Abstract
At present, humanity is confronting with a novel life-threatening challenge from the COVID-19 pandemic infectious disease caused by the novel coronavirus SARS-CoV-2. To date, the various transmission modes of SARS-CoV-2 have not been completely determined. Food products might be carriers for SARS-CoV-2. The COVID-19 pandemic not only can spread through the respiratory tract like SARS and MERS but also the presence of the SARS-CoV-2 RNA in feces of several patients, shows the possibility of their fecal-oral route spread. Besides, people with gastric problems, including gastric intestinal metaplasia and atrophic gastritis, may be susceptible to this kind of COVID-19 infection. Accordingly, food may act as a potential vehicle of SARS-CoV-2 due to whether carry-through or carry-over contaminations. Considering carry-over, SARS-CoV-2 spread from personnel to food products or food surfaces is feasible. Beyond that, some shreds of evidence showed that pigs and rabbits can be infected by SARS-CoV-2. Thus, viral transmission through meat products may be conceivable, indicating carry-through contamination. As the spread rate of SARS-CoV-2 is high and its stability in different environments, especially food processing surfaces, is also remarkable, it may enter foods in whether industrialized processing or the traditional one. Therefore, established precautious acts is suggested to be applied in food processing units. The present review elucidates the risk of various staple food products, including meat and meat products, dairy products, bread, fruits, vegetables, and ready-to-eat foods as potential carriers for transmission of SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, Food, Transmission, Foodborne
Abbreviations
- NoV
norovirus
- HAV
hepatitis A virus
- EV
enterovirus
- HRV
human rotavirus
- HEV
hepatitis E virus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- MERS-CoV
middle-east respiratory syndrome coronavirus
- COVID-19
Coronavirus disease 2019
- ACE-2
angiotensin converting-enzyme 2
- RV
rotavirus
- GAP
good agricultural practices
1. Introduction
At present, notwithstanding food seems safer than ever, foodborne diseases are considered as one of the most predominant agents of morbidity and mortality (Bosch, Pintó, & Guix, 2016). Food-related outbreaks can be struck by the ingestion of food comprising microbial agents (e.g., bacteria, viruses, and parasites), or chemical toxicants or biological toxins (Bari & Yeasmin, 2018). Progressively, viruses can be highlighted as significant causative agents of foodborne infections throughout the world (Miranda & Schaffner, 2019). Viruses are not able to propagate or manufacture toxicants in the food; thus, foods may work as vehicles for their transition (Petrović & D'Agostino, 2016). Even though human norovirus (NoV) and hepatitis A virus (HAV) are the most common foodborne viral infections, but other viruses, including enterovirus (EV), human rotavirus (RV), hepatitis E virus (HEV), astrovirus, Aichi virus, sapovirus, coronavirus, parvovirus, and human adenovirus have potential to transmit by food (Petrović & D'Agostino, 2016).
Coronaviruses are RNA viruses belong to the family Coronaviridae, order Nidovirales that can cause disparate infectious diseases such as respiratory, enteric, hepatic, and neurologic disorders in different hosts, including humans, pigs, cows, cats, dogs, horses, mice, and birds. Coronaviruses are categorized into 4 genera: alpha-CoVs, beta-CoVs, gamma-CoVs, and delta-CoVs (H. Li, Liu, Yu, Tang, & Tang, 2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is considered as third zoonotic beta-CoVs, which have known in the 21st century. SARS-CoV and middle-east respiratory syndrome (MERS-CoV), formerly recognized beta-CoVs, made important epidemics in the globe. The latter three viruses chiefly affect the lungs and give rise to pulmonary infection. The principal confirmed transmission routes of the latter viruses are animal-to-person and person-to-person which can occur through droplets formed by a cough or sneeze as well as close contact with spatters of infected persons. However, the fecal-oral route of transmission was observed in other members of this family such as canine coronavirus (He et al., 2020), equine coronavirus (Nemoto, Schofield, & Cullinane, 2019), and feline coronavirus (C. Li et al., 2019). Although the COVID-19 is generally known by respiratory symptoms, many studies have shown that some patients, contracting this infection with gastrointestinal symptoms, have viral particles in their feces (Hindson, 2020). This observation strengthens the conjecture of the fecal-oral route of SARS-CoV-2 transmission. The result of an investigation on the stability of SARS-CoV-2 in aerosols and on varied inanimate surfaces manifested that the SARS-CoV-2 can be viable in aerosols for up to 3 h and on some inanimate surfaces such as copper, cardboard, and plastic or stainless steel for up to 4, 24, and 72 h, respectively, indicating the high stability of SARS-CoV-2 in the environmental condition (van Doremalen et al., 2020). Because of this evidence, food matrices may act as a potential vehicle of SARS-CoV-2. Foodborne viral infections commonly can be transferred through the fecal-oral route (Miranda & Schaffner, 2019). Contamination of foods may happen via three pathways, including within the food processing, infected food staffs, and ingestion of animal-based foods that have a zoonotic virus. Notwithstanding the recent FDA's statement, no scientific evidence exists to show a relationship between SARS-CoV-2 and food products. The administration of meat or other parts of infectious animals may cause zoonotic foodborne illnesses (Ceylan, Meral, & Cetinkaya, 2020). In China, between early July to mid-August 2020, at least 9 occurrences of food contamination by SARS-CoV-2 reported on the packaging materials, storage environment, and surface of imported frozen raw foods (Han, Zhang, He, & Jia, 2020). SARS-CoV-2 can be infective on contaminated food products; However, persisting the infectivity after consumption of foods, and subsequent viral invasion to body tissues has not been notified (Thippareddi, Balamurugan, Patel, Singh, & Brassard, 2020).
To our knowledge, the risk of SARS-CoV-2 transmission through food products has been evaluated in neither original articles nor review ones. Therefore, the main purpose of the present paper is to investigate the risk of various staple food products, including meat and meat products, dairy products, bread, fruits, and vegetables as potential vehicles for transmission of SARS-CoV-2.
2. COVID-19
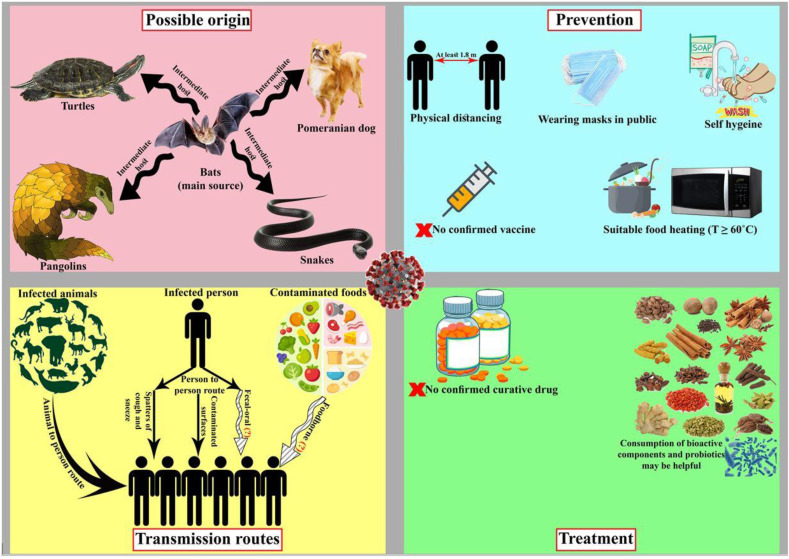
The global population is now facing with unaccustomed species of coronavirus, known as SARS-CoV-2, which caused not only great human fatalities but also a world economic crisis. Coronaviruses are RNA viruses belong to the family Coronaviridae, order Nidovirales that cause respiratory infection diseases such as MERS and SARS. These viruses are transmitted typically between animals and humans. SARS-CoV-2, which gives rise to COVID-19, is a new species of the coronavirus and causes a global life-threatening disorder to human health (Bandyopadhyay, 2020; Tang et al., 2020). Before December 2019, the known coronaviruses, which cause infection in humans are including; the alpha-CoVs, the beta-CoVs, SARS-CoV, and MERS-CoV (Gulati et al., 2020). The novel SARS-CoV-2 leads 2019 coronavirus disease, which first emerged in China and now spread worldwide (Lodigiani et al., 2020). Civet cats and camels were responsible for the transmission of SARS and MERS to humankind, respectively; however, bats were a natural source of both. Through phylogenesis, SARS-CoV-2 with 89% similarity, is correlated to SARS (Jalava, 2020; Pressman, Naidu, & Clemens, 2020) Some recent investigations propose bats as a possibly original reservoir of the COVID-19 outbreaks, but the intermediate host of SARS-CoV-2 is secret. Several animals such as snakes, pangolins, turtles, and Pomeranian dogs, were suggested as a possible intermediate host of the novel coronavirus (Dhama et al., 2020; Rodriguez-Morales et al., 2020).
Relatively little is known about the nature of the novel COVID-19, but gradually it will quickly increase. At the beginning of the new epidemic, for predicting this new disease behavior and its clinical signs and treatment, former evidence from past global epidemics of SARS and MERS was used. The COVID-19 symptoms usually appear after an incubation period of about 5–6 days and up to 14 days in some cases (Q. Li et al., 2020, Li et al., 2020, Li et al., 2020). These signs are widely different and may be similar to other viral infections such as influenza (Jalava, 2020). The most common symptoms which observed related to COVID-19 include dry cough, fever, dyspnea, headache, and myalgia or fatigue. Conversely, more rarely signs at the time of hospitalization are hemoptysis, shortness of breath, and diarrhea (Xu et al., 2020).
The specific receptor for the attachment of SARS-CoV-2 is found to be angiotensin converting-enzyme 2 (ACE-2) prevailing predominantly in the pulmonary epithelial cells (Zhou et al., 2020). The viral nucleic acids invade the cells immediately after attaching the spike protein to the receptor. Then, SARS-CoV-2 utilizes the cell factory to manufacture viral particles to be proliferated to infect other host cells. To maintain the organ entity, the immune mechanism enters to the scene, and an unbalanced immune response results in submerging of the alveoli with fluid, and subsequently impairing the epithelial cells of the alveoli, interfering O2 exchange which leads to acute respiratory distress syndrome, multi-organ breakdown, and eventually death (Huang et al., 2020; N.; Zhu et al., 2020). Interestingly, in addition to pulmonary epithelial cells, the ACE-2 receptor was observed in epithelial cells of other orangs such as the gastrointestinal tract (GIT) and liver, which implied that these orangs could be served as possible targets of SARS-CoV-2 invasion (Ding & Liang, 2020). In a study on the patients who tested COVID-19 positive obtained from stool samples, but negative from respiratory samples, the tissue specimens from GIT were collected (Uno, 2020). It was reported that the ACE-2 receptor and SARS-CoV-2 nucleocapsid protein were chiefly observed in the cytoplasm of epithelial cells of the stomach, duodenum, and rectum. Therefore, the fecal-oral route for SARS-CoV-2 transmission could be possible, and more studies should be conducted to evaluate the risk of infection under this condition.
The actions necessary to prevent the spread of this infection are maintaining personal and respiratory hygiene, and physical distancing. The first reported reproduction number of COVID-19 infection is between 1.4 and 6.5, with an average of 3.6 compared with Ebola, MERS, and H1N1, which were less than 2.7, indicating the COVID-19 infection is highly contagious. This value can be decreased through different mitigation strategies such as travel restriction and physical distancing. It is extremely recommended to lockdown offices and academic institutions and stop unnecessary gatherings with a large number of people. Otherwise, it is recommended to work with the minimum number of personnel (Bandyopadhyay, 2020).
3. Foodborne viral infections
Food contaminations may occur anywhere of the food supply chain, including pre-harvest, post-harvest, manufacturing, distribution, and just prior to consumption (Mousavi Khaneghah et al., 2020). Source of plentiful outbreaks linked to the consumption of various types of contaminated food sectors such as deli meat, vegetables, berries, shellfish, and a numerous of ready to eat foods (e.g., sandwiches, bread rolls, bakery products, cold meat, pastries, and ice cubes (Petrović & D'Agostino, 2016).
The fecal-oral route is considered as a frequent pathway for transmission of foodborne viral infection. This transmission pathway usually occurs through the ingestion of viruses that present as a very small piece in feces by other people as well as it can be transmitted to people by ingestion of vomitus pieces that have been scattered from infected people (Miranda & Schaffner, 2019). Table 1 demonstrates a brief outlook on foodborne viruses. In the following section, the main foodborne viruses would be discussed.
Table 1.
Foodborne virusesa.
| Virus | Genomic type | Family | Disease | Transmission | Main contaminated foods | Clinical symptoms |
|---|---|---|---|---|---|---|
| Norovirus | Single-stranded RNA | Calciviridae | Gastroenteritis |
|
Bottled water, imported frozen raspberries, oysters, Frozen strawberries, and lettuce, crustaceans, shellfish, mollusks, and leafy greens | Nausea, vomiting, non-bloody watery diarrhea, abdominal pain, and occasionally low-grade fever |
| ||||||
| HAV | Single-stranded RNA | Picornaviridae | Hepatitis A |
|
Seafood, strawberries, frozen berries, pomegranate arils, hamburgers, green onions, milk, and ice-slush beverages | Low-grade fever, fatigue, nausea, anorexia, malaise, myalgia, and vomiting |
| ||||||
| Human rotavirus | Double stranded RNA | Reoviridae | Rotaviral enteritis |
|
Water | Nausea, vomiting, watery diarrhea, and low-grade fever |
| ||||||
| HEV | Single-stranded RNA | Hepeviridae | Hepatitis E |
|
Water, vegetable strawberries, raspberries, shellfish (oysters, bivalves, and mussels), undercooked meat (pork, wild boar, or Sika deer) | Malaise, fever, body aches, nausea, vomiting, dark-colored urine, and jaundice |
| ||||||
| ||||||
|
| Virus | Genomic type | Family | Disease | Transmission | Main contaminated foods | Clinical symptoms |
|---|---|---|---|---|---|---|
| Astrovirus | Positive-sense single-stranded RNA virus | Astroviridae | Astroviral diarrhea |
|
Water, leafy green vegetables and soft fruits, shellfish (clams, mussels, and oysters), salads, sandwiches, garnishes, and turkeys | Gastroenteritis, mild watery diarrhea, abdominal pain, loss of appetite, vomiting, and fever |
| Adenovirus | Double-stranded DNA virus | Adenoviridae | Respiratory tract infections |
|
Water, ice, sausage dish, shellfish (clams, mussels, and oysters), strawberries, frozen berries, and leafy green vegetables | upper and lower respiratory tract infections, gastroenteritis, ocular infections, hemorrhagic cystitis, hepatitis, hemorrhagic colitis, pancreatitis, nephritis, or meningoencephalitis |
| Gastroenteritis | ||||||
| Ocular infections | ||||||
| Aichi virus | Positive-sense | Picornaviridae | Gastroenteritis |
|
Oysters and seafood | Nausea, diarrhea, abdominal pain, vomiting, and fever |
| single-stranded RNA | ||||||
| Sapovirus | Linear positive-sense single-stranded RNA | Caliciviridae | Gastroenteritis |
|
Salad, river water, and Oyster | Diarrhea, fever, and vomiting |
| ||||||
| ||||||
| Human parvovirus | Single-stranded, mostly negative-sense DNA viruses | Parvoviridae | Erythema infectiosum |
|
Shellfish | Neutropenia, fever, lymphopenia, and thrombocytopenia |
|
||||||
|
||||||
|
| Virus | Genomic type | Family | Disease | Transmission | Main contaminated foods | Clinical symptoms |
|---|---|---|---|---|---|---|
| SARS-CoV | Positive-sense single-stranded RNA | Coronaviridae | Respiratory infection |
|
Bats, civets, or raccoons | Diarrhea, fecal shedding, fever, fatigue, headaches, chills, muscle pain, loss of appetite, dry cough, and breathing difficulties |
| Gastroenteritis |
|
|||||
|
||||||
| MERS | Positive-sense single-stranded RNA | Coronaviridae | Respiratory infection |
|
Dromedary and bats | Fever, shortness of breath, and cough |
| Gastroenteritis |
|
|||||
|
||||||
| SARS-CoV-2 | Positive-sense single-stranded RNA | Coronaviridae | Respiratory infection |
|
Bats, pigs, and rabbits, pangolins, turtles, and snakes | Fever, dry cough, fatigue, diarrhea, aches and pains, sore throat, conjunctivitis, headache, a rash on the skin, or discoloration of fingers or toes, loss of taste or smell, breathing difficulties, chest pain or pressure |
| Gastroenteritis |
|
|||||
|
References: (Liu, 2019; Long et al., 2017; Percival et al., 2014).
3.1. Hepatitis a virus (HAV)
HAV is a nonenveloped RNA virus, a member of the Picornaviridae family, is the causative agent of acute inflammatory condition of the liver (Hirneisen et al., 2010). Among all Seven HAV genotypes have been recognized, three genotypes infect humans (I, II, and III). HAV is responsible for an acute self-limited infection, which is typically mild and subclinical in infants. This virus infects people living in areas with poor sanitation, so the risk is highest in developing countries. Transmission is most commonly person-to-person, but can occur as the foodborne and waterborne spread, among injection drug users and rarely by infected blood products. HAV infection generally is characterized by acute onset and early signs, including low-grade fever, anorexia, malaise, myalgia, and vomiting (Long, Prober, & Fischer, 2017).
It is estimated that HAV infection annually resulted in roughly 1.5 million clinical cases in worldwide, and death occurs in about 0.2% of clinical cases; however, the real statistics of infected people is about 10 times higher due to under underreporting (Franco, Meleleo, Serino, Sorbara, & Zaratti, 2012). A relatively low number of viral particles as low as 10 to 100 can cause HAV infection in humans. The incubation period generally ranges between 15 and 50 days, during which the viral particles are shed by an infected person. The prevalence of HAV is correlated with sanitation, unavailability of safe drinking water, and socioeconomic condition (Agrawal et al., 2019). Although most outbreaks of HAV take place during the establishment of food products and due to cross-contamination by a food handler, contamination of food before distribution lead to more widespread foodborne outbreaks. Decrementation of the foodborne transmission route of HAV may be feasible through appropriate food handler sanitation, the enhancement in the production of foods, and periodic vaccination of personnel at risk for HAV (Shah et al., 2020).
3.2. Norovirus (NoV)
NoV, also known as winter vomiting bug, is a non-enveloped RNA virus belonging to the Caliciviridae family with arch-like capsomeres on the capsids. Human NoVs, which are responsible for the foodborne disease, are the main cause of gastroenteritis outbreaks throughout the world (Lane, Husemann, Holland, & Khaled, 2019). According to the amino-acid sequence of NoV protein structure, NoVs have been divided into seven groups based on their genotypes: GI to GVII (Vinjé, 2015); GI and GII are the most related genogroups to human diseases, while type Ⅳ has rarely been implicated in outbreaks. G II.4 strains are the major cause of human NoV outbreaks, with up to 80% of infections. Multiple routes of transmission are identified such as person-to-person and upon exposure to contaminated food, fomites, water, or aerosolized vomitus particles. Studies showed that the common sources of norovirus outbreaks are contaminated shellfish, produce water, and ready-to-eat (RTE) foods (Robilotti, Deresinski, & Pinsky, 2015). NoV infection usually manifests as acute-onset vomiting with non-bloody, watery diarrhea, after an incubation period of 12–48 h. Other symptoms include nausea, abdominal cramps, and occasionally low-grade fever.
It is estimated that human NoV infection annually leads to almost 700 million cases and 220,000 deaths in the globe (Bartsch, Lopman, Ozawa, Hall, & Lee, 2016). In general, NoVs possess a low infectious dose of about 10 viral particles. The principal origin of NoV foodborne outbreaks is food products washed with tap water or processed by food handlers who have poor self-hygiene, and ingesting raw foods without appropriate cooking treatment. These food products include salads, vegetables, fruits, sandwiches, shellfish, and oysters (P. K. S. Chan, Kwan, & Chan, 2017).
3.3. Rotavirus (RV)
RV is a double-stranded RNA virus belongs to the Reoviridae family, with at least 7 different antigens (A to G) (Mizukoshi et al., 2014). The major cause of RV infection in humans is associated with rotavirus Group A; however, people have also been infected with RV Groups B and C. Rotavirus is the main causative agent of infantile diarrhea and the most common cause of hospitalization due to infantile gastroenteritis in industrialized countries. While the mortality rate due to RV infection is very low in developed countries, RV is the main cause of mortality in developing nations. RV infection causes non-bloody diarrhea, often with fever, and clinical signs typically last between 3 and 8 days. The most vulnerable population that is affected is children less than 5 years of age. RVs are transmitted by the fecal-oral route, or via aerosol droplets between people. The seasonality of rotavirus infection is less evident in tropical climates, but the disease is still more prevalent in the cooler and drier months (Long et al., 2017; Percival, Yates, Williams, Chalmers, & Gray, 2014).
Before the application of RV vaccination, this disease in the US annually caused 3 million disease episodes, involving half a million outpatient visits, and 60,000 rehabilitation, but resulting only in 20–40 deaths. Fortunately, the statistics suggested a decreasing trend of annual mortality rate caused by RV infection from 528,000 in 2000 to 215,000 in 2013 (Liu, 2019). Similar to NoV, RV also has a low infectious dose of about 10 viral particles. It is worthy of note that RV has high stability on hands at an infectious dose for about 1–4 h. Besides, washing hands with soap and water is not effective for RV removal. Thus, there is the risk of contamination of food products by food handlers who are shedding the viral particles. It has been appraised that about 4% of RV cases may source from food products, especially shellfish, contaminated during initial processing phases or from water (Sánchez-Uribe et al., 2013). Alcohol-based disinfectants are highly suggested as a practical way to alleviate RV infectivity (Golin, Choi, & Ghahary, 2020).
3.4. Other foodborne viruses
Other foodborne viruses such as adenovirus, Aichi virus, HEV, astrovirus, and enterovirus are not as prevalent as the main three viruses discussed above. The infection caused by latter viruses often leads to less severe illnesses, compared to HAV, NoV, and RV, which are usually self-limited. Besides, some of them may be endemic for a specific region, e.g. Aichi virus (Liu, 2019). Contaminated sea creatures such as oyster and shellfish as well as some other ready to eat foods are the main vehicle for the transmission of these viruses (Benabbes, Anga, Faouzi, Rhaissi, & Nourlil, 2013; La Rosa et al., 2018; Randazzo et al., 2018). In addition to GIT-related symptoms, adenoviruses can be causative agents for minor to severe respiratory infections in humans (Liu, 2019). As these enteric viruses incline to be spread from person-to-person, food products can be considered an ideal vehicle for their transmission. Establishment of good hygiene practices during food processing by food handlers, and maintaining self-hygiene by consumers prior to the ingestion of food products can be notable advice to hamper the risk of infection outbreaks.
4. Various transmission routes of SARS-CoV-2 and the probable function of foods as carriers
These viruses have zoonotic origins and are initially transmitted from animals to humans. Fig. 1 illustrates the recent discoveries about the novel coronavirus. Asymptomatic and pre-symptomatic patients can transmit the infection, thus making it difficult to control the outbreak (Limeres Posse, Diz Dios, & Scully, 2017). Based on the current information taken from several studies, a wide range of contamination of the seafood market might be the initiation source of the COVID-19 outbreak in Wuhan, China (Jalava, 2020). To date, the various transmission modes of SARS-CoV-2 have not been completely determined. This infection can spread through the respiratory tract like SARS and MERS (J. F. W. Chan et al., 2020). Person-to-person spread, which can occur through droplets formed by a cough or sneeze as well as contact with spatters of infected persons, is considered as an essential factor for the universal extension of the outbreak (Dhama et al., 2020; Pressman et al., 2020). The result of an investigation on the stability of SARS-CoV-2 in aerosols and on varied inanimate surfaces manifested that the SARS-CoV-2 can be viable in aerosols for up to 3 h and on some inanimate surfaces such as copper, cardboard, and plastic or stainless steel for up to 4, 24, and 72 h, respectively (van Doremalen et al., 2020). Although CoVs possess the capability to be infectious on food and/or food packaging from several days to several weeks, the infectivity potency declines as time passes (D. Li, Zhao, & Tan, 2020). So far, any document has not been reported about the transmission of SARS-CoV-2 through foods and/or food packaging materials (Pressman et al., 2020). Nevertheless, the probability of foodborne transmission cannot be excluded because of a study has demonstrated that the nucleic acid of SARS-CoV-2 has been found in the fecal paradigms of COVID-19 patients, whilst they indicated neither gastrointestinal signs nor correlation to the severity of lung infections (Dhama et al., 2020; Zhang, Wang, & Xue, 2020). The existence of the SARS-CoV-2 RNA in feces proposes the possibility of fecal-oral spread (Gao, Chen, & Fang, 2020). Accordingly, foodborne outbreaks principally may be caused by not only carry-over contaminations, for instance, food contamination through preparation by infected personnel, and contamination of food products within the manufacturing procedure, but also carrying-through contaminations, e.g. ingestion of meats, organs, and other products of animal origin harboring a zoonotic virus (Velebit et al., 2019). A recently published review suggested that foods may carry SARS-CoV-2 (Duda-Chodak, Lukasiewicz, Zięć, Florkiewicz, & Filipiak-Florkiewicz, 2020). However, the risk of SARS-CoV-2 survival under conventional processing and storage conditions of food products has not been discussed yet. To the best of our knowledge, the risk of fecal-oral route infection by SARS-CoV-2 in people contracted gastric diseases, e.g., gastric intestinal metaplasia and atrophic gastritis, is perturbing (Uno, 2020). In other words, elderly people with the mentioned diseases usually have higher pH values in the stomach (even 5 to 7), so the virus may survive the gastric condition and subsequently absorb to ACE-2 receptors. Then, COVID-19 with gastrointestinal symptoms may happen. All in all, the risk of COVID-19 infection through the fecal-oral route, and particularly by SARS-CoV-2 contaminated foods, should not be underrated.
Fig. 1.
Recent discoveries about SARS-CoV-2. The items which were determined by (?) mark was under preliminary studies.
4.1. Effect of food composition on the survival of SARS-CoV-2
Although there is no direct evidence about the impact of food components, e.g., water activity (aw), pH, protein, fat, sugar, and minerals, on SARS-CoV-2 stability, the general data about the effect of these intrinsic factors on food-borne viruses might come in handy. Coronaviruses are generally susceptible to acidic pH; however, it was suggested that SARS-CoV-2 is stable in a wide pH range from 3 up to 10 (Chin et al., 2020; Goli, 2020). Then, it must have been stable in almost all food products. Straube et al. (2010) reported that the avian influenza virus was inactivated in solutions containing 0.15, and 0.2% d,l-lactic acid, pH range 4.40–4.70, and 3.80 to 3.91, respectively, as opposed to 0.1% lactic acid solution (pH = 5.80 to 5.99) (Straube et al., 2010). Fortunately, lower pH values decline the heat stability of viruses (Goli, 2020). In other words, it was observed that coronaviruses are more stable at 4 °C than higher temperatures (Kampf, Todt, Pfaender, & Steinmann, 2020). As the pH value decreases, the detrimental effects of higher temperatures on the virus will be more pronounced. As was stated, the higher aw in herbs, fruits, and vegetables might promote the sensitivity of enteric viruses to heat treatment (Bozkurt, D'Souza, & Davidson, 2015). Besides, a direct correlation between food protein content, and viral stability against thermal treatment was also observed. For instance, Rabenau et al. (2005) discerned that heat treatment of SARS-CoV in solutions with and without protein (20% fetal calf serum) led to about 1.93 and 5.01 log reduction in viral titer, respectively (Rabenau et al., 2005). Similarly, higher fat contents exert noticeable protection effects on viruses. More to the point, the thermal treatment of milk constituting different fat levels from 1 to 18% at 71 °C resulted in an increment on D-value of HAV from 1.64 to 3.16, respectively (Bertrand et al., 2014; Bozkurt et al., 2015). Increasing the sugar level of food products would also contribute to enhancing viral heat resistance (Bertrand et al., 2014).
4.2. Various food products and SARS-CoV-2
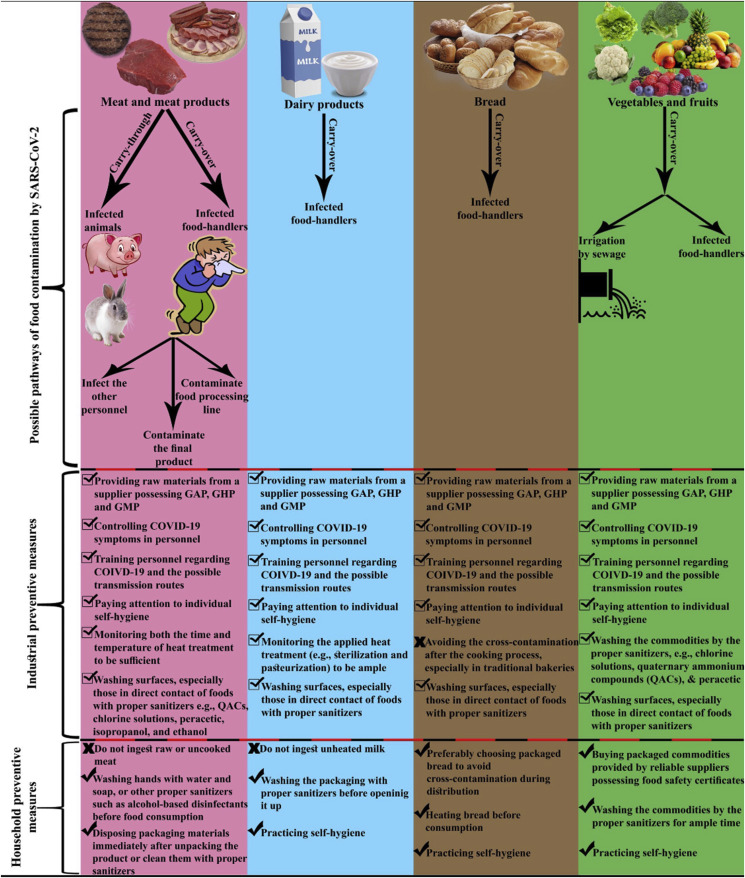
In the following section, the risk of some staple foods, including meat and meat products, dairy products, bread, vegetable, fruits, and RTE as potential carriers for SARS-CoV-2 transmission would be discussed. Fig. 2 illustrates the possible pathways of food contamination by SARS-CoV-2 and strategies to reduce viral transmission risk to various kinds of food products.
Fig. 2.
The risk of contamination of various food products by SARS-CoV-2 and suggested strategies to mitigate this risk.
4.2.1. Meat and meat products
Meat and meat products bestow invaluable amounts of macronutrients such as proteins, and essential lipids as well as micronutrients such as iron and some vitamins to human beings which are crucial for maintaining well-being (Kulczyński, Sidor, & Gramza-Michałowska, 2019). Notwithstanding all the benefactions which the consumption of meat brings us, it has always been a source of deleterious food pathogens, including Listeria monocytogenes (M. Zhu, Du, Cordray, & Ahn, 2005), Salmonella spp. (Knudsen, Sommer, Sørensen, Olsen, & Aabo, 2011), Escherichia coli O157: H7 (King et al., 2014), and HEV (Birgen, Njue, Kaindi, Ogutu, & Owade, 2020). Therefore, meat and meat products should be considered as a potential origin of outbreaks in case of poor hygiene conditions of processing as well as cross-contamination. Meats such as seafood, beef, poultry, and pork are rich sources of heparin and heparan (Pressman et al., 2020). The latter compounds are involved in SARS-CoV-2 viral attachment onto target cells (Mycroft-West et al., 2020); thus, the viral transmission through meat and meat products may be feasible. It is recommended that raw meat properly heat treated (˃ 60 °C for at least 30 min) before consumption. Besides, the consumption of game meat should be restricted (J. Li, Guan, et al., 2020). A recent study has demonstrated that SARS-CoV-2 can infect pig and rabbit cells under in vitro conditions (Chu et al., 2020). Thus, it is safer for wholesale meat distributors and retailers to provide raw meat from units possessing good manufacturing practices, good hygiene practices, and good agricultural practices (GAP) certificates as carry-through contamination of meat by SARS-CoV-2 can be possible.
In the commercial processing point of view, sausages and hamburgers are the most popular meat products. Sausages are usually trade as raw, precooked or cooked, and/or smoked. During the cooking process, sausages are heat-treated until the core temperature reaches 74–76 °C (Sukumaran et al., 2018). This thermal process seemed to be appropriate for SARS-CoV-2 elimination. One study suggested that heat treatment at 70 °C for only 5 min decreased SARS-CoV-2 viral load from about 6 log TCID50/mL to an undetectable number, whereas the viral count maintained roughly unchanged at 4 °C for 14 days (Chin et al., 2020). Therefore, substantial caution is required to be taken during the processing of raw and precooked sausages as they do not receive adequate heat treatment. The food production units should screen the COVID-19 symptoms among the personnel, especially those who are in direct contact with the processing line. The SARS-CoV-2 viral load may also be destructed during the heat processing of hamburgers (until the cold point reaches 72 °C) (Lima Filho et al., 2019). Detection of SARS-CoV-2 on the imported frozen chicken wing from Brazil is considered the first recognition of novel coronavirus on actual foods (Han et al., 2020). Besides, hamburgers are fired prior to consumption, which minimizes the risk of SARS-CoV-2 transmission. It is worth mentioning that freezing temperature during the storage of hamburgers may not be effective on the reduction of the SARS-CoV-2 viral load. Thus, personnel should be cautious not to contaminate the product during transportation and cold storage.
4.2.2. Dairy products
Milk is the most consumed dairy product in the globe (FAO, 2013). Although milk and milk products are lucrative sources of nutraceuticals such as proteins, calcium, and vitamin D, they might be contaminated to some pathogenic microorganisms, including Listeria monocytogenes, Salmonella spp., Campylobacter jejuni, and E. coli O157: H7 (Keba et al., 2020). Thus, heat treatment of milk is mandatory in most countries during industrial processing, as it ensures the safety of milk consumption. However, lots of people in the world may prefer to consume milk on-farm due to a lack of trust in industrial processing and possible adulteration (Viktoria Rampl, Eberhardt, Schütte, & Kenning, 2012). During the COVID-19 pandemic, the ingestion of raw milk without any thermal treatment is not recommended, due to the risk of cross-contamination by food handlers. In other words, SARS-CoV-2 may be spread through raw milk and subsequently infect the consumer. Furthermore, household heating of raw milk is not a good alternative, as the nutritional value of milk may considerably decrease. Therefore, the consumption of commercially heat-treated milk whether pasteurized or sterilized could be a reasonable choice. However, considering the high-temperature short-time (HTST) pasteurization of milk (about 71 °C, 15 s), the risk of SARS-CoV-2 survival exists as the time of heat treatment may not be ample for the destruction of viral particles. In this context, the ultra-high temperature (UHT) treatment (130–150 °C, 3–5 s) of milk may be more effective in the reduction of the viral load because of the high temperature (S, 2020). Besides, the latter process has lower detrimental effects on milk nutritional value compared to household heating. It is worth mentioning that it was reported some milk components such as lactoferrin, lactadherin, and glycomacropeptide possess antiviral role against various viruses, especially HIV (Giansanti, Panella, Leboffe, & Antonini, 2016), so that it also may have antiviral activity against SARS-CoV-2, which is suggested to be investigated.
Yogurt is another popular dairy product. It is expected that the SARS-CoV-2 viral load considerably decreases to a non-detectable value during the heat treatment of yogurt milk (90 °C, 15 min) before the fermentation stage (Mortazavian, Ghorbanipour, Mohammadifar, & Mohammadi, 2011). However, the risk of cross-contamination after heat treatment should be considered. By the end of fermentation, the final pH reaches a value of about 4.5. The metabolites which are produced by starter culture, including lactic acid, H2O2, and bacteriocins have significant inhibitory effects against various types of bacterial pathogens such as Salmonella spp., and Staphylococcus aureus (Azizkhani, Saris, & Baniasadi, 2020). It was observed that SARS-CoV-2 was stable in a great pH spectrum ranged from 3 to 10 (Chin et al., 2020). It may briefly explain the reason for the higher spread rate of SARS-CoV-2 in contrast to the other CoVs including SARS-CoV and MERS-CoV (Aboubakr, Sharafeldin, & Goyal, 2020, pp. 1–17). Thus, there is a risk of SARS-CoV-2 survival and maintaining its infectivity during refrigerated storage. It is noteworthy that some studies suggested the antiviral role of lactic acid bacteria metabolites (Tachedjian, Aldunate, Bradshaw, & Cone, 2017). Following the WHO statement, SARS-CoV-2 can be remained stable for up to 2 years at −20 °C. From detecting at least nine occurrences of food contamination by SARS-CoV-2 on imported frozen foods in China (Han et al., 2020), it can be concluded that several frozen dairy products such as ice cream, frozen yogurt, and frozen dairy desserts may act as carriers for the novel coronavirus. Therefore, more studies are needed to be conducted to evaluate the risk of transmission through dairy products.
Taken together, as SARS-CoV-2 majorly transmitted through humans, the industrialized food production units with lower staff as well as more machinery processing would distinctively reduce the risk of food contamination by SARS-CoV-2.
4.2.3. Bread
There are various types of bread in the world formulated by different cereals such as wheat, rye, oatmeal, and rice. Each of these bakery products has its specifications and particular nutritional values (Rasane, Jha, Sabikhi, Kumar, & Unnikrishnan, 2013). Bakery units usually are classified into two groups; traditional and commercial. Kimura et al. (2005) reported a Salmonellosis outbreak among people who ingested food from restaurants in Southern California and Arizona. The results of investigations determined that the source of the outbreak was the bread which was prepared by an ill food handler in a commercial bakery unit (Kimura et al., 2005). Therefore, the risk of carried-over contaminations via bread, which is processed, whether in commercial or traditional units, may cause concerns. Although the cooking process of bread may devastate SARS-CoV-2, the cross-contamination of the final product by infected food handlers can be possible. Besides, the contamination risk is hypothesized to be higher in traditional bread processing units compared to industrial ones. The precautions would be monitoring the food handlers in case of presence of COVID-19 symptoms, maintaining self-hygiene by food handlers, and household heating the bread before consumption.
4.2.4. Vegetable and fruit
The consumption of vegetables and fruits is critical for preserving human well-being. These nature-originated products are often considered as healthy by consumers. Hence, excessive cautions should be taken to safely distribute them among the population. To our knowledge, negligence in planting to harvest process would give rise to catastrophic outbreaks (Herman, Hall, & Gould, 2015). The contamination of vegetables and fruits are often struck by irrigation with sewage (Rusiñol et al., 2020). As evidence shows that COVID-19 infected patients can excrete viral particles, there are concerns about the presence and stability of SARS-CoV-2 in sewage. In other words, if plans and trees are irrigated by sewage constituting SARS-CoV-2, then there will be the risk of COVID-19 outbreaks which were spread through contaminated vegetables and fruits. It is wrong to presume that sunlight would disinfect the SARS-CoV-2 as the main component of sunlight is in the ultraviolet A spectrum (320–400 nm), which is almost ineffective against SARS-CoV-2 (Seyer & Sanlidag, 2020). Precautions can be implemented via several procedures, including providing the latter commodities from orchards, farms, and greenhouses possessing GAP certificate, avoiding the ingestion of unwashed fruits and vegetables, and peeling the skin of these commodities such as banana, orange, lemon, onion, and potato. Concerning commodities that cannot be peeled such as parsley, basil, chives, and berries, sufficient washing practice with appropriate disinfectants such as free chlorine solution (about 30 mg/L), Chlorine Dioxide (diluted by water at 1:2.5 ratio), and sodium hypochlorite (0.25%) (Quevedo-león et al., 2020). It was reported that the efficiency of washing in the removal of viral particles from fruits and vegetables was highly associated with their surface roughness. One study reported that washing raspberries with chlorinated water were less effective in the removal of HAV compared to other types of berries and vegetables (Butot, Putallaz, Amoroso, & Sánchez, 2009). This could be ascribable to the presence of crevices and hair-like projections which protect viral particles against the detrimental situations.
4.2.5. Other foods
Nuts such as almond, walnut, peanut, pistachio, and hazelnut are considered microbiologically safe from the viewpoint of consumers due to their low moisture content. However, there have been several outbreaks associated with these food products (Brar & Danyluk, 2018; Keller, 2014). The cross-contamination of nuts may occur at any point in production, including harvesting, processing, transportation, or ingestion (Brar & Danyluk, 2018). Nut-related outbreaks majorly transpired by pathogens such as S. aureus, Salmonella spp., L. monocytogenes, E. coli O157: H7, Cronobacter, Clostridium perfringens, C. botulinum, and Bacillus cereus (Brar & Danyluk, 2018; Brar & Danyluk, 2018). The infiltration of pathogens into the inner layers of nuts results in higher thermal stability in comparison with superficial pathogens (Brar & Danyluk, 2018). Moreover, owing to the high-fat content of nuts, more intense heat treatment is required to inhibit the bacteria (Brar & Danyluk, 2018). As SARS-CoV-2 possess high stability in environmental condition, the carry-over contamination of nuts may happen. Confectionary stores are considered as very crowded places with lots of people. In this condition, if nuts were exposed to air, then they would be contaminated by the infected individuals. As the nuts usually are not further processed at home, the risk of viral spread through nuts may increase. Thus, the nuts in confectionary should not be exposed to the direct contact of the public. Furthermore, the same precautions should be established for dried fruits, date palms, and the other RTE food products, as the carry-over contamination may happen (Beuchat & Mann, 2014). Food handlers shall be trained to carefully process foods in a way that avert any chances of cross-contamination during processing.
4.2.6. Street-vended foods and restaurants
Street food is considered as a major origin of cost-effective and ready to eat food or beverages as well as an important segment of the urban food supply that usually sold and made via peddlers in the public regions. A wide variety of foods such as meat, fish, fruits, vegetables, grains, cereals, frozen products, and beverages recognize as street food. Owing to vendors do not own adequate information toward food safety, this type of food is not trusty and may cause illnesses originating from foods throughout the world. The quality of raw materials to food manufacturing and storage may affect street-vended food safety (Ranka, 2020).
Even though food contamination can take place at any point from farm to fork, restaurant food handlers are an ordinary root of foodborne outbreaks. Diverse pathogens, including Salmonella spp. (Eikmeier, Medus, & Smith, 2018), Legionella anisa (Smith et al., 2015), E. coli O157: H7 (Hussein & Bollinger, 2005), Giardiasis (Ryan, Hijjawi, Feng, & Xiao, 2019), and norovirus (Angelo, Nisler, Hall, Brown, & Gould, 2017) have been hitherto reported to be associated with restaurant-originated foodborne outbreaks. It was reported that 66% of outbreaks in the USA from 2006 to 2007 were associated with restaurants. Several factors, such as food handler health, food preparation practices, and contamination prior to reaching the restaurant, are reported as the main etiology of restaurant-related outbreaks (Gould, Rosenblum, Nicholas, Phan, & Jones, 2013). Amongst the latter factors, food handler hygiene and health was estimated to possess the highest influence (64% of cases) on food contamination in the USA (Gould et al., 2013). Therefore, these statistics profoundly highlight the great impact of food handlers on the occurrence of food-associated outbreaks. Besides, the airborne spread of SARS-CoV-2 is also possible, particularly in indoor environments (Morawska & Cao, 2020). Thus, eating foods in indoor and busy restaurants next to lots of people would remarkably increase the risk of COVID-19 infection.
Some approaches, including sanitation, wearing masks, separation of kitchen from the public, sitting consumer six feet away from the person next to him, and preferably not eating foods in public or at least ingesting foods in open-air restaurants are recommended during the COVID-19 pandemic.
5. Conclusions
The globe populations are now facing with unaccustomed species of coronavirus, known as SARS-CoV-2, which caused not only great human fatalities, but also a world economic crisis. Some strategies and guidelines, including quarantine, social and physical distancing, have been proposed to manage and control the viral pandemic. As there was no confirmed vaccine or medication for the treatment of SARS-CoV-2 infection, preventive actions such as washing hands (for at least 20 s), wearing masks in the public, maintain respiratory system hygiene, and avoiding from direct contact with individuals who are having symptoms, should be considered. SARS-CoV-2 is predominantly an airborne infection, and recent studies have suggested that contracting the infection through food and/or food packaging may not be possible. However, this suggestion should not be taken for granted, as there are mere studies about this context, and SARS-CoV-2 possesses high stability in environmental conditions. Furthermore, as viral particles were observed in fecal samples collected from COVID-19 positive patients, the fecal-oral transmission route should be studied under more investigations. It is worthy of note that both carry-through and carry-over contaminations may occur for meat and meat products as there are pieces of evidence suggesting that SARS-CoV-2 infection can be transpired in pigs and rabbits. Besides, infected food handlers can contaminate various foods, including meat products, dairy products, bread, fruits, vegetables, and packaging materials. Concerning the food processing approach, industrial production may have some advantages over traditional one, especially because of the substitution of staff by machinery, reduction of direct contacts of food handlers with products, and maintain nutritional value to a large extent. Principally, the manufacturers of RTE and frozen products, including ice cream and frozen yogurt, should be cautious about the cross-contamination of these products by SARS-CoV-2 as they could not be further processed at home. Regarding food preparation units, ISO-22000, and HACCP guidelines are suggested to be precisely implemented. Beyond that, the efficiency of novel technologies such as pulsed electric field, cold plasma, and high-pressure processing, are suggested to be investigated in the elimination of SARS-CoV-2 as these techniques possess minor deleterious impacts on the nutritional values of food products. People have to pay attention to the hygiene instructions advised by WHO while shopping for food commodities. Consumers are suggested to immediately dispose of the packaging materials after unpacking the food products, and not to ingest raw foods, especially meat products. Therefore, various cooking approaches should be implemented at temperatures higher than 60 °C for at least 30 min. Furthermore, we propose the daily ingestion of foods containing bioactive components and probiotics, as the role of these compounds in reinforcing the immune system has been well established. More studies are suggested to be conducted considering the food, especially products from the animals as a possible vehicle for SARS-CoV-2 to shed light on the exact transmission modes of this virus.
Declaration of competing interest
There are none to declare.
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Transboundary and Emerging Diseases; 2020. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A., Singh S., Kolhapure S., Hoet B., Arankalle V., Mitra M. Increasing burden of hepatitis A in adolescents and adults and the need for long-term protection: A review from the Indian subcontinent. Infectious Disease and Therapy. 2019;8(4):483–497. doi: 10.1007/s40121-019-00270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo K.M., Nisler A.L., Hall A.J., Brown L.G., Gould L.H. Epidemiology of restaurant-associated foodborne disease outbreaks, United States, 1998-2013. Epidemiology and Infection. 2017;145(3):523–534. doi: 10.1017/S0950268816002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizkhani M., Saris P.E.J., Baniasadi M. An in-vitro assessment of antifungal and antibacterial activity of cow, camel, Ewe, and goat milk kefir and probiotic yogurt. Journal of Food Measurement and Characterization. 2020 doi: 10.1007/s11694-020-00645-4. [DOI] [Google Scholar]
- Bandyopadhyay S. Coronavirus disease 2019 (COVID-19): We shall overcome. Clean Technologies and Environmental Policy. 2020;22(3):545–546. doi: 10.1007/s10098-020-01843-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M.L., Yeasmin S. Elsevier Inc; 2018. Foodborne diseases and responsible agents. Food safety and preservation. [DOI] [Google Scholar]
- Bartsch S.M., Lopman B.A., Ozawa S., Hall A.J., Lee B.Y. Global economic burden of norovirus gastroenteritis. PloS One. 2016;11(4):1–16. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabbes L., Anga L., Faouzi A., Rhaissi H., Nourlil J. Detection OF human enterovirus and adenovirus IN shellfish collected IN Morocco mediterranean coast. Journal of Microbiology, Biotechnology and Food Sciences. 2013;3(2):97–100. doi: 10.15414/jmbfs.2017.7.2.97-100. [DOI] [Google Scholar]
- Bertrand I., Bosch A., Gantzer C., Maul A., Pinto R., Stein J. United Kingdom Food Standards Agency; 2014. A critical review of the effect of heat, pH and water activity on the survival of Hepatitis A and E viruses.https://www.food.gov.uk/sites/default/files/FS101074_Hepatitis_virus_survival_review_-FINAL.pdf [Google Scholar]
- Beuchat L.R., Mann D.A. Survival of salmonella on dried fruits and in aqueous dried fruit homogenates as affected by temperature. Journal of Food Protection. 2014;77(7):1102–1109. doi: 10.4315/0362-028X.JFP-13-549. [DOI] [PubMed] [Google Scholar]
- Birgen B.J., Njue L.G., Kaindi D.W.M., Ogutu F.O., Owade J.O. Quantitative versus qualitative risk assessment of meat and its products: What is feasible for sub-saharan african countries? Critical Reviews in Food Science and Nutrition. 2020:1–13. doi: 10.1080/10408398.2020.1812505. [DOI] [PubMed] [Google Scholar]
- Bosch A., Pintó R.M., Guix S. Foodborne viruses. Current Opinion in Food Science. 2016;8:110–119. doi: 10.1016/j.cofs.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt H., D'Souza D.H., Davidson P.M. Thermal inactivation of foodborne enteric viruses and their viral surrogates in foods. Journal of Food Protection. 2015;78(8):1597–1617. doi: 10.4315/0362-028X.JFP-14-487. [DOI] [PubMed] [Google Scholar]
- Brar P.K., Danyluk M.D. Nuts and grains: Microbiology and preharvest contamination risks. Preharvest Food Safety. 2018;(April):105–121. doi: 10.1128/microbiolspec.pfs-0023-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar P.K., Danyluk M.D. In: Preharvest food safety. Thakur S., Kniel K.E., editors. ASM Press; Washington, DC: 2018. Nuts and grains: Microbiology and preharvest contamination risks; pp. 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butot S., Putallaz T., Amoroso R., Sánchez G. Inactivation of enteric viruses in minimally processed berries and herbs. Applied and Environmental Microbiology. 2009;75(12):4155–4161. doi: 10.1128/AEM.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan Z., Meral R., Cetinkaya T. Relevance of SARS-CoV-2 in food safety and food hygiene: Potential preventive measures, suggestions and nanotechnological approaches. VirusDisease. 2020;31(2):154–160. doi: 10.1007/s13337-020-00611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K.S., Kwan H.S., Chan M.C.W. In: 1st ed. Chan P.K.S., Kwan H.S., Chan M.C.W., editors. Vol. 91. Academic Press; London: 2017. The norovirus features, detection, and prevention of foodborne disease. (Foreign affairs). [DOI] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1):e10. doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Chan J.F.-W., Yuen T.T.-T., Shuai H., Yuan S., Wang Y., et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. The Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/s2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Kumar Patel S., Sharun K., Pathak M., Tiwari R., Iqbal Yatoo M., et al. SARS-CoV-2: Jumping the species barrier, lessons from SARS and MERS, its zoonotic spillover, transmission to humans, preventive and control measures and recent developments to counter this pandemic virus. Jphres.Org. 2020:2020040011. doi: 10.20944/preprints202004.0011.v1. SARS-CoV-2: Jumping the Species Barrier, Les. April. [DOI] [Google Scholar]
- Ding S., Liang T.J. Is SARS-CoV-2 also an enteric pathogen with potential fecal–oral transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020;159(1):53–61. doi: 10.1053/j.gastro.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda-Chodak A., Lukasiewicz M., Zięć G., Florkiewicz A., Filipiak-Florkiewicz A. Covid-19 pandemic and food: Present knowledge, risks, consumers fears and safety. Trends in Food Science & Technology. 2020;105:145–160. doi: 10.1016/j.tifs.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikmeier D., Medus C., Smith K. Incubation period for outbreak-associated, non-typhoidal salmonellosis cases, Minnesota. Epidemiology and Infection. 2018;146(4):423–429. doi: 10.1017/S0950268818000079. 2000-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO Dairy production and products. 2013. http://www.fao.org/dairy-production-products/products/en/
- Franco E., Meleleo C., Serino L., Sorbara D., Zaratti L. Hepatitis A: Epidemiology and prevention in developing countries. World Journal of Hepatology. 2012;4(3):68–73. doi: 10.4254/wjh.v4.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q.Y., Chen Y.X., Fang J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. Journal of Digestive Diseases. 2020;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti F., Panella G., Leboffe L., Antonini G. Lactoferrin from milk: Nutraceutical and pharmacological properties. Pharmaceuticals. 2016;9(4):1–15. doi: 10.3390/ph9040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goli M. Review of novel human β-coronavirus (2019-nCoV or SARS-CoV-2) from the food industry perspective—appropriate approaches to food production technology. Food Sciences and Nutrition. 2020;(August):1–10. doi: 10.1002/fsn3.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golin A.P., Choi D., Ghahary A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. American Journal of Infection Control. 2020;48(9):1062–1067. doi: 10.1016/j.ajic.2020.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould L.H., Rosenblum I., Nicholas D., Phan Q., Jones T.F. Contributing factors in restaurant-Associated foodborne disease outbreaks, foodnet sites, 2006 and 2007. Journal of Food Protection. 2013;76(11):1824–1828. doi: 10.4315/0362-028X.JFP-13-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati A., Pomeranz C., Qamar Z., Thomas S., Frisch D., George G., et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic aishwarya. The American Journal of the Medical Sciences. 2020;360(1):5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Zhang X., He S., Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environmental Chemistry Letters. 2020 doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman K.M., Hall A.J., Gould L.H. Outbreaks attributed to fresh leafy vegetables, United States, 1973-2012. Epidemiology and Infection. 2015;143(14):3011–3021. doi: 10.1017/S0950268815000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H.-J., Zhang W., Liang J., Lu M., Wang R., Li G., et al. Etiology and genetic evolution of canine coronavirus circulating in five provinces of China, during 2018–2019. Microbial Pathogenesis. 2020;145:1–7. doi: 10.1007/s00134-020-05991-x.Bizzarro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson J. COVID-19: Faecal–oral transmission? Nature Reviews | GastrOenterOlOGy & HepatOlOGy. 2020;17(259) doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirneisen K.A., Black E.P., Cascarino J.L., Fino V.R., Hoover D.G., Kniel K.E. Viral inactivation in foods: A review of traditional and novel food-processing technologies. Comprehensive Reviews in Food Science and Food Safety. 2010;9(1):3–20. doi: 10.1111/j.1541-4337.2009.00092.x. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein H.S., Bollinger L.M. Prevalence of Shiga toxin-producing Escherichia coli in beef. Meat Science. 2005;71(4):676–689. doi: 10.1016/j.meatsci.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Jalava K. First respiratory transmitted food borne outbreak? International Journal of Hygiene and Environmental Health. 2020 doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keba A., Rolon M.L., Tamene A., Dessie K., Vipham J., Kovac J., et al. Review of the prevalence of foodborne pathogens in milk and dairy products in Ethiopia. International Dairy Journal. 2020;109:104762. doi: 10.1016/j.idairyj.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.E. In: Matthews K.R., Sapers G., Gerba C., editors. Vol. 2011. Academic Press; San Diego, CA: 2014. Tree fruits and nuts: Outbreaks, contamination sources, prevention, and remediation; pp. 291–312. (The produce contamination problem). [DOI] [Google Scholar]
- Kimura A.C., Palumbo M.S., Meyers H., Abbott S., Rodriguez R., Werner S.B. A multi-state outbreak of Salmonella serotype Thompson infection from commercially distributed bread contaminated by an ill food handler. Epidemiology and Infection. 2005;133(5):823–828. doi: 10.1017/S0950268805004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.A., Loukiadis E., Mariani-Kurkdjian P., Haeghebaert S., Weill F.X., Baliere C., et al. Foodborne transmission of sorbitol-fermenting Escherichia coli O157:[H7] via ground beef: An outbreak in northern France, 2011. Clinical Microbiology and Infections. 2014;20(12):O1136–O1144. doi: 10.1111/1469-0691.12736. [DOI] [PubMed] [Google Scholar]
- Knudsen G.M., Sommer H.M., Sørensen N.D., Olsen J.E., Aabo S. Survival of Salmonella on cuts of beef carcasses subjected to dry aging. Journal of Applied Microbiology. 2011;111(4):848–854. doi: 10.1111/j.1365-2672.2011.05094.x. [DOI] [PubMed] [Google Scholar]
- Kulczyński B., Sidor A., Gramza-Michałowska A. Characteristics of selected antioxidative and bioactive compounds in meat and animal origin products. Antioxidants. 2019;8(9) doi: 10.3390/antiox8090335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Proroga Y.T.R., De Medici D., Capuano F., Iaconelli M., Della Libera S., et al. First detection of hepatitis E virus in shellfish and in seawater from production areas in southern Italy. Food and Environmental Virology. 2018;10(1):127–131. doi: 10.1007/s12560-017-9319-z. [DOI] [PubMed] [Google Scholar]
- Lane D., Husemann E., Holland D., Khaled A. Understanding foodborne transmission mechanisms for norovirus: A study for the UK's food standards agency. European Journal of Operational Research. 2019;275(2):721–736. doi: 10.1016/j.ejor.2018.11.070. [DOI] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New England Journal of Medicine. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li J., Justin), Xie X., Cai X., Huang J., et al. Game consumption and the 2019 novel coronavirus. The Lancet Infectious Diseases. 2020;20(3):275–276. doi: 10.1016/S1473-3099(20)30063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Liu Q., Kong F., Guo D., Zhai J., Su M., et al. Circulation and genetic diversity of Feline coronavirus type I and II from clinically healthy and FIP-suspected cats in China. Transboundary and Emerging Diseases. 2019;66(2):763–775. doi: 10.1111/tbed.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. International Journal of Antimicrobial Agents. 2020;55(5):105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Filho T., Della Lucia S.M., Minim L.A., Gamba M.M., Lima R.M., Minim V.P.R. Directional hedonic thresholds for sodium concentration in hamburger. Food Quality and Preference. 2019;78(June):103722. doi: 10.1016/j.foodqual.2019.103722. [DOI] [Google Scholar]
- Limeres Posse J., Diz Dios P., Scully C. 2017. Viral diseases transmissible by kissing. Saliva protection and transmissible diseases. [DOI] [Google Scholar]
- Liu D. In: Liu D., editor. CRC Press; Boca Raton, FL: 2019. Handbook of foodborne diseases. (Taylor & francis group). [DOI] [Google Scholar]
- Li D., Zhao M.Y., Tan T.H.M. What makes a foodborne virus: Comparing coronaviruses with human noroviruses. Current Opinion in Food Science. 2020;42:1–7. doi: 10.1016/j.cofs.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research. 2020;191(April):9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.S., Prober C.G., Fischer M. Principles and practice of pediatric infectious diseases. Principles and Practice of Pediatric Infectious Diseases. 2017:1–1662. doi: 10.1086/516117. [DOI] [Google Scholar]
- Miranda R.C., Schaffner D.W. Virus risk in the food supply chain. Current Opinion in Food Science. 2019;30:43–48. doi: 10.1016/j.cofs.2018.12.002. January. [DOI] [Google Scholar]
- Mizukoshi F., Kuroda M., Tsukagoshi H., Sekizuka T., Funatogawa K., Morita Y., et al. A food-borne outbreak of gastroenteritis due to genotype G1P[8] rotavirus among adolescents in Japan. Microbiology and Immunology. 2014;58(9):536–539. doi: 10.1111/1348-0421.12176. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavian A.M., Ghorbanipour S., Mohammadifar M.A., Mohammadi M. Biochemical properties and viable probiotic population of yogurt at different bacterial inoculation rates and incubation temperatures. The Philippine Agricultural Scientist. 2011;94(2):155–160. [Google Scholar]
- Mousavi Khaneghah A., Abhari K., Eş I., Soares M.B., Oliveira R.B.A., Hosseini H., et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends in Food Science & Technology. 2020;95(November 2019):205–218. doi: 10.1016/j.tifs.2019.11.022. [DOI] [Google Scholar]
- Mycroft-West C., Su D., Elli S., Guimond S., Miller G., Turnbull J.…Skidmore M. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv. 2020:1–9. doi: 10.1101/2020.02.29.971093. [DOI] [Google Scholar]
- Nemoto M., Schofield W., Cullinane A. The first detection of equine coronavirus in adult horses and foals in Ireland. Viruses. 2019;11(10):1–7. doi: 10.3390/v11100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival S., Yates M.V., Williams D.W., Chalmers R.M., Gray N.F. In: 2nd ed. Percival S., Yates M.V., Williams D.W., Chalmers R.M., Gray N.F., editors. Academic Press is; London: 2014. Microbiology of waterborne diseases: Microbiological aspects and risks. (Microbiology of waterborne diseases: Microbiological aspects and risks). [DOI] [Google Scholar]
- Petrović T., D'Agostino M. Viral contamination of food. Antimicrobial Food Packaging. 2016:65–79. doi: 10.1016/B978-0-12-800723-5.00005-X. [DOI] [Google Scholar]
- Pressman P., Naidu A.S., Clemens R. COVID-19 and food safety: Risk management and future considerations. Nutrition Today. 2020;(December 2019):1–8. [Google Scholar]
- Quevedo-león R., Bastías-Montes J., Espinoza-Tellez T., Ronceros B., Balic I., Muñoz O. Inactivation of Coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Scientia Agropecuaria. 2020;11(2):257–266. doi: 10.17268/sci.agropecu.2020.02.14. [DOI] [Google Scholar]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Medical Microbiology and Immunology. 2005;194(1–2):1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Vásquez-García A., Bracho M.A., Alcaraz M.J., Aznar R., Sánchez G. Hepatitis E virus in lettuce and water samples: A method-comparison study. International Journal of Food Microbiology. 2018;277(December 2017):34–40. doi: 10.1016/j.ijfoodmicro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Ranka S. How corona virus could affect the culture of eating special reference to street Food : The new normal. IOSR Journal of Business and Management. 2020;22(6):1–7. doi: 10.9790/487X-2206060107. [DOI] [Google Scholar]
- Rasane P., Jha A., Sabikhi L., Kumar A., Unnikrishnan V.S. Nutritional advantages of oats and opportunities for its processing as value added foods - a review. Journal of Food Science & Technology. 2013;52(2):662–675. doi: 10.1007/s13197-013-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robilotti E., Deresinski S., Pinsky B.A. Norovirus. Clinical Microbiology Reviews. 2015;28(1):134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Bonilla-Aldana D.K., Balbin-Ramon G.J., Rabaan A.A., Sah R., Paniz-Mondolfi A., et al. History is repeating itself: Probable zoonotic spillover as the cause of the 2019 novel coronavirus epidemic. Infezioni in Medicina, Le. 2020;28(1):3–5. [PubMed] [Google Scholar]
- Rusiñol M., Hundesa A., Cárdenas-Youngs Y., Fernández-Bravo A., Pérez-Cataluña A., Moreno-Mesonero L., et al. Vol. 710. Science of the Total Environment; 2020. Microbiological contamination of conventional and reclaimed irrigation water: Evaluation and management measures. December 2019. [DOI] [PubMed] [Google Scholar]
- Ryan U., Hijjawi N., Feng Y., Xiao L. Giardia: An under-reported foodborne parasite. International Journal for Parasitology. 2019;49(1):1–11. doi: 10.1016/j.ijpara.2018.07.003. [DOI] [PubMed] [Google Scholar]
- S S. UHT processing - best technology for shelf - life extension of milk. International Journal of Food Science, Nutrition and Dietetics. 2020;9:1–2. doi: 10.19070/2326-3350-200009e. [DOI] [Google Scholar]
- Sánchez-Uribe E., Esparza-Aguilar M., Gastañaduy P.A., Desai R., Patel M., Richardson V. Risk factors associated with rotavirus gastroenteritis during a community outbreak in chiapas , Mexico during the postvaccination era. Journal of the Pediatric Infectious Diseases Society. 2013;2(1):15–20. doi: 10.1093/jpids/pis077. [DOI] [PubMed] [Google Scholar]
- Seyer A., Sanlidag T. Solar ultraviolet radiation sensitivity of SARS-CoV-2. The Lancet Microbe. 2020;1(1):e8–e9. doi: 10.1016/s2666-5247(20)30013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N., Faridi M.M.A., Mitra M., Bavdekar A., Karadkhele A., Puppalwar G., et al. Review of long term immunogenicity and tolerability of live hepatitis A vaccine. Human Vaccines & Immunotherapeutics. 2020:1–6. doi: 10.1080/21645515.2020.1741997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.S., Ritger K., Samala U., Black S.R., Okodua M., Miller L., et al. Legionellosis outbreak associated with a hotel fountain. Open Forum Infectious Diseases. 2015;2(4):1–7. doi: 10.1093/o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube J., Manteufel J., Heinze J., Fehlhaber K., Truyen U., Albert T. Low pathogenic avian influenza viruses (H3N8, H5N6): In vitro influence of D,L-lactic acid and sodium chloride on infectivity and virus persistence in short fermented raw poultry sausage. Food and Environmental Virology. 2010;2(2):74–82. doi: 10.1007/s12560-010-9030-9. [DOI] [Google Scholar]
- Sukumaran A.T., Holtcamp A.J., Englishbey A.K., Campbell Y.L., Kim T., Schilling M.W., et al. Effect of deboning time on the growth of Salmonella, E. coli, aerobic, and lactic acid bacteria during beef sausage processing and storage. Meat Science. 2018;139(January):49–55. doi: 10.1016/j.meatsci.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Tachedjian G., Aldunate M., Bradshaw C.S., Cone R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Research in Microbiology. 2017;168(9–10):782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., Yao X., Wu X., et al. On the origin and continuing evolution of SARS-CoV-2. National Science Review. 2020;(February):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thippareddi H., Balamurugan S., Patel J., Singh M., Brassard J. Coronaviruses – potential human threat from foodborne transmission? Lebensmittel-Wissenschaft & Technologie. 2020;134(July):110147. doi: 10.1016/j.lwt.2020.110147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y. Why does SARS-CoV-2 invade the gastrointestinal epithelium? Gastroenterology. 2020. [DOI] [PMC free article] [PubMed]
- Velebit B., Djordjevic V., Milojevic L., Babic M., Grkovic N., Jankovic V., et al. The common foodborne viruses: A review. IOP Conference Series: Earth and Environmental Science. 2019;333(1) doi: 10.1088/1755-1315/333/1/012110. [DOI] [Google Scholar]
- Viktoria Rampl L., Eberhardt T., Schütte R., Kenning P. Consumer trust in food retailers: Conceptual framework and empirical evidence. International Journal of Retail & Distribution Management. 2012;40(4):254–272. doi: 10.1108/09590551211211765. [DOI] [Google Scholar]
- Vinjé J. Advances in laboratory methods for detection and typing of norovirus. Journal of Clinical Microbiology. 2015;53(2):373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368(January):1–7. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.C., Wang S.B., Xue Y.D. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. Journal of Medical Virology. 2020;92(6):680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X., Lou, Wang X.G., Hu B., Zhang L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Du M., Cordray J., Ahn D.U. Control of Listeria monocytogenes contamination in ready-to-eat meat products. Comprehensive Reviews in Food Science and Food Safety. 2005;4(2):34–42. doi: 10.1111/j.1541-4337.2005.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]