Abstract
BACKGROUND
Endoscopic drainage of walled-off necrosis (WON) is still a challenge due to stent-associated problems. We explored endoscopic gastric fenestration (EGF) as an innovative alternative intervention.
AIM
To assess the feasibility, efficacy and safety of EGF for WON.
METHODS
Between March 2019 and March 2020, five patients with symptomatic WON in close contact with the stomach wall were treated by EGF. Endoscopic ultrasound (EUS) was used to select appropriate sites for gastric fenestration, which then proceeded layer by layer as in endoscopic submucosal dissection. Both the stomach muscularis propria and pseudocyst capsule were penetrated. Fenestrations were expanded up to 1.5-3 cm for drainage or subsequent necrosectomy.
RESULTS
EGF failed in Case 1 due to nonadherence of WON to the gastric wall. EGF was successfully implemented in the other four cases by further refinement of fenestration site selection according to computed tomography, endoscopy and EUS features. The average procedure time for EGF was 124 min (EUS assessment, 32.3 min; initial fenestration, 28.8 min; expanded fenestration, 33 min), and tended to decrease as experience gradually increased. The diameter of the fenestration site was 1.5-3 cm, beyond the caliber of a lumen-apposing metal stent (LAMS), to ensure effective drainage or subsequent necrosectomy. Fenestration sites showed surprising capacity for postoperative self-healing within 1-3 wk. No EGF-related complications were seen. WON disappeared within 3 wk after EGF. In Case 3, another separate WON, treated by endoscopic LAMS drainage, recurred within 4 d after LAMS removal due to stent-related hemorrhage, and resolved slowly over almost 3 mo. No recurrences were observed in the five patients.
CONCLUSION
EGF is an innovative and promising alternative intervention for WON adherent to the gastric wall. The challenge resides in the gauging of actual adherence and in selecting appropriate fenestration sites.
Keywords: Endoscopic gastric fenestration, Walled-off necrosis, Lumen-apposing metal stents, Stent-related complications
Core Tip: Endoscopic drainage of walled-off necrosis (WON) is still a challenge due to stent-associated problems. Endoscopic gastric fenestration may be an innovative alternative intervention for WON adherent to the gastric wall, and might outperform lumen-apposing metal stent drainage, with lower cost and no stent-related complications. The challenge is to select appropriate fenestration. We established some characteristics for suitable fenestration sites: Computed tomography: Intimate contact between the stomach and encapsulated WON without clear layers; endoscopy: Intense inflammation (edema, erosion or ulceration) of gastric mucosa; endoscopic ultrasound: Modest abutment (generally < 1 cm) of the stomach and WON without clear layers.
INTRODUCTION
Walled-off necrosis (WON) is a local complication of acute pancreatitis in which a mature, encapsulated collection of partially liquefied necrotic pancreatic or peripancreatic tissue develops a well-defined inflammatory wall[1]. Evidence-based multidisciplinary guidelines issued by the European Society of Gastrointestinal Endoscopy (ESGE) currently stipulate that in the absence of clinical improvement, endoscopic drainage is now the first-line procedure for symptomatic WON, with endoscopic necrosectomy or minimally invasive methods (rather than open surgery) constituting the next therapeutic step[2]. Although previous studies have shown that endoscopic and surgical remedies are comparable in instances of pancreatic pseudocyst[3-7], endoscopic treatment of symptomatic WON (especially infected lesions) is more of a challenge. The ESGE recommends either plastic or lumen-apposing metal stent (LAMS) placement for initial endoscopic transmural drainage[2]. Unfortunately, plastic stents have proven significantly less effective overall in the setting of WON (as opposed to pancreatic pseudocyst) due to their small calibers. Metal stents are now increasingly used for draining WON endoscopically, despite current controversial reports (vs plastic stents)[8-13], and the sparseness of pertinent long-term data. Furthermore, certain complications of stenting, namely delayed bleeding, stent migration, and jaundice-producing biliary strictures, have occurred significantly more often when using metal (vs plastic) stents, especially > 3 wk after intervention[13-18]. Finally, the costs entailed seem considerably higher for procedures involving LAMSs rather than plastic stents, which clearly affects therapeutic choice[13].
In weighing these factors, we questioned whether bridging of the gastrointestinal tract and WON by stents is a requirement for adequate endoscopic drainage. A more direct method, akin to surgical cystogastrostomy, is so-called endoscopic gastric fenestration (EGF). This approach calls for portals of reasonable magnitude to ensure effective drainage, and it may eliminate the need for and consequences of stenting, with substantial monetary savings. It is imperative that intimate contact exists between WON and the gastrointestinal wall. The fundamental technical difficulties are then gauging adherence (with certainty) and identifying appropriate sites for fenestration. Emergency EGF for recurrent pancreatic pseudocyst has already been performed in China[19]. We thus considered EGF a viable technique in selected instances of WON, applying it to five qualifying patients treated in our department. Here, we provide preliminary accounts of this technique as a promising new intervention for WON.
MATERIALS AND METHODS
Patient selection and evaluation
We enrolled five patients with symptomatic WON after necrotizing pancreatitis (NP) for EGF drainage between March 2019 and March 2020 at the First Medical Center, Chinese PLA General Hospital in Beijing, China. The inclusion criteria were as follows: (1) Preoperative enhanced computed tomography (CT) or magnetic resonance imaging (MRI) confirmed abutment of necrotic pseudocysts against the gastric wall; and (2) Preoperative assessment precluded contraindications for endoscopy and anesthesia.
All patients agreed to the requisite examinations and gave signed written informed consent prior to endoscopic treatment. The study was approved by the Ethics Committee of the PLA General Hospital (s-2019-298-02).
Procedures
All endoscopic procedures were performed by Li W, an endoscopist with > 20 years’ experience in advanced endoscopic techniques who first performed the natural orifice transluminal endoscopic surgery study in China. Patients were placed in the left-lateral position and underwent tracheal intubation and intravenous anesthesia routinely to avoid aspiration. Before EGF, endoscopic ultrasonography (EUS; GF-UCT260, Olympus, Japan) was performed initially to assess the adherence of WON to the gastric wall. Accurate measurements were obtained under EUS guidance, adjusting the probe to avoid undue compression of the stomach and WON. The fenestration sites were usually the most obvious compression areas in the stomach in close contact with WON, and were marked prospectively by Dual knife (Olympus) or biopsy forceps under EUS guidance.
The fenestration procedure was divided into 2 parts: Initial fenestration by endoscopic submucosal dissection (ESD) and expanded fenestration. Selected sites in the stomach were incised layer by layer as in ESD until gastric muscularis propria and adherent WON capsules were both penetrated. Then, the “windows” were expanded to 1.5-3 cm by a Dual knife, insulated-tip diathermic (IT) knife II (Olympus) or electric snare (Cook, United States) (so-called expanded fenestration). Expanded fenestration was performed with greater precision under EUS guidance and with respect to spatial orientations of WON, rather than blindly expanded. Finally, fluid drainage and subsequent necrosectomy (if necessary) of WON were performed by endoscopic entry into WON through the fenestration sites.
Standard postoperative treatments were fasting, intravenous nutritional support, use of proton pump inhibitors, and antibiotic treatment (3 d). If nasal-cyst tubing was placed, passed via fenestration fistula into WON intraoperatively, analytes in drainage fluid (e.g., amylase and lipase) were regularly assayed. CT scans and gastroscopy were usually performed within the first and second week after EGF, repeating endoscopic necrosectomy if needed. Moreover, CT scans and endoscopic follow-up were also performed 2-3 mo after discharge to assess the presence or recurrence of WON, and the healing of fenestration sites. All five patients were followed up by outpatient appointment and telephone consultation for 5-16 mo after discharge.
Evaluation data
The primary outcome measures included: Clinical symptoms, imaging and endoscopic characteristics, procedure-related outcome data (including the time of EUS assessment and fenestration procedures), procedure-related complications, postoperative management, endoscopic procedural cost, overall cost of hospitalization and follow-up, hospital stay, follow-up time and recurrence.
RESULTS
Baseline characteristics
The baseline characteristics of the five cases are listed in Table 1. The average diameter of WON was 13.2 cm (range 9.3-19.5 cm), and multiple WON cysts were observed in two patients. Endoscopic procedures were performed > 4 wk after NP onset. The chief complaints were pancreatic pain and gastric outlet obstruction. EGF was performed 17 mo (afflicted the longest) after NP onset in Case 3. WON was asymptomatic under conservative management for the initial first year, but gradually enlarged and caused abdominal distension. Endoscopic drainage was proposed 6 mo before EGF, while a fistula was revealed in the stomach that indicated spontaneous rupture of WON into the stomach. Abdominal distension was relieved, and no further intervention was performed at that time. However, the WON re-expanded after transient decline, and the patient suffered intracystic infection and hemorrhage 19 d prior to EGF. Intracystic hemorrhage was successfully controlled by emergency intravascular embolization, while the infection persisted and indicated refractoriness to carbapenem antibiotics.
Table 1.
The baseline characteristics of all five patients in this study
|
Characteristics
|
Case 1
|
Case 2
|
Case 3
|
Case 4
|
Case 5
|
| Age/sex | 56/female | 63/male | 45/male | 72/male | 64/female |
| Etiology of NP | High fat diet and cholelithiasis | Cholelithiasis | High fat diet and cholelithiasis | High fat diet | High fat diet and cholelithiasis |
| Time interval between NP onset and endoscopic procedures (mo) | 3 | 1.3 | 17 | 3.5 | 1.7 |
| Clinical symptoms | Pancreatic pain and gastric outlet obstruction | Pancreatic pain and gastric outlet obstruction | Intra-cystic infection and hemorrhage | Gastric outlet obstruction | Pancreatic pain and gastric outlet obstruction |
| Diameter of WON (cm) | 10.3 | 13 | 13.9 | 9.3 | 19.5 |
| Multiple cysts of WON | No | No | 2 cysts without communication | 3 cysts with communication | No |
NP: Necrotizing pancreatitis; WON: Walled-off necrosis.
Endoscopic procedure characteristics
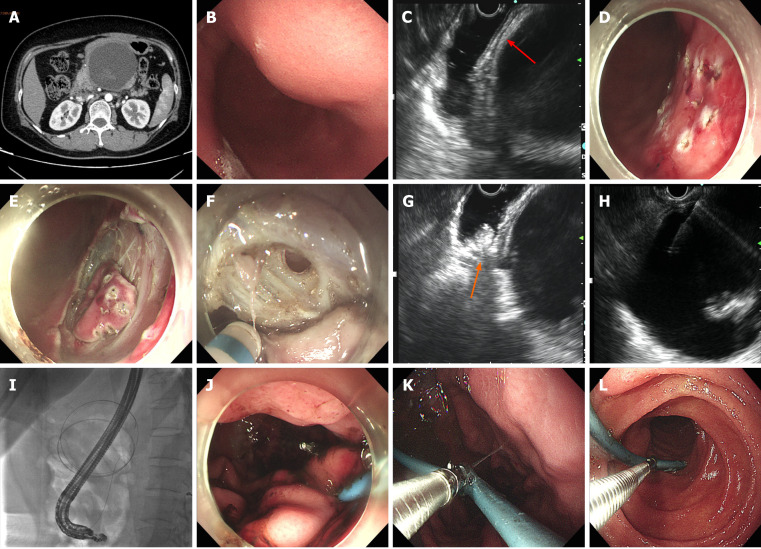
Case 1 failed EGF due to nonadherence of encapsulated WON to the gastric wall. Subsequent EUS and X-ray fluoroscopy showed maneuvering of WON > 10 cm from the gastric wall, precluding plastic or metal stenting. A nasal-cyst drainage tube was inserted instead, and the incised muscularis propria of the stomach was closed by metal clips (Figure 1). The total procedure time was 178 min and the endoscopic procedural cost was US $3549.1 (Table 2).
Figure 1.
Case 1 (failed fenestration) with indwelling nasal-cyst drainage tube. A: Closely connected walled-off necrosis (WON) and gastric wall (preoperative computed tomography scan); B: Smooth, compressive indentation of stomach by WON; C: Endoscopic ultrasound (EUS) showed closely connected WON and gastric wall (with clear layers, red arrow); D and E: Incising the selected sites layer by layer by an endoscopic submucosal dissection approach; F: Nonadherence of WON and stomach after incising gastric muscularis propria; G: WON mobilization far from fenestration site (orange arrow) under EUS guidance; H: Needle puncture into WON from gastric wall; I: Visible separation of WON and stomach by X-ray fluoroscopy after inserting the guidewire into WON; J: Indwelling nasal-cyst drainage tube passed through the stomach into WON and closing the incised gastric muscularis propria by metal clips; K and L: Nasal-cyst drainage tube was cut off and we reverted to internal drainage 15 d later.
Table 2.
The main endoscopic procedural characteristics of all five patients in this study
|
Characteristics
|
Case 1
|
Case 2
|
Case 3
|
Case 4
|
Case 5
|
| Successful EGF | No. Non-adherence of encapsulated WON to the gastric wall. Stent placement also failed, so a nasocystic drainage tube was inserted instead | Yes | Yes | Yes | Yes |
| Fenestration sites | Posterior wall between gastric antrum and body | Upper posterior wall of gastric body | Greater curvature of gastric fundus | Posterior wall of gastric antrum | Posterior wall between gastric antrum and body |
| Diameter of fenestration sites (cm) | - | 2 | 1.5 | 2.5 | 3 |
| Total procedure time (min) | 178 | 162 | 117 | 94 | 123 |
| EUS assessment time (min) | 80 | 53 | 20 | 33 | 23 |
| Total fenestration time (min) | 35 | 75 | 60 | 42 | 70 |
| Initial fenestration (by ESD approach) time (min) | 35 | 52 | 19 | 16 | 28 |
| Expanded fenestration time (min) | - | 23 | 41 | 26 | 42 |
| WON fluid drainage time (min) | - | 5 | 17 | 13 | 20 |
| WON exploration and necrosectomy time (min) | - | 27 | 20 | 6 | 10 |
| Intraoperative fluid collection of WON (mL) | 40 cloudy brown liquid | 500 light gray liquid | 1000 cloudy brown liquid | 400 light gray liquid | 1300 yellowish pus |
| Endoscopic procedural cost ($) | 3549.1 | 2136.4 | 2381.4 | 2096.7 | 1941.5 |
EGF: Endoscopic gastric fenestration; WON: Walled-off necrosis; EUS: Endoscopic ultrasound; ESD: Endoscopic submucosal dissection.
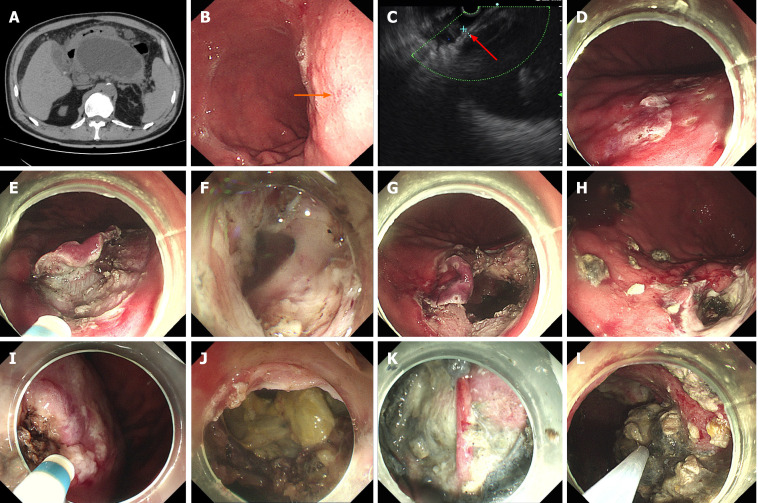
EGF was successfully performed in the other four patients after further refinement of fenestration site selection (Figure 2A-C). Details of the endoscopic procedures are shown in Table 2. The average procedural cost of EGF was US $2139. The total average procedural time was 124 min, including 32.3 min for EUS assessment, 28.8 min for initial fenestration and 33 min for expanded fenestration. The diameter of fenestration sites was 1.5-3 cm. In the first successful case of EGF, initial fenestration area of the stomach by ESD was large (Figure 2E), and expanded fenestration was performed within the initial ESD wound (Figure 2G). As experience of the technique was gained, the initial fenestration area by ESD was narrowed gradually (Figure 2I), and the expanded fenestration area was enlarged up to 2.5-3 cm (Figure 2J). The procedural time for fluid drainage and necrosectomy depended on the size and necrosis status of WON (Table 2). A nasocystic tube was placed in Cases 2 and 3 but not in Cases 4 and 5.
Figure 2.
Endoscopic gastric fenestration technique. A: Closely connected walled-off necrosis (WON) and gastric wall lacking clear layers (black arrow, preoperative computed tomography scan); B: Compressive indentation of stomach by WON, with intense inflammation (orange arrow); C: Endoscopic ultrasound assessment and selection of fenestration site, abutment < 1 cm in combined thickness without clear layers (red arrow); D: Marking of prospective fenestration; E: Initial fenestration by endoscopic submucosal dissection; F: Penetration of WON capsule, releasing fluid content; G: Expanded fenestration; H: Self-healing of fenestration as seen by postoperative endoscopy (1 wk after endoscopic gastric fenestration); I: Narrowed area of initial fenestration; J: Enlarged expanded fenestration up to 3 cm; K: Necrotic tissue and exposed blood vessel in WON; L: Debridement of necrotic tissue.
Postoperative characteristics
The detailed postoperative characteristics and data are shown in Table 3. The initial three patients fasted for 7 d, while the latter two patients fasted for only 1 d. In Case 1, external drainage of the nasocystic tube was reverted to internal drainage 15 d later (Figure 1K and L). The patient suffered recurrent infection of WON during initial internal drainage, which fortunately responded well to antimicrobial treatment. It took up to 3 mo for WON to disappear. In the other four cases, no EGF-related complications were observed, and postoperative endoscopy (with endoscopic necrosectomy if necessary) showed surprising self-healing of the fenestration (Figure 2H) regardless of whether the nasal-cyst tube was indwelling. WON disappeared within 3 wk after EGF.
Table 3.
The main postoperative characteristics of all five patients in this study
|
Characteristics
|
Case 1
|
Case 2
|
Case 3
|
Case 4
|
Case 5
|
| Time of postoperative fasting (d) | 7 | 7 | 7 | 1 | 1 |
| Time of nasal-cyst tube indwelling (d) | 15 | 8 | 7 | - | - |
| Average daily fluid collection via nasal cyst tube (mL) | 172 | 339 | 97 | - | - |
| Postoperative endoscopic management | The nasocystic tube was reverted to internal drainage 15 d later, and finally removed 3 mo later | One more necrosectomy 1 wk later | A LAMS was placed in another separate WON 16 d after EGF, but removed 1 wk later due to stent-related hemorrhage | One more necrosectomy 1 wk later | One more necrosectomy 1 wk later |
| Complications | Recurrent infection of WON during initial internal drainage | None | EGF: None.LAMS drainage: Stent-related hemorrhage | None | None |
| Total endoscopic procedural cost during hospitalization and follow-up ($) | 4182.6 | 2427.2 | 5852.7 | 2408.3 | 2265.9 |
| Overall cost of hospitalization and follow-up ($) | 14684.2 | 7349.1 | 20198.3 | 10504.5 | 12641.4 |
| Postoperative hospital stay (d) | 24 | 12 | 36 | 8 | 9 |
| Time to WON disappearance (d) | 92 | 20 | WON underwent EGF: 14WON underwent LAMS drainage: 84 | 14 | 21 |
| Time of follow-up (mo) | 16 | 13 | 12 | 6 | 5 |
| Recurrence of WON | No | No | No | No | No |
LAMS: Lumen-apposing metal stent; WON: Walled-off necrosis; EGF: Endoscopic gastric fenestration.
In Case 3, another separate WON (noncommunicating with the EGF-treated WON) continued to enlarge, and fever returned after EGF. EUS assessment showed nonadherence of WON to the gastric wall; thus, a LAMS (16 mm–2 cm; Micro-Tech, China) was placed for drainage (operating time, 71 min; procedural cost, US $2941.1) 16 d after EGF. The LAMS had to be removed 1 wk later due to stent-related hemorrhage. WON had almost disappeared in CT scans before LAMS removal, but reappeared 4 d after LAMS removal and was finally resolved 3 mo later.
The average postoperative hospital stay and overall cost of all five cases was 17.8 d (range, 8–36 d) and US $13075.5 (range, US $7349.1–20198.3), respectively. Regardless of EGF failure (Case 1) and endoscopic LAMS drainage (Case 3), the average postoperative hospital stay and overall cost of EGF was 9.7 d (range, 8-12 d) and US $10 165.0 (range, US $7349.1-12641.4), respectively. Endoscopic monitoring 2-3 mo after discharge showed that the fenestration sites were well healed. All five patients were followed up for 5-16 mo. No recurrences were observed. All five patients expressed satisfaction with endoscopic treatment and their recuperative status.
DISCUSSION
Currently, endoscopic drainage has become the first-line approach for treating symptomatic WON, comparing favorably with minimally invasive surgical intervention[20-22]. Although traditional endoscopic drainage involves stenting of some sort to ensure a patent fistula and effective drainage, the inefficiency of plastic stents[10,11], the complications (especially delayed bleeding) that may develop[13,14,17], and cost[13] of LAMS devices are problematic. Liu et al[19] reported the emergency use of endoscopic gastric mural fenestration under EUS and CT guidance to treat a recurrent pancreatic pseudocyst. After full-thickness incision and partial resection of the gastric wall, their patient experienced rapid resolution of symptoms (abdominal distension and dyspnea). Post-fenestration CT and upper gastrointestinal endoscopy both confirmed a smaller pseudocyst cavity.
In our study, we restricted EGF to patients with WON close to the gastric wall under EUS investigation. The challenge of this technique resides in the gauging of actual adherence and in selecting appropriate sites for fenestration. As an initial study of EGF, the technical procedures are still being developed. In Case 1, both preoperative CT/MRI and EUS imaging confirmed closeness of WON to the gastric wall, which proved erroneous once the gastric muscularis propria was incised. Subsequent EUS and X-ray fluoroscopy showed maneuvering of WON > 10 cm from the gastric wall, precluding even plastic or metal stenting. This preoperative oversight prolonged operating time, increased cost, and undermined drainage. In addition, there were also some perforations in the stomach after incision of the gastric muscularis propria, increasing the risk of postoperative peritonitis. We further refined fenestration site selection and finally EGF was successfully implemented in the subsequent four cases. We compared CT scan, endoscopy and EUS features of Case 1 with those of the other four successfully treated patients, and preliminarily established the following characteristics for selecting suitable fenestration sites: (1) Intimate contact between the stomach wall and encapsulated WON on preoperative CT scanning, lacking clear layers; (2) Intense inflammation (i.e., edema, erosion or ulceration) of gastric mucosa, detectable by endoscopy; and (3) Modest abutment (generally < 1 cm altogether) of the stomach and WON, determined by EUS, again without clear layers. Given these features, adherence between WON and the gastric wall is likely.
Once successfully executed, we expanded fenestrations beyond the caliber of a LAMS (up to 1.5-3 cm) to ensure effective drainage or subsequent necrosectomy. We found that the fenestration procedure was related to the location, opening diameter, inflammation and blood supply of the fenestration site. Although the procedural time of EGF in our study was still longer than that of LAMS drainage[13], it tended to decrease as experience in the technique was accumulated, without considering the increased bleeding control time due to intense inflammation and rich blood supply in Case 5. The total procedural time might be limited to 60-90 min or less when the technique is matured in the near future. The fenestration sites displayed surprising capacity for self-healing and resolution of WON in the ensuing 1-3 wk. We have since realized that fenestration size may need to fluctuate, depending on the dimensions of WON and the necrotic tissues amassed. In the first successful case of EGF (Case 2), initial fenestration area of the stomach by ESD was large, and expanded fenestration was performed within the initial ESD wound. As experience of the technique was gained, we found it was unnecessary to resect such a large area of gastric mucosa by ESD during initial fenestration. The initial fenestration area was minimized, while the subsequent expanded fenestration was enlarged with greater precision under EUS guidance and with respect to spatial orientations of WON, rather than blindly expanded, thus avoiding intra-abdominal extravasation of gastric juice.
Postoperative treatments are still being developed in this initial case series. Case 1 who failed EGF fasted for 1 wk postoperatively to avoid metal clips shedding and postoperative perforation. For Case 2 and 3, a nasocystic tube was placed to avoid complete healing of the fenestration fistula and poor drainage of the WON. In addition, both patients fasted for 1 wk until postoperative endoscopy showed surprising self-healing of fenestration fistula, as well as necrotic tissue attachment at the fistula that prevented food from entering the WON. For Case 4 and 5, fenestration fistula was expanded up to 2.5-3 cm to ensure adequate drainage, so a nasocystic tube was no longer necessary. We also tried to restore diet 1 d after EGF, according to the initial experience of EGF and previous experience of endoscopic LAMS drainage. Both patients had no discomfort after eating, so we initially suggested that the diet could be restored as soon as possible if no complications were seen after EGF.
Previous studies have indicated that direct endoscopic necrosectomy is not required in all patients with WON[2,20]. In our study, one or two sessions of necrosectomy were performed in each patient. During EGF, necrosectomy was performed selectively according to the extent of necrosis in WON. There was virtually no solid necrotic tissue remaining in WON on endoscopic and CT monitoring 7 d after EGF, which indicated spontaneous drainage of necrotic tissue through the sufficiently large fenestration fistula. Sometimes, necrotic tissue was seen by postoperative endoscopy attached to the fistula, but it rarely affected drainage of WON. Necrosectomy after EGF was performed mainly to remove the necrotic tissue attached to the fenestration fistula, with the primary purpose of obtaining more postoperative data, such as healing of the fistula. Therefore, necrosectomy was not required in all patients who underwent EGF, and the number of necrosectomy procedures was determined by the extent of necrosis in WON.
In this study, the average overall and procedural cost of EGF was US $10165.0 and US $2139, respectively. Overall cost included cost of the procedure, postprocedural hospitalization, readmission, pharmacy, anesthesia, radiology, and laboratory and other support. It should be noted that as a preliminary study, we arranged detailed postoperative examinations and treatments to obtain more postoperative data, including gastroscopy, necrosectomy and CT scans, which would prolong postoperative hospitalization and overall cost, and some of them might be omitted in the future as experience of the technique is gained. Specifically, Case 3 underwent both EGF and LAMS drainage in succession, inadvertently providing a self-comparison. EGF eliminated the need for and consequences of stenting, and achieved efficient drainage of WON without complications or recurrence. However, initial success after LAMS placement was curtailed by stent-related hemorrhage, forcing removal 1 wk later. Recurrence of WON appeared within 4 d after LAMS removal, prolonging hospital stay and increasing postoperative hospitalization cost. The average endoscopic procedural cost of EGF drainage seemed less than that of a LAMS approach in our study (US $2139 vs $2941.1). At present, the cost of endoscopic treatment for WON differs among studies. The overall cost of LAMS drainage was US $20029-53117, and that of plastic stent drainage was US $15941–57486[13,23,24]. Bang et al[13] reported that the procedural cost of LAMS and plastic stent was US $12155 and US $6609, respectively. There are few data on the cost of LAMS in China, but a multicenter randomized controlled trial (LVPWON trial) has been designed to determine whether LAMS is effective, safe and superior to plastic stenting for WON drainage[25]. We realize that it is inappropriate to compare the cost of EGF and LAMS only based on this study; thus, we intend to conduct a prospective study to compare EGF with endoscopic LAMS/plastic stent drainage in the future, which could provide more convincing evidence.
CONCLUSION
In conclusion, our findings suggest that EGF is an innovative and promising intervention in patients with WON, perhaps outperforming endoscopic LAMS placement if WON is adherent to the gastric wall. A larger patient sample or series of cases must be recruited for controlled trials to better assess the potential benefits.
ARTICLE HIGHLIGHTS
Research background
Endoscopic drainage of walled-off necrosis (WON) is still a challenge due to stent-associated problems.
Research motivation
We explored endoscopic gastric fenestration (EGF) as an innovative alternative intervention for WON.
Research objectives
In this retrospective study, we report our preliminary experience in assessing the feasibility, efficacy and safety of EGF for WON.
Research methods
Five patients with symptomatic WON in close contact with the stomach wall were treated by EGF. Endoscopic ultrasound (EUS) was used to select appropriate sites for gastric fenestration, which then proceeded layer by layer as in endoscopic submucosal dissection. Both stomach muscularis propria and pseudocyst capsule were penetrated. Fenestrations were expanded up to 1.5-3 cm for drainage or subsequent necrosectomy. The detail procedure-related outcome data (including the time of EUS assessment and fenestration procedures), procedure-related complications, postoperative management, procedural cost, overall cost of hospitalization and follow-up, hospital stay, follow-up time and recurrence were recorded.
Research results
EGF failed in Case 1 due to nonadherence of WON to the gastric wall. EGF was successfully implemented in the subsequent four cases. The average procedural time of EGF was 124 min (EUS assessment, 32.3 min; initial fenestration, 28.8 min; expanded fenestration, 33 min), and tended to decrease as experience of the technique was gained. No EGF-related complications were observed. WON disappeared within 3 wk after EGF. In Case 3, WON, treated by endoscopic lumen-apposing metal stent (LAMS) drainage, recurred within a few days after LAMS removal due to stent-related hemorrhage and showed slow resolution for almost 3 mo. No recurrences were observed in all five patients.
Research conclusions
EGF is an innovative and promising alternative intervention for WON adherent to the gastric wall, and might outperform endoscopic LAMS drainage, involving less cost and no stent-related complications.
Research perspectives
The challenge of this technique resides in the gauging of actual adherence and in selecting appropriate sites for fenestration. We intend to conduct a prospective study to compare EGF with endoscopic LAMS/plastic stent drainage in the future.
ACKNOWLEDGEMENTS
Guo X, Zhang XL, Li MY and Yan B participated in the patients' hospitalization management; Zhang ZX, Sun LH and Yang T provided care for the study patients.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of the PLA General Hospital, No. s2019-298-02.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent.
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Society of Digestive Endoscopology.
Peer-review started: July 23, 2020
First decision: August 8, 2020
Article in press: September 18, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Jha AK, Mendoza Ladd A, Rawat K, Sugimoto M S-Editor: Yan JP L-Editor: Webster JR P-Editor: Wang LL
Contributor Information
Fang Liu, Department of Gastroenterology and Hepatology, Medical School of Chinese PLA, Beijing 100853, China; Department of Gastroenterology and Hepatology, The First Medical Center, Chinese PLA General Hospital, Beijing 100853, China.
Liang Wu, Department of International Center for Diagnosis and Treatment of Liver Disease, The Fifth Medical Center, Chinese PLA General Hospital, Beijing 100039, China.
Xiang-Dong Wang, Department of Gastroenterology and Hepatology, The First Medical Center, Chinese PLA General Hospital, Beijing 100853, China.
Jian-Guo Xiao, Department of Critical Care Medicine, The First Medical Center, Chinese PLA General Hospital, Beijing 100853, China.
Wen Li, Department of Gastroenterology and Hepatology, The First Medical Center, Chinese PLA General Hospital, Beijing 100853, China. liwen2000@yahoo.com.
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut . 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy . 2018;50:524–546. doi: 10.1055/a-0588-5365. [DOI] [PubMed] [Google Scholar]
- 3.Teoh AY, Dhir V, Jin ZD, Kida M, Seo DW, Ho KY. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc . 2016;8:310–318. doi: 10.4253/wjge.v8.i6.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farias GFA, Bernardo WM, De Moura DTH, Guedes HG, Brunaldi VO, Visconti TAC, Gonçalves CVT, Sakai CM, Matuguma SE, Santos MELD, Sakai P, De Moura EGH. Endoscopic versus surgical treatment for pancreatic pseudocysts: Systematic review and meta-analysis. Medicine (Baltimore) . 2019;98:e14255. doi: 10.1097/MD.0000000000014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saul A, Ramirez Luna MA, Chan C, Uscanga L, Valdovinos Andraca F, Hernandez Calleros J, Elizondo J, Tellez Avila F. EUS-guided drainage of pancreatic pseudocysts offers similar success and complications compared to surgical treatment but with a lower cost. Surg Endosc . 2016;30:1459–1465. doi: 10.1007/s00464-015-4351-2. [DOI] [PubMed] [Google Scholar]
- 6.Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013; 145: 583-90. :e1. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Feng T, Ji W. Endoscopic versus surgical treatment for pancreatic pseudocyst. Dig Endosc . 2016;28:83–91. doi: 10.1111/den.12542. [DOI] [PubMed] [Google Scholar]
- 8.Petrone MC, Archibugi L, Forti E, Conigliaro R, Di Mitri R, Tarantino I, Fabbri C, Larghi A, Testoni SGG, Mutignani M, Arcidiacono PG. Novel lumen-apposing metal stent for the drainage of pancreatic fluid collections: An Italian multicentre experience. United European Gastroenterol J . 2018;6:1363–1371. doi: 10.1177/2050640618785078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE, Taylor LJ, Munigala S, Bhat YM. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos) Gastrointest Endosc . 2016;83:699–707. doi: 10.1016/j.gie.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SB, Lee IS, Choi MG. Metal versus plastic stents for drainage of pancreatic fluid collection: A meta-analysis. United European Gastroenterol J . 2018;6:729–738. doi: 10.1177/2050640618761702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, Isby L, Kahaleh M, Karia K, Yoo J, Ofosu A, Ng B, Sharaiha RZ. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc . 2017;85:758–765. doi: 10.1016/j.gie.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Bang JY, Hawes R, Bartolucci A, Varadarajulu S. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc . 2015;27:486–498. doi: 10.1111/den.12418. [DOI] [PubMed] [Google Scholar]
- 13.Bang JY, Navaneethan U, Hasan MK, Sutton B, Hawes R, Varadarajulu S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut . 2019;68:1200–1209. doi: 10.1136/gutjnl-2017-315335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeissig S, Sulk S, Brueckner S, Matthes K, Hohmann M, Reichel S, Ellrichmann M, Arlt A, Will U, Hampe J. Severe bleeding is a rare event in patients receiving lumen-apposing metal stents for the drainage of pancreatic fluid collections. Gut . 2019;68:945–946. doi: 10.1136/gutjnl-2018-316581. [DOI] [PubMed] [Google Scholar]
- 15.Wundsam HV, Spaun GO, Bräuer F, Schwaiger C, Fischer I, Függer R. Evolution of Transluminal Necrosectomy for Acute Pancreatitis to Stent in Stent Therapy: Step-Up Approach Leads to Low Mortality and Morbidity Rates in 302 Consecutive Cases of Acute Pancreatitis. J Laparoendosc Adv Surg Tech A . 2019;29:891–899. doi: 10.1089/lap.2018.0768. [DOI] [PubMed] [Google Scholar]
- 16.Mehta N, Abushahin A, Franco M, Stevens T, Chahal P, Vargo J, Bhatt A. Endoscopic retrieval of a lumen-apposing metal stent complicated by inward migration after cystogastrostomy. Endoscopy . 2018;50:E286–E288. doi: 10.1055/a-0640-2786. [DOI] [PubMed] [Google Scholar]
- 17.Bang JY, Hasan M, Navaneethan U, Hawes R, Varadarajulu S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut . 2017;66:2054–2056. doi: 10.1136/gutjnl-2016-312812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa JM, Gonçalves BM, Costa RS, Costeira F, Dias N, Gonçalves R, Soares JB. Endoscopic management of stent displacement after pancreatic pseudocyst drainage. Endoscopy . 2018;50:E304–E306. doi: 10.1055/a-0655-1912. [DOI] [PubMed] [Google Scholar]
- 19.Liu BR, Song JT, Zhang XY. Video of the Month: Emergency Endoscopic Fenestration for Treatment of a Recurrence Pancreatic Pseudocyst. Am J Gastroenterol . 2015;110:644. doi: 10.1038/ajg.2015.13. [DOI] [PubMed] [Google Scholar]
- 20.Bang JY, Arnoletti JP, Holt BA, Sutton B, Hasan MK, Navaneethan U, Feranec N, Wilcox CM, Tharian B, Hawes RH, Varadarajulu S. An Endoscopic Transluminal Approach, Compared With Minimally Invasive Surgery, Reduces Complications and Costs for Patients With Necrotizing Pancreatitis. Gastroenterology 2019; 156: 1027-1040. :e3. doi: 10.1053/j.gastro.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Garg PK, Meena D, Babu D, Padhan RK, Dhingra R, Krishna A, Kumar S, Misra MC, Bansal VK. Endoscopic versus laparoscopic drainage of pseudocyst and walled-off necrosis following acute pancreatitis: a randomized trial. Surg Endosc . 2020;34:1157–1166. doi: 10.1007/s00464-019-06866-z. [DOI] [PubMed] [Google Scholar]
- 22.van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet . 2018;391:51–58. doi: 10.1016/S0140-6736(17)32404-2. [DOI] [PubMed] [Google Scholar]
- 23.Neermark S, Rasmussen D, Rysgaard S, Gluud LL, Novovic S, Schmidt PN. The cost of endoscopic treatment for walled-off pancreatic necrosis. Pancreatology . 2019;19:828–833. doi: 10.1016/j.pan.2019.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Chen YI, Barkun AN, Adam V, Bai G, Singh VK, Bukhari M, Gutierrez OB, Elmunzer BJ, Moran R, Fayad L, El Zein M, Kumbhari V, Repici A, Khashab MA. Cost-effectiveness analysis comparing lumen-apposing metal stents with plastic stents in the management of pancreatic walled-off necrosis. Gastrointest Endosc 2018; 88: 267-276. :e1. doi: 10.1016/j.gie.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Zhu HY, Xie P, Song YX, Li ZS, Jin ZD, Du YQ. Lumen-apposing metal stents (LAMS) versus plastic stents for EUS-guided drainage of walled-off necrosis (WON) (LVPWON): study protocol for a multicenter randomized controlled trial. Trials . 2018;19:549. doi: 10.1186/s13063-018-2901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]