Key Points
Question
Do vitamin D, omega-3, and a strength-training exercise program alone or in combination prevent 6 health outcomes among relatively healthy adults aged 70 years or older?
Findings
In this randomized trial that included 2157 adults aged 70 years or older, 3-year treatment with vitamin D3 (2000 IU/d), with omega-3 fatty acids (1 g/d), or with a strength-training exercise program did not result in statistically significant differences in improvement in systolic or diastolic blood pressure, nonvertebral fractures, physical performance, infection rate, or cognition.
Meaning
These findings do not support the use of vitamin D, omega-3, or a strength-training exercise program for these clinical outcomes among relatively healthy older adults.
Abstract
Importance
The benefits of vitamin D, omega-3 fatty acids, and exercise in disease prevention remain unclear.
Objective
To test whether vitamin D, omega-3s, and a strength-training exercise program, alone or in combination, improved 6 health outcomes among older adults.
Design, Setting, and Participants
Double-blind, placebo-controlled, 2 × 2 × 2 factorial randomized clinical trial among 2157 adults aged 70 years or older who had no major health events in the 5 years prior to enrollment and had sufficient mobility and good cognitive status. Patients were recruited between December 2012 and November 2014, and final follow-up was in November 2017.
Interventions
Participants were randomized to 3 years of intervention in 1 of the following 8 groups: 2000 IU/d of vitamin D3, 1 g/d of omega-3s, and a strength-training exercise program (n = 264); vitamin D3 and omega-3s (n = 265); vitamin D3 and exercise (n = 275); vitamin D3 alone (n = 272); omega-3s and exercise (n = 275); omega-3s alone (n = 269); exercise alone (n = 267); or placebo (n = 270).
Main Outcomes and Measures
The 6 primary outcomes were change in systolic and diastolic blood pressure (BP), Short Physical Performance Battery (SPPB), Montreal Cognitive Assessment (MoCA), and incidence rates (IRs) of nonvertebral fractures and infections over 3 years. Based on multiple comparisons of 6 primary end points, 99% confidence intervals are presented and P < .01 was required for statistical significance.
Results
Among 2157 randomized participants (mean age, 74.9 years; 61.7% women), 1900 (88%) completed the study. Median follow-up was 2.99 years. Overall, there were no statistically significant benefits of any intervention individually or in combination for the 6 end points at 3 years. For instance, the differences in mean change in systolic BP with vitamin D vs no vitamin D and with omega-3s vs no omega-3s were both −0.8 (99% CI, –2.1 to 0.5) mm Hg, with P < .13 and P < .11, respectively; the difference in mean change in diastolic BP with omega-3s vs no omega-3s was –0.5 (99% CI, –1.2 to 0.2) mm Hg; P = .06); and the difference in mean change in IR of infections with omega-3s vs no omega-3s was –0.13 (99% CI, –0.23 to –0.03), with an IR ratio of 0.89 (99% CI, 0.78-1.01; P = .02). No effects were found on the outcomes of SPPB, MoCA, and incidence of nonvertebral fractures). A total of 25 deaths were reported, with similar numbers in all treatment groups.
Results
Among 2157 randomized participants (mean age, 74.9 years; 61.7% women), 1900 (88%) completed the study. Median follow-up was 2.99 years. Overall, there were no statistically significant benefits of any intervention individually or in combination for the 6 end points at 3 years. A total of 25 deaths were reported, with similar numbers in all treatment groups.
| Mean change at 3 y in | Vitamin D vs no vitamin D | Omega-3s vs no omega-3s | Strength exercise vs control | |||
|---|---|---|---|---|---|---|
| Difference (99% CI) | P value | Difference (99% CI) | P value | Difference (99% CI) | P value | |
| Systolic BP, mm Hg | –0.8 (–2.1 to 0.5) | .13 | –0.8 (–2.1 to 0.5) | .11 | 0.5 (–0.8 to 1.9) | .30 |
| Diastolic BP, mm Hg | 0 (–0.7 to 0.8) | .88 | –0.5 (–1.2 to 0.2) | .06 | 0.3 (–0.4 to 1.0) | .32 |
| SPPB, points | –0.1 (–0.3 to 0.1) | .26 | –0.0 (–0.2 to 0.2) | .76 | –0.1 (–0.3 to 0.1) | .25 |
| MoCA, points | –0.1 (–0.4 to 0.1) | .11 | –0.1 (–0.3 to 0.2) | .52 | 0.0 (–0.2 to 0.2) | .96 |
| Nonvertebral fractures, IR ratio | 1.03 (0.75-1.43) | .79 | 1.18 (0.85-1.63) | .19 | 1.06 (0.77-1.47) | .62 |
| Infections, IR ratio | 0.95 (0.84-1.08) | .33 | 0.89 (0.78-1.01) | .02 | 1.04 (0.92-1.18) | .38 |
Conclusions and Relevance
Among adults without major comorbidities aged 70 years or older, treatment with vitamin D3, omega-3s, or a strength-training exercise program did not result in statistically significant differences in improvement in systolic or diastolic blood pressure, nonvertebral fractures, physical performance, infection rates, or cognitive function. These findings do not support the effectiveness of these 3 interventions for these clinical outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT01745263
This randomized trial compares the effects of vitamin D, omega-3s, and a strength-training exercise program alone or in combination vs no supplementation and control exercise on changes in blood pressure, physical and cognitive function, nonvertebral fractures, and infections over 3 years among older adults.
Introduction
The numbers of older adults and adults with age-related chronic diseases are expected to more than double between 2019 and 2050.1,2 Vitamin D,3 omega-3 fatty acids,4,5 and exercise6 may prevent age-related chronic diseases. However, despite evidence that older adults do not receive sufficient vitamin D,3 omega-3s,4,5 or exercise,6 recent evidence from larger clinical trials showed no benefit of these interventions in relatively healthy older adults.7,8,9,10
The DO-HEALTH trial tested whether vitamin D, omega-3s, and a strength-training exercise program, alone or in combination, would improve 6 primary end points reflecting 5 health domains (cardiovascular health, bone health, muscle health, brain health, and immunity) among adults without major comorbidities aged 70 years or older, compared with placebo or attention control.
Methods
Study Design
This randomized, double-blind, placebo-controlled trial with a 2 × 2 × 2 factorial design had 3 primary treatment comparisons: (1) 2000 IU/d of vitamin D compared with placebo vitamin D; (2) 1 g/d of omega-3s (330 mg of eicosapentaenoic acid [EPA] plus 660 mg of docosahexaenoic acid [DHA] from marine algae) compared with placebo omega-3s; and (3) a strength-training exercise program of 30 minutes 3 times per week compared with an attention control exercise program focused on joint flexibility of 30 minutes 3 times a week.
The trial was performed at 7 centers in Switzerland, France, Germany, Portugal, and Austria. The study protocol and statistical analysis plan (Supplement 1) was approved by ethics and regulatory agencies of all 5 countries and have been previously published.11 A data and safety monitoring board oversaw the study.
Study Participants
Participants provided written informed consent. Participants were at least 70 years old and community dwelling. Inclusion criteria were no major health events (ie, cancer or myocardial infarction) in the 5 years prior to enrollment, sufficient mobility to come to the study centers without help, and a Mini-Mental State Examination (MMSE) score of at least 24. Recruitment was conducted with the goal of including at least 40% of participants with a history of falling in the prior 12 months to increase representation of older adults at higher risk of frailty. Individuals who took more than 1000 IU/d of vitamin D in supplements during the 36 months prior to enrollment or who were unwilling to limit vitamin D supplement intake to 800 IU/d and calcium supplementation to 500 mg/d during trial participation were excluded. Individuals who took omega-3 supplements during the 3 months prior to enrollment and/or were unwilling to avoid them during the trial were excluded.
Randomization and Masking
Participants were randomized to 1 of 8 treatment groups (Figure 1) using block randomization (block sizes of 16 individuals) stratified by recruitment center, prior fall, sex, and age (70-84 years or ≥85 years). A central randomization center in Switzerland, supported by trial software, was responsible for the blinding, treatment allocation, and study intervention labeling. Participants received 2 gel capsules per day (vitamin D or placebo and omega-3s or placebo), identical in size, appearance, taste, and weight. All capsules had coatings to prevent unblinding by aftertaste. DSM Nutritional Products AG provided the ingredients for the capsules, which were produced by Swisscaps.
Figure 1. Flow of Participants in the DO-HEALTH Randomized Clinical Trial.
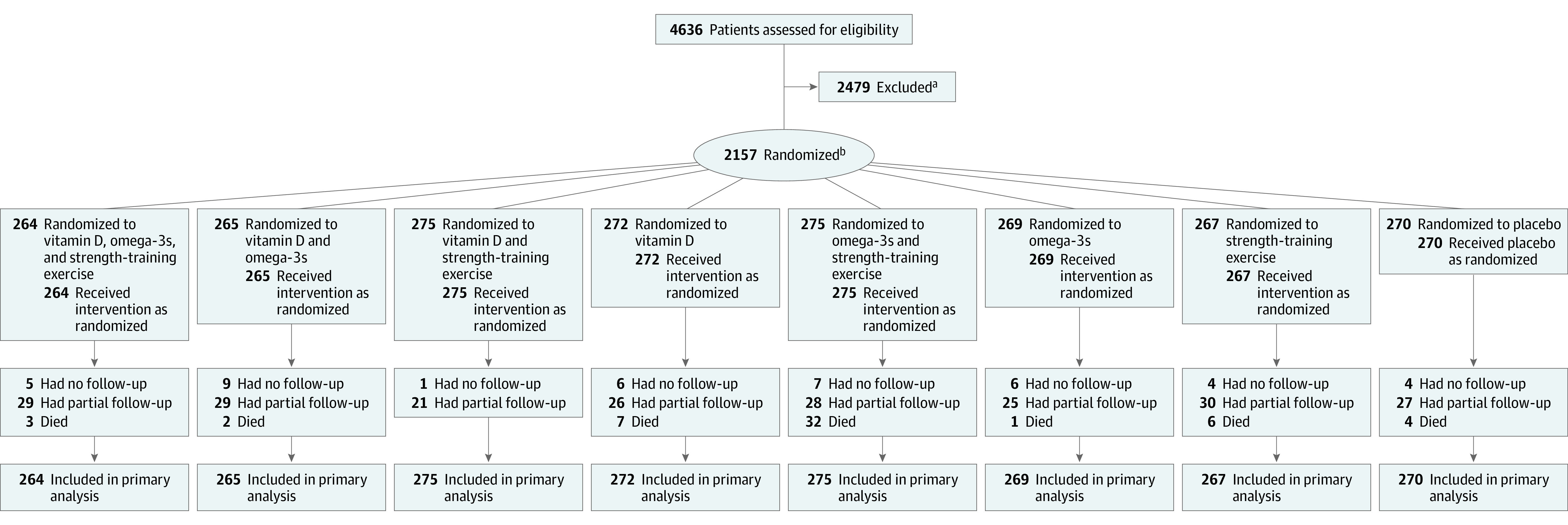
Altogether, 1075 participants who received vitamin D were included in the primary analysis (21 had no follow-up, 105 had partial follow-up, and 12 died); 1081 who did not receive vitamin D were included (21 had no follow-up, 110 had partial follow-up, and 13 died); 1073 who received omega-3s were included (27 had no follow-up, 111 had partial follow-up, and 8 died); 1084 who did not receive omega-3s were included (15 had no follow-up, 104 had partial follow-up, and 17 died); 1081 who received strength-training exercise were included (17 had no follow-up, 108 had partial follow-up, and 11 died); and 1076 who received control exercise were included (25 had no follow-up, 107 had partial follow-up, and 14 died).
aReasons for exclusion were as follows: 1268 unwilling to consent; 315 diagnoses of cancer (except nonmelanoma skin cancer, myocardial infarction, stroke, transient ischemic attack, or angina pectoris or history of coronary artery intervention); 280 unwilling to limit calcium supplements to ≤500 mg/d and vitamin D supplements to ≤800 IU/d and refrain from omega-3 supplements; 82 aged <70 years; 45 unable to swallow capsules; 34 not community dwelling; 31 participation in another clinical trial; 28 epilepsy and/or taking antiepileptic drugs; 20 hypercalcemia (serum calcium level >2.6 mmol/L); 18 insufficiently mobile; 15 living with a DO-HEALTH participant; 14 history of granulomatous disease (tuberculosis, sarcoidosis); 13 hypoparathyroidism/primary hyperparathyroidism; 11 Mini-Mental State Examination score <24 points; 6 major visual/hearing impairment; 4 severe renal impairment (creatinine clearance <15 mL/min); 3 severe liver disease; 3 fell more than 3 times in the last month; 2 hemiplegia or other gait impairment; 1 osteitis deformans (Paget disease); and 286 other reasons(medical condition that would make the results of the tests and assessments unreliable and/or would put too much burden on the participant and/or lead to safety concerns, eg, regular alcohol consumption and/or opioid abuse).
bStratified block randomization on recruitment center, prior fall, sex, and age.
All examinations and assessments were performed by trained and certified study staff using standardized methods. Participants, staff dispensing study pills and collecting outcomes, and data analysts were masked to group assignment. A physiotherapist not involved in the assessments provided instructions on the exercise programs.
Procedures
Participants were followed up for 3 years, both in yearly clinical visits (baseline and 12, 24, and 36 months) and with telephone calls every 3 months to collect information on fractures, infections, falls, adverse events, and health care use.
DSM Nutritional Products R&D Analytics performed 25-hydroxyvitamin D (25[OH]D) (25 measurements and the Research Toxicology Center performed polyunsaturated fatty acid measurements by sensitive and selective assays based on liquid chromatography coupled to a mass spectrometry detection system at baseline and at 12, 24, and 36 months. Mass spectrometry detection systems were monitored with standard, quality control, and human National Institute of Standards and Technology plasma reference samples.
Outcomes
Six primary outcomes were measured: systolic and diastolic blood pressure change (for cardiovascular health), nonvertebral fractures (for bone health), the Short Physical Performance Battery (SPPB)12 (for muscle health), the Montreal Cognitive Assessment (MoCA)13 (for brain health), and infections (for immune system health). All 4 continuous outcomes (systolic and diastolic blood pressure, the SPPB, and the MoCA) were assessed at baseline and at 12, 24, and 36 months. Systolic and diastolic blood pressure were measured 3 times after a 5-minute rest in a seated position using a standardized and validated protocol.14 The mean of the last 2 measurements was used in analyses.14 The SPPB is a performance-based test that includes 3 components: walking velocity, time for 5 repeated chair stands, and a balance test.12 A 0- to 4-point score is assigned to each test (4 = best), and scores are summed to yield an overall score ranging from 0 to 12 (12 = best).12 The MoCA is a 1-page test that measures several cognitive domains (visuospatial/executive skills, attention, naming, memory, delayed recall, attention, language, abstraction, and orientation to time and place). The MoCA score ranges from 0 to 30 (30 = best) and was chosen for its relatively high sensitivity for mild cognitive impairment.13,15,16 Rates of nonvertebral fractures and infections were assessed every 3 months (by telephone or clinical visit). Fractures were confirmed by x-ray reports or medical records describing an x-ray report or repair of the fracture. Every 3 months, participants were asked whether any infection with or without fever had occurred and date(s) of any vaccinations. Infections were assessed with a questionnaire developed in 2 pilot trials.17,18 Infections were verified by an independent physician using all available information, including symptoms, treatment, and primary care clinician diagnosis and hospitalization record, if available. Comorbidity was assessed using a validated self-administered questionnaire.19 Quality of life and self-rated health were measured with the EuroQol 5 Dimensions 3 Levels instrument.20 Physical activity and metabolic equivalent tasks were assessed with the Nurses’ Health Study questionnaire21 and the Compendium of Physical Activities.22 Several secondary end points were explored in ancillary studies (eTable 1 in Supplement 2) and will be reported elsewhere.
Sample Size and Statistical Analysis
The trial was designed to enroll 2152 participants, anticipating that 32% would be lost to follow-up and assuming no statistical interactions between the 3 treatment groups based on their distinct mechanistic pathways. With an effective sample size of 1807 before accounting for dropout, the trial was designed to have greater than 90% power at the .01 level to detect the following: a reduction in systolic/diastolic blood pressure by 6 mm Hg/3 mm Hg (based on prior small, short-term, randomized trials of vitamin D14,23,24); a 52% reduction in the 3-year incidence of nonvertebral fractures from 14% to 6.7% (based on a prior meta-analysis examining the relationship between vitamin D supplementation and fracture reduction25 and on a trial of vitamin D supplementation after hip fracture26); a reduction in SPPB score from 7.52 to 7.12 (based on the defined minimum clinically important difference in older adults of 0.3 to 0.8 points27); a mean difference of 0.7 in MoCA score (based on a prior 6-year prospective study that documented a decrease of 0.7 points on the MMSE per year comparing older adults with severe vitamin D deficiency (25[OH]D <10 ng/mL) with those without vitamin D deficiency (25[OH]D ≥30 ng/mL)28); and a reduction in the 3-year incidence of infections by 15% (based on several smaller trials14,17,23,24,25,26). At the time of the trial preparation, the effect of vitamin D or omega-3s on MoCA was not available. The cognitive function tests MoCA and MMSE have been well correlated (r = 0.53-0.87).13,15,16 After the start of the trial, the minimal clinically important difference for systolic/diastolic blood pressure reduction to reduce stroke was reported as 7.1 mm Hg/2.4 mm Hg29 and a minimum clinically important difference for the MoCA was defined as an improvement of 1.7 points over 3.5 years.30
The factorial design was chosen to evaluate both main and combined effects of the interventions in prespecified primary analyses. For each study outcome, the assumption of no effect modification between interventions was examined by including interaction terms between each combination of interventions into a regression model. Significant treatment interactions (P < .10) were found for the SPPB and infections, and therefore, treatment indicators were included for each of the 8 combinations of treatments in the regression models for the SPPB and infections. The regression models for blood pressure, nonvertebral fractures, and MoCA included indicators only for the 3 interventions. For fractures and infections, an offset was used in the Poisson regression models so that counts were representative of the actual amount of follow-up time. All patients were analyzed according to their randomization group, regardless of adherence to their assigned treatment. The analysis data set included all randomized patients with all of their available data.
Baseline clinical and demographic characteristics were compared between treatment groups using standardized mean differences. Linear regression models were used for all continuous outcomes, adjusting for correlation between serial measurements from the same participant. For the SPPB, the correlation was estimated and adjusted for using generalized estimating equations. For other continuous outcomes, the correlation was included in the structure of the model residuals. Initial models included indicators for the intervention groups, indicators for time, and interactions between interventions and time. Nonsignificant interaction terms were removed, leaving the main effect of each intervention to represent the consistent mean effect of the intervention across all 3 years. All models were adjusted for age, sex, prior fall, body mass index, study site, and the appropriate baseline outcome measure. For the outcomes of nonvertebral fractures and infections, a Poisson regression model was used with each participant’s number of fractures or infections as the outcome. The same fixed-adjustment covariates as above were included, along with each participant’s time in the study as an exposure offset.
Predefined subgroup analyses are presented by sex, age (70-84 years or ≥85 years), vitamin D deficiency at baseline (serum 25[OH]D levels <20 or ≥20 ng/mL), and median baseline polyunsaturated fatty acid levels (DHA plus EPA <100 or ≥100 μg/mL). Because of the limited number of participants older than 85 years (98/2157 [4.5%]), a post hoc decision was made to examine age subgroups aged 70-74 years and aged 75 years or older. Other planned subgroup analyses (by body mass index, physical activity, prior fall, prior fracture, fracture risk, baseline symptomatic osteoarthritis of the knee, and baseline calcium and protein intake) are not reported here.
In a post hoc analysis, missing follow-up data were imputed using multiple imputation, and study site was treated as a random effect (eTable 2 in Supplement 2). Ten imputed data sets were created, and values after death were not imputed.
All analyses were performed using SAS version 9.4 (SAS Institute Inc). To account for multiple comparisons, the significance threshold was set at P < .01, and findings are presented with 99% confidence intervals.
Results
Recruitment and Participant Characteristics
From December 2012 to November 2014, 2157 participants were enrolled: 1006 from Switzerland (552 from Zurich, 253 from Basel, and 201 from Geneva), 350 from Berlin, Germany, 200 from Innsbruck, Austria, 300 from Toulouse, France, and 301 from Coimbra, Portugal. The mean age of participants was 74.9 (SD, 4.4) years, including 57.3% who were aged 70 to 74 years, 38.1% aged 75 to 84 years, and 4.5% aged 85 years or older. Participants included 61.7% women. The median follow-up time was 2.99 years (range, 0-3.49 years); the final date of follow-up was November 17, 2017. At baseline, 10.9% took 800 IU/d or more of vitamin D, 40.7% had vitamin D deficiency (25([OH]D <20 ng/mL), and 41.9% had a fall in the year prior to enrollment.
Baseline characteristics were balanced by treatment group (Table 1). Participants had a relatively low comorbidity score (mean, 3.3 [SD, 3.0]; maximum score of 36, with lower scores indicating less comorbidity), good cognitive health (mean MMSE score, 28.5 [SD, 1.5]; maximum score of 30), and good mobility (median baseline SPPB score, 11.0 [interquartile range, 10.0-12.0]), and 82.6% were engaged in moderate to high physical activity based on the Nurses’ Health Study questionnaire. At baseline, 39.2% of participants reported a diagnosis of hypertension and 49.6% used antihypertensive medication. During follow-up, 1.2% of participants (n = 25) died and 1.0% (n = 22) were admitted to a nursing home (eTable 3 in Supplement 2).
Table 1. Baseline Characteristics of the DO-HEALTH Study Samplea.
| Characteristics | Vitamin D | Omega-3s | Exercise | Overall total (n = 2157) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D (n = 1076) | No vitamin D (n = 1081) | SMD | Omega-3s (n = 1073) | No omega-3s (n = 1084) | SMD | Strength-training exercise (n = 1081) | Control exercise (n = 1076)b | SMD | ||
| Age, mean (SD), y | 75.0 (4.5) | 74.9 (4.4) | 0.03 | 74.7 (4.3) | 75.2 (4.6) | 0.10 | 75.0 (4.5) | 74.9 (4.4) | 0.02 | 74.9 (4.4) |
| Age categories, No. (%), y | 0.05 | 0.10 | 0.02 | |||||||
| 70-74 | 606 (56.3) | 631 (58.4) | 635 (59.2) | 602 (55.5) | 622 (57.5) | 615 (57.2) | 1237 (57.3) | |||
| 75-84 | 417 (38.8) | 405 (37.5) | 398 (37.1) | 424 (39.1) | 408 (37.7) | 414 (38.5) | 822 (38.1) | |||
| ≥85 | 53 (4.9) | 45 (4.2) | 40 (3.7) | 58 (5.4) | 51 (4.7) | 47 (4.4) | 98 (4.5) | |||
| BMI, mean (SD)c | 26.5 (4.4) [n = 1075] | 26.2 (4.2) | 0.08 | 26.3 (4.2) [n = 1072] | 26.4 (4.3) | 0.02 | 26.3 (4.2) | 26.4 (4.4) [n = 1075] | 0.02 | 26.3 (4.3) [n = 2156] |
| Sex, No. (%) | ||||||||||
| Women | 667 (62.0) | 664 (61.4) | 0.01 | 668 (62.3) | 663 (61.2) | 0.02 | 665 (61.5) | 666 (61.9) | 0.01 | 1331 (61.7) |
| Men | 409 (38.0) | 417 (38.6) | 405 (37.7) | 421 (38.8) | 416 (38.5) | 410 (38.1) | 826 (38.3) | |||
| Education, mean (SD), y | 12.7 (4.4) [n = 1074] | 12.6 (4.2) [n = 1081] | 0.02 | 12.6 (4.2) [n = 1071] | 12.7 (4.4) [n = 1084] | 0.02 | 12.6 (4.2) [n = 1079] | 12.6 (4.4) [n = 1076] | 0.00 | 12.6 (4.3) [n = 2155] |
| MoCA score, mean (SD)d | 25.8 (3.3) [n = 1074] | 25.5 (3.4) [n = 1079] | 0.07 | 25.6 (3.4) [n = 1072] | 25.7 (3.3) [n = 1081] | 0.01 | 25.7 (3.3) [n = 1080] | 25.6 (3.4) [n = 1073] | 0.01 | 25.7 (3.4) [n = 2153] |
| MMSE score, mean (SD)e | 28.5 (1.5) | 28.5 (1.5) | 0.02 | 28.5 (1.5) | 28.5 (1.5) | 0.05 | 28.4 (1.5) | 28.5 (1.5) | 0.06 | 28.5 (1.5) |
| Comorbidity score, mean (SD)f | 3.3 (3.1) [n = 1075] | 3.3 (3.0) [n = 1080] | 0.02 | 3.3 (3.1) [n = 1072] | 3.3 (2.9) [n = 1083] | 0.00 | 3.2 (3.0) [n = 1079] | 3.4 (3.1) | 0.06 | 3.3 (3.0) [n = 2155] |
| Self-reported hypertension, No. (%) | 427 (39.7) [n = 1075] | 417 (38.6) [n = 1080] | 0.02 | 414 (38.6) [n = 1072] | 430 (39.7) [n = 1083] | 0.02 | 405 (37.5) [n = 1079] | 439 (40.8) | 0.07 | 844 (39.2) [n = 2155] |
| Use of antihypertensive drugs, No. (%) | 548 (50.9) | 521 (48.2) | 0.06 | 509 (47.4) | 560 (51.7) | 0.09 | 516 (47.7) | 553 (51.4) | 0.07 | 1069 (49.6) |
| Health-related quality of life score, median (IQR)g | 0.90 (0.89-1.00) [n = 1075] | 0.91 (0.89-1.00) [n = 1079] | 0.01 | 1.00 (0.89-1.00) [n = 1072] | 0.89 (0.89-1.00) [n = 1082] | 0.10 | 0.91 (0.89-1.00) [n = 1080] | 0.89 (0.89-1.00) [n = 1074] | 0.03 | 0.91 (0.89-1.00) [n = 2154] |
| Self-rated health score, mean (SD)h | 81.0 (15.1) [n = 1075] | 81.5 (14.7) [n = 1079] | 0.04 | 81.2 (14.9) [n = 1072] | 81.2 (14.9) [n = 1082] | <0.001 | 80.9 (14.8) [n = 1080] | 81.5 (15.1) [n = 1074] | 0.04 | 81.2 (14.9) [n = 2154] |
| SPPB score, median (IQR)i | 12.0 (10.0-12.0) [n = 1073] | 11.0 (10.0-12.0) [n = 1080] | 0.03 | 11.0 (10.0-12.0) [n = 1071] | 11.0 (10.0-12.0) [n = 1082] | 0.02 | 11.0 (10.0-12.0) [n = 1080] | 11.0 (10.0-12.0) [n = 1073] | 0.03 | 11.0 (10.0-12.0) [n = 2153] |
| Prior fall, No. (%)j | 446 (41.4) | 457 (42.3) | 0.02 | 441 (41.1) | 462 (42.6) | 0.03 | 450 (41.6) | 453 (42.1) | 0.01 | 903 (41.9) |
| Repeated chair test, median (IQR), sk | 10.6 (8.8-13.4) [n = 1066] | 10.9 (8.8-13.5) [n = 1065] | 0.03 | 10.6 (8.8-13.4) [n = 1056] | 10.8 (8.8-13.6) [n = 1075] | 0.02 | 10.7 (8.9-13.6) [n = 1069] | 10.8 (8.7-13.4) [n = 1062] | 0.06 | 10.7 (8.8-13.5) [n = 2131] |
| Vitamin D supplement use ≥800 IU/d, No. (%) | 110 (10.2) | 126 (11.7) | 0.05 | 123 (11.5) | 113 (10.4) | 0.03 | 127 (11.7) | 109 (10.1) | 0.05 | 236 (10.9) |
| Vitamin D severe deficiency (<12 ng/mL), No. (%) | 127 (11.9) [n = 1066] | 114 (10.6) [n = 1074] | 0.04 | 128 (12.0) [n = 1063] | 113 (10.5) [n = 1077] | 0.05 | 110 (10.3) [n = 1071] | 131 (12.3) [n = 1069] | 0.06 | 241 (11.3) [n = 2140] |
| Vitamin D deficiency (<20 ng/mL), No. (%) | 427 (40.1) [n = 1066] | 445 (41.4) [n = 1074] | 0.03 | 422 (39.7) [n = 1063] | 450 (41.8) [n = 1077] | 0.04 | 422 (39.4) [n = 1071] | 450 (42.1) [n = 1069] | 0.06 | 872 (40.7) [n = 2140] |
| Serum 25-hydroxyvitamin D concentration, mean (SD), ng/mLl | 22.4 (8.4) [n = 1066] | 22.4 (8.5) [n = 1074] | 0.01 | 22.4 (8.4) [n = 1063] | 22.4 (8.4) [n = 1077] | 0.00 | 22.8 (8.6) [n = 1071] | 22.0 (8.2) [n = 1069] | 0.10 | 22.4 (8.4) [n = 2140] |
| Serum DHA concentration, mean (SD), μg/mLm | 78.1 (37.9) [n = 1064] | 78.1 (35.9) [n = 1073] | 0.00 | 78.9 (37.2) [n = 1059] | 77.3 (36.6) [n = 1078] | 0.04 | 78.2 (36.5) [n = 1069] | 78.0 (37.4) [n = 1068] | 0.00 | 78.1 (36.9) [n = 2137] |
| Serum EPA concentration, median (IQR), μg/mLm | 24.8 (17.4-37.7) [n = 1064] | 26.2 (18.6-37.7) [n = 1073] | 0.02 | 26.1 (18.5-37.7) [n = 1059] | 25.3 (17.6-37.9) [n = 1078] | 0.01 | 25.1 (17.5-37.6) [n = 1069] | 25.9 (18.6-38.1) [n = 1068] | 0.02 | 25.5 (18.1-37.7) [n = 2137] |
| Physical activity level, No. (%)n | 0.10 | 0.05 | 0.05 | |||||||
| None | 207 (19.3) | 168 (15.6) | 190 (17.7) | 185 (17.1) | 179 (16.6) | 196 (18.2) | 375 (17.4) | |||
| 1-2 times per wk | 318 (29.6) | 334 (30.9) | 311 (29.0) | 341 (31.5) | 323 (29.9) | 329 (30.6) | 652 (30.3) | |||
| ≥3 times per wk | 550 (51.2) [n = 1075] | 578 (53.5) [n = 1080] | 570 (53.2) [n = 1071] | 558 (51.5) | 578 (53.5) [n = 1080] | 550 (51.2) [n = 1075] | 1128 (52.3) [n = 2155] | |||
| Physical activity volume, mean (SD), MET-h/wko | 35.4 (32.0) | 38.5 (34.1) [n = 1080] | 0.09 | 38.2 (34.1) [n = 1072] | 35.7 (32.0) | 0.07 | 37.0 (33.4) [n = 1080] | 36.9 (32.7) | 0.00 | 36.9 (33.1) [n = 2156] |
| Current smoking, No. (%) | 63 (5.9) | 63 (5.8) | 0.00 | 64 (6.0) | 62 (5.7) | 0.01 | 58 (5.4) | 68 (6.3) | 0.04 | 126 (5.8) |
| Systolic blood pressure, mean (SD), mm Hg | 144.2 (18.6) [n = 1070] | 142.9 (18.1) [n = 1075] | 0.07 | 143.2 (18.3) [n = 1069] | 143.9 (18.4) [n = 1076] | 0.04 | 143.7 (18.5) [n = 1075] | 143.4 (18.2) [n = 1070] | 0.02 | 143.5 (18.3) [n = 2145] |
| Diastolic blood pressure, mean (SD), mm Hg | 76.0 (10.1) [n = 1070] | 75.7 (10.0) [n = 1075] | 0.03 | 75.8 (9.7) [n = 1069] | 75.9 (10.4) [n = 1076] | 0.01 | 75.9 (10.3) [n = 1075] | 75.8 (9.8) [n = 1070] | 0.01 | 75.9 (10.0) [n = 2145] |
Abbreviations: IQR, interquartile range; SMD, standardized mean difference
Medians and IQRs are presented for variables with skewness >1.5. Percentages are rounded to 1 decimal, which could lead to percentage sums of 100.1% or 99.9%.
Flexibility was the control exercise program.
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Higher BMI values reflect overweight (≥25) and obesity (≥30).
The Montreal Cognitive Assessment (MoCA) is a screening test for mild cognitive dysfunction and has a range of 0 to 30 points, in which higher scores are better and scores greater than 26 suggest normal cognitive function.
The Mini-Mental State Examination (MMSE) was used to measure cognitive impairment and has a range of 0 to 30 points, in which higher scores are better and scores greater than 24 suggest normal cognitive function.
Comorbidity was measured by the Self-Administered Comorbidity Questionnaire, which assesses current medical comorbidities (12 comorbidities by 3 dimensions: presence, medication, and limitation of activities). It has a range of 0 to 36 points, in which lower scores are better.
Health-related quality of life was assessed by the EuroQol 5 Dimensions 3 Levels (EQ-5D-3L). Scores range from less than 0 to a maximum of 1 point, in which 0 means a health state equivalent to death, negative values are equivalent to a health state worse than death, and 1 is equivalent to perfect health.
Self-rated health was assessed by the EQ-5D-3L vertical visual analog scale, which ranges from 0 to 100 points, in which higher scores are better.
The Short Physical Performance Battery (SPPB) assesses lower extremity function. Scores range from 0 to 12, in which higher scores are better.
Prior fall in the 1-year period before study start.
Repeated chair stands assess reaction time; the score is the time (in seconds) to complete 5 repeated chair stands. It is part of the SPPB, and lower values (less time to complete the stands) are better.
Normal low value for serum 25-hydroxyvitamin D concentration is ≥20 ng/mL.
There is no consensus on normal values for serum docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA) concentrations.
Physical activity level was based on the Nurses’ Health Study questionnaire on physical activity.
Weekly volume of physical activity was estimated based on the Nurses’ Health Study questionnaire on physical activity, in which energy expenditure of different activities in metabolic equivalent tasks (METs) of activities based on the Compendium of Physical Activities were summed over the previous week.
Retention and Adherence
The withdrawal rate was 11.9%, with no difference in withdrawal rates across the 8 treatment groups (P = .52) (Figure 1). Follow-up data were missing for 9.6%. A total of 85.8% of participants took at least 80% of their total study pills (eTable 4 in Supplement 2). At year 3, participants randomized to receive vitamin D had higher mean serum concentrations of 25(OH)D than those not randomized to receive vitamin D (37.6 vs 24.4 ng/mL, respectively). Those randomized to receive omega-3s had higher concentrations of DHA and EPA compared with those not randomized to receive omega-3s (135.6 vs 76.3 μg/mL for DHA and 64.7 vs 33.8 μg/mL for EPA at 3 years) (eTable 5 and eFigure 1 in Supplement 2).
Based on self-report every 3 months throughout the 3-year follow-up (eTable 4 in Supplement 2), at years 1, 2, and 3, 70.3% of participants performed the exercise programs at least twice per week and 61.8% of participants performed the exercise programs at least 3 times per week.
Primary End Points
The mean systolic blood pressure was 143.5 (99% CI, 142.5-144.6) mm Hg at baseline and declined by 8.2 (99% CI, –8.9 to –7.5) mm Hg across all participants over 3-year follow-up. The mean diastolic blood pressure was 75.9 (99% CI, 75.3-76.4) mm Hg at baseline and declined by 3.1 (99% CI, –3.5 to –2.8) mm Hg across all participants over 3-year follow-up. There was no statistically significant difference for any of the 3 treatments compared with their placebo/control in systolic or diastolic blood pressure over 3-year follow-up (Table 2). The mean difference in systolic blood pressure over 3-year follow-up among participants randomized to vitamin D vs no vitamin D was –0.8 mm Hg (99% CI, –2.1 to 0.5 mm Hg; P = .13). The difference in diastolic blood pressure among participants randomized to omega-3s vs no omega-3s was −0.5 mm Hg (99% CI, –1.2 to 0.2 mm Hg; P = .06).
Table 2. Results for Blood Pressure, Physical Performance, and Cognitive End Pointsa.
| Vitamin D | Omega-3s | Exercise | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D (n = 1076) | No vitamin D (n = 1081) | Difference (99% CI) | P value | Omega-3s (n = 1073) | No omega-3s (n = 1084) | Difference (99% CI) | P value | Strength-training exercise (n = 1081) | Control exercise (n = 1076)b | Difference (99% CI) | P value | |
| Systolic blood pressure, mm Hg (99% CI) | ||||||||||||
| Unadjusted at baseline | 144.2 (142.7 to 145.6) | 142.9 (141.5 to 144.3) | 1.2 (–0.8 to 3.3) | .12 | 143.2 (141.8 to 144.6) | 143.9 (142.4 to 145.3) | –0.7 (–2.7 to 1.4) | .41 | 143.7 (142.2 to 145.2) | 143.4 (141.9 to 144.8) | 0.3 (–1.7 to 2.4) | .70 |
| Adjusted change from baselinec | ||||||||||||
| Year 1 | –8.1 (–9.3 to –7.0) | –7.8 (–9.0 to –6.7) | –0.3 (–2 to 1.4) | .64 | –8.1 (–9.2 to –6.9) | –7.9 (–9.1 to –6.7) | –0.1 (–1.8 to 1.5) | .82 | –7.5 (–8.7 to –6.3) | –8.4 (–9.6 to –7.3) | 0.9 (–0.7 to 2.6) | .15 |
| Year 2 | –8.8 (–10 to –7.6) | –7.9 (–9.1 to –6.7) | –0.9 (–2.6 to 0.8) | .18 | –9.0 (–10.2 to –7.8) | –7.7 (–8.9 to –6.5) | –1.3 (–3.0 to 0.4) | .05 | –8.2 (–9.4 to –7.0) | –8.5 (–9.7 to –7.3) | 0.3 (–1.4 to 2.0) | .60 |
| Year 3 | –9.1 (–10.4 to –7.8) | –7.7 (–9 to –6.5) | –1.3 (–3.2 to 0.5) | .18 | –9.0 (–10.4 to –7.7) | –7.8 (–9.1 to –6.5) | –1.3 (–3.1 to 0.6) | .08 | –8.3 (–9.6 to –7.0) | –8.5 (–9.8 to –7.2) | 0.2 (–1.7 to 2.0) | .80 |
| Averaging across 3 y | –8.6 (–9.6 to –7.7) | –7.9 (–8.8 to –6.9) | –0.8 (–2.1 to 0.5) | .13 | –8.7 (–9.6 to –7.7) | –7.8 (–8.8 to –6.9) | –0.8 (–2.1 to 0.5) | .11 | –8.0 (–8.9 to –7.0) | –8.5 (–9.4 to –7.6) | 0.5 (–0.8 to 1.9) | .30 |
| Diastolic blood pressure, mm Hg (99% CI) | ||||||||||||
| Unadjusted at baseline | 76.0 (75.2 to 76.8) | 75.7 (74.9 to 76.5) | 0.3 (–0.8 to 1.4) | .48 | 75.8 (75.1 to 76.6) | 75.9 (75.1 to 76.8) | –0.1 (–1.2 to 1.0) | .78 | 75.9 (75.1 to 76.7) | 75.8 (75.1 to 76.6) | 0.1 (–1.1 to 1.2) | .89 |
| Adjusted change from baselinec | ||||||||||||
| Year 1 | –2.9 (–3.5 to –2.2) | –3.1 (–3.7 to –2.4) | 0.2 (–0.7 to 1.1) | .56 | –3.1 (–3.8 to –2.5) | –2.8 (–3.4 to –2.1) | –0.4 (–1.3 to 0.5) | .27 | –2.6 (–3.2 to –2.0) | –3.3 (–3.9 to –2.7) | 0.7 (–0.2 to 1.6) | .05 |
| Year 2 | –3.2 (–3.8 to –2.5) | –3.1 (–3.8 to –2.5) | –0.1 (–1.0 to 0.9) | .89 | –3.4 (–4.1 to –2.8) | –2.8 (–3.5 to –2.2) | –0.6 (–1.5 to 0.3) | .08 | –3.2 (–3.8 to –2.5) | –3.1 (–3.8 to –2.4) | –0.1 (–1.0 to 0.9) | .87 |
| Year 3 | –3.4 (–4.1 to –2.7) | –3.3 (–4.0 to –2.6) | –0.1 (–1.0 to 0.9) | .84 | –3.7 (–4.4 to –3) | –3 (–3.7 to –2.3) | –0.7 (–1.6 to 0.3) | .08 | –3.3 (–4.0 to –2.6) | –3.4 (–4.1 to –2.7) | 0.1 (–0.9 to 1.0) | .87 |
| Averaging across 3 y | –3.1 (–3.6 to –2.6) | –3.2 (–3.7 to –2.7) | 0 (–0.7 to 0.8) | .88 | –3.4 (–3.9 to –2.9) | –2.9 (–3.4 to –2.4) | –0.5 (–1.2 to 0.2) | .06 | –3.0 (–3.5 to –2.5) | –3.3 (–3.8 to –2.8) | 0.3 (–0.4 to 1.0) | .32 |
| MoCA score (99% CI)d | ||||||||||||
| Unadjusted at baseline | 25.8 (25.5 to 26.0) | 25.5 (25.3 to 25.8) | 0.2 (–0.1 to 0.6) | .11 | 25.6 (25.4 to 25.9) | 25.7 (25.4 to 25.9) | –0.0 (–0.4 to 0.4) | .91 | 25.7 (25.4 to 25.9) | 25.6 (25.4 to 25.9) | 0.0 (–0.3 to 0.4) | .85 |
| Adjusted change from baselinec,e | ||||||||||||
| Year 1 | 0.3 (0.1 to 0.5) | 0.5 (0.3 to 0.7) | –0.2 (–0.5 to 0) | .03 | 0.4 (0.2 to 0.5) | 0.4 (0.3 to 0.6) | –0.1 (–0.4 to 0.2) | .40 | 0.4 (0.2 to 0.6) | 0.4 (0.2 to 0.6) | 0.0 (–0.3 to 0.2) | .69 |
| Year 2 | 0.5 (0.3 to 0.7) | 0.6 (0.4 to 0.8) | –0.1 (–0.4 to 0.2) | .25 | 0.5 (0.3 to 0.7) | 0.6 (0.4 to 0.8) | –0.1 (–0.4 to 0.2) | .48 | 0.6 (0.4 to 0.8) | 0.5 (0.3 to 0.6) | 0.2 (–0.1 to 0.4) | .13 |
| Year 3 | 0.4 (0.2 to 0.6) | 0.5 (0.3 to 0.7) | 0.0 (–0.3 to 0.2) | .73 | 0.5 (0.3 to 0.7) | 0.4 (0.2 to 0.6) | 0.0 (–0.3 to 0.3) | .90 | 0.4 (0.2 to 0.6) | 0.5 (0.3 to 0.7) | –0.1 (–0.4 to 0.2) | .40 |
| Averaging across 3 y | 0.4 (0.2 to 0.5) | 0.5 (0.4 to 0.7) | –0.1 (–0.4 to 0.1) | .11 | 0.4 (0.3 to 0.6) | 0.5 (0.3 to 0.6) | –0.1 (–0.3 to 0.2) | .52 | 0.5 (0.3 to 0.6) | 0.5 (0.3 to 0.6) | 0.0 (–0.2 to 0.2) | .96 |
| SPPB score (99% CI)f | n = 272 | n = 270 | n = 269 | n = 270 | n = 267 | n = 270 | ||||||
| Unadjusted at baseline | 10.9 (10.7 to 11.1) | 10.9 (10.7 to 11.1) | –0.1 (–0.4 to 0.2) | .52 | 10.9 (10.7 to 11.1) | 10.9 (10.7 to 11.1) | 0.0 (–0.3 to 0.4) | .79 | 10.8 (10.5 to 11.0) | 10.9 (10.7 to 11.1) | 0.0 (–0.3 to 0.3) | .88 |
| Adjusted change from baselinec,g | ||||||||||||
| Year 1 | –0.2 (–0.4 to –0.0) | –0.0 (–0.2 to 0.1) | –0.2 (–0.5 to 0.1) | .05 | –0.1 (–0.2 to 0.1) | –0.0 (–0.2 to 0.1) | –0.1 (–0.3 to 0.2) | .46 | –0.1 (–0.3 to 0.1) | –0.0 (–0.2 to 0.1) | –0.1(–0.3 to 0.2) | .42 |
| Year 2 | –0.1 (–0.2 to 0.1) | –0.0 (–0.2 to 0.1) | –0.0 (–0.3 to 0.2) | .82 | –0.1 (–0.3 to 0.1) | –0.0 (–0.2 to 0.1) | –0.1 (–0.3 to 0.2) | .40 | –0.1 (–0.2 to 0.1) | –0.0 (–0.2 to 0.1) | –0.0 (–0.3 to 0.2) | .72 |
| Year 3 | –0.1 (–0.3 to 0.1) | –0.1 (–0.3 to 0.1) | –0.0 (–0.3 to 0.2) | .74 | –0.0 (–0.2 to 0.2) | –0.1 (–0.3 to 0.1) | 0.1 (–0.2 to 0.3) | .45 | –0.3 (–0.5 to –0.0) | –0.1 (–0.3 to 0.1) | –0.1 (–0.4 to 0.1) | .19 |
| Averaging across 3 y | –0.1 (–0.3 to 0.0) | –0.1 (–0.2 to 0.1) | –0.1 (–0.3 to 0.1) | .26 | –0.1 (–0.2 to 0.1) | –0.1 (–0.2 to 0.1) | –0.0 (–0.2 to 0.2) | .76 | –0.1 (–0.3 to 0.0) | –0.1 (–0.2 to 0.1) | –0.1 (–0.3 to 0.1) | .25 |
Abbreviations: MoCA, Montreal Cognitive Assessment; SPPB, Short Physical Performance Battery.
Sample sizes reflect the number of participants randomized to each treatment group.
Flexibility was the control exercise program.
Linear regression models were used for all continuous outcomes, adjusting for correlation between serial measurements from the same participant. For the SPPB, the correlation was estimated and adjusted for using generalized estimating equations. For blood pressure and MoCA, the correlation was included in the structure of the model residuals. Models were adjusted for the fixed effects of age, sex, prior falls, body mass index, number of visits, study site, and corresponding baseline measure. Results for year 1 are the adjusted average change between year 1 follow-up outcomes and baseline (randomization) outcomes. Similarly, year 2 results reflect the adjusted average change between year 2 follow-up outcomes and baseline, and year 3 results reflect changes between year 3 follow-up outcomes and baseline. Changes across all 3 years are the weighted, adjusted average of the 3 yearly effects just described and conceptually capture the average follow-up outcome minus the baseline outcome: ([year 1 + year 2 + year 3]/3 − baseline).
The MoCA was used to assess cognitive decline. It is a 1-page, 30-point test that covers several cognitive domains (visuospatial/executive skills, attention, naming, memory, delayed recall, attention, language, abstraction, and orientation to time and place), in which positive changes indicate improvement in cognitive function over time.
A significant time × treatment interaction (P = .04) was found only for the effect of the strength-training exercise on the MoCA, so the effects of the strength-training exercise on the MoCA should be viewed on a year-by-year basis rather than across all 3 years.
The SPPB is a brief performance-based test that includes walking speed, repeated chair stands, and a balance test to assess muscle function. Its 3 components are scored between 0 and 4, with 4 indicating the highest level of performance, and are summed to yield an overall score with a maximum of 12 points. Negative changes indicate a decrease in muscle function over time. Comparisons for the SPPB were vitamin D vs placebo, omega-3s vs placebo, and strength-training exercise program vs control exercise.
Significant treatment interactions were found for the SPPB. Therefore, treatment indicators were included for each of the 8 combinations of treatments in the regression models and for each intervention (with neither of the other 2 interventions present) compared with the 270 participants who received none of the 3 interventions.
Among all participants, SPPB scores declined (–0.07; 99% CI, –0.12 to –0.01; P = .001) and MoCA scores improved (0.5; 99% CI, 0.3-0.6; P < .001) during follow-up. Overall, 256 nonvertebral fractures were recorded, consistent with a yearly incidence rate of 0.04 (99% CI, 0.04-0.05) per person-year. There were no statistically significant differences for any of the 3 treatments compared with their respective placebo/control in the SPPB, the MoCA, or nonvertebral fractures (Table 2 and Table 3).
Table 3. Results for Fracture and Infection End Points.
| Vitamin D | Omega-3s | Exercise | ||||
|---|---|---|---|---|---|---|
| Vitamin D | No vitamin D | Omega-3s | No omega-3s | Strength-training exercise | Control exercisea | |
| Nonvertebral fractures over 3 yb | ||||||
| Crude estimates | ||||||
| No. of participantsc | 1076 | 1081 | 1073 | 1084 | 1081 | 1076 |
| No. of fractures | 129 | 127 | 136 | 120 | 133 | 123 |
| Incidence rate per person-y (99% CI) | 0.04 (0.03-0.05) | 0.04 (0.03-0.05) | 0.05 (0.04-0.06) | 0.04 (0.03-0.05) | 0.04 (0.04-0.06) | 0.04 (0.03-0.05) |
| Absolute difference (99% CI)d | 0.00 (–0.01 to 0.01) | 0.00 (–0.00 to 0.02) | 0.00 (–0.01 to 0.01) | |||
| Incidence rate ratio (99% CI) | 1.02 (0.74-1.40) | 1.17 (0.85-1.62) | 1.07 (0.77-1.47) | |||
| P value | .89 | .21 | .60 | |||
| Adjusted estimates | ||||||
| Adjusted incidence rate per person-y, (99% CI) | 0.04 (0.03-0.05) | 0.04 (0.03-0.05) | 0.04 (0.03-0.05) | 0.04 (0.03-0.05) | 0.04 (0.03-0.05) | 0.04 (0.03-0.05) |
| Adjusted incidence rate ratio (99% CI)e | 1.03 (0.75-1.43) | 1.18 (0.85-1.63) | 1.06 (0.77-1.47) | |||
| P value | .79 | .19 | .62 | |||
| All infections over 3 yf | ||||||
| Crude estimates | ||||||
| No. of participantsc | 272 | 270 | 269 | 270 | 267 | 270 |
| No. of infections | 786 | 825 | 716 | 825 | 841 | 825 |
| Incidence rate per person-y (99% CI) | 1.04 (0.95-1.14) | 1.10 (1.00-1.20) | 0.97 (0.88-1.06) | 1.10 (1.00-1.20) | 1.14 (1.04-1.24) | 1.10 (1.00-1.20) |
| Absolute difference (99% CI)d | –0.06 (–0.16 to 0.05) | –0.13 (–0.23 to –0.03) | 0.04 (–0.07 to 0.15) | |||
| Incidence rate ratio (99% CI) | 0.95 (0.84-1.0) | 0.88 (0.77-1.01) | 1.04 (0.92-1.18) | |||
| P value | .30 | .01 | .44 | |||
| Adjusted estimates | ||||||
| Adjusted incidence rate per person-y (99% CI) | 1.01 (0.92-1.10) | 1.06 (0.96-1.15) | 0.94 (0.85-1.03) | 1.06 (0.96-1.15) | 1.10 (1.01-1.20) | 1.06 (0.96-1.15) |
| Adjusted incidence rate ratio (99% CI)e | 0.95 (0.84-1.08) | 0.89 (0.78-1.01) | 1.04 (0.92-1.18) | |||
| P value | .33 | .02 | .38 | |||
Flexibility was the control exercise program.
Fractures were assessed every 3 months and confirmed by x-ray reports or medical records that described an x-ray report of the fracture or noted repair of the fracture.
Sample sizes reflect the number of participants randomized to each treatment group.
Estimates are from unadjusted Poisson regression models of nonvertebral fracture and infection counts across 3 years.
Rates and P values from Poisson regression models of nonvertebral fracture and infection counts across 3 years. Models were adjusted for the fixed effects of age, sex, prior falls, body mass index, and study site. A significant treatment interaction between vitamin D and omega-3s was found for infections (P = .01). Therefore, treatment indicators were included for each of the 8 combinations of treatments in the regression models for infections.
Infections were assessed via questionnaire every 3 months and verified by an independent physician using all available information, including symptoms present, treatment received, and general practitioner diagnosis and hospitalization record, if available. Comparison groups for infections are vitamin C vs placebo, omega-3s vs placebo, and strength-training exercise program vs control exercise.
Overall, 6233 infections were documented during follow-up (yearly incidence rate, 1.04 [99% CI, 1.01-1.08] per person-year). There was no statistically significant effect of any of the 3 treatments on the mean number of infections compared with their placebo. There was no statistically significant effect of omega-3s on the incidence of infections compared with placebo (716 infections [11.49% of the 6233 total infections] among 269 participants receiving omega-3s; 825 infections [13.24%] among 270 receiving placebo; incidence rate ratio [IRR], 0.89; 99% CI, 0.78-1.01; P = .02) (Table 3). In a post hoc analysis, the effect of treatment interventions was examined for the most frequently occurring types of infections. Results showed statistically significant effects of omega-3s for upper respiratory tract infections (1291 infections [46.86%] among 1073 participants receiving omega-3s; 1464 infections [53.14%] among 1084 not receiving omega-3s; IRR, 0.90; 99% CI, 0.81-0.99; P = .005) (eTable 6 in Supplement 2) and for urinary tract infections (275 infections [44.35%] among 1073 receiving omega-3s;345 infections [55.65%] among 1084 not receiving omega-3s; IRR, 0.38; 99% CI, 0.23-0.62; P < .001).
Prespecified Subgroup Analyses
To avoid overtesting, only the 2 primary outcomes with the lowest P values, systolic blood pressure and infections, were tested for combined intervention effects (Figure 2) and by subgroups of sex and age (70-74 years vs 75 years or older) (eFigure 2A and eTable 7 in Supplement 2). Similarly, subgroup analyses defined by baseline blood levels of the nutrients were limited to the effects of vitamin D and omega-3s on systolic blood pressure and infections (eFigure 2B in Supplement 2). Subgroup analyses were preplanned but considered exploratory.
Figure 2. Effects in the 8 Treatment Groups on Systolic Blood Pressure and Infections Over 3 Years.
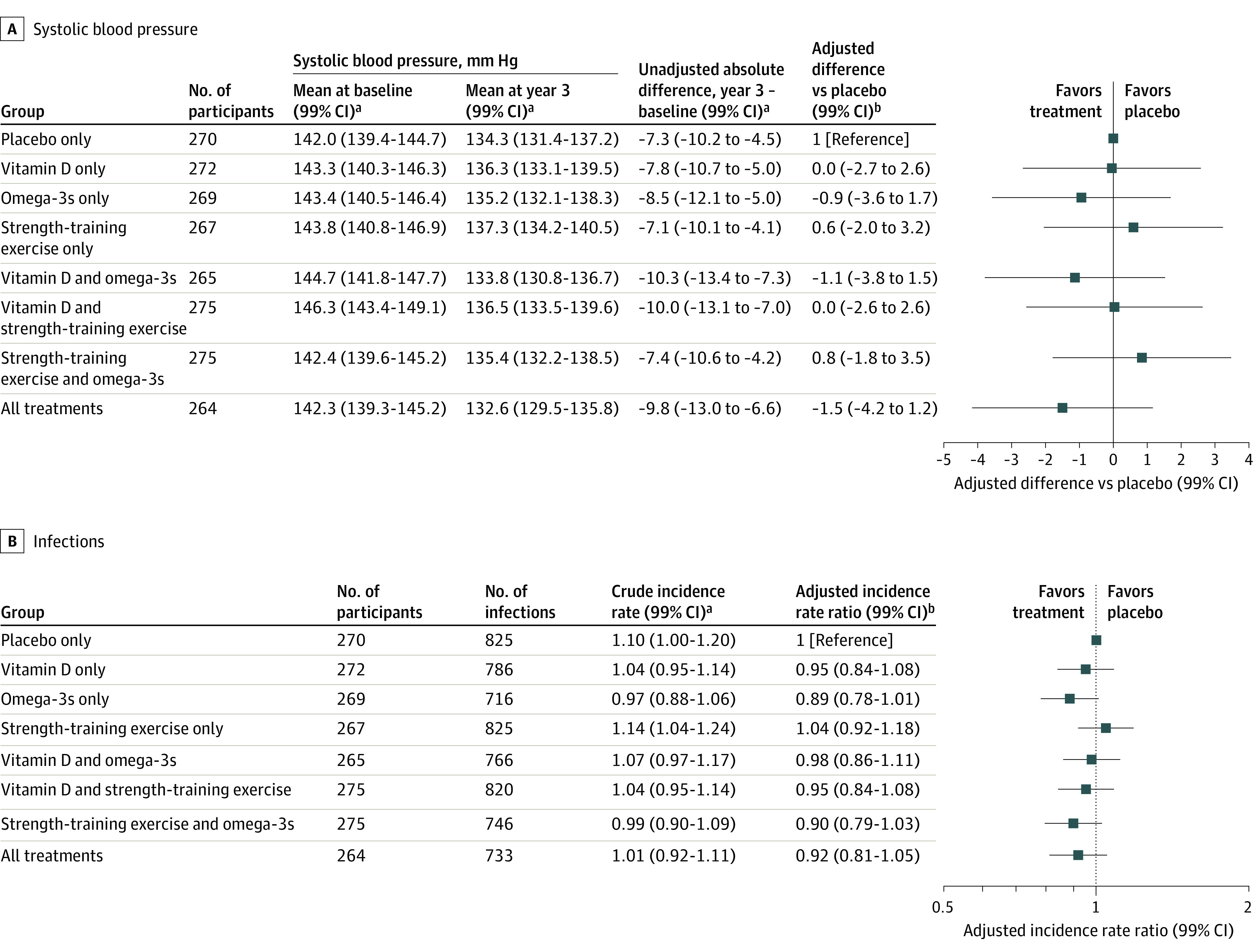
The 2 primary outcomes with the greatest intervention effects, systolic blood pressure and infections, were examined for combined intervention effects. A, Differences in systolic blood pressure are presented for the 8 randomized treatment groups (different from the treatment main effects results presented in Table 2) in the primary analysis, adjusted for the fixed effects of age, sex, prior fall, number of visit, body mass index, study site, and baseline systolic blood pressure. The plot shows differences of least-square means from a repeated-measures linear regression model with changes from baseline at 1, 2, and 3 years as outcomes compared with changes in the placebo group. B, Infections are presented for the 8 randomized treatment groups in the primary analysis, adjusted for the fixed effects of age, sex, prior fall, body mass index, study site, and offset of log person-years. The plot shows the incidence rate ratio per person-year from a Poisson regression model with infection count across 3 years as the outcome. Infections were assessed via questionnaire every 3 months and verified by an independent physician using all available information, including symptoms present, treatment received, and general practitioner diagnosis and hospitalization record, if available.
aUnadjusted values.
bAdjusted values.
Combined Effects
Figure 2 shows the combined effects of the interventions based on the 8 treatment groups, along with each treatment group compared with placebo. For both systolic blood pressure and infections, there were no statistically significant benefits in any of the treatment combinations compared with placebo.
Subgroups
For systolic blood pressure change, there was a statistically significant interaction between vitamin D and sex (P = .01), with men benefiting more from vitamin D (–2.5 [99% CI, –4.5 to –0.4] mm Hg; P = .002) (eFigure 2A in Supplement 2). The interaction between the strength-training exercise program and sex was also significant (P = .001), with a significant increase in systolic blood pressure among women (1.9 [99% CI, 0.1-3.6] mm Hg; P = .005).
The effect of vitamin D on systolic blood pressure change was also examined in the subgroup of participants with baseline 25(OH)D levels of less than 20 ng/mL vs participants with baseline levels of 20 ng/mL or greater (eFigure 2B in Supplement 2). There was no statistically significant interaction for the effect of vitamin D on systolic blood pressure change according to baseline vitamin D level. There was also no statistically significant interaction for the effect of omega-3s on systolic blood pressure change according to baseline blood levels of DHA and EPA.
For infections, there was a statistically significant interaction for vitamin D and age in which younger participants (aged 70-74 years) had fewer infections in response to vitamin D (IRR, 0.84; 99% CI, 0.71-0.99; P = .007) (eFigure 2A in Supplement 2). Men benefited from omega-3s for the outcome of a reduced infection rate (IRR, 0.78; 99% CI, 0.61-0.99; P = .008). However, there was a statistically significant interaction for the effect of omega-3s on infection by baseline DHA and EPA levels, with a greater reduction in infections in the group with higher baseline DHA and EPA levels (≥100 μg/mL; IRR, 0.82; 99% CI, 0.68-0.99; P = .007) (eFigure 2B in Supplement 2). For the strength-training exercise program, there were no differential benefits by sex. However, there was a significant increase in infection rate among older participants randomized to the strength-training exercise program compared with placebo (IRR, 1.27; 99% CI, 1.03-1.56; P = .003). Although some of the subgroup comparisons had statistically significant results of tests for interaction, given the null main effect and the large number of statistical comparisons, these should be considered hypothesis generating.
Adverse Events
Nineteen participants (8 receiving vitamin D and 11 not receiving vitamin D) had measured calcium levels of 2.6 mmol/L or greater, and 5 participants (2 receiving vitamin D and 3 not receiving vitamin D) had parathyroid hormone levels of 15 ng/L or less (eTables 8 and 9 in Supplement 2).
Discussion
In this 5-country European trial of 2157 adults aged 70 years or older without major comorbidities, vitamin D, omega-3s, and a strength-training exercise program, individually or in combination, did not improve 6 primary health end points, including systolic and diastolic blood pressure, nonvertebral fractures, lower extremity function as measured by the SPPB, cognitive decline as measured by the MoCA, and rate of infections. Study strengths included high adherence to all 3 interventions, minimal mortality and loss to follow-up, and biomarkers suggesting adherence of participants to study medications.
The effect of vitamin D on blood pressure was tested in this trial based on preclinical studies documenting that vascular smooth muscle cells, endothelial cells, and cardiomyocytes expressed the vitamin D receptor.31,32 Additional preclinical evidence showed that vitamin D regulates the renin-angiotensin-aldosterone system via suppression of renin gene expression33 and that vitamin D receptor–knockout mice have hypertension.33 However, clinical trials of vitamin D and hypertension have shown mixed results.34,35,36
A potential benefit of omega-3s on systolic blood pressure was supported by a 2015 meta-analysis of 70 small randomized clinical trials (mean follow-up of 69 days) suggesting that omega-3s (EPA plus DHA) at a mean dosage of 3.8 g/d, compared with placebo, was associated with a 1.52–mm Hg (99% CI, −2.25 to −0.79 mm Hg) reduction of systolic blood pressure.37 A 2018 update of this meta-analysis that included 50 trials of 4 weeks’ duration or longer showed that omega-3s was associated with a 2.20–mm Hg (99% CI, –3.17 to –1.22 mm Hg) reduction of systolic blood pressure.38 Results reported herein showing no statistically significant benefit of 1 g/d of omega-3s on blood pressure may in part be explained by the relatively small dosage of omega-3s.37
Regarding the outcome of infections, 2 meta-analyses of randomized clinical trials suggested that administration of omega-3s (mainly as fish oil) reduced rates of postoperative infectious complications in critically ill or surgical patients.39,40 However, among older adults without major comorbidities followed up for 3 years, omega-3s did not reduce infections overall.
This strength-training exercise program had no statistically significant effect on any of the outcomes tested. However, this study’s findings do not invalidate previous beneficial effects of the same exercise program on fall prevention among frail older adults with acute hip fracture and a mean age of 84 years,26 the well-documented benefit of exercise on fall-related fracture prevention among community-dwelling older adults in general, or the benefits of physical activity on healthy aging.41
Limitations
This study has several limitations. First, 83% of participants were already engaging in moderate to high physical activity at baseline, and there may have been little potential for further benefit from additional exercise. The healthy study population may also explain the smaller number of fractures than anticipated. Similarly, more participants with baseline values near the maximum values for the SPPB and MoCA measures may have reduced the chance to detect a benefit of the interventions on these outcomes and limited generalizability of the study’s findings to older adults without major comorbidities. Second, the overall improvement of cognitive function may be explained by a learning effect. Third, only 40.7% of participants had 25(OH)D levels of less than 20 ng/mL at baseline, and according to current guidelines, all were allowed to take 800 IU/d of supplemental vitamin D outside the study medication. Fourth, given the large number of randomization groups and comparisons (3 main treatment groups × 6 clinical end points), even the P = .01 significance threshold may have been too liberal. Fifth, even for pairwise comparisons in which the P value was between .01 and <.05, the magnitude of the difference was small and likely not clinically meaningful.
Conclusions
Among adults aged 70 years or older without major comorbidities, treatment with vitamin D3 (2000 IU/d), omega-3 fatty acids (1 g/d), or a strength-training exercise program did not result in statistically significant differences in improvement in systolic or diastolic blood pressure, nonvertebral fractures, physical performance, infection rates, or cognitive function. These findings do not support the effectiveness of these 3 interventions for these clinical outcomes.
Trial Protocol
eTable 1. Pre-defined Primary, Secondary, Exploratory, and Biomarkers Outcomes in DO-HEALTH
eTable 2. Post Hoc Analysis Using Multiply Imputed Data and Site Random Effects
eTable 3. Mortality and Nursing Home Admission in the 3-Year Follow-up of DO-HEALTH by Treatment Group
eTable 4. Adherence to the Study Medication and Exercise Programs [n, (%)] in DO-HEALTH by Treatment Group
eTable 5. Average Absolute Changes in Serum 25(OH)D, DHA, and EPA Levels in the Main Effects Groups
eTable 6. Effects of Treatments on Type of Infections in the 3-Year Follow-up of DO-HEALTH by Treatment Group
eTable 7. P-Values for Interactions
eTable 8. Mean Calcium, Parathyroid Hormone (PTH), and Estimated Glomerular Filtration Rate (eGFR) Levels in the 3 Year Follow-up of DO-HEALTH by Treatment Group
eTable 9. Adverse Events in the DO-HEALTH by Treatment Group
eFigure 1. Serum Changes in 25(OH)D, DHA and EPA by the 8 Treatment Arms
eFigure 2A. Subgroup Analysis of Effect of Treatment on Systolic Blood Pressure and Infections by Sex and Age
eFigure 2B. Subgroup Analysis of Effect of Treatment on Systolic Blood Pressure and Infections by Baseline Levels of 25(OH)D and Median Levels of DHA and EPA
Data Sharing Statement
References
- 1.Aloia JF, Mikhail M, Fazzari M, Islam S, Ragolia L, Guralnik J. Physical performance and vitamin D in elderly black women—the PODA randomized clinical trial. J Clin Endocrinol Metab. 2019;104(5):1441-1448. doi: 10.1210/jc.2018-01418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization World Report on Ageing and Health. World Health Organization; 2015. [Google Scholar]
- 3.Lips P, Cashman KD, Lamberg-Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):23-P54. doi: 10.1530/EJE-18-0736 [DOI] [PubMed] [Google Scholar]
- 4.Givens DI, Gibbs RA. Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them. Proc Nutr Soc. 2008;67(3):273-280. doi: 10.1017/S0029665108007167 [DOI] [PubMed] [Google Scholar]
- 5.Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology Fatty Acids and Outcomes Research Consortium . Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155-1166. doi: 10.1001/jamainternmed.2016.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalache A, Gatti A. Active ageing: a policy framework. Adv Gerontol. 2003;11:7-18. [PubMed] [Google Scholar]
- 7.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine omega-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. 2015;175(5):703-711. doi: 10.1001/jamainternmed.2015.0225 [DOI] [PubMed] [Google Scholar]
- 10.Scragg RKR. Overview of results from the Vitamin D Assessment (ViDA) study. J Endocrinol Invest. 2019;42(12):1391-1399. doi: 10.1007/s40618-019-01056-z [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA, de Godoi Rezende Costa Molino C, Rival S, et al. ; DO-HEALTH Research Group . DO-HEALTH: vitamin D3-omega 3-home exercise-healthy aging and longevity trial—design of a multinational clinical trial on healthy aging among European seniors. Contemp Clin Trials. Published online August 25, 2020. doi: 10.1016/j.cct.2020.106124 [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94. doi: 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160-169. doi: 10.1002/jbmr.551 [DOI] [PubMed] [Google Scholar]
- 15.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the southeastern US. Int J Geriatr Psychiatry. 2009;24(2):197-201. doi: 10.1002/gps.2101 [DOI] [PubMed] [Google Scholar]
- 16.Markwick A, Zamboni G, de Jager CA. Profiles of cognitive subtest impairment in the Montreal Cognitive Assessment (MoCA) in a research cohort with normal Mini-Mental State Examination (MMSE) scores. J Clin Exp Neuropsychol. 2012;34(7):750-757. doi: 10.1080/13803395.2012.672966 [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175-183. doi: 10.1001/jamainternmed.2015.7148 [DOI] [PubMed] [Google Scholar]
- 18.Bischoff-Ferrari HA, Orav EJ, Egli A, et al. Recovery after unilateral knee replacement due to severe osteoarthritis and progression in the contralateral knee: a randomised clinical trial comparing daily 2000 IU versus 800 IU vitamin D. RMD Open. 2018;4(2):e000678-e000678. doi: 10.1136/rmdopen-2018-000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156-163. doi: 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 20.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991-999. doi: 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Herrmann SD, et al. l. The Compendium of Physical Activities Tracking Guide Accessed April 1, 2020. https://sites.google.com/site/compendiumofphysicalactivities/
- 23.Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709-710. doi: 10.1016/S0140-6736(05)60827-6 [DOI] [PubMed] [Google Scholar]
- 24.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633-1637. doi: 10.1210/jcem.86.4.7393 [DOI] [PubMed] [Google Scholar]
- 25.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40-49. doi: 10.1056/NEJMoa1109617 [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Dawson-Hughes B, Platz A, et al. Effect of high-dosage cholecalciferol and extended physiotherapy on complications after hip fracture: a randomized controlled trial. Arch Intern Med. 2010;170(9):813-820. doi: 10.1001/archinternmed.2010.67 [DOI] [PubMed] [Google Scholar]
- 27.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13(6):538-544. doi: 10.1007/s12603-009-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141. doi: 10.1001/archinternmed.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassere MN, Johnson KR, Schiff M, Rees D. Is blood pressure reduction a valid surrogate end point for stroke prevention? an analysis incorporating a systematic review of randomised controlled trials, a by-trial weighted errors-in-variables regression, the surrogate threshold effect (STE) and the Biomarker-Surrogacy (BioSurrogate) Evaluation Schema (BSES). BMC Med Res Methodol. 2012;12(1):27. doi: 10.1186/1471-2288-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan K, Rossetti H, Hynan LS, et al. Changes in Montreal Cognitive Assessment scores over time. Assessment. 2017;24(6):772-777. doi: 10.1177/1073191116654217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chonchol M, Cigolini M, Targher G. Association between 25-hydroxyvitamin D deficiency and cardiovascular disease in type 2 diabetic patients with mild kidney dysfunction. Nephrol Dial Transplant. 2008;23(1):269-274. doi: 10.1093/ndt/gfm537 [DOI] [PubMed] [Google Scholar]
- 32.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39-48. doi: 10.1016/j.pbiomolbio.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 33.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90(1-5):387-392. doi: 10.1016/j.jsbmb.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 34.Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med. 2018;5:87-87. doi: 10.3389/fcvm.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beveridge LA, Struthers AD, Khan F, et al. ; D-PRESSURE Collaboration . Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745-754. doi: 10.1001/jamainternmed.2015.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swart KM, Lips P, Brouwer IA, et al. Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: an individual participant data meta-analysis of randomized controlled trials. Am J Clin Nutr. 2018;107(6):1043-1053. doi: 10.1093/ajcn/nqy078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27(7):885-896. doi: 10.1093/ajh/hpu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AbuMweis S, Jew S, Tayyem R, Agraib L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J Hum Nutr Diet. 2018;31(1):67-84. doi: 10.1111/jhn.12493 [DOI] [PubMed] [Google Scholar]
- 39.Li NN, Zhou Y, Qin XP, et al. Does intravenous fish oil benefit patients post-surgery? a meta-analysis of randomised controlled trials. Clin Nutr. 2014;33(2):226-239. doi: 10.1016/j.clnu.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 40.Pradelli L, Mayer K, Muscaritoli M, Heller AR. Omega-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care. 2012;16(5):R184. doi: 10.1186/cc11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE Study Investigators . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. JAMA. 2014;311(23):2387-2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Pre-defined Primary, Secondary, Exploratory, and Biomarkers Outcomes in DO-HEALTH
eTable 2. Post Hoc Analysis Using Multiply Imputed Data and Site Random Effects
eTable 3. Mortality and Nursing Home Admission in the 3-Year Follow-up of DO-HEALTH by Treatment Group
eTable 4. Adherence to the Study Medication and Exercise Programs [n, (%)] in DO-HEALTH by Treatment Group
eTable 5. Average Absolute Changes in Serum 25(OH)D, DHA, and EPA Levels in the Main Effects Groups
eTable 6. Effects of Treatments on Type of Infections in the 3-Year Follow-up of DO-HEALTH by Treatment Group
eTable 7. P-Values for Interactions
eTable 8. Mean Calcium, Parathyroid Hormone (PTH), and Estimated Glomerular Filtration Rate (eGFR) Levels in the 3 Year Follow-up of DO-HEALTH by Treatment Group
eTable 9. Adverse Events in the DO-HEALTH by Treatment Group
eFigure 1. Serum Changes in 25(OH)D, DHA and EPA by the 8 Treatment Arms
eFigure 2A. Subgroup Analysis of Effect of Treatment on Systolic Blood Pressure and Infections by Sex and Age
eFigure 2B. Subgroup Analysis of Effect of Treatment on Systolic Blood Pressure and Infections by Baseline Levels of 25(OH)D and Median Levels of DHA and EPA
Data Sharing Statement


