Abstract
BACKGROUND:
As the geriatric population grows, the need for hospitals performing high quality emergency general surgery (EGS) on older patients will increase. Identifying clusters of high-performing geriatric emergency general surgery hospitals would substantiate the need for in-depth analyses of hospital-specific structures and practices that benefit older EGS patients. The objectives of this study were therefore to identify clusters of hospitals based on mortality performance for geriatric patients undergoing common EGS operations and to determine if hospital performance was similar for all operation types.
METHODS:
Hospitals in the California State Inpatient Database were included if they performed a range of eight common EGS operations in patients 65 years or older, with a minimum requirement of three of each operation performed over 2 years. Multivariable beta regression models were created to define hospital-level risk-adjusted mortality. Centroid cluster analysis was used to identify groups of hospitals based on mortality and to determine if mortality-performance differed by operation.
RESULTS:
One hundred seven hospitals were included, performing a total of 24,279 operations in older patients. Hospitals separated into three distinct clusters: high, average, and low performers. The high-performing hospitals had survival rates 1 to 2 standard deviations better than the low-performers (p < 0.001). For each cluster, high performance in any one EGS operation consistently translated into high performance across all EGS operations.
CONCLUSION:
Hospitals conducting EGS operations in the geriatric patient population cluster into three distinct groups based on their survival performance. High-performing hospitals significantly outperform the average and low performers across every operation. The high-performers achieve reliable, high-quality results regardless of operation type. Further qualitative research is needed to investigate the perioperative drivers of hospital performance in the geriatric EGS population.
LEVEL OF EVIDENCE:
Study Type Prognostic, level III.
Keywords: Emergency general surgery, geriatric, hospital performance, cluster analysis
As the geriatric population grows, the need for hospitals performing high-quality emergency general surgery (EGS) on older patients is increasing.1,2 A growing body of literature has been devoted to the study of the geriatric EGS population at the patient level, primarily aimed at aiding risk stratification and the prediction of individual outcomes.3-6 Although hospital structure and processes have been assessed in nonsurgical diseases among geriatric patients,7,8 much less attention has been focused on the study of institutional characteristics that influence the perioperative care of older EGS patients.9
Previous research examining hospital performance demonstrates that institutions can vary significantly across a variety of metrics, leading to a dichotomy of high performance and low-performance hospitals.10-12 The studied factors that contribute to a hospital's performance—such as mortality outcome— are complex and multifactorial, and include institutional culture, proficiency, and the interconnectedness of organizational structure and silos.13,14
In centers that care for high acuity and time-dependent conditions, such as those treating the geriatric EGS population, institutional culture and high-functioning habits are crucial to patient outcomes.15 Identifying high-performance practices and institutions (“high performance” in this context meaning low mortality) may help address the challenge of caring for high acuity conditions in an aging population. The objectives of this study were therefore to identify clusters of hospitals based on mortality performance for geriatric patients undergoing common EGS operations, and to determine if hospital performance was similar for all operation types.
METHODS
Data Source
Patients who underwent one of eight common EGS operations over a 2-year period (2010–2011) were identified from within the California State Inpatient Database (SID). The SID contains patient level factors including demographics, comorbidities, payor status, procedures, length of stay and in-hospital mortality. It is published by the Agency for Healthcare Research and Quality as a part of the Healthcare Cost and Utilization project. The SID was utilized to determine at which hospitals patients underwent analyzed operations, a list of which was then utilized to pair the database to information contained in the American Healthcare Association (AHA) Annual Survey of Hospitals database. The AHA contains hospital level variables including bed size, technology status, and trauma center or academic teaching affiliations. The setting for this study, California, was chosen based on the heterogeneity of its population, geography, and healthcare settings in an attempt to generate conclusions that were as generalizable as possible.
Patient Selection
Adults 65 years or older, who underwent any of eight commonly performed EGS operations and were captured in the SID, were included in this analysis. Operations were identified based on their International Classification of Disease, 9th edition procedure and diagnosis codes (listed in Supplemental Digital Content, Appendix A, http://links.lww.com/TA/B341). The operations analyzed included both open and laparoscopic appendectomy, cholecystectomy, colectomy, inguinal hernia repair, ventral hernia repair, lysis of adhesions, enterectomy, and repair of a perforated viscus.
Patients who were transferred between analyzed institutions were excluded due to the difficulty of ascribing responsibility for patient outcomes between transferring versus receiving institutions. Likewise, patients who underwent more than one of the index operations were only analyzed based on the outcome of their first operation. Hospitals included for analysis performed at least three of each procedure over the 2-year study period. The rationale for these inclusion criteria was to ensure that the analysis was performed among institutions that performed a variety of these operations relatively commonly and to exclude institutions for whom these operations are exceedingly rare. We further excluded hospitals classified as specialty rehabilitation hospitals, pediatric hospitals, and Veteran's Administration hospitals.
Analyzed Variables
Variables utilized in this analysis can be divided into two categories: patient-level and hospital-level variables. Patient-level variables included age, sex, race, ethnicity, payor status, operation performed, Van Walraven comorbidity score, and in-hospital mortality. The Van Walraven Comorbidity Score was utilized due to its previous validation as a superior modification of the Elixhauser score for use in administrative data sets.16-18 Hospital-level variables available in the AHA data set that were utilized for analysis included total hospital operative volume for each of the analyzed operations, trauma center status (defined as either state or American College of Surgeons Level I or II status), affiliation with a medical school, and high-technology capability (defined as having performed general cardiac surgery, cardiac transplantation, or liver transplantation).
STATISTICAL METHODS
Analysis was performed in three distinct steps.
Part I: Risk-Adjustment of Mortality Rates
Part I consisted of the construction of operation specific risk-adjusted models for the proportion of patients who died at each analyzed hospital. As each operation was modeled separately, each hospital had eight separate risk-adjusted death rates corresponding to each analyzed operation. Beta-logistic generalized linear regression models were constructed at the hospital level for each of the analyzed operations to model risk-adjusted mortality. As the hospital was the unit of analysis, the risk-adjustment consisted of mortality proportions (ratio of numbers of deaths versus total numbers of operations performed) for each hospital. Beta regression is commonly used to model a continuous dependent variable with a limited range from 0 to 1, often representing probabilities or proportions.19 The Beta distribution was utilized as it accounts for the continuous dependent proportion random variable. The logit link function allows for model coefficients to be interpreted on the natural-log odds scale, which can then be exponentiated to produce odds ratios. The combination of the Beta probability distribution coupled to the logit link allows for capture of the expected sigmoidal relationship between mortality proportions and the linear function of the predictors, ensuring risks adjustments will properly be within the (0,1) range. Modeling adjusted for mortality over the 2-year period accounting for both patient case-mix as well as hospital-level variables.
Part II: Cluster Analysis
Part II consisted of inputting these eight mortality rates into a cluster analysis to compare performance by operation type across hospitals. K-means cluster analysis was performed among the eight adjusted hospital mortality proportions to identify subgroups of hospitals with similar mortality performance across operation type. The criteria for similar mortality performance were defined as statistically distinct clusters that also allowed for meaningful and useful clinical interpretation. Each of the eight outcomes was standardized to have a mean equal to zero and a variance of one. Standardizing the means at zero simplifies interpretation: positive/negative values are dichotomized to above/below average. Setting all variances equal to one attached equal weights across operation types in the cluster solutions' algorithm, preventing those procedures with the highest variability of mortality from having undue influence on the cluster solution. An R-squared statistic was calculated to assess the relative fit of each cluster model and test performance of increasing or decreasing the number of clusters modeled. Within-cluster variation refers to the variability of mortality for each operation across hospitals within that cluster. The wider the operation-specific mortality rate ranges across all hospitals in a cluster, the greater the within-cluster variation. Larger within-cluster variation is an indication of greater heterogeneity in standardized hospital mortality rates in that cluster compared with other clusters.
Part III: Covariates of Cluster Membership
Part III used generalized multinomial logistic regression analyses to determine factors that led to differences in hospital cluster performance. We considered two types of covariates: those that were institutional characteristics of the hospitals (trauma center status, high-technology status, medical school affiliation, and number of beds >100) and those that were characteristics of the patient case-mix of the hospitals (mean age at admission, mean comorbidity severity score, % sex, % race, % payor status). Multinomial logistic regression models were analyzed to determine what institutional hospital-level factors were significantly associated with cluster assignment. Cluster membership was treated as a simple categorical variable without attention paid to performance. Factors included for analysis included trauma center status, high-technology capability, and teaching center status.
All data were analyzed using SAS 9.4 (SAS Institute, Cary, NC). A p value less than 0.05 was considered statistically significant. This study was approved by the Yale University Human Research Protection Program Institutional Review Board for biomedical research, known as the Human Investigation Committee.
RESULTS
The analysis encompassed 107 hospitals performing a total of 24,279 operations over the 2-year study period. The most common operations analyzed were cholecystectomy followed by colectomy. The total breakdown of operations performed is as follows: appendectomy, 2,837 (12%); cholecystectomy, 9,957 (41%); colectomy, 4,051 (17%); inguinal hernia repair, 1,188 (5%); lysis of adhesions, 2,343 (10%); repair of perforated viscus, 681 (3%), small bowel resection, 2,416 (10%); ventral hernia repair, 806 (3%).
Cluster modeling returned three distinct clusters based on their mortality performance: 31.7% of hospitals were in the highest-performing cluster (n = 34), 29.9% were in the middle-performing cluster (n = 32), and 38.3% were in the lowest performing cluster (n = 41).
The centroids of the clusters of hospitals that emerged indicated that performance level for one operation type was consistent with performance level across all other operation types (see Fig. 1). Specifically, a hospital's high-performance level in one EGS operation type was consistently observed with high performance on all other EGS operation types. Likewise, the centroids of the low-performing cluster remained low for all eight types of operations, regardless of the complexity of the procedure performed. There was not one operation type where a centroid of the low-performing cluster of hospitals (meaning poorly performing) was better than a corresponding centroid of the average cluster or high-performing cluster of hospitals.
Figure 1.
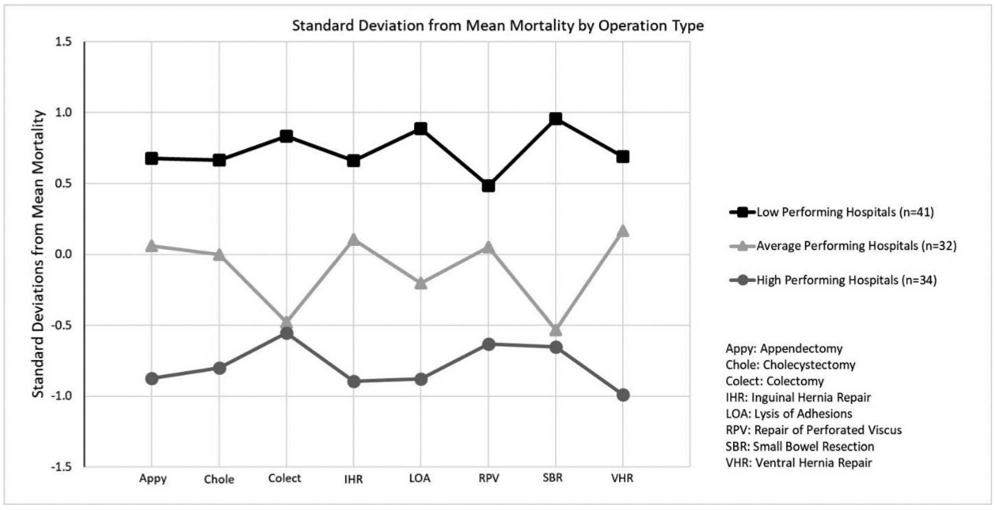
Standard deviation from mean mortality of the low-, average-, and high-performing clusters stratified by operation type.
The high-performing hospitals (see Fig. 1) demonstrated their greatest deviation below mean mortality (indicating better performance than the mean) among ventral hernia repair (−0.990) and least deviation from average when performing colectomy (−0.551). Conversely, the low-performing cohort demonstrated its greatest deviation above mean mortality (meaning worse than the mean) when performing small bowel resection (0.958) and least deviation when performing repair of perforated viscus (0.483). High-performing hospitals exceeded low-performing hospitals by over one standard deviation across all operations with the greatest observed difference among adhesiolysis (1.764), and ventral hernia repair (1.681). The smallest observed difference between the high and low performers occurred among repair of perforated viscus (1.113).
The within-cluster variance was smallest at high-performing hospitals (3.74) compared with average-performing hospitals (3.94) and low-performing hospitals (5.67). This means that high-performing hospitals tended to have lower variability in mortality outcome compared to the average- and low-performing institutions. Further cluster-specific standardized mortality by procedure types are displayed in Table 1. The three-cluster solution is easily interpretable and meaningful and explains 43.4% of the total variance.
TABLE 1.
Procedure Specific Standard Deviation From Mean Mortality Stratified by Cluster
| Operation | Low-Performing Hospitals | Average-Performing Hospitals | High-Performing Hospitals |
|---|---|---|---|
| Appendectomy | 0.677 | 0.061 | −0.873 |
| Cholecystectomy | 0.664 | −0.001 | −0.799 |
| Colectomy | 0.832 | −0.480 | −0.551 |
| Inguinal hernia repair | 0.660 | 0.105 | −0.895 |
| Adhesiolysis | 0.887 | −0.204 | −0.877 |
| Repair of perforated viscus | 0.483 | 0.050 | −0.630 |
| Small-bowel resection | 0.958 | −0.534 | −0.653 |
| Ventral hernia repair | 0.691 | 0.167 | −0.990 |
| Within class variance | 5.752 | 3.759 | 3.860 |
Hospital-level characteristics differed only by total number of procedures performed by cluster as demonstrated in Table 2. High-performing hospitals performed more procedures compared with both average- and poor-performing hospitals. Average-performing centers performed more procedures than poor-performing institutions (p < 0.001). There were no significant differences between clusters with regards to trauma center status, high-technology status, medical school affiliation, or bed size.
TABLE 2.
Hospital Characteristics Stratified by Cluster
| N = 107 | Low-Performing Hospitals (n = 41) | Average-Performing Hospitals (n = 32) | High-Performing Hospitals (n = 34) | p value |
|---|---|---|---|---|
| Procedure count | ||||
| Mean (SD) | 143.59 (47.44) | 211.34 (43.54) | 342.03 (98.56) | <0.001 |
| Median (range) | 147.0 (41.0–228.0) | 208.5 (131.0–301.0) | 305.5 (225.0–656.0) | <0.001 |
| Trauma center status | ||||
| Missing | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0.24 |
| No | 34 (82.93%) | 22 (68.75%) | 23 (67.65%) | |
| Yes | 7 (17.07%) | 10 (31.25%) | 11 (32.35%) | |
| High-Technology Hospital | ||||
| Missing | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0.12 |
| No | 11 (26.83%) | 5 (15.63%) | 3 (8.82%) | |
| Yes | 30 (73.17%) | 27 (84.38%) | 31 (91.18%) | |
| Medical school affiliation | ||||
| Missing | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0.37 |
| Yes | 13 (31.71%) | 8 (25.00%) | 14 (41.18%) | |
| No | 28 (68.29%) | 24 (75.00%) | 20 (58.82%) | |
| More than 100 beds | ||||
| Missing | 12 (29.27%) | 7 (21.88%) | 2 (6.90%) | 0.11 |
| No | 8 (27.59%) | 6 (24.00%) | 27 (93.10%) | |
| Yes | 21 (72.41%) | 19 (76.00%) | 2 (6.90%) | |
Individual patient characteristics also differed when stratified across performance categories (Table 3). Patients cared for at high-performing hospitals were older than those cared for at average- or low-performing hospitals (p < 0.001). Hospitals in the lowest cluster cared for the lowest percentage of white patients (49.6%) and highest percentage of black (8.0%) and other-race patients (42.4%) (p < 0.001). The lowest-performing cluster of hospitals also cared for the highest percentage of patients with Medicaid (6.3%) and “other’ insurance status (1.3%) (p < 0.001). The clusters did not significantly differ by sex or median Wan Walraven Comorbidity Score.
TABLE 3.
Patient Characteristics Stratified by Cluster
| N = 24,279 | Low-Performing Hospitals (n = 5,887) |
Average-Performing Hospitals (n = 6,763) |
High-Performing Hospitals (n = 11,629) |
p value |
|---|---|---|---|---|
| Age in years at admission | ||||
| Mean (SD) | 76.35 (7.49) | 76.54 (7.62) | 76.83 (7.64) | <0.001 |
| Median (range) | 76.0 (65.0–101.0) | 76.0 (65.0–99.0) | 77.0 (65.0–101.0) | <0.001 |
| Van Walraven Comorbidity score | ||||
| n (n Missing) | 5,887 (0) | 6,763 (0) | 1,1629 (0) | |
| Mean (SD) | 5.99 (7.43) | 5.66 (7.18) | 5.93 (7.36) | <0.021 |
| Median (range) | 5.0 (−11.0–42.0) | 4.0 (−14.0–41.0) | 5.0 (−14.0–42.0) | <0.06 |
| Indicator of sex | ||||
| Missing | 31 (0.53%) | 22 (0.33%) | 19 (0.16%) | 0.91 |
| Male | 2,605 (44.48%) | 2,981 (44.22%) | 5,171 (44.54%) | |
| Female | 3,251 (55.52%) | 3,760 (55.78%) | 6,439 (55.46%) | |
| Race | ||||
| Missing | 211 (3.58%) | 229 (3.39%) | 233 (2.00%) | <0.001 |
| White | 2,816 (49.61%) | 4,713 (72.13%) | 8,174 (71.73%) | |
| Black | 454 (8.00%) | 144 (2.20%) | 468 (4.11%) | |
| Other | 2,406 (42.39%) | 1,677 (25.67%) | 2,754 (24.17%) | |
| Payer status | ||||
| Missing | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | <0.001 |
| Medicare | 4,900 (83.23%) | 5,933 (87.73%) | 9,986 (85.87%) | |
| Medicaid | 373 (6.34%) | 294 (4.35%) | 309 (2.66%) | |
| Private insurance | 539 (9.16%) | 458 (6.77%) | 1,237 (10.64%) | |
| Other | 75 (1.27%) | 78 (1.15%) | 97 (0.83%) | |
After adjusting for covariates, multinomial logistic regression did not find that trauma center status, high-technology status or teaching hospital affiliation were associated with cluster assignment. These results are displayed in Table 4.
TABLE 4.
Multinomial Logistic Regression Modeling Cluster Assignment
| Variables | Cluster Type | Odds Ratio | 95% CI | p value | |
|---|---|---|---|---|---|
| Trauma center status | 0.36 | ||||
| Low vs. average | 0.429 | 0.131 | 1.405 | 0.16 | |
| Average vs. high | 1.279 | 0.420 | 3.890 | 0.67 | |
| Low vs. high | 0.548 | 0.174 | 1.726 | 0.31 | |
| High technology | 0.23 | ||||
| Low vs. average | 0.308 | 0.076 | 1.247 | 0.10 | |
| High vs. average | 0.539 | 0.113 | 2.561 | 0.44 | |
| Low vs. high | 0.572 | 0.170 | 1.932 | 0.37 | |
| Teaching hospital | 0.37 | ||||
| Low vs. average | 0.846 | 0.309 | 2.316 | 0.75 | |
| High vs. average | 0.463 | 0.154 | 1.388 | 0.17 | |
| Low vs. high | 1.829 | 0.607 | 5.508 | 0.28 | |
DISCUSSION
Among hospitals performing a wide range of common EGS operations in the geriatric population, mortality performance separates into three distinct clusters: high performers, average performers, and low (poor) performers. Hospital performance is operation independent and highly consistent, meaning that hospitals which attain excellent performance for a single operation maintain that same low-mortality success across all EGS operations; likewise, poor performers maintain their poor performance across all analyzed procedures.
Given their increased rates of comorbidities and decreased physiologic reserve, the care of older patients suffering from high-acuity, time-dependent EGS conditions can be challenging, resulting in suboptimal outcomes.20,21 This study's result-that a large, high-performing cluster of hospitals consistently outperforms other lesser clusters-suggests that even with this challenging patient-population, certain institutions are highly reliable. One potential conclusion is that these hospitals have systems of care in place that explain their superior results and their ability to protect patients despite unexpected events.22 Based on what we know about high reliability organizations, these high-performing centers are likely to have protocols in place that contribute to improved results, as well as institutional processes that promote a culture of improvement, feedback, and communication.23,24
Despite the observation that those hospitals in the top performing cluster are highly reliable with low variability in their outcomes, our study failed to identify specific hospital-level factors that contributed to cluster assignment. Although quality improvement and hospital practices such as those previously cited can be inferred to exist in the top-performing cluster, it is unknown what systems of care most contribute to a hospital's success in the perioperative management of the geriatric EGS patient population.
That there exists such a difference in hospital performance without a clear answer as to what drives the observed difference is a call for more study into the subject. This research effort may need to use several methodologies to distinguish what habits top performing hospitals benefit from, and what the consequences are for deficiency at the low-performing hospitals. Importantly, this effort will have to distinguish what hospital traits are unique to improving the care of the geriatric EGS patient and cannot simply focus on already identified strategies that improve outcomes in the elective population.
Research into what drives improved performance may be aided by the size of the clusters observed in this study. Rather than finding that both high and lower performers make up a small percentage of the healthcare landscape as observed in other studies (<10%),25 the proportions seen in our study were 32% and 38%, respectively. Because so many examined hospitals were represented in the best and worst performing clusters, it should be easier to identify similar traits across institutions that influence patient care.
Potential factors related to improved performance in this study include those related to EGS patients in general and those factors related to the care of geriatric patients in particular. Examples of potential institutional characteristics that may improve outcomes among EGS patients include: thorough and timely diagnosis facilitated by a well-equipped emergency department, hospitals with an acute care surgery practice paradigm, institutions with a chief quality officer, standardized algorithms and processes of care for perioperative geriatric patient management, and access to expert services such as fully staffed intensive care units, specialist anesthesia, and accredited nursing.
Geriatric specific processes of cares may be as or more important. One major example is specialist geriatric consultation services, which have a proven benefit in both surgical patients26-28 as well as those facing complex management decisions.29,30 The effect of such services may be observed in two ways: (1) improved preoperative optimization and postoperative care of the geriatric EGS patient resulting in improved outcomes and (2) improved preoperative risk stratification and clarification of management goals, which can lower potential postoperative mortality by foregoing aggressive treatments.
This study has several limitations. As a retrospective study, it is subject to all potential biases of a study of its type. Although every effort was made to account for and model patient and hospital-level variation, our analysis is limited to the administrative data sets available to us. Particularly lacking in these databases are the ability to generate validated frailty risk scores to account for the impact frailty has on geriatric surgery patient outcomes. Likewise, it is unknown what institutional factors (such as geriatric consult-liaison services) studied hospitals have used within their institutions and thus these were not accounted for. Additionally, some hospitals and hospital systems may benefit from robust and well-managed prehospital systems of care while others may not—it is unknown to what extent this potential unmeasured variable has influenced our results. Finally, our study was limited to centers in a set geographic region that performed at least three of eight common EGS operations over a 2-year period and thus may not be valid when applied to other settings.
In conclusion, hospitals separate into three distinct clusters based on their in-hospital mortality performance for performing EGS operations in the geriatric population. These clusters maintain their performance rank reliably across operations. The hospital-level factors that drive these differences could not be elucidated in this study. Further study is needed to determine which hospital-level factors drive hospital performance and to investigate what systemwide processes will most improve geriatric patient care.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Becher acknowledges that this publication was made possible by the support of: the Yale Center for Clinical Investigation CTSA grant KL2 TR001862 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH); and the American Association for the Surgery of Trauma (AAST) Emergency General Surgery Research Scholarship Award. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the AAST or the NIH. Dr. Gill acknowledges the support of the Academic Leadership Award (K07AG043587) and Claude D. PepperOlder Americans Independence Center (P30AG021342) from the National Institute on Aging.
Footnotes
Presented at: The 32nd Eastern Association for the Surgery of Trauma Annual Scientific Assembly, Austin, TX, January 15 to 19, 2019.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
DISCLOSURE
The authors declare no conflicts of interest.
Contributor Information
Michael P. DeWane, Section of General Surgery, Trauma, and Surgical Critical Care, Department of Surgery, Yale School of Medicine, New Haven, Connecticut..
Nitin Sukumar, Yale Center for Analytical Sciences, Yale School of Public Health; New Haven, Connecticut..
Marilyn J. Stolar, Yale Center for Analytical Sciences, Yale School of Public Health; New Haven, Connecticut..
Thomas M. Gill, Section of Geriatrics, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut..
Adrian A. Maung, Section of General Surgery, Trauma, and Surgical Critical Care, Department of Surgery, Yale School of Medicine, New Haven, Connecticut..
Kevin M. Schuster, Section of General Surgery, Trauma, and Surgical Critical Care, Department of Surgery, Yale School of Medicine, New Haven, Connecticut..
Kimberly A. Davis, Section of General Surgery, Trauma, and Surgical Critical Care, Department of Surgery, Yale School of Medicine, New Haven, Connecticut..
Robert D. Becher, Section of General Surgery, Trauma, and Surgical Critical Care, Department of Surgery, Yale School of Medicine, New Haven, Connecticut..
REFERENCES
- 1.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. US: United States Census Bureau, Economics and Statistics Administration; 2014. [Google Scholar]
- 2.Desserud KF, Veen T, Søreide K. Emergency general surgery in the geriatric patient. Br J Surg. 2016;103(2):e52–e61. [DOI] [PubMed] [Google Scholar]
- 3.Sparkes T, Jones G, Evans C. Outcomes following emergency general surgery in nonagenarians. Int J Surg. 2012;10(8):S90. [Google Scholar]
- 4.Goeteyn J, Evans LA, De Cleyn S, Fauconnier S, Damen C, Hewitt J, Ceelen W. Frailty as a predictor of mortality in the elderly emergency general surgery patient. Acta Chir Belg. 2017;117(6):370–375. [DOI] [PubMed] [Google Scholar]
- 5.Khan M, Azim A, O'Keeffe T, Jehan F, Kulvatunyou N, Santino C, Tang A, Vercruysse G, Gries L, Joseph B. Geriatric rescue after surgery (GRAS) score to predict failure-to-rescue in geriatric emergency general surgery patients. Am J Surg. 2018;215(1):53–57. [DOI] [PubMed] [Google Scholar]
- 6.Frailty and sarcopenia outcomes in emergency general surgery—ICH GCP—clinical trials registry [internet]. [cited 2018 Dec 27]. Available from: https://ichgcp.net/clinical-trials-registry/NCT03534765.
- 7.Wang Y, Eldridge N, Metersky ML, Sonnenfeld N, Fine JM, Pandolfi MM, Eckenrode S, Bakullari A, Galusha DH, Jaser L, Verzier NR, Nuti SV, Hunt D, Normand SL, Krumholz HM. Association between hospital performance on patient safety and 30-day mortality and unplanned readmission for Medicare fee-for-service patients with acute myocardial infarction. J Am Heart Assoc. 2016;5(7):pii: e003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkassabany NM, Passarella M, Mehta S, Liu J, Neuman MD. Hospital characteristics, inpatient processes of care, and readmissions of older adults with hip fractures. J Am Geriatr Soc. 2016;64(8):1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta A, Dultz LA, Joseph B, Canner JK, Stevens K, Jones C, Haut ER, Efron DT, Sakran JV. Emergency general surgery in geriatric patients: a statewide analysis of surgeon and hospital volume with outcomes. J Trauma Acute Care Surg. 2018;84(6):864–875. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Dimick JB. Understanding and reducing variation in surgical mortality. Annu Rev Med. 2009;60(1):405–415. [DOI] [PubMed] [Google Scholar]
- 11.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Suter LG, Bernheim SM, Drye EE, Krumholz HM. Hospital readmission performance and patterns of readmission: retrospective cohort study of Medicare admissions. BMJ. [Internet]. 2013. November 20 [cited 2018 May 8];347 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3898430/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009; 250(6):1029–1034. [DOI] [PubMed] [Google Scholar]
- 13.Taylor N, Clay-Williams R, Hogden E, Braithwaite J, Groene O. High performing hospitals: a qualitative systematic review of associated factors and practical strategies for improvement. BMC Health Serv Res. [Internet]. 2015. June 24 [cited 2018 May 8];15 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4478709/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewster AL, Cherlin EJ, Ndumele CD, Collins D, Burgess JF, Charns MP, Bradley EH, Curry LA. What works in readmissions reduction: how hospitals improve performance. Med Care. 2016;54(6):600–607. [DOI] [PubMed] [Google Scholar]
- 15.Pearse RM, Dana EC, Lanigan CJ, Pook JA. Organisational failures in urgent and emergency surgery. A potential peri-operative risk factor. Anaesthesia. 2001;56(7):684–689. [DOI] [PubMed] [Google Scholar]
- 16.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. [DOI] [PubMed] [Google Scholar]
- 17.Ladha KS, Zhao K, Quraishi SA, Kurth T, Eikermann M, Kaafarani HM, Klein EN, Seethala R, Lee J. The Deyo-Charlson and Elixhauser-van Walraven comorbidity indices as predictors of mortality in critically ill patients. BMJ Open. 2015;5(9):e008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson NR, Fan Y, Dalton JE, Jehi L, Rosenbaum BP, Vadera S, Griffith SD. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care. 2015;53(4):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrari S, Cribari-Neto F. Beta regression for modelling rates and proportions. J Appl Stat. 2004;31(7):799–815. [Google Scholar]
- 20.Ukkonen M, Kivivuori A, Rantanen T, Paajanen H. Emergency abdominal operations in the elderly: a multivariate regression analysis of 430 consecutive patients with acute abdomen. World J Surg. 2015;39(12):2854–2861. [DOI] [PubMed] [Google Scholar]
- 21.Rangel EL, Cooper Z, Olufajo OA, Reznor G, Lipsitz SR, Salim A, Kwakye G, Calahan C, Sarhan M, Hanna JS. Mortality after emergency surgery continues to rise after discharge in the elderly: predictors of 1-year mortality. J Trauma Acute Care Surg. 2015;79(3):349–358. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan JL, Rivard PE, Shin MH, Rosen AK. Applying the high reliability health care maturity model to assess hospital performance: a VA case study. Jt Comm J Qual Patient Saf. 2016;42(9):389–AP12. [DOI] [PubMed] [Google Scholar]
- 23.Weick KE. Organizational culture as a source of high reliability. Calif Manage Rev. 1987;29(2):112–127. [Google Scholar]
- 24.Chassin MR, Loeb JM. High-reliability health care: getting there from here. Milbank Q. 2013;91(3):459–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingraham AM, Cohen ME, Bilimoria KY, Raval MV, Ko CY, Nathens AB, Hall BL. Comparison of 30-day outcomes after emergency general surgery procedures: potential for targeted improvement. Surgery. 2010;148(2):217–238. [DOI] [PubMed] [Google Scholar]
- 26.Olufajo OA, Tulebaev S, Javedan H, Gates J, Wang J, Duarte M, Kelly E, Lilley E, Salim A, Cooper Z. Integrating geriatric consults into routine care of older trauma patients: one-year experience of a level I trauma Center. J Am Coll Surg. 2016;222(6):1029–1035. [DOI] [PubMed] [Google Scholar]
- 27.Lenartowicz M, Parkovnick M, McFarlan A, Haas B, Straus SE, Nathens AB, Wong CL. An evaluation of a proactive geriatric trauma consultation service. Ann Surg. 2012;256(6):1098–1101. [DOI] [PubMed] [Google Scholar]
- 28.Braude P, Goodman A, Elias T, Babic-Illman G, Challacombe B, Harari D, Dhesi JK. Evaluation and establishment of a ward-based geriatric liaison service for older urological surgical patients: proactive care of older people undergoing surgery (POPS)-urology. BJU Int. 2017;120(1):123–129. [DOI] [PubMed] [Google Scholar]
- 29.Schiphorst AH, Ten Bokkel Huinink D, Breumelhof R, Burgmans JP, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3): 365–370. [DOI] [PubMed] [Google Scholar]
- 30.Verweij NM, Souwer ETD, Schiphorst AHW, Maas HA, Portielje JEA, Pronk A, van den Bos F, Hamaker ME. The effect of a geriatric evaluation on treatment decisions for older patients with colorectal cancer. Int J Colorectal Dis. 2017;32(11):1625–1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


