Key Points
Question
What is the effect of e-cigarettes, added to individual counseling, on smoking cessation?
Findings
In this randomized clinical trial that was terminated early after enrolling 376 of a planned 486 participants, individuals randomized to nicotine e-cigarettes plus counseling, compared with counseling alone, had significantly greater 7-day point prevalence abstinence at 12 weeks (21.9% vs 9.1%, respectively), although the difference was no longer statistically significant at 24 weeks.
Meaning
Interpretation is limited by early trial termination, and further research is needed regarding long-term efficacy of e-cigarettes for smoking cessation.
Abstract
Importance
Electronic cigarettes (e-cigarettes) for smoking cessation remain controversial.
Objective
To evaluate e-cigarettes with individual counseling for smoking cessation.
Design, Setting, and Participants
A randomized clinical trial enrolled adults motivated to quit smoking from November 2016 to September 2019 at 17 Canadian sites (801 individuals screened; 274 ineligible and 151 declined). Manufacturing delays resulted in early termination (376/486 participants, 77% of target). Outcomes through 24 weeks (March 2020) are reported.
Interventions
Randomization to nicotine e-cigarettes (n = 128), nonnicotine e-cigarettes (n = 127), or no e-cigarettes (n = 121) for 12 weeks. All groups received individual counseling.
Main Outcomes and Measures
The primary end point was point prevalence abstinence (7-day recall, biochemically validated using expired carbon monoxide) at 12 weeks, changed from 52 weeks following early termination. Participants missing data were assumed to be smoking. The 7 secondary end points, examined at multiple follow-ups, were point prevalence abstinence at other follow-ups, continuous abstinence, daily cigarette consumption change, serious adverse events, adverse events, dropouts due to adverse effects, and treatment adherence.
Results
Among 376 randomized participants (mean age, 52 years; 178 women [47%]), 299 (80%) and 278 (74%) self-reported smoking status at 12 and 24 weeks, respectively. Point prevalence abstinence was significantly greater for nicotine e-cigarettes plus counseling vs counseling alone at 12 weeks (21.9% vs 9.1%; risk difference [RD], 12.8 [95% CI, 4.0 to 21.6]) but not 24 weeks (17.2% vs 9.9%; RD, 7.3 [95% CI, –1.2 to 15.7]). Point prevalence abstinence for nonnicotine e-cigarettes plus counseling was not significantly different from counseling alone at 12 weeks (17.3% vs 9.1%; RD, 8.2 [95% CI, –0.1 to 16.6]), but was significantly greater at 24 weeks (20.5% vs 9.9%; RD, 10.6 [95% CI, 1.8 to 19.4]). Adverse events were common (nicotine e-cigarette with counseling: 120 [94%]; nonnicotine e-cigarette with counseling: 118 [93%]; counseling only: 88 [73%]), with the most common being cough (64%) and dry mouth (53%).
Conclusions and Relevance
Among adults motivated to quit smoking, nicotine e-cigarettes plus counseling vs counseling alone significantly increased point prevalence abstinence at 12 weeks. However, the difference was no longer significant at 24 weeks, and trial interpretation is limited by early termination and inconsistent findings for nicotine and nonnicotine e-cigarettes, suggesting further research is needed.
Trial Registration
ClinicalTrials.gov Identifier: NCT02417467
This randomized trial compares the effect of using e-cigarettes with individual counseling vs individual counseling alone for smoking cessation on point prevalence abstinence at 12 weeks.
Introduction
The long-term health effects of inhaling combustible tobacco are well established, and many individuals who smoke cigarettes have tried to quit.1 In a US survey study conducted in 2010-2011 (response rate, 63%) of 8263 persons who reported having made a quit attempt in the past year, even with the use of at least 5 weeks of pharmacologic and/or behavioral therapy, more than 70% reported having returned to smoking.2 Many smokers have adopted the use of electronic cigarettes (e-cigarettes) to attempt to quit. However, the efficacy of e-cigarettes for smoking cessation remains controversial. A small number of clinical trials using e-cigarettes has been reported, some suggesting modest improvements in various smoking outcomes with e-cigarette use.3,4,5,6,7,8,9,10,11 Nevertheless, data concerning smoking abstinence with e-cigarette use among motivated quitters in the general population are limited, particularly with respect to e-cigarettes alone (rather than in combination with an approved cessation therapy). Therefore, additional data are needed concerning the short-term use of e-cigarettes for smoking cessation.
Methods
Study Design and Population
The methods of the E3 Trial (Evaluating the Efficacy of e-Cigarette Use for Smoking Cessation) have been previously described,12 and the protocol, including statistical analysis plan, is provided in Supplement 1. This study was a multicenter randomized clinical trial examining the efficacy of e-cigarettes for smoking cessation in a general population. The trial was conducted according to all applicable regulatory requirements, including approval by the research ethics boards of participating centers, and written informed consent of participants. Individuals who were 18 years of age and older, smoked a mean of 10 cigarettes or more per day, and had a moderate or strong desire and intention to attempt to quit (Motivation to Stop Scale level 5 or higher13) were enrolled from November 2016 to September 2019 at 17 centers in Canada (Figure 1). We excluded individuals who had used a smoking cessation therapy in the past 30 days, an e-cigarette in the past 60 days, or had ever used an e-cigarette for 7 days consecutively or more. Other exclusion criteria included a history of bipolar disorder, psychosis, or schizophrenia; current cancer or in remission for less than 1 year; condition with a prognosis of less than 1 year; less than 1 month following a major cardiac event; or use of noncigarette tobacco products or marijuana smoking. Self-reported information on race/ethnicity (White/Black/other) was collected at baseline (after randomization) because these data have the potential to improve the understanding of disparities in health and research participation.
Figure 1. Randomization and Follow-up of Study Participants.

e-Cigarette indicates electronic cigarette.
aSubgroups sum to greater than 425 because screened individuals could have more than 1 reason for exclusion.
bPrecluding medical conditions included history of psychosis, schizophrenia, or bipolar disorder (n = 35; 6%); current cancer or in remission for less than 1 year (n = 22; 4%); condition with a prognosis of less than 1 year (n = 7; 1%); and less than 1 month following a major cardiac event (n = 5; 1%).
cOther reasons included pregnant/lactating women (n = 3; 0.5%) and unable to provide informed consent in English or French (n = 2; 0.3%)
dIncludes all participants. Participants were analyzed according to the group to which they were randomized. Participants who were lost to follow-up or withdrew were assumed to have returned to smoking at their baseline level.
eFor participants who were lost to follow-up, vital status was obtained if possible from medical record review or alternate contact.
Participants were randomized to 1 of 3 treatment groups: (1) nicotine e-cigarettes plus counseling, (2) nonnicotine e-cigarettes plus counseling, or (3) counseling alone. Individual counseling was selected as the comparator to provide good assay sensitivity for the primary and secondary outcomes, while ensuring that all participants received evidence-based therapy. The nonnicotine e-cigarettes plus counseling group was included to examine the behavioral aspect of e-cigarettes. Eligible participants were randomized via an online central randomization system. The system used a computer-generated randomization list containing permuted blocks of 6 and 9, stratified by center. Participants, investigators, and study personnel were blinded to nicotine content in the e-cigarette groups. Due to a prolonged and unforeseen delay in e-cigarette manufacturing, enrollment was paused on September 27, 2019, and then terminated on November 14, 2019. Given reduced power, the timing of the primary end point was changed from 52 weeks to 12 weeks on December 4, 2019 (rationale has been previously described12 and is provided in the eMethods in Supplement 2).
Interventions
Participants randomized to e-cigarettes were supplied with 12 weeks of e-cigarettes (eFigure in Supplement 2). e-Cigarettes consisted of a rechargeable base with prefilled, disposable, tobacco-flavored liquid cartridges (15 or 0 mg nicotine/mL), which were produced specifically for use in clinical studies (purchased from NJOY Inc, Scottsdale, Arizona). At baseline, participants received 21 cartridges, with additional cartridges supplied as needed to complete the treatment period. Nicotine and nonnicotine e-cigarettes were identical in appearance. Participants were instructed to use their e-cigarettes as desired because the number of sessions and puffs was expected to vary based on individuals’ habits and level of nicotine dependence. The protocol did not specify a schedule for e-cigarette tapering given individual variability in use; however, participants were aware that they would return their e-cigarettes after 12 weeks.
Participants received individual smoking cessation and relapse prevention counseling (minimum 30 minutes at baseline, 10 minutes during telephone follow-ups, and 15-20 minutes at clinic visits). Trained research personnel provided counseling using a number of approaches (eg, development/revision of a quit plan, encouragement of self-monitoring, review of triggers and challenges, coping skills).12 Participants randomized to e-cigarettes were asked about e-cigarette use and counseled regarding adherence and challenges with use. Quit plans were individualized; therefore, participants were not required to quit immediately at the baseline visit (eg, participants could choose to gradually reduce conventional cigarette smoking over the treatment period).
Follow-up
Follow-up was conducted by telephone at weeks 1, 2, 8, and 18, and at clinic visits at weeks 4, 12, 24, and 52. Self-reported smoking (7-day recall), adherence, and adverse events (AEs) were assessed during follow-up contacts. In addition to the Fagerström Test for Nicotine Dependence (FTND; completed at baseline to assess nicotine dependence),14 participants completed the Glover-Nilsson Smoking Behavioral Questionnaire (to assess behavioral dependence on smoking)15 and the Beck Depression Inventory II (BDI-II; to assess depressive symptoms) during clinic visits.16 At clinic visits, self-reported smoking abstinence was biochemically validated using exhaled carbon monoxide level of 10 ppm or less (Micro 3 and 4 Smokerlyzer, Bedfont Scientific Ltd, Rochester, United Kingdom).17 For visits that would otherwise not be completed (eg, participants unwilling or unable to complete follow-up), we requested minimal high-priority data only (vital and smoking status, and serious AEs [SAEs]). If it was not possible to collect any data, participants were considered lost to follow-up.
End Point Assessment
The primary end point was point prevalence smoking abstinence at 12 weeks following randomization, defined as self-reported abstinence in the past 7 days with exhaled carbon monoxide level of 10 ppm or less. The 7 secondary end points, examined at multiple follow-ups, were point prevalence abstinence at other follow-ups, continuous abstinence, daily cigarette consumption change from baseline at all follow-ups (1, 2, 4, 8, 12, 18, 24, and 52 weeks), SAEs, AEs, dropouts due to AEs, and treatment adherence. Follow-up for 52 weeks was completed in September 2020 but outcomes through 24 weeks (March 2020) are reported herein. Continuous abstinence was defined as self-reported abstinence at all follow-ups since baseline, with exhaled carbon monoxide level of 10 ppm or less at clinic visits. SAEs were adjudicated by an end points evaluation committee, and the trial was monitored by an external data and safety monitoring board, which conferred before enrollment of the first participant and every 6 months thereafter.
Power and Sample Size Calculations
The original sample size of 486 (162 participants/group) was estimated to have more than 80% power to detect a 12% or greater absolute difference in point prevalence abstinence at 52 weeks (2-tailed α of .05), assuming an abstinence rate of 10% for counseling alone. While smaller differences may be clinically relevant, 12% was selected as the smallest difference feasible to evaluate. This treatment effect is consistent with other general population trials of nicotine replacement therapies (NRTs).18 Because the estimate of power at 52 weeks was less than 68% due to early termination, the timing of the primary end point was changed to 12 weeks (eMethods in Supplement 2).
Statistical Analyses
Participants were analyzed according to their group of randomization. Similar to other smoking cessation trials, analyses assumed that participants who withdrew or were lost to follow-up returned to smoking at their baseline level. Descriptive analyses examined baseline characteristics, e-cigarette adherence, blinding, self-reported use of nonstudy cessation therapies, AEs, and SAEs. Discrete data were described using counts and proportions. Continuous data were described using means and SDs or, in the presence of skewed distributions, medians and interquartile ranges.
The primary analysis compared point prevalence abstinence at 12 weeks for nicotine e-cigarettes plus counseling vs counseling alone. Secondary analyses compared the other groups in pairwise comparisons. Point prevalence abstinence, continuous abstinence, and change in daily cigarette consumption were evaluated at all follow-ups. For each pairwise comparison, risk differences (RDs) with corresponding 95% CIs were calculated based on the binomial distribution. Statistical significance was defined as a CI not including the null value. Given the potential for type I error due to multiple comparisons, analyses of secondary end points are considered exploratory.
We conducted prespecified sensitivity analyses to examine the effect of our assumption that participants who withdrew or were lost to follow-up returned to smoking: (1) a complete case analysis and (2) multiple imputation to impute missing smoking abstinence and reduction data. Multiple imputation was performed using the fully conditional specification approach with 5 imputed data sets and results combined using the Rubin rules (eMethods in Supplement 2). Other prespecified sensitivity analyses examined the effect of imbalances in baseline participant characteristics using multiple logistic regression models to estimate odds ratios and 95% CIs for point prevalence abstinence at 12 and 24 weeks, adjusting for characteristics for which the absolute value of the standardized difference was 0.1 or greater. We conducted additional post hoc analyses: (1) to examine potential clustering by site using generalized linear mixed models with a random effect for site to estimate odds ratios and 95% CIs for point prevalence abstinence at 12 and 24 weeks, and (2) to compare the baseline characteristics of participants with self-reported smoking data at 12 weeks (primary end point) with those of participants without self-reported smoking data. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute).
Results
Baseline Characteristics
A total of 376 participants (77% of 486 target sample size) were randomized (mean [SD] age, 52 [13] years; 47% women) (Table 1). Participants smoked a mean (SD) of 21 (11) cigarettes/d at baseline for a mean (SD) of 35 (14) years. Most had previously tried to quit (91%), had used pharmacologic or behavioral therapy (80%), and had at least moderate FTND-defined nicotine dependence (83%) and Glover-Nilsson–defined dependence on smoking behaviors (83%). Baseline characteristics were generally well-balanced between the study groups, with the most prominent difference in participants having previously tried an e-cigarette (43%, 38%, and 27% of the nicotine e-cigarettes plus counseling, nonnicotine e-cigarettes plus counseling, and counseling alone groups, respectively).
Table 1. Baseline Characteristics of Participants by Treatment Group.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Nicotine e-cigarettes plus individual counseling (n = 128) | Nonnicotine e-cigarettes plus individual counseling (n = 127) | Individual counseling alone (n = 121) | |
| Demographic characteristics | |||
| Age, mean (SD), y | 53 (13) | 52 (13) | 53 (12) |
| Sex | |||
| Male | 63 (49) | 71 (56) | 64 (53) |
| Female | 65 (51) | 56 (44) | 57 (47) |
| Self-reported race/ethnicity | |||
| White | 120 (94) | 111 (87) | 104 (86) |
| Black | 1 (1) | 7 (6) | 3 (2) |
| Othera | 7 (6) | 9 (7) | 14 (12) |
| Education | |||
| More than high school | 80 (63) | 79 (62) | 74 (61) |
| Smoking characteristics | |||
| Years smoked, mean (SD) | 35 (14) | 35 (14) | 35 (13) |
| Cigarettes/d at baseline, mean (SD) | 21 (9) | 21 (11) | 21 (11) |
| Previously attempted to quit | 116 (91) | 118 (93) | 108 (89) |
| No. of serious attempts to quit, median (IQR) | 2 (1-5) | 2 (1-4) | 3 (1-4) |
| Previously used abstinence aids for smoking cessationb | 101 (79) | 100 (79) | 99 (82) |
| Previously tried an e-cigarette | 55 (43) | 48 (38) | 33 (27) |
| Other smoker(s) at home | 40 (31) | 45 (35) | 36 (30) |
| Other lifestyle characteristics | |||
| Body mass index ≥30c | 48 (38) | 46 (36) | 45 (37) |
| Alcoholic drinks/wk, mean (SD) | 4 (6) | 4 (8) | 3 (5) |
| Questionnaires | |||
| Motivation to Stop Scaled | n = 128 | n = 127 | n = 121 |
| Mean score (SD) | 6.0 (0.8) | 6.1 (0.8) | 6.3 (0.8) |
| 5 (“I want to stop smoking and hope to soon”) | 41 (32) | 38 (30) | 30 (25) |
| 6 (“I really want to stop smoking and intend to in the next 3 months”) | 45 (35) | 39 (31) | 28 (23) |
| 7 (“I really want to stop smoking and intend to in the next months”) | 42 (33) | 50 (39) | 63 (52) |
| Fagerström Test for Nicotine Dependencee | n = 127 | n = 127 | n = 120 |
| Mean score (SD) | 6 (2) | 6 (2) | 6 (2) |
| Mild | 19 (15) | 25 (20) | 21 (18) |
| Moderate | 59 (47) | 57 (45) | 45 (46) |
| Severe | 49 (39) | 45 (35) | 44 (37) |
| Glover-Nilsson Smoking Behavioral Questionnairef | n = 127 | n = 127 | n = 119 |
| Mean score (SD) | 21 (8) | 20 (8) | 20 (8) |
| Mild | 17 (13) | 25 (20) | 22 (19) |
| Moderate | 59 (46) | 55 (43) | 53 (45) |
| Strong | 43 (34) | 36 (28) | 33 (28) |
| Very strong | 9 (7) | 11 (9) | 11 (9) |
| Beck Depression Inventory IIg | n = 128 | n = 127 | n = 118 |
| Mean score (SD) | 11 (9) | 10 (9) | 11 (10) |
| Minimal | 86 (67) | 92 (72) | 78 (66) |
| Mild | 19 (15) | 19 (15) | 18 (15) |
| Moderate | 16 (13) | 12 (9) | 14 (12) |
| Severe | 7 (6) | 4 (3) | 8 (7) |
| Medical historyh | |||
| Elevated cholesterol | 47 (37) | 50 (40) | 46 (38) |
| Depressioni | 45 (35) | 42 (33) | 36 (30) |
| Hypertension | 42 (32) | 41 (32) | 33 (27) |
| Respiratory problems | 31 (24) | 40 (32) | 34 (28) |
| Asthma | 14 (11) | 18 (14) | 20 (17) |
| Chronic obstructive pulmonary disease | 13 (10) | 14 (11) | 11 (9) |
| Chronic bronchitis | 10 (8) | 10 (8) | 10 (8) |
| Emphysema | 5 (4) | 5 (4) | 5 (4) |
| Otherj | 2 (2) | 5 (4) | 4 (3) |
| More than 1 respiratory problem | 11 (9) | 9 (7) | 11 (9) |
| Heart disease | 22 (17) | 22 (17) | 23 (19) |
| Diabetes | 16 (13) | 24 (19) | 22 (18) |
| Cancer | 11 (9) | 12 (9) | 14 (12) |
Abbreviations: e-cigarette, electronic cigarette; IQR, interquartile range.
Participants were asked to select “White,” “Black,” or “Other, specify.” Self-reported responses to “Other” included Arab, Asian, East Indian, Filipino, Indigenous, Israeli, Italian, Moroccan, Nepalese, Spanish, Trinidadian, Tunisian, and Urdu.
Previously used abstinence aids includes bupropion, counseling, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine patch, nicotine quickmist, varenicline, and other aids (acupuncture, apps, hypnosis, laser).
Calculated as weight in kilograms divided by height in meters squared.
Possible scores range between 1 and 7, with higher scores indicating stronger motivation to quit smoking. Potential participants completed this 1-item scale during screening, and must have selected level 5 or higher to be eligible for the trial, indicating a moderate or strong desire and intention to attempt to quit.
Possible scores range between 0 and 10, with higher scores indicating a stronger dependence on nicotine. Mild: 0-3; moderate: 4-6; and severe: greater than or equal to 7.
Possible scores range between 0 and 44, with higher scores indicating greater behavioral dependence on smoking. Mild: 0-12; moderate: 12-22; strong: 12-33; and very strong: greater than or equal to 34.
Possible scores range between 0 and 63, with higher scores indicating greater depressive symptoms. Minimal: 0-13; mild: 14-19; moderate: 20-28; and severe: greater than or equal to 29.
Medical history was self-reported.
Defined as prior use of medication for depression.
Other (respiratory problems) includes chronic pneumonia, shortness of breath, and sleep apnea.
Smoking Abstinence and Reduction
Self-reported smoking data were available for 299 (80%) and 278 (74%) participants at 12 and 24 weeks, respectively (Figure 1). Among participants who self-reported no smoking in the past week, abstinence was biochemically validated for 53 of 61 (87%) and 46 of 60 (77%) participants at 12 and 24 weeks, respectively. All reported comparisons not labeled primary are prespecified secondary analyses. Inferential statistics are provided for all smoking abstinence and reduction comparisons in eTables 1-3 in Supplement 2.
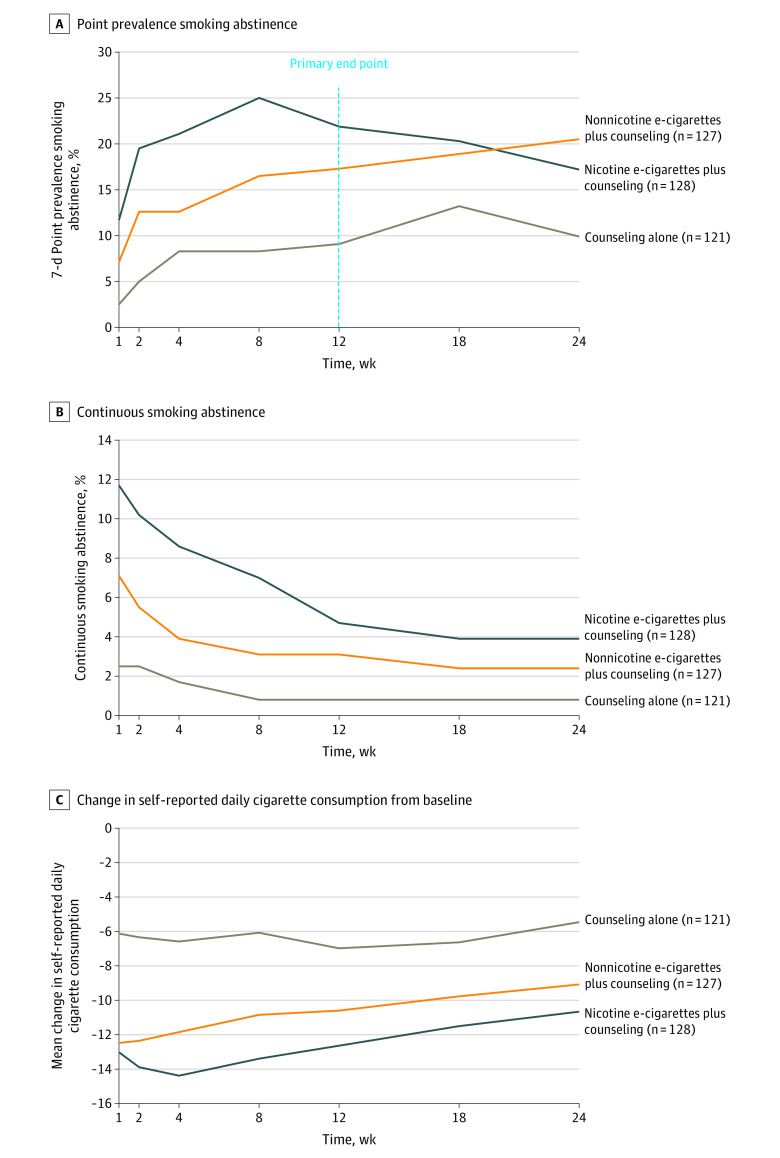
At the primary end point of 12 weeks, 7-day point prevalence abstinence was significantly greater among participants randomized to nicotine e-cigarettes plus counseling compared with counseling alone (21.9% vs 9.1%; RD, 12.8 [95% CI, 4.0 to 21.6]) (Figure 2A). This difference was no longer statistically significant at 24 weeks (17.2% vs 9.9%; RD, 7.3 [95% CI, –1.2 to 15.7]). There was no statistically significant difference in point prevalence abstinence between participants randomized to nonnicotine e-cigarettes plus counseling compared with counseling alone at 12 weeks (17.3% vs 9.1%; RD, 8.2 [95% CI, –0.1 to 16.6]). However, nonnicotine e-cigarettes plus counseling significantly increased abstinence, compared with counseling alone, at 24 weeks (20.5% vs 9.9%; RD, 10.6 [95% CI, 1.8 to 19.4]). There were no significant differences in point prevalence abstinence between the nicotine and nonnicotine e-cigarettes plus counseling groups at 12 weeks (21.9% vs 17.3%; RD, 4.6 [95% CI, –5.2 to 14.3]) or 24 weeks (17.2% vs 20.5%; RD, –3.3 [95% CI, –12.9 to 6.3]). Continuous abstinence was low across groups, with no statistically significant differences between any treatment groups at 12 or 24 weeks (Figure 2B).
Figure 2. Smoking Abstinence and Reduction by Treatment Group.
Participants who withdrew consent or were lost to follow-up were considered to have returned to smoking at their baseline level. See Supplement 2 for results of all statistical comparisons. The primary end point was point prevalence abstinence at 12 weeks. All other reported smoking abstinence and reduction outcomes were prespecified secondary end points. A, Participants were considered abstinent if they abstained from smoking in the 7 days before the visit through a self-report of 0 cigarettes smoked/d, with an expired carbon monoxide reading less than or equal to 10 ppm at clinic visits at 4, 12, and 24 weeks. Expired carbon monoxide readings were available for 93%, 87%, and 77% of self-reported abstinent participants at 4, 12, and 24 weeks, respectively. B, Participants were considered continuously abstinent if they reported smoking 0 cigarettes in the 7 days before each follow-up since randomization, with expired carbon monoxide readings less than or equal to 10 ppm at clinic visits at 4, 12, and 24 weeks. Expired carbon monoxide readings were available for 78%, 82%, and 78% of continuously self-reported abstinent participants at 4, 12, and 24 weeks, respectively. C, Change in the mean number of self-reported cigarettes smoked per day in the past week. e-Cigarette indicates electronic cigarette.
Reduction in mean self-reported daily cigarette consumption from baseline was significantly greater among participants randomized to nicotine e-cigarettes plus counseling compared with counseling alone at 12 weeks (–12.6 vs –7.0; RD, –5.7 [95% CI, –8.0 to –3.3]) and 24 weeks (–10.7 vs –5.5; RD, –5.2 [95% CI, –7.6 to –2.8]) (Figure 2C). Reduction in mean self-reported daily cigarette consumption was also significantly greater among participants randomized to nonnicotine e-cigarettes plus counseling compared with counseling alone at 12 weeks (–10.6 vs –7.0; RD, –3.6 [95% CI, –6.3 to –1.0]) and 24 weeks (–9.1 vs –5.5; RD, –3.6 [95% CI, –6.3 to –1.0]). Change in mean self-reported daily cigarette consumption was not significantly different between the nicotine and nonnicotine e-cigarettes plus counseling groups at 12 weeks (–12.6 vs –10.6; RD, –2.0 [95% CI, –4.7 to 0.6]) or 24 weeks (–10.7 vs –9.1; RD, –1.6 [95% CI, –4.3 to 1.1]).
Sensitivity Analyses
Prespecified sensitivity analyses were conducted examining the effect of the assumption that participants who withdrew or were lost to follow-up had returned to smoking. These included a complete case analysis and multiple imputation to impute missing smoking abstinence and reduction data (eTables 1-3 in Supplement 2). Point estimates were attenuated in these sensitivity analyses and 95% CIs were wider, suggesting that the abstinence and reduction analyses were sensitive to the missing data assumptions and should, therefore, be interpreted with caution. To examine the effect of imbalances in baseline participant characteristics between groups, prespecified logistic regression models at 12 and 24 weeks were constructed to adjust for baseline characteristics for which the absolute value of the standardized difference was 0.1 or greater (eTable 4 in Supplement 2); these results were similar to those of our main analyses (eTable 5 in Supplement 2).
Post Hoc Analyses
Additional post hoc analyses were conducted. The effect of clustering by site was assessed using generalized linear mixed models, which included site as a random effect; these results were similar to our main analyses at 12 and 24 weeks (eTable 6 in Supplement 2). Additional analyses (eTable 7 in Supplement 2) found that participants for whom we were unable to obtain self-reported smoking data at 12 weeks were more likely to be older (mean age, 54 vs 52 years), male (57% vs 52%), White (96% vs 87%), have completed high school or less (45% vs 36%), have smoked for more years (mean, 37 vs 34) and more heavily (mean, 22 vs 21 cigarettes/d), have made at least 1 previous quit attempt (97% vs 89%), have previously used a smoking cessation therapy (87% vs 78%), have higher FTND-defined nicotine dependence (mean score, 6.2 vs 5.6), and live with another smoker (42% vs 30%), and were less likely to have previously used an e-cigarette (30% vs 38%) than participants who provided self-reported smoking data. These participants reported fewer depressive symptoms at baseline (mean BDI-II score, 9 vs 11), but were more likely to have a body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 or greater (41% vs 36%), self-reported history of heart disease (28% vs 15%), hypertension (35% vs 30%), and asthma (18% vs 13%) than participants who returned for follow-up.
Adherence, Blinding, and Nonstudy Smoking Cessation Therapies
At 12 weeks, 75 of 110 participants (68%) in the nicotine e-cigarettes plus counseling group with adherence data reported using their e-cigarette in the previous week for a mean (SD) of 6 (2) days and 11 (10) sessions/d compared with 54 of 101 participants (54%) in the nonnicotine e-cigarettes plus counseling group for a mean (SD) of 5 (2) days and 6 (6) sessions/d. Participants were asked to guess their assigned treatment group at 12 weeks; 54 of 109 (50%) correctly guessed their allocation in the nicotine e-cigarettes plus counseling group vs 45 of 99 (46%) in the nonnicotine e-cigarettes plus counseling group.
Through 12 weeks, participants (including those who withdrew or were lost to follow-up) received a mean (SD) of 97 (38) minutes of individual counseling. Participants randomized to nicotine e-cigarettes plus counseling received a mean (SD) of 18 (6) minutes of counseling per follow-up contact through 12 weeks, participants randomized to nonnicotine e-cigarettes plus counseling received a mean (SD) of 17 (5) minutes, and participants randomized to counseling alone received a mean (SD) of 14 (7) minutes. Among only participants who returned for follow-up, the mean (SD) duration of counseling per follow-up contact was 20 (7), 19 (5), and 22 (10) minutes for the nicotine e-cigarettes plus counseling, nonnicotine e-cigarettes plus counseling, and counseling alone groups, respectively.
Among all 376 participants, 60 (16%) reported using at least 1 nonstudy smoking cessation therapy at any follow-up through 12 weeks (nicotine e-cigarettes plus counseling: 16 [13%]; nonnicotine e-cigarettes plus counseling: 18 [14%]; counseling alone: 26 [22%]) (eTable 8 in Supplement 2). Nonstudy therapies included e-cigarettes (9%), NRTs (6%), and varenicline or bupropion (1%). Participants who reported the use of at least 1 nonstudy therapy increased to 147 (39%) through 24 weeks (60 nicotine e-cigarettes plus counseling participants [47%], 45 nonnicotine e-cigarettes plus counseling participants [35%], 42 counseling alone participants [35%]). The use of nonstudy e-cigarettes through 24 weeks increased to 47 participants in the nicotine e-cigarettes plus counseling group (37%), 29 in the nonnicotine e-cigarettes plus counseling group (23%), and 20 in the counseling alone group (17%) (eTable 9 in Supplement 2).
Adverse Events
AEs were commonly reported among the 376 participants (Table 2), including cough (242, 64%), dry mouth (201, 54%), rhinitis (188, 50%), and headache (185, 49%). Cough was reported by 95 nicotine e-cigarettes plus counseling participants (74%), 81 nonnicotine e-cigarettes plus counseling participants (64%), and 66 counseling alone participants (55%). Occurrence of other AEs was comparable between the nicotine and nonnicotine e-cigarettes plus counseling groups, but more frequent compared with the counseling alone group.
Table 2. Adverse Events During the 12-Week Treatment Period by Treatment Group.
| No. (%) | |||
|---|---|---|---|
| Nicotine e-cigarettes plus individual counseling (n = 128) | Nonnicotine e-cigarettes plus individual counseling (n = 127) | Individual counseling alone (n = 121) | |
| Serious adverse eventsa | |||
| Participants with a serious adverse event | 1 (0.8) | 4 (3.1) | 2 (1.7) |
| Death | 0 | 0 | 0 |
| Respiratoryb | 1 (0.8) | 0 | 0 |
| Cardiovascularc | 0 | 1 (0.8) | 1 (0.8) |
| Neuropsychiatric | 0 | 0 | 0 |
| Otherd | 0 | 3 (2.4) | 1 (0.8) |
| Mild adverse events | |||
| Participants with an adverse event | 120 (94) | 118 (93) | 88 (73) |
| Cough | 95 (74) | 81 (64) | 66 (55) |
| Dry mouth | 72 (56) | 74 (58) | 55 (46) |
| Headache | 70 (55) | 69 (54) | 46 (38) |
| Rhinitis | 70 (55) | 67 (53) | 51 (42) |
| Throat irritation | 70 (55) | 53 (42) | 30 (25) |
| Dyspnea | 53 (41) | 61 (48) | 43 (36) |
| Sore throat | 44 (34) | 39 (31) | 21 (17) |
| Light headedness | 42 (33) | 34 (27) | 28 (23) |
| Dizziness | 39 (31) | 31 (24) | 37 (31) |
| Mouth irritation | 38 (30) | 24 (19) | 15 (12) |
| Nausea | 37 (29) | 30 (24) | 20 (17) |
| Indigestion | 31 (24) | 33 (26) | 28 (23) |
| Mouth ulcers | 19 (15) | 16 (13) | 7 (6) |
| Vertigo | 16 (13) | 11 (9) | 9 (7) |
Abbreviation: e-cigarette, electronic cigarette.
The denominator used to calculate percentages is the total number of participants randomized to each group. Only the first event for each participant in each category was counted (ie, the numbers represent the number of participants experiencing an event in each category, rather than the absolute number of events). Serious adverse events and adverse events were obtained via self-report at clinic and telephone follow-ups. All documentation obtained pertaining to each reported serious adverse event was independently evaluated by an end points evaluation committee, which determined its potential causal relationship with the study intervention.
One participant in the nicotine e-cigarettes plus counseling group was hospitalized with a chronic obstructive pulmonary disease exacerbation secondary to pneumonia 12 days after being randomized into the trial and had used their e-cigarette in the day preceding the event.
One participant in the nonnicotine e-cigarettes plus counseling group experienced a myocardial infarction 84 days after randomization and had used their e-cigarette in the day preceding the event. One participants in the counseling alone group had critical ischemia in their left leg due to a superficial femoral artery occlusion 43 days after randomization.
Includes 3 participants in the nonnicotine e-cigarettes plus counseling group. One participant experienced both appendicitis and a neoplastic cecal lesion during the treatment period, the second participant experienced epistaxis 39 days after randomization, and the third participant experienced noncardiac chest pain 88 days after randomization. All 3 participants had used their e-cigarette in the day preceding the events. In the counseling group, 1 participant had a urinary tract infection 16 days after randomization.
A total of 8 SAEs occurred in 7 participants during the 12-week treatment period (1 [1%] nicotine e-cigarettes plus counseling participant, 4 [3%] nonnicotine e-cigarettes plus counseling participants, and 2 [2%] counseling alone participants) (Table 2; eTable 10 in Supplement 2). One participant in the nicotine e-cigarettes plus counseling group had a chronic obstructive pulmonary disease exacerbation 12 days after beginning study e-cigarette use. Seven additional SAEs occurred in 6 participants between the 12- and 24-week follow-ups (2 [2%] nicotine e-cigarettes plus counseling participants, 2 [2%] nonnicotine e-cigarettes plus counseling participants, and 2 [2%] counseling alone participants) (eTables 11-12 in Supplement 2).
Discussion
In this randomized clinical trial, nicotine e-cigarettes plus counseling, compared with counseling alone, significantly increased point prevalence abstinence at 12 weeks among adults motivated to quit smoking. However, the difference was no longer statistically significant at 24 weeks. Nonnicotine e-cigarettes plus counseling significantly increased point prevalence abstinence compared with counseling alone at 24 weeks, although the difference was less than the 12% difference that the study was powered to detect (10.6%). Overall, the findings regarding abstinence with e-cigarettes plus counseling were modest and inconsistent.
Several e-cigarette clinical trials have been previously reported.3,4,5,6,7,8,9,10,11 While some trials suggested modest improvements in smoking outcomes, the trials varied greatly in their research questions, designs, and populations. Only 3 e-cigarette trials were previously conducted in North America, all in the United States.9,10,11 Two of these trials were small (n <100) and of very short treatment duration (3 weeks), although both observed a reduction in self-reported cigarettes smoked with e-cigarette use.9,11 The third trial did not find the provision of free e-cigarettes to employees to be more efficacious for smoking cessation than other therapies and/or financial incentives.10
Only 2 previous randomized clinical trials found e-cigarette use significantly increased abstinence among motivated smokers in a general population.3,8 Hajek et al3 examined e-cigarettes alone, while Walker et al8 examined e-cigarettes in combination with a conventional smoking cessation therapy. Conducted in the United Kingdom, the study by Hajek et al3 (n = 886) found that nicotine e-cigarettes doubled continuous smoking abstinence at 1 year compared with traditional NRTs (18.0% vs 9.9%) among highly motivated adults. However, among abstinent participants, 80% in the nicotine e-cigarette group were still using an e-cigarette at 1 year.
These data suggest that smokers may be displacing their nicotine addiction from conventional cigarettes to e-cigarettes. While participants in the present trial returned their study-provided e-cigarette at 12 weeks, 37% of participants randomized to nicotine e-cigarettes plus counseling reported nonstudy e-cigarette use at 24 weeks (along with 23% of participants in the nonnicotine e-cigarettes plus counseling group and 17% of participants in the counseling alone group). Displacement of nicotine addiction to e-cigarettes during the treatment period may explain the decline in smoking abstinence after 12 weeks among participants randomized to nicotine e-cigarettes plus counseling. These findings suggest that nicotine-tapering strategies used for other NRTs (eg, nicotine patch) could be investigated in future studies of e-cigarettes. While fixed dosing is difficult owing to the nature of e-cigarettes, a gradual reduction in nicotine e-liquid concentration could theoretically achieve a similar effect. While both nicotine and nonnicotine e-cigarettes plus counseling resulted in a sustained reduction in self-reported daily cigarette consumption compared with counseling alone, the health benefits of smoking reduction (vs complete abstinence) are controversial.19 However, studies have shown that individuals who reduce their daily cigarette consumption are more likely to successfully quit in future attempts.20
e-Cigarette safety is an ongoing concern. A large number of e-cigarette, or vaping, product use–associated lung injury cases have been reported,21 most of which were attributed to e-cigarette liquid containing vitamin E acetate and/or tetrahydrocannabinol.22 Therefore, most commercially available e-cigarettes are unlikely to cause e-cigarette, or vaping, product use–associated lung injury unless modified by users. However, a number of studies have reported adverse pulmonary effects related to long-term e-cigarette use: bronchoscopy studies suggest that chronic e-cigarette use alters human bronchial epithelial proteome23; increases neutrophil elastase and matrix metalloprotease levels24; and causes an innate immune response involving increased neutrophilic activation and altered mucin secretion.25 If e-cigarettes are used for smoking cessation, they should be used for the shortest duration possible.
Limitations
This trial had several limitations. First, given the unexpected early termination of the trial after the recruitment of 77% of the target sample, the power to detect differences between groups was reduced. Second, there were low rates of continuous abstinence, which required self-reported abstinence at all follow-ups. Quit plans were individualized, and participants were not required to quit immediately at the baseline visit. Consequently, most participants who achieved abstinence did so during the treatment period (rather than at baseline). Third, there was differential lost-to-follow-up rates between groups, with counseling alone participants having a higher rate than the e-cigarettes plus counseling groups. These participants were assumed to have returned to smoking (standard practice in smoking cessation trials). This assumption could have biased the findings in favor of the e-cigarettes plus counseling groups. However, participants in the counseling alone group were more likely to use a nonstudy cessation therapy during the 12-week treatment period, including e-cigarettes, which could have diluted the observed treatment effect. Fourth, there were no statistical adjustments for multiple comparisons; the conclusions are based on the primary end point of point prevalence abstinence at 12 weeks. Secondary end points should be considered hypothesis-generating. Fifth, the e-cigarette used in this trial was produced specifically for use in clinical studies, which may limit the trial’s external validity to commercially available devices.
Conclusions
Among adults motivated to quit smoking, nicotine e-cigarettes plus counseling vs counseling alone significantly increased point prevalence abstinence at 12 weeks. However, the difference was no longer significant at 24 weeks, and trial interpretation is limited by early termination and inconsistent findings for nicotine and nonnicotine e-cigarettes, suggesting further research is needed.
Trial Protocol
eMethods.
eTable 1. Risk Differences (95% CI) for 7-Day Point Prevalence Smoking Abstinence Between Treatment Groups
eTable 2. Risk Differences (95% CI) for Continuous Smoking Abstinence Between Treatment Groups
eTable 3. Risk Differences (95% CI) for Change in Self-Reported Daily Cigarette Consumption from Baseline Between Treatment Groups
eTable 4. Balance of Baseline Characteristics of Participants By Treatment Group
eTable 5. Sensitivity Analyses for Point Prevalence Abstinence at 12 and 24 Weeks, Adjusting for Imbalances in Baseline Participant Characteristics
eTable 6. Post Hoc Analyses for Point Prevalence Abstinence at 12 and 24 Weeks, Accounting for Clustering by Site
eTable 7. Description of Baseline Characteristics of Participants by Self-Reported Smoking Data Availability at 12 Weeks
eTable 8. Use of Non-Study Smoking Cessation Therapy During the 12-Week Treatment Period by Treatment Group
eTable 9. Use of Non-Study Smoking Cessation Therapy Through the 24 Week Follow-up by Treatment Group
eTable 10. Serious Adverse Events Experienced During the 12-Week Treatment Period by Treatment Group
eTable 11. Summary of Serious Adverse Events Occurring Between 12 Weeks (End of Treatment Period) and 24 Weeks by Treatment Group
eTable 12. Serious Adverse Events Occurring Between 12 Weeks (End of Treatment Period) and 24 Weeks by Treatment Group
eFigure. e-Cigarette Used in the E3 Trial
Data Sharing Statement
References
- 1.National Center for Chronic Disease Prevention The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Siahpush M, Shaikh RA, McCarthy M, Sikora Kessler A, Tibbits M, Singh GK. Association between duration of use of pharmacotherapy and smoking cessation: findings from a national survey. BMJ Open. 2015;5(1):e006229. doi: 10.1136/bmjopen-2014-006229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629-637. doi: 10.1056/NEJMoa1808779 [DOI] [PubMed] [Google Scholar]
- 4.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637. doi: 10.1016/S0140-6736(13)61842-5 [DOI] [PubMed] [Google Scholar]
- 5.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S-H, Ahn S-H, Cheong Y-S. Effect of electronic cigarettes on smoking reduction and cessation in Korean male smokers: a randomized controlled study. J Am Board Fam Med. 2019;32(4):567-574. doi: 10.3122/jabfm.2019.04.180384 [DOI] [PubMed] [Google Scholar]
- 7.Masiero M, Lucchiari C, Mazzocco K, et al. E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tob Res. 2019;21(1):119-126. doi: 10.1093/ntr/nty047 [DOI] [PubMed] [Google Scholar]
- 8.Walker N, Parag V, Verbiest M, Laking G, Laugesen M, Bullen C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir Med. 2020;8(1):54-64. doi: 10.1016/S2213-2600(19)30269-3 [DOI] [PubMed] [Google Scholar]
- 9.Carpenter MJ, Heckman BW, Wahlquist AE, et al. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1795-1803. doi: 10.1158/1055-9965.EPI-17-0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018;378(24):2302-2310. doi: 10.1056/NEJMsa1715757 [DOI] [PubMed] [Google Scholar]
- 11.Tseng T-Y, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943. doi: 10.1093/ntr/ntw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hébert-Losier A, Filion KB, Windle SB, Eisenberg MJ. A randomized controlled trial evaluating the efficacy of e-Cigarette use for smoking cessation in the general population: E3 trial design. CJC Open. 2020;2(3):168-175. doi: 10.1016/j.cjco.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel K, Brown J, Willemsen MC, West R, Kotz D. External validation of the Motivation To Stop Scale (MTSS): findings from the International Tobacco Control (ITC) Netherlands Survey. Eur J Public Health. 2017;27(1):129-134. [DOI] [PubMed] [Google Scholar]
- 14.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 15.Glover ED, Nilsson F, Westin A, Glover PN, Laflin MT, Persson B. Developmental history of the Glover-Nilsson Smoking Behavioral Questionnaire. Am J Health Behav. 2005;29(5):443-455. doi: 10.5993/AJHB.29.5.7 [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. Psychological Corporation; 1996;78:490-498. [Google Scholar]
- 17.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149-159. doi: 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179(2):135-144. doi: 10.1503/cmaj.070256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med. 2015;13:257. doi: 10.1186/s12916-015-0505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371-381. doi: 10.1037/0022-006X.72.3.371 [DOI] [PubMed] [Google Scholar]
- 21.Ellington S, Salvatore PP, Ko J, et al. ; Lung Injury Response Epidemiology/Surveillance Task Force . Update: product, substance-use, and demographic characteristics of hospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury: United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(2):44-49. doi: 10.15585/mmwr.mm6902e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blount BC, Karwowski MP, Shields PG, et al. ; Lung Injury Response Laboratory Working Group . Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. doi: 10.1056/NEJMoa1916433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A, Coakley RC, Mascenik T, et al. Chronic e-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018;198(1):67-76. doi: 10.1164/rccm.201710-2033OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Coakley RD, Ghio AJ, et al. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med. 2019;200(11):1392-1401. doi: 10.1164/rccm.201903-0615OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reidel B, Radicioni G, Clapp PW, et al. e-Cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197(4):492-501. doi: 10.1164/rccm.201708-1590OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eTable 1. Risk Differences (95% CI) for 7-Day Point Prevalence Smoking Abstinence Between Treatment Groups
eTable 2. Risk Differences (95% CI) for Continuous Smoking Abstinence Between Treatment Groups
eTable 3. Risk Differences (95% CI) for Change in Self-Reported Daily Cigarette Consumption from Baseline Between Treatment Groups
eTable 4. Balance of Baseline Characteristics of Participants By Treatment Group
eTable 5. Sensitivity Analyses for Point Prevalence Abstinence at 12 and 24 Weeks, Adjusting for Imbalances in Baseline Participant Characteristics
eTable 6. Post Hoc Analyses for Point Prevalence Abstinence at 12 and 24 Weeks, Accounting for Clustering by Site
eTable 7. Description of Baseline Characteristics of Participants by Self-Reported Smoking Data Availability at 12 Weeks
eTable 8. Use of Non-Study Smoking Cessation Therapy During the 12-Week Treatment Period by Treatment Group
eTable 9. Use of Non-Study Smoking Cessation Therapy Through the 24 Week Follow-up by Treatment Group
eTable 10. Serious Adverse Events Experienced During the 12-Week Treatment Period by Treatment Group
eTable 11. Summary of Serious Adverse Events Occurring Between 12 Weeks (End of Treatment Period) and 24 Weeks by Treatment Group
eTable 12. Serious Adverse Events Occurring Between 12 Weeks (End of Treatment Period) and 24 Weeks by Treatment Group
eFigure. e-Cigarette Used in the E3 Trial
Data Sharing Statement