Abstract
Introduction
The International Cancer Benchmarking Partnership demonstrated international differences in ovarian cancer survival, particularly for women aged 65–74 with advanced disease. These findings suggest differences in treatment could be contributing to survival disparities.
Objective
To compare clinical practice guidelines and patterns of care across seven high-income countries.
Methods
A comparison of guidelines was performed and validated by a clinical working group. To explore clinical practice, a patterns of care survey was developed. A questionnaire regarding management and potential health system-related barriers to providing treatment was emailed to gynecological specialists. Guideline and survey results were crudely compared with 3-year survival by ‘distant’ stage using Spearman’s rho.
Results
Twenty-seven guidelines were compared, and 119 clinicians completed the survey. Guideline-related measures varied between countries but did not correlate with survival internationally. Guidelines were consistent for surgical recommendations of either primary debulking surgery or neoadjuvant chemotherapy followed by interval debulking surgery with the aim of complete cytoreduction. Reported patterns of surgical care varied internationally, including for rates of primary versus interval debulking, extensive/‘ultra-radical’ surgery, and perceived barriers to optimal cytoreduction. Comparison showed that willingness to undertake extensive surgery correlated with survival across countries (rs=0.94, p=0.017). For systemic/radiation therapies, guideline differences were more pronounced, particularly for bevacizumab and PARP (poly (ADP-ribose) polymerase) inhibitors. Reported health system-related barriers also varied internationally and included a lack of adequate hospital staffing and treatment monitoring via local and national audits.
Discussion
Findings suggest international variations in ovarian cancer treatment. Characteristics relating to countries with higher stage-specific survival included higher reported rates of primary surgery; willingness to undertake extensive/ultra-radical procedures; greater access to high-cost drugs; and auditing.
Keywords: ovarian cancer, surgery, surgical oncology, medical oncology
HIGHLIGHTS.
International differences in ovarian cancer treatment may contribute to differences in stage-specific survival between countries.
Differences were found in rates of primary surgery, willingness to undertake extensive/‘ultra-radical’ surgery, and in types of systemic therapies recommended across clinical guidelines.
Differences in perceived barriers to providing optimal treatment were also reported.
Introduction
Ovarian cancer is the sixth most common cancer among women and has the highest mortality rate of all gynecological cancers internationally.1 Previous findings from the International Cancer Benchmarking Partnership (ICBP) demonstrated that, while differences in stage at diagnosis for ovarian cancer partly explained the survival gap, differences in survival also existed within each stage of disease.2 This a global collaboration of clinicians, policymakers, researchers, and cancer data experts, seeking to explain cancer survival differences between high-income countries with comprehensive cancer registry coverage, similar national health system expenditure, and universal access to healthcare. The ICBP SurvMark-2 project recently demonstrated international differences in ovarian cancer survival within age and stage groups, particularly for older women (65–74 years) with advanced disease, where 3-year net survival ranged from 52% (Norway) to 29% (Ireland).3 Notwithstanding improvements in ovarian cancer outcomes internationally,4 variations in age- and stage-specific survival suggest differences in treatment may exist.
Clinical practice guidelines are designed to ensure that patients receive optimal care, typically based on the best available evidence, and offer a way of exploring treatment differences by comparing recommendations internationally.5 One also investigates patterns of care to explore how they align with guideline recommendations and how they may be influenced by health system-related factors. Most women with ovarian cancer are diagnosed with advanced stage disease (III-IV), for which optimal treatment is cytoreductive surgery and chemotherapy. Surgical options consist of either primary debulking surgery, or neoadjuvant chemotherapy followed by interval debulking and may involve extensive (‘ultra-radical’) procedures.6 Despite a lack of consensus regarding primary versus interval debulking, and lack of prospective evidence supporting extensive/ultra-radical surgery, the goal of surgery remains no residual macroscopic disease, which is associated with improved survival.7
Systemic therapies play an important role in ovarian cancer treatment, with the use of carboplatin and paclitaxel chemotherapy now well-established.8 Intra-peritoneal chemotherapy remains controversial due to toxicity and, despite early promise, later trials failed to show improved survival.9 Radiotherapy may also be used in specific types of ovarian cancer and for palliation of advanced disease.10 Choice of therapies has further evolved through BRCA-mutation testing and the success of poly (ADP-ribose) polymerase (PARP) inhibitors in clinical trials.11
Health system-related factors influence the type and quality of treatment patients receive. This includes resources needed for surgery, such as sufficient operating theater time and intensive care unit beds,12 13 funding for expensive anti-cancer drugs, and the use of national audits to inform change and improve outcomes.14 Due to the lack of uniform and robust clinical data available to directly compare clinical practices among different countries, we have used guidelines and a validated questionnaire to indirectly explore differences in patterns of care and how these relate to survival for women with ovarian cancer in seven high-income countries. This study is the first to compare international clinical practice for ovarian cancer treatment.
Methods
A document search was performed using PubMed; guideline-specific databases (Guidelines International Network); and online government portals. Search terms included specific disease name and relevant jurisdiction (‘ovarian cancer treatment guidelines OR pathway in Canada’). Inclusion criteria were guidelines widely used in routine ovarian cancer treatment as validated by working group members from each country, and guideline revisions from the past 20 years to highlight the frequency of updates over time. Information was extracted for jurisdiction; organization(s); publication year(s); and treatment modality. A working group of 19 clinicians was formed to validate the guideline comparison and provide additional insight into clinical practice differences.
A questionnaire was developed based on a previous survey conducted by Farrell et al investigating changes in surgical practice for ovarian cancer in Australia and New Zealand15 and extended to include additional questions. The questionnaire consisted of 34 questions divided into four sections: 1. respondent characteristics, 2. surgical practice, 3. systemic therapy, and 4. health system-related factors, and was validated and tested by a clinical working group. The gynecological oncologists/specialist surgeons, medical oncologists, clinical oncologists, and general gynecologists were chosen to receive the questionnaire using existing local networks available to the working group members. Working group members identified distribution lists which included those actively involved in the treatment of ovarian cancer. Country-specific response rates were calculated by dividing the number of responses by the number of questionnaires distributed in each country. Denominators were either database confirmed or estimated by multiplying the number of centers treating ovarian cancer in the country by the approximate number of specialists in each center. Survey-specific response rates were also calculated using the number of responses from each country. All data were maintained in Microsoft Excel (version 1803).
In all tables, figures, and supplementary materials, countries are ordered using the latest 3-year survival figures (2010–2014) from highest (Norway, 57.2%) to lowest (Ireland, 44.8%).3 Comparisons of 3-year survival by ‘distant’ stage (2010–2014) were performed using Spearman's rho in R Studio (version 3.5.1). Information gathered from guidelines and the survey relate to the current time period (~2019) and does not align with the 2010–2014 survival data.
Results
Twenty-seven guidelines were identified (online supplementary material 1). Comparisons are presented by treatment modality with additional textual information in Table 1. Guideline measures were compared across countries (online supplementary table 1). Most countries had a single organization producing guidelines except the UK (n=6). The UK also had the most documents identified (n=9) and the most recently published guideline (2018). Denmark had the most revisions, updating the same guideline four times since 2003. When crudely compared, correlations between guideline measures and survival for each country were all non-significant (online supplementary figures). A total of 119 clinicians completed the patterns of care survey. Respondent characteristics are summarized in Table 2. Country-specific response rates ranged from 100% (Norway) to 10% (Australia). Survey-specific response rates also varied between countries, ranging from 3% to 27% (for Ireland and Canada, respectively) (online supplementary table 2). Only survey results from sections 1 and 4 are reported here.
Table 1.
Summary of guideline recommendations for surgery and systemic therapies
| Country | Guideline | Modality | Additional information | ||||||
| Lymph node staging | Primary debulking surgery | Interval debulking surgery* | IP chemo-therapy | Bevacizumab | Radiotherapy | PARP inhibitors | |||
| Norway | NDH | R | R | R | N | R | N | R | |
| Australia | CA | R | R | R | R† | R | N | R | Can be considered in stage III women optimally debulked; should be provided in a center with appropriate expertize, and potential toxicities should be fully explained† |
| Denmark | DGCG | R† | R | R | N | R | N | R | Systematic lymphadenectomy also recommended† |
| Canada | AHS | R | R | R | R | N† | T‡ | R | More trials needed before this regimen can be adopted† Consider in select cases to improve local control, at the discretion of the radiation oncologist‡ |
| BCC | R | R | R | R | R | T† | R | Post-operative radiation therapy only recommended for clear cell, endometrioid, and mucinous tumors.† | |
| CCO | R | R | R | R | R | N | R | ||
| UK | NICE | R | R | R | N† | N‡ | N | R | Do not offer, except in clinical trial† Not recommended for first-line treatment in combination with carboplatin and paclitaxel‡ |
| BGCS | R | R | R | N† | N | N | R | Can be offered in clinical trial where appropriate expertize and resources exist† | |
| Scotland | SIGN | R | R | R | R† | R | N | R | Can be considered provided a regimen of proven benefit in a clinical trial compared with intravenous therapy is used and delivered in a center with appropriate expertize and where the potential toxicities are fully explained† |
| Wales | SWCN | R | R | R | N† | N | N | N | Concerns regarding associated morbidity and technical difficulties; not considered standard practice in the UK† |
| NWCN | R | R | R | N | N | N | N | ||
| Northern Ireland | NICaN | – | – | – | N† | N | N | R | Not currently recommended outside of clinical trial† |
| New Zealand | NZMH | R | R | R | N | N | N | N | |
| Ireland | NCCP | – | – | – | – | – | – | – | |
| Inter-national | NCCN | R | R | R | R | R | N | R | |
| ESMO | R | R | R | N† | R | N | R | Not adopted as standard of care due to a lack of trials with IV control arm; only recommended in clinical trials† | |
| ESGO | R | R | R | – | – | – | – | ||
Northern Ireland guidelines only contain recommendations for systemic anti-cancer therapy; ESGO only contain recommendations for surgery.
In Ireland, a clinical practice guideline for the management of ovarian cancer did not exist at the time of collection (as of August 2019); a guideline for diagnosis and staging has since been published by Ireland’s National Cancer Control Program (NCCP), with others in development.
*Neoadjuvant chemotherapy followed by interval debulking surgery.
†Signifies additional information relating to the value shown earlier in the same row.
N, not recommended/mentioned; R, recommended; T, textual information.
Table 2.
Survey respondent characteristics
| Category | Country, n (%) | ||||||
| Norway | Australia* | Denmark | Canada | UK | New Zealand | Ireland | |
| Total, n | 18 | 13 | 16 | 32 | 30 | 7 | 3 |
| Age | |||||||
| 35–39 | 2 (11) | 1 (8) | 0 | 3 (9) | 2 (7) | 2 (29) | 1 (33) |
| 40–49 | 2 (11) | 3 (23) | 6 (37) | 14 (44) | 10 (33) | 1 (14) | 0 |
| 50–59 | 8 (44) | 7 (54) | 8 (50) | 8 (25) | 13 (43) | 4 (57) | 1 (33) |
| 60–69 | 6 (33) | 2 (15) | 2 (12) | 7 (22) | 5 (17) | 0 | 1 (33) |
| ≥70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Years in practice | |||||||
| <5 | 4 (22) | 2 (15) | 6 (37) | 4 (12) | 5 (17) | 2 (29) | 1 (33) |
| 5–9 | 2 (11) | 3 (23) | 3 (19) | 8 (25) | 4 (13) | 0 | 0 |
| 10–14 | 0 | 3 (23) | 4 (25) | 5 (16) | 4 (13) | 1 (14) | 0 |
| 15–19 | 6 (33) | 3 (23) | 3 (19) | 3 (9) | 12 (40) | 3 (43) | 1 (33) |
| >20 | 6 (33) | 2 (15) | 0 | 12 (38) | 5 (17) | 1 (14) | 1 (33) |
| Specialty | |||||||
| Gynecological oncologist/specialist surgeons | 18 (100) | 10 (77) | 10 (63) | 28 (87) | 19 (63) | 2 (28) | 1 (33) |
| Medical oncologist | 0 | 3 (23) | 5 (31) | 4 (13) | 6 (20) | 5 (72) | 2 (67) |
| Clinical/radiation oncologist | 0 | 0 | 0 | 0 | 3 (10) | 0 | 0 |
| OBGYN | 0 | 0 | 1 (6) | 0 | 2 (7) | 0 | 0 |
*Including clinicians from New South Wales, Western Australia, and Victoria only.
ijgc-2020-001403supp001.pdf (120.2KB, pdf)
ijgc-2020-001403supp002.pdf (43.8KB, pdf)
ijgc-2020-001403supp003.pdf (48.6KB, pdf)
ijgc-2020-001403supp004.pdf (64.1KB, pdf)
ijgc-2020-001403supp005.pdf (72.3KB, pdf)
Surgery
Guideline recommendations for surgery remained consistent. All guidelines recommend surgical staging, including pelvic and para-aortic lymph node sampling either randomly or by resection of suspicious nodes. Danish guidelines also recommend systematic lymphadenectomy for early-stage disease. All guidelines containing surgical recommendations included the options of primary debulking surgery (unless contra-indicated) and neoadjuvant chemotherapy followed by interval debulking, with the aim of complete cytoreduction, if feasible (online supplementary glossary of surgical terms). These guidelines also considered cytoreductive surgery for ‘relapsed’/‘recurrent’ disease depending on disease-free interval and patient performance status (results not shown).
ijgc-2020-001403supp006.pdf (48.9KB, pdf)
When surveyed, clinicians reported differences in patterns of surgical care. Norwegian clinicians reported the highest rates of primary surgery in patients with advanced epithelial ovarian cancer, whereas those from the UK reported the lowest rates of primary and highest rates of interval surgery (Figure 1). In the same patient population, all Norwegian and Australian respondents either agreed or strongly agreed with ultra-radical surgery, whereas clinicians from Canada and the UK agreed with ultra-radical surgery to a lesser extent, with some respondents either disagreeing or strongly disagreeing with this approach (Figure 2). When crudely compared, willingness to undertake extensive/ultra-radical surgery correlated with 3-year survival by distant stage (rs=0.94, p=0.017).
Figure 1.
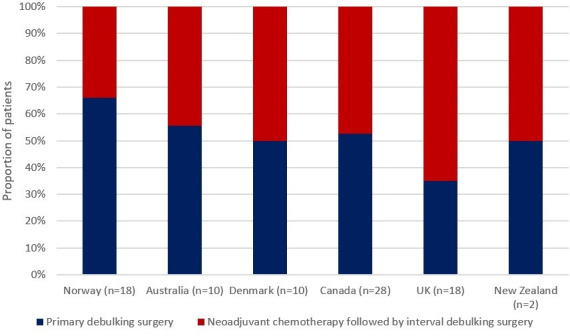
Survey question: 'What percentage of your patients with advanced epithelial ovarian cancer who had surgery underwent primary debulking followed by chemotherapy? What percentage underwent neoadjuvant chemotherapy followed by interval debulking?'. Median results presented for each country. n=number of respondents.
Figure 2.
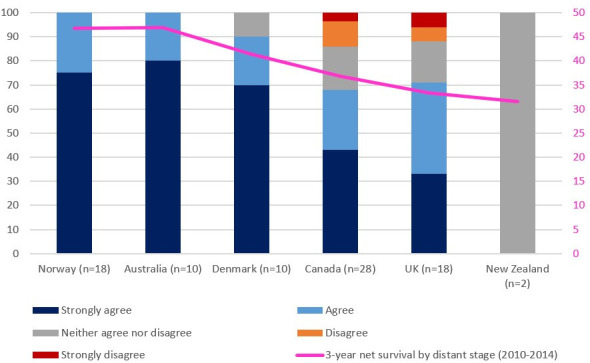
Survey question: 'To what extent do you agree with ‘ultra-radical’ surgery for patients with advanced ovarian cancer, either by referring them or performing the operation with your own team?'. n=number of respondents. Results compared against 3-year net survival in patients with ‘distant’ stage disease according to ICBP SurvMark-2 results (rs=0.94, p=0.017).
Clinicians across all countries reported ‘medical co-morbidities’ as a perceived barrier to achieving optimal debulking in patients with advanced disease. Norwegian clinicians were least likely to report data for an ‘older patient population’. UK clinicians reported ‘a lack of supportive care (intensive care unit beds)’ more often than clinicians from other countries and were less likely to report ‘non-resectable metastasis outside abdominal cavity’; a barrier frequently reported by clinicians elsewhere (online supplementary material 2).
ijgc-2020-001403supp007.pdf (190.4KB, pdf)
Systemic/Radiation Therapy
All guidelines recommended six cycles of platinum-based chemotherapy consisting of carboplatin and paclitaxel, most additionally recommending docetaxel, gemcitabine, or liposomal doxorubicin in cases of hypersensitivity and/or allergy to paclitaxel (results not shown). Differences were seen in guideline recommendations for other types of systemic therapy. Canadian, Australian, and Scottish guidelines recommend intra-peritoneal chemotherapy, whereas Danish and other UK guidelines do not recommend it outside of clinical trials. Norwegian and New Zealand guidelines omit guidance on intra-peritoneal chemotherapy. Norwegian, Australian, Danish, Canadian, and Scottish guidelines recommend considering bevacizumab, whereas guidelines from New Zealand and the UK (Wales and Northern Ireland) do not. PARP inhibitors, including olaparib and/or niraparib, were recommended in all countries except New Zealand. Most of these guidelines recommended PARP inhibitors as maintenance treatment for relapsed platinum-sensitive BRCA mutation-positive advanced ovarian cancer. Some more recent guidelines from the UK (2019) and Ontario, Canada (2018) also recommend olaparib in newly diagnosed advanced disease.
Differences in radiotherapy were found. Cancer Australia’s optimal care pathway states that “some women may benefit from radiation treatment”, and British Columbia guidelines recommend radiotherapy on an individual basis for clear cell ovarian cancer. Alberta’s guideline indicates that radiation oncologists should consider radiotherapy in the context of palliation for selected cases to improve local control. All other guidelines did not contain radiotherapy recommendations.
Health System-Related Factors
Differences in perceived health system-related barriers to accessing optimal treatment were reported (Figure 3). Norwegian clinicians most commonly reported restrictions in prescribing high-cost medications. Canadian clinicians often reported a ‘lack of patient access to clinical trials’. From the UK, a ‘lack of hospital staffing’ was commonly reported followed by ‘delays in treatment’. In New Zealand, clinicians often reported a lack of resources and funding for second-line drugs. Clinicians from Australia, Canada, the UK, New Zealand, and Ireland also reported a lack of treatment monitoring’. Danish clinicians most often reported perceiving ‘no barriers’.
Figure 3.
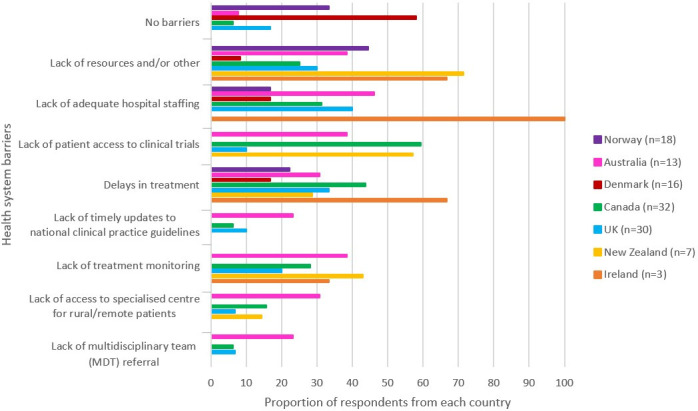
Survey question: 'What do you consider health system barriers to accessing optimal treatment in your patient population?' n=number of respondents.
Discussion
This study suggests international differences in ovarian cancer treatment. Differences were seen in guidelines measures, including the number of documents published and revisions made. Although these did not correlate with survival when crudely compared, further research exploring the complex relationship between international guidelines and outcomes is needed. Despite consistency across guidelines, reported surgical practices varied. While all guidelines recommend primary or interval debulking for patients with advanced disease, clinicians from countries with higher survival (Norway and Australia) reported higher rates of primary debulking. Although complete primary debulking has been associated with higher survival in late-stage patients,16 a lack of consensus still exists, with an Australian retrospective study finding that an increasing shift towards interval debulking was associated with increased survival.17 Commentators argue that primary debulking should still be considered the treatment of choice for fit patients with advanced resectable disease, whereas interval debulking is more suitable for patients with poorer performance and nutritional status, who are more likely to develop post-operative morbidity and mortality.18 Furthermore, most guidelines did not explicitly recommend extensive/ultra-radica’ surgery and yet clinicians from higher performing countries were more likely than those from lower performing countries to agree with ‘ultra-radical’ surgery.
Norwegian clinicians were least likely to perceive age as a barrier to achieving optimal cytoreduction and Norway demonstrated the highest survival in elderly patients with distant stage disease. In the UK, where clinicians perceived a lack of supportive care, survival for these patients was lower. These barriers could make clinicians less willing to operate on some patients, instead preferring a palliative option. Patients with advanced ovarian cancer are more likely to have severe co-morbidities and higher mortality,19 and historically, elderly patients were shown to be less likely to receive comprehensive surgical treatment.20 A Dutch study recently found that older patients and those with advanced disease were significantly less likely to receive any cancer-directed treatment.21 Moreover, available resources and operating theater time may influence a surgeons’ ability to perform extensive surgery and could impact patient outcomes.13 Importantly, it is this sub-category of elderly patients with advanced disease where survival is lowest and where significant differences exist.3
Other factors affecting surgical outcome include centralization of services,22 patient selection, discussion at multidisciplinary meetings and adequate pre-operative staging.23 It is noteworthy that the two lowest performing countries, New Zealand and Ireland, had not implemented centralization in the 2010–2014 time period.24 25 Moreover, some higher and lower performing countries centralized around the same time, including Norway (1995),26 the UK (1999),27 and Denmark (2001),28 indicating that other factors could be playing a role within centralized services, including access to specialist surgery.29 Since surgical outcome is a key prognostic factor for women with advanced ovarian cancer,7 differences in surgical practice may be contributing to survival variations and warrant further investigation within countries.
Differences were found in recommended systemic therapies. Intra-peritoneal chemotherapy remains contentious, with recent trial results failing to show a survival benefit in women with newly diagnosed advanced ovarian cancer.9 Disparities in its use may also stem from issues associated with increased resources and catheter-related complications, and that to overcome these would require time and specialist training.30 The benefits of bevacizumab are debated since it is yet to demonstrate improvements in overall survival.31 Given international differences in national health services spending,32 inequalities in access to high-cost drugs like bevacizumab may reflect different levels of available investment. Bevacizumab is not recommended in some of the lowest performing countries (Wales, Northern Ireland, and New Zealand), as it is not funded. New Zealand’s funding decisions for new medicines are taken by Pharmac, the national regulatory body who declined bevacizumab based on several decision criteria.33 The Cancer Drugs Fund in England and Scottish Medicines Consortium in Scotland fund bevacizumab for selected patients. PARP inhibitors have shown survival benefit in patients with a BRCA mutation with relapsed disease and, as of December 2019, are now recommended in all countries.34 Their recent introduction does not align with the 2010–2014 study period, but PARP inhibitors will probably influence future survival analyses.
Different health system-related barriers to providing optimal treatment were perceived by clinicians internationally. For example, restrictions to drug prescribing were reported in Norway. In a qualitative study, Norwegian oncologists recently described distrust in their centralized drug review process, which has led to inequities in drug availability due to the privatization of high-cost medications.35 In Australia, insufficient hospital staffing was reported as a perceived barrier to providing optimal treatment. This has been reported by Australian health professionals previously, relating to a lack of staff with specialized expertize outside metropolitan centers.36 Similarly, in New Zealand, where a lack of resources was reported, a previous clinical audit suggested inadequate theater space could be impacting patient waiting times and outcomes.28 In Canada, a perceived lack of patient access to clinical trials was reported. This may correspond with previous findings describing the barriers faced by Canadian physicians when participating in clinical research.37
Moreover, clinicians from countries with higher survival, such as Denmark and Norway, were more likely to report having no barriers to providing optimal treatment, whereas clinicians from countries with lower survival often reported a lack of treatment monitoring (via national and/or local audit) as a perceived barrier. One example of a current national auditing system is the Danish Gynecological Cancer Database, which has collected data on all women with ovarian cancer treated at Danish hospitals since 2005.38 Another example is the introduction of Scotland’s quality performance indicators.39 National auditing for ovarian cancer has been recommended as a method of investigating treatment disparities and informing quality improvements.13 Given challenges in comparing ovarian cancer treatment rates internationally, this study supports existing calls for improved data collection at local and national levels. This is particularly pertinent in countries with lower survival, where national audits are not routinely conducted, and highlights a key area of improvement for policy and practice.
We note some limitations in this study. We acknowledge low survey response rates from some countries, which may not reflect true patterns of care. Comparatively, in a review of international surveys for patterns of surgery in advanced ovarian cancer, response rates ranged from 30% to 81%.40 Discrepancies between country- and survey-specific response rates are also noted. Countries with higher denominators often had lower response rates, yet also received a larger number of responses. This has the potential to bias conclusions drawn from the survey about national practice patterns. Questionnaires were distributed to either membership societies or specialist hospital departments. Questionnaires distributed via society mailing lists were sent to a wider demographic of clinicians, some of whom may not have responded. Therefore some country-specific response rates may appear disproportionately lower than others.
We were not able to determine the demographic characteristics of non-responders and were not able to exclude responder bias. The varying responsibilities of specialists internationally may hinder the interpretation of survey results. Gynecological oncologists in certain countries cover both surgery and medical oncology (Norway and Canada), but in others provide specialist surgical care only (Denmark and the UK). The questionnaire did not ask clinicians what proportion of their practice is spent treating ovarian cancer, and some respondents may be treating fewer patients with ovarian cancer than others, potentially affecting the results.
Information was not collected on the willingness of clinicians not to operate on patients. Rates of patients receiving no treatment were also not accounted for. An existing study has shown that a large proportion of patients with ovarian cancer receive no treatment at all, often because patients choose not to have treatment.23 This group are likely to be older with more co-morbidities, have poorer outcomes, and could partly explain international survival variations. The guideline recommendations and survey results relate to the current time-period (~2019), which must be considered when comparing these findings with ICBP SurvMark-2 results (2010–2014).
Despite consistency across guidelines, surgical practice varied internationally, particularly in rates of primary versus interval debulking, views towards extensive/ultra-radical surgery, and perceived barriers to achieving optimal cytoreduction. These differences are probably due to a combination of patient, clinician, and health system-related factors. Given the importance of surgical outcome in survival for patients with advanced ovarian cancer, differences in surgical practice could be a key driver of international disparities. Differences in recommendations for systemic/radiation therapies were apparent and may reflect inequalities in levels of investment available to health systems to fund expensive drugs. In an effort to internationally benchmark ovarian cancer treatment, we indicate certain characteristics relating to countries with higher stage-specific survival including higher reported rates of primary debulking; willingness to undertake ultra-radical procedures; greater access to high-cost drugs; and auditing. Treatment differences noted between countries warrant further investigation at local levels to determine their severity and potential impact on patient outcomes, particularly for older women with advanced disease, and in countries with lower stage-specific survival.
Acknowledgments
We thank Lucie Hooper, Shanta Keshwala, and Charlotte Lynch of Cancer Research UK for managing the program. We also thank the International Cancer Benchmarking Partnership Program Board for their advice; and the Exploratory Modules Academic Reference Group for providing independent peer review and advice on the methodological approach and interpretation of findings.
Footnotes
Twitter: @GreggNelsonERAS
Contributors: CHN, JBu, RF, andSH: methodology, validation, data collection, analysis, writing - original draft and editing. AA, JBe, SF, MF-K-F, CG, NFH, LH, CKH, GK, JK, OM, GN, AN, DO, TS, and PHS: validation, data collection, analysis, writing - review & editing. CJC: methodolgy, writing - review and editing. PAC: methodology, validation, data collection, analysis, writing - review and editing. EZ: methodology, analysis.
Funding: This study was funded by the Canadian Partnership Against Cancer; Cancer Council Victoria; Cancer Institute New South Wales; Cancer Research UK; Danish Cancer Society; National Cancer Registry Ireland; The Cancer Society of New Zealand; National Health Service England; Norwegian Cancer Society; Public Health Agency Northern Ireland, on behalf of the Northern Ireland Cancer Registry; The Scottish Government; Western Australia Department of Health; and Wales Cancer Network.
Disclaimer: The findings and interpretations in this article are those of the authors and do not necessarily represent the views of any government agency or funder. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. This manuscript includes anonymous survey data at country level. Survey data at states/provinces/nation level are available on request from ICBP@cancer.org.uk.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Maringe C, Walters S, Butler J, et al. Stage at diagnosis and ovarian cancer survival: evidence from the International cancer benchmarking partnership. Gynecol Oncol 2012;127:75–82. 10.1016/j.ygyno.2012.06.033 [DOI] [PubMed] [Google Scholar]
- 3. Cabasag CJ, Butler J, Arnold M, et al. Exploring variations in ovarian cancer survival by age and stage (ICBP SurvMark-2): a population-based study. Gynecol Oncol 2020;157:234–44. 10.1016/j.ygyno.2019.12.047 [DOI] [PubMed] [Google Scholar]
- 4. Arnold M, Rutherford M, Bardot A, et al. Progress in cancer control: survival, mortality and incidence in countries 1995-2014. Lancet Oncol 2019;20:1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolters R, Regierer AC, Schwentner L, et al. A comparison of international breast cancer guidelines - do the national guidelines differ in treatment recommendations? Eur J Cancer 2012;48:1–11. 10.1016/j.ejca.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 6. National Institute of Health and Care Excellence (NICE) Ultra-radical (extensive) surgery for advanced ovarian cancer. Interventional Procedures Guidance [IPG47]. Available: https://www.nice.org.uk/guidance/IPG470
- 7. Chang S-J, Hodeib M, Chang J, et al. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol 2013;130:493–8. 10.1016/j.ygyno.2013.05.040 [DOI] [PubMed] [Google Scholar]
- 8. Clamp AR, James EC, McNeish IA, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019;394:2084–95. 10.1016/S0140-6736(19)32259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 2019;37:1380–90. 10.1200/JCO.18.01568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fields EC, McGuire WP, Lin L, et al. Radiation treatment in women with ovarian cancer: past, present, and future. Front Oncol 2017;7:177. 10.3389/fonc.2017.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495–505. 10.1056/NEJMoa1810858 [DOI] [PubMed] [Google Scholar]
- 12. Hall M, Savvatis K, Nixon K, et al. Maximal-effort cytoreductive surgery for ovarian cancer patients with a high tumor burden: variations in practice and impact on outcome. Ann Surg Oncol 2019;26:2943–51. 10.1245/s10434-019-07516-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Department of Health The Health of the 51%: Women. Annual report of the Chief Medical Officer, 2014, 2015. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/595439/CMO_annual_report_2014.pdf
- 14. Prades J, Manchon-Walsh P, Solà J, et al. Improving clinical outcomes through centralization of rectal cancer surgery and clinical audit: a mixed-methods assessment. Eur J Public Health 2016;26:538–42. 10.1093/eurpub/ckv237 [DOI] [PubMed] [Google Scholar]
- 15. Farrell R, Liauw WS, Brand AH. Ovarian cancer surgery in Australia and New Zealand: a survey to determine changes in surgical practice over 10 years. Int J Gynecol Cancer 2018;28:945–50. 10.1097/IGC.0000000000001247 [DOI] [PubMed] [Google Scholar]
- 16. Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 17. Nicklin JL, McGrath S, Tripcony L, et al. The shift toward neo-adjuvant chemotherapy and interval debulking surgery for management of advanced ovarian and related cancers in a population-based setting: impact on clinical outcomes. Aust N Z J Obstet Gynaecol 2017;57:651–8. 10.1111/ajo.12665 [DOI] [PubMed] [Google Scholar]
- 18. Hacker NF. Neoadjuvant chemotherapy for advanced epithelial ovarian cancer. Who really benefits? Aust N Z J Obstet Gynaecol 2017;57:585–7. 10.1111/ajo.12737 [DOI] [PubMed] [Google Scholar]
- 19. Tetsche MS, Dethlefsen C, Pedersen L, et al. The impact of comorbidity and stage on ovarian cancer mortality: a nationwide Danish cohort study. BMC Cancer 2008;8:31. 10.1186/1471-2407-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer 2007;109:2031–42. 10.1002/cncr.22604 [DOI] [PubMed] [Google Scholar]
- 21. Zijlstra M, Timmermans M, Fransen H, et al. Treatment patterns and associated factors in patients with advanced epithelial ovarian cancer: a population-based study. Int J Gynecol Cancer 2019;29:1032–7. 10.1136/ijgc-2019-000489 [DOI] [PubMed] [Google Scholar]
- 22. Woo YL, Kyrgiou M, Bryant A, et al. Centralisation of services for gynaecological cancers - a Cochrane systematic review. Gynecol Oncol 2012;126:286–90. 10.1016/j.ygyno.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 23. Querleu D, Planchamp F, Chiva L, et al. European Society of Gynaecologic Oncology quality indicators for advanced ovarian cancer surgery. Int J Gynecol Cancer 2016;26:1354–63. 10.1097/IGC.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 24. Ha M, Gangji A. Faster cancer treatment pathway in gynaecological malignancy: a repeat clinical audit. N Z Med J 2018;131:45–55. [PubMed] [Google Scholar]
- 25. Department of Health Diagnosis and staging of patients with ovarian cancer: national clinical guideline No.20. Available: https://www.hse.ie/eng/services/list/5/cancer/profinfo/guidelines/diagnosis-and-staging-of-patients-with-ovarian-cancer.pdf
- 26. Aune G, Torp SH, Syversen U, et al. Ten years' experience with centralized surgery of ovarian cancer in one health region in Norway. Int J Gynecol Cancer 2012;22:226–31. 10.1097/IGC.0b013e31823589ef [DOI] [PubMed] [Google Scholar]
- 27. Melvilla A, Eastwood A, Kleijnen J. Guidance of commissioning cancer services. improving outcomes in gynaecological cancer – the manual. Department of Health, 1999. https://www.vaco.co.uk/vacofrontpage/ImprovingOutcomesinGynaecologicalCancers-TheManaul.pdf. [Google Scholar]
- 28. Edwards HM, Noer MC, Sperling CD, et al. Survival of ovarian cancer patients in Denmark: results from the Danish Gynaecological Cancer Group (DGCG) database, 1995-2012. Acta Oncol 2016;55(Suppl 2):36–43. 10.1080/0284186X.2016.1182641 [DOI] [PubMed] [Google Scholar]
- 29. Butler J, Gildea C, Poole J, et al. Specialist surgery for ovarian cancer in England. Gynecol Oncol 2015;138:700–6. 10.1016/j.ygyno.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 30. Gourley C, Walker J, Mackay H. Update of intraperitoneal chemotherapy for the treatment of epithelial ovarian cancer. Am Soc Clin Oncol Educ Book 2016:35143–51. [DOI] [PubMed] [Google Scholar]
- 31. Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 32. Papanicolas I, Mossialos E, Gundersen A, et al. Performance of UK National Health Service compared with other high income countries: observational study. BMJ 2019;367. 10.1136/bmj.l6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. PHARMAC Cancer treatments subcommittee of PTAC meeting held 18 September 2015. Available: https://www.pharmac.govt.nz/assets/ptac-cancer-treatments-subcommittee-minutes-2015-09.pdf
- 34. PHARMAC Decision to fund two new cancer treatments: olaparib and fulvestrant, 2019. Available: https://www.pharmac.govt.nz/news/notification-2019-12-05-olaparib-fulvestrant/
- 35. Feiring E, Wang H. Rationing cancer treatment: a qualitative study of perceptions of legitimate limit-setting. BMC Health Serv Res 2018;18:342. 10.1186/s12913-018-3137-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crawford-Williams F, Goodwin B, March S, et al. Cancer care in regional Australia from the health professional's perspective. Support Care Cancer 2018;26:3507–15. 10.1007/s00520-018-4218-x [DOI] [PubMed] [Google Scholar]
- 37. Mahmud A, Zalay O, Springer A, et al. Barriers to participation in clinical trials: a physician survey. Curr Oncol 2018;25:119–25. 10.3747/co.25.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sørensen SM, Bjørn SF, Jochumsen KM, et al. Danish gynecological cancer database. Clin Epidemiol 2016;8:485–90. 10.2147/CLEP.S99479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. NHS National Services Scotland Ovarian cancer quality performance indicators, 2018. Available: https://www.isdscotland.org/Health-Topics/Quality-Indicators/Publications/2018-02-20/2018-02-20-Ovarian-QPI-Report.pdf
- 40. Park SJ, Kim J, Kim SN, et al. Practice patterns of surgery for advanced ovarian cancer: analysis from international surveys. Jpn J Clin Oncol 2019;49:137–45. 10.1093/jjco/hyy175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ijgc-2020-001403supp001.pdf (120.2KB, pdf)
ijgc-2020-001403supp002.pdf (43.8KB, pdf)
ijgc-2020-001403supp003.pdf (48.6KB, pdf)
ijgc-2020-001403supp004.pdf (64.1KB, pdf)
ijgc-2020-001403supp005.pdf (72.3KB, pdf)
ijgc-2020-001403supp006.pdf (48.9KB, pdf)
ijgc-2020-001403supp007.pdf (190.4KB, pdf)


