,24) |
| OAK | Benefit regardless of it | SCC, NSCC | IIIB/IV | To compare atezolizumab (fixed dose 1,200 mg every 3 weeks) with docetaxel 75 mg per square meter of body-surface every 3 area weeks | 15.7 (95% CI 12.6–18.0) vs. 10.3 (8.8–12.0) months (HR 0.74, 95% CI 0.58–0.93; P=0.0102) | Similar in both groups | Similar in both groups | 37 vs. 54% | Improved (statistically significant) | (23,24) |
ABCP, atezolizumab+ccarboplatin+paclitaxel+bevacizumab; ACP, atezolizumab+carboplatin+paclitaxel; AE, adverse events; BCP, carboplatin+paclitaxel+bevacizumab; ChT, chemotherapy; CI, confidence interval; G, grade; HR, hazard ratio; IT, immunotherapy; NSCC, non-squamous cell cancer; OS, overall survival; ORR, overall response rate; PFS, progression-free survival; PDL-1, programmed cell death protein ligand 1; QoL, quality of life; SCC, squamous cell cancer.
In the second line of treatment, the superiority of antibodies [nivolumab (19,21), pembrolizumab (23), atezolizumab (25)] over docetaxel chemotherapy was demonstrated in terms of parameters such as OS, hazard ratio (HR), ORR and treatment toxicity. All patients benefited from the treatment, regardless of PD-L1 expression and histology (Table II). Second-line immunotherapy did not only affect PFS, but is typical of it. The significant improvement in the quality of life of patients treated with immunotherapy is also noteworthy (22,24,26).
Based on these study results (Table II), it can be concluded that the immunotherapy is a therapy that allows patients with metastatic lung cancer to have longer OS compared with standard chemotherapy and, in addition, the immunotherapy is a therapy with considerably lower toxicity. However, the immunotherapy offers benefits to patients whose immune system is functional, and a simple clinical exponent of this functionality is the general condition of the patient. Therefore, the immunotherapy should be used as early as possible, in the first- or second-line palliative systemic treatment, when patients are in very good general condition.
So far, no randomized clinical trial has emerged for immunotherapy and BSC with intent as in the study by Temel et al (3,6) for chemotherapy and BSC. We do not know exactly what is the impact (e.g., on OS or QoL) of early hospice care implementation to immunotherapy compared to the group of patients treated only with immunotherapy. Based on the observation of patients treated with chemotherapy and concurrent EPC, it may be suspected that such patients have a chance of an early initiation of treatment AE induced by immunotherapy, which may translate into survival. However, there is no literature data on this subject.
5. Immunotherapy vs. palliative care
Immunotherapy and opioids
The most common symptoms in patients with advanced lung cancer are pain, cough and dyspnea. Management of them is the object of daily practice in EPC, and their treatment consists primarily in the use of opioids, various steroids and antibiotics (31). A range of data from immunotherapy studies indicates that these drugs considerably lower the efficacy of this method of treatment.
Opioids are drugs with proven immunosuppressive effects (32). Their influence on the immune system is multidirectional (Fig. 1B). From the perspective of the application of the immunotherapy in the treatment of cancers, the most important seems to be the effect on T cells, which are the main effectors of action of anti-PD1 and anti-PDL1 antibodies and their influence on the hypothalamic-pituitary-adrenal axis. Opioids interact with μ receptors located on T cells, and the effect of this interaction is impairment of T cell viability and their proliferation caused by activation factors (32). Immunosuppression induced by opioids is a result of their effect on the hypothalamic-pituitary-adrenal axis. Corticotropin-releasing hormone stimulates the anterior pituitary to produce adrenocorticotropic hormone (ACTH), which in turn stimulates the adrenal cortex to produce glucocorticoids. These influence various elements of the innate and adaptive immune system, suppress cellular immunity and contribute to the tolerance of different antigens by altering the function of T and B cells (32). The effect of opioids on this axis depends on the time of drug administration during the day and on the duration of their use. Opioids change the concentration of ACTH as well as of glucocorticoids. Such biological mechanisms of action translate into worse immunotherapy outcomes in patients on concomitant opioids. It was demonstrated that patients treated with opioids from the beginning of the immunotherapy and patients who, during the immunotherapy, required the increasing of doses, had a significantly lower survival rate (median OS 4.9 vs. 16.5 months, P=0.0030) (33,34). It should be noted that the strongest immunosuppressive properties have morphine and fentanyl, weaker oxycodone and tramadol and the weakest buprenorphine (33). With respect to the last medication, no immunosuppressive effect was reported (33).
Immunotherapy and glucocorticoids
Another group of drugs, often recommended by EPC practitioners and causing suppression of the immune system, are glucocorticoids. The mechanism of their immunosuppressive effects is described above. The registration studies for pembrolizumab in the first-line therapy as well as for nivolumab and atezolizumab in the second-line therapy excluded patients receiving corticosteroids. The exclusion criterion was the administration of prednisone at the dose of 10 mg or more per day or a different glucocorticoid at an equivalent dose. Over time, the immunotherapy went from clinical trials to daily practice and became a standard therapy. Hence the immunotherapy in patients receiving corticosteroids has began to be used. Indications for use of glucocorticoids in patients with lung cancer include alleviation of cancer-related symptoms as well as treatment of comorbidities, e.g., chronic obstructive pulmonary disease (COPD). They are also used in the treatment of adverse effects of the immunotherapy. The study by Arbour et al (35) assessed the results of the actual population of patients undergoing the immunotherapy and receiving steroids and undergoing the immunotherapy and not receiving steroids. In the group of 640 patients, 90 patients (14%) received prednisone at a dose of ≥10 mg/day (or a different steroid at an equivalent dose) at the beginning of the immunotherapy. The most common indications for steroid use were dyspnea (33%), fatigue (21%) and cerebral metastases (19%). In patients receiving steroids, more frequently there were more than two factors of an unfavorable prognosis, the performance status ≥2 (according to the ECOG) and the presence of cerebral metastases (P<0.01 for both factors). In the pooled analysis, patients receiving steroids had a lower ORR (7 vs. 18%), significantly lower PFS (P<0.001) and OS (P<0.001) compared to patients not receiving corticosteroids (35). The very important issue is the time of corticosteroids treatment initiation. Patients who received and discontinued corticosteroids days 1 to 30 before to the initiation of PD-L1 had intermediate PFS and OS compared with those who received corticosteroids from the beginning of ICI treatment and those who received no corticosteroids within 30 days of the start of therapy. The authors of the publication performed a multifactorial analysis which included the history of tobacco smoking, performance, history of cerebral metastases and the use of corticosteroids at the beginning of the immunotherapy. The result of the analysis showed that the use of corticosteroids was an independent prognostic factor associated with lower PFS and OS rates (35). The disadvantage of the analysis is that it did not take into account the expression of PDL1 or TMB. In addition, the study included a group of patients who discontinued corticosteroids 1–30 days prior to the immunotherapy. They obtained indirect PFS and OS values compared to patients receiving and not receiving steroids at the beginning of the therapy (35). An important aspect of the use of corticosteroids in patients with lung cancer undergoing the immunotherapy is the treatment of immunotherapy-related AE. Although biological mechanisms described above indicate a reduction in the function of the immune system due to steroid therapy, many studies have demonstrated that patients undergoing the immunotherapy and steroid therapy achieve higher survival rates, as opposed to chemotherapy (1,2,6,25). These observations raise a number of questions. Do glucocorticoids actually decrease the efficacy of the immunotherapy? Are they used for a more aggressive disease, in patients with a worse prognosis, who do not benefit from the immunotherapy, which could indicate their prognostic significance? One study performed a retrospective analysis of 424 patients with NSCLC treated with immune-checkpoint inhibitors, with regard to the use of steroids (36). The highest survival rate was achieved by patients who did not take steroids and were indirectly treated with steroids from the beginning of the immunotherapy and the shortest survival rate by patients who started corticosteroids in the first eight weeks of the immunotherapy (median OS 13.83 vs. 4.2 vs. 2.2 months respectively, P=0.0001). The analysis of indications for steroid use showed that patients who received them for non-neoplastic reasons reached the survival rate comparable to those who did not receive steroids (median OS of 13.4 vs. 13.8 months, respectively; P<0.0001). In the group of patients receiving corticosteroids due to cancer-related problems, median OS was 1.9 months (P<0.0001) (36). The results of the study support the hypothesis that the use of steroids can have the prognostic significance and indicate a group with an unfavorable prognosis. The prudent use of steroids in immunotherapy patients is recommended in the absence of conclusive data. The most commonly used steroid is dexamethasone at a dose of 4 to 16 mg, mostly due to pain (including headache), nausea, weakness and lack of appetite (37–39). The literature data indicates that glucocorticoids are used in palliative care in over half of the patients (39–41). The literature does not include any clear-cut information about whether EPC contributes to the earlier and more frequent use of steroids in metastatic lung cancer patients. However, it seems that due to the nature of the most common symptoms in this population, this may potentially affect the efficacy of the immunotherapy.
Immunotherapy and antibiotics
The third group of drugs that may affect the efficacy of the immunotherapy is antibiotics. Based on numerous studies regarding the correlation between intestinal microflora and the immune system, it has been found that intestinal homeostasis prevents systemic inflammation and reduces the ability of neoplastic cells to escape immune surveillance (40,41). It has been hypothesized that modulation of intestinal microflora by antibiotics affects the immune system and may be associated with the efficacy of action of anti-PD-1 and anti-PDL1 antibodies. Host intestinal microbiota and immune system create numerous interactions which modulate the local and systemic immune system (42). Antibiotics cause dysbiosis, i.e. reduce the diversity of intestinal microflora, which promotes chronic inflammatory conditions (43,44). The study by Derosa et al (44) assessed the effect of using antibiotics on the efficacy of the immunotherapy. The administration of antibiotics from 30 to 60 days prior to the immunotherapy was taken into account. It was noted that 20% of patients with NSCLC received antibiotics 30 days prior to the immunotherapy. Compared to the patients not taking antibiotics, that group had a significantly shorter time from progression [median PFS 1.9 vs. 3.8 months; HR 1.5, 95% confidence interval (CI) 1.0–2.2, P=0.03] and shorter OS (median OS 7.9 vs. 24.6 months; HR 4.4; 95% CI 2.6–7.7, P<0,01). In the group of patients who received antibiotics 60 days prior to the immunotherapy, no differences in the ORR and PFS were observed, but shorter OS was noted (median OS 9.8 vs. 21.9 months; HR 2.0, 95% CI 1.3–3.2, P<0.01). A multifactorial analysis showed that the antibiotic therapy was an independent factor associated with a lower survival rate (HR 2.5, 95% CI 1.6–3.7; P<0.01) (44). The use of the antibiotic therapy seems to be an indication of unfavorable prognosis in patients undergoing the immunotherapy regardless of conventional prognostic markers. Do antibiotics make the treatment less effective? Do they indicate a group of patients with a worse prognosis, with a greater mass of the neoplastic disease, more problems and comorbidities which are more difficult to manage? There are no clear answers to these questions. The EPC recommendations list antibiotics for various problems, including cough or fever (31,45). However, it is likely that such patients more often require the antibiotic therapy due to their predisposition to pneumonia and exacerbation of comorbidities such as COPD (31,45,46). The doubt may arise whether EPC, through the frequent use of antibiotics, decreases the efficacy of the immunotherapy. Literature data indicates that up to 86% of patients who undergo palliative care receive antibiotics, which are even abused in the last few weeks of their lives (45–47). However, there is no data on whether the EPC of lung cancer patients affects their too early use and, consequently, perhaps the efficacy of the immunotherapy.
6. Conclusions
EPC has a well-established role in the treatment of patients with metastatic NSCLC. However, the studies that have become the cornerstone of the worldwide guidelines in this respect did not include patients treated with anti-PD1 and anti-PDL1 antibodies. Drugs that are often used in hospice care probably worsen the efficacy of the immunotherapy or indicate the worst prognostic group of patients who would not benefit from the immunotherapy. Insufficient scientific evidence in this area of knowledge requires special consideration in everyday clinical practice and implementation of EPC in lung cancer patients undergoing the immunotherapy.
Controlled clinical studies involving patients undergoing the immunotherapy and EPC could help remove these doubts.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- AE
adverse events
- ChT
chemotherapy
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- ECOG
Eastern Cooperative Oncology Group
- EPC
early palliative care
- EOL-care
end-of-life care
- FACIT-Sp
the functional assessment of chronic illness therapy-spiritual well-being
- FACT-G
the functional assessment of cancer therapy-general
- FACT-L
the functional assessment of cancer therapy-lung
- HADS
Hospital Anxiety and Depression scale
- G
grade
- HR
hazard ratio
- IT
immunotherapy
- LCS
lung-cancer subscale
- NSCLC
non-small cell lung cancer
- NSCC
non-squamous cell cancer
- ORR
overall response rate
- OS
overall survival
- PFS
progression-free survival
- PHQ-9
patient health questionnaire-9
- PD-1
programmed cell death protein 1
- PDL-1
programmed cell death protein ligand 1
- QoL
quality of life
- QUAL-E
quality of life at the end of life
- SCC
squamous cell cancer
- TOI
trial outcome index
- TMB
tumor mutation burden
- VEGF
vascular endothelial growth factor
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors contributions
MP was responsible for the concept of the manuscript, collecting and interpreting data on immunotherapy of NSCLC, design and production of the figure. PP was responsible for collecting and interpreting data on palliative care. BR was supervisor of the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, et al. Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann C, Swami N, Krzyżanowska M, Hannon B, Leighl N, Oza A, Moore M, Rydall A, Rodin G, Tannock I, et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 5.King JD, Eickhoff J, Traynor A, Campbell TC. Integrated onco-palliative care associated with prolonged survival compared to standard care for patients with advanced lung cancer: A retrospective review. J Pain Symptom Manage. 2016;51:1027–1032. doi: 10.1016/j.jpainsymman.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, Back AL, Kamdar M, Jacobsen J, Chittenden EH, et al. Effects of early palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol. 2017;35:834–841. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN): NCCN Guidelines-Non-small Cell Lung Cancer, Version 3.2020. https://www.nccn.org/global. [Feb 11;2020 ];
- 8.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer JR, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18:1600–1609. doi: 10.1016/S1470-2045(17)30690-3. [DOI] [PubMed] [Google Scholar]
- 12.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 13.Garassino M, Rodriguez-Abreu D, Gadgeel, Esteban E, Felip E, De Angelis F, Domine M, Hochmair M, Powell SF, Cheng SY, et al. Health-related quality of life (HRQoL) in the KEYNOTE-189 study of pembrolizumab (pembro) or placebo (pbo) + pemetrexed (pem) + platinum (plt) for metastatic NSCLC. J Clin Oncol. 2019;36 [Google Scholar]
- 14.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, et al. Nivolumab plus Ipilimumab in advanced non-small cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 16.Reck M, Schenker M, Lee KH, Provencio M, Nishio M, Lesniewski-Kmak K, Sangha R, Ahmed S, Raimbourg J, Feeney K, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small cell lung cancer with high tumour mutational burden: Patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137–147. doi: 10.1016/j.ejca.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. IMpower150 study group Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 18.Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, Shtivelband M, González Larriba JL, Rothenstein J, Früh M, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without bevacizumab versus bevacizumab plus chemotherapy in non-small cell lung Cancer. J Clin Oncol. 2020;38:2530–2542. doi: 10.1200/JCO.19.03158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M, Taylor F, Penrod JR, DeRosa M, Morrissey L, Dastani H, Orsini L, Gralla RJ. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: Results from the CheckMate 017 study. J Thorac Oncol. 2018;13:194–204. doi: 10.1016/j.jtho.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reck M, Brahmer J, Bennett B, Taylor F, Penrod JR, DeRosa M, Dastani H, Spigel DR, Gralla RJ. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30. doi: 10.1016/j.ejca.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 24.Barlesi F, Garon EB, Kim DW, Felip E, Han JY, Kim JH, Ahn MJ, Fidler MJ, Gubens MA, de Castro G, Jr, et al. Health-related quality of life in KEYNOTE-010: A phase II/III study of pembrolizumab versus docetaxel in patients with previously treated advanced, programmed death ligand 1-expressing NSCLC. J Thorac Oncol. 2019;14:793–801. doi: 10.1016/j.jtho.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. OAK study group Atezolizumab versus docetaxel in patients with previously treated non-small cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bordoni R, Ciardiello F, von Pawel J, Cortinovis D, Karagiannis T, Ballinger M, Sandler A, Yu W, He P, Matheny C, et al. Patient-reported outcomes in OAK: A phase III study of atezolizumab versus docetaxel in advanced non-small cell lung cancer. Clin Lung Cancer. 2018;19:441–449.e4. doi: 10.1016/j.cllc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, et al. Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 28.Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, Ferrell BR, Loscalzo M, Meier DE, Paice JA, et al. American society of clinical oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 29.Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: Potential mechanisms of prolonged survival. Chron Respir Dis. 2013;10:35–47. doi: 10.1177/1479972312471549. [DOI] [PubMed] [Google Scholar]
- 30.Saito AM, Landrum MB, Neville BA, Ayanian JZ, Earle CC. The effect on survival of continuing chemotherapy to near death. BMC Palliat Care. 2011;10:14. doi: 10.1186/1472-684X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watsob MS, Lucas CF, Hoy AM, Back IN. 1st edition. Oxford University Press; Oxford: 2005. Oxford Handbook of Palliative Care. [Google Scholar]
- 32.Zajączkowska R, Leppert W, Mika J, Kocot-Kępska M, Woroń J, Wrzosek A, Wordliczek J. Perioperative immunosuppression and risk of cancer progression: The impact of opioids on pain management. Pain Res Manag. 2018;2018:9293704. doi: 10.1155/2018/9293704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldi S, Marcantognini G, Fiordoliva I, di Pietro Paolo M, Morgese F, Toriniai M, Burattini L, Berardi R. Presented at MASCC/ISOO Annual Meeting on Supportive Care in Cancer. San Francisco, CA: 2019. The negative prognostic role of opioids in patients with NSCLC treated with immunotherapy. [Google Scholar]
- 34.Bironzo P, Pignataro D, Audisio M, Tagliamento M, Paratore C, Tabbò F, Bungaro M, Zichi C, Filippis M, Rapetti S, et al. Association between opioids and outcome of 1st line immunotherapy in advanced NSCLC patients: A retrospective evaluation. JTO. 2019;14:s713. [Google Scholar]
- 35.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 36.De Giglio A, Mezquita L, Auclin E, Blanc-Durand F, El-Amarti L, Caramella C, Martinez Bernal G, Hendriks L, Ferrara R, Naltet C, et al. Impact of early introduction of steroid on immune-checkpoint inhibitors (ICI) in patients with advanced non- small cell lung cancer treated. Ann Oncol. 2019;30((s11)):xi16. doi: 10.1093/annonc/mdz449. [DOI] [Google Scholar]
- 37.Shih A, Jackson KC., II Role of corticosteroids in palliative care. J Pain Palliat Care Pharmacother. 2007;21:69–76. doi: 10.1080/J354v21n04_14. [DOI] [PubMed] [Google Scholar]
- 38.Barghi K, Edmonds KP, Ajayi TA, Atayee RB. Prescribing trends of palliative care teams use of dexamethasone for cancer-related pain. J Pain Palliat Care Pharmacother. 2018;32:37–43. doi: 10.1080/15360288.2018.1460436. [DOI] [PubMed] [Google Scholar]
- 39.North West Clinical Senates: Guidelines for the use of corticosteroids in palliative care. http://www.nwcscnsenate.nhs.uk/files/2814/3394/6186/Corticosterioids.pdf. [May 18;2020 ];
- 40.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165:276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012;33:459–466. doi: 10.1016/j.it.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34:260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 44.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell lung cancer. Ann Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg JH, Albrecht JS, Fromme EK, Noble BN, McGregor JC, Comer AC, Furuno JP. Antimicrobial use for symptom management in patients receiving hospice and palliative care: A systematic review. J Palliat Med. 2013;16:1568–1574. doi: 10.1089/jpm.2013.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helde-Frankling M, Bergqvist J, Bergman P, Björkhem-Bergman L. Antibiotic treatment in end-of-life cancer patients-A retrospective observational study at a palliative care center in Sweden. Cancers (Basel) 2016;8:84. doi: 10.3390/cancers8090084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juthani-Mehta M, Malani PN, Mitchell SL. Antimicrobials at the end of life: An opportunity to improve palliative care and infection management. JAMA. 2015;314:2017–2018. doi: 10.1001/jama.2015.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.