Highlights
-
•
Neonatal hypoglycemic brain injury was the commonest cause of Infantile Spasms (IS).
-
•
Comprehensive genetic evaluation was performed in presumed genetic IS patients.
-
•
Molecular diagnosis was achieved in 44% of presumed genetic patients.
-
•
Longer lead time to treatment was significantly associated with resistant spasms.
Keywords: Infantile spasms, Etiology, Hypoglycemia, Genetic, Lead time
Abstract
This study explores the etiology and lead time to treatment for infantile spasm (IS) patients and their effect on treatment responsiveness, in a limited resource setting. Patients with IS onset age ≤12 months’, seen over 3 years were recruited retrospectively. Clinical information, neuroimaging and genetic results retrieved. Patients categorized into three primary etiological groups: Structural (including Structural Genetic), Genetic, and Unknown. The effect of etiology and lead time from IS onset to initiating appropriate treatment on spasm resolution, evaluated. Total 113 patients were eligible. Mean IS onset age was 6.86(±4.25) months (M: F 3.3:1). Patients were grouped into: Structural 85, Genetic 11 and Unknown 17. Etiology was ascertained in 94/113 (83.1%) with neonatal hypoglycemic brain injury (NHBI) being the most common (40/113, 36%). A genetic etiology identified in 17 (including 6 Structural Genetic, of which five had Tuberous Sclerosis). Structural group was less likely to be treatment resistant (p = 0.013, OR 0.30 [0.12–0.76]). Median treatment lead time – 60 days. Longer lead time to treatment was significantly associated with resistant spasms (χ2 for trend = 10.0, p = 0.0015). NHBI was the commonest underlying cause of IS. There was significant time lag to initiating appropriate treatment, affecting treatment responsiveness.
1. Introduction
Infantile spasms (IS) are a seizure type manifesting in first year of life, as a component of an age-dependent epilepsy syndrome [1]. IS has many underlying causes [2], [3], [4], [5], [6], [7], [8]. Studies from resource- limited settings have highlighted neonatal asphyxial brain injury as the commonest acquired cause for IS [4], [5], [6]. Neonatal hypoglycemic brain injury (NHBI) is also being increasingly recognized as an important acquired cause of IS [9], [10], [11].
Genetic defects have been shown to be another major cause of IS [7], [8]. However patients with IS of unknown etiology in published Indian studies [4], [5], [6] have not been investigated using comprehensive next generation genetic technologies. Hence etiological data after detailed genetic evaluation of IS patients from developing healthcare setups, still remains limited [8], [12].
Studies have suggested that shorter lead time to treatment is associated with a better treatment response and subsequent improved developmental outcome [13], [14]. Unfortunately, diagnosis and treatment of IS is often delayed in both developed and developing countries, despite a characteristic hypsarrythmic EEG pattern [4], [5], [15]. Also only few large scale studies have assessed the effect of etiology on treatment outcome, suggesting a better outcome for patients in the unknown group [13], [14]. A recent study though concluded that those in Genetic/Unknown group do not necessarily have a favorable outcome [16].
In this study from a pediatric neurology clinic from Jaipur in North India, we have comprehensively investigated the etiologies of IS by accessing patients’ clinical information including perinatal histories, reviewing the available neuroimaging findings, and retrieving genetic testing results. We have also evaluated associations of etiology and lead time to specific treatment with resolution of spasms.
2. Methods
2.1. Patient selection
In this retrospective cohort study, IS patients with an onset age of <12 months with EEG evidence of hypsarrythmia or its variants [17], seen over a 3-year period between March 2015-March 2018 were eligible.
2.2. Setting
This study was conducted in the pediatric neurology clinic at Santokba Durlabhji hospital, a tertiary care teaching hospital in Jaipur, North India. Our center is a secondary and tertiary care referral centre for city of Jaipur (Rajasthan), in addition to being a tertiary centre for outlying districts of Rajasthan, and adjoining districts of neighbouring states, Haryana and Punjab.
2.3. Assessment of case records
The following variables were summarized from each case record: Birth details (gestation, birth weight, APGAR), adverse perinatal events (delayed cry, neonatal encephalopathy, neonatal seizures, intensive care admission, feeding issues, blood glucose reports & cerebrospinal fluid results), IS onset age and the age of initial presentation to a pediatric neurologist. If any relevant information was unavailable, it was sought from the families via follow-up visits or calls.
2.4. Investigations
2.4.1. Neuroimaging
Brain Magnetic Resonance Imaging (MRI) was retrieved and findings were confirmed independently by two radiologists. All MRI scans at our centre are performed on a 3 Tesla machine with standard imaging protocols. If patient already has a 1.5 Tesla MRI performed according to standard imaging protocols, prior to visiting us, the neuroimaging is not repeated.
2.4.2. Metabolic testing
All our IS patients without an obvious acquired postnatal cause or a cortical malformation, routinely have a metabolic screen performed including serum ammonia, serum lactate and tandem mass spectroscopy (TMS). These results were retrieved.
2.4.3. Genetic testing
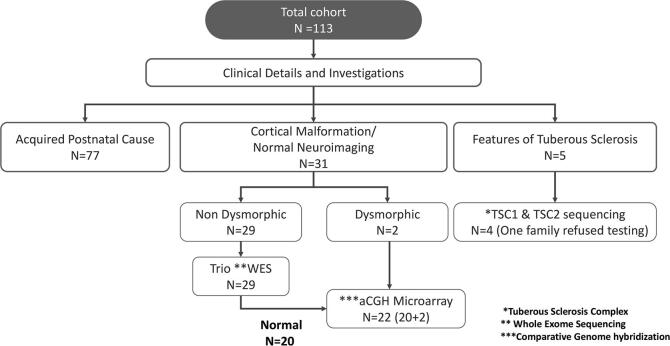
For patients in whom an acquired postnatal cause could not be determined from the history and neuroimaging, genetic testing was performed according to the protocol in Fig. 1.
Fig. 1.
Genetic testing protocol for infantile spasms patients with presumed genetic etiology.
Next Generation Sequencing with copy number variations (CNVs) was done to identify the disease causal variant using the whole exome capture kit (Exome research panel, Integrated DNA Technologies). The variants were analyzed using Varminer (in-house analysis tool) and interpreted based on American College of Medical Genetics 2015 guidelines [18]. All pathogenic and likely pathogenic variants were included in the final analysis.
Patients who remained without an etiological diagnosis after trio whole exome sequencing (WES) then had array comparative genome hybridization (array aCGH) analysis (affymetrix CytoScan™ 750 K). An exception was for patients with dysmorphic features, who had array aCGH as first line genetic test (Fig.1).
2.5. Etiology definitions:
Neonatal asphyxiation and brain injury had a history of resuscitation at birth followed by neonatal encephalopathy with APGAR ≤ 5 at 5 min and/or MRI showing features of hypoxic ischemic encephalopathy or its sequelae [19], [20].
Neonatal hypoglycemic brain injury (NHBI) had feeding issues in the postnatal period with lethargy, apnea and/or seizures associated with a documented blood sugar level of <47 mg% (<2.6 mmol/l), or MRI features to suggest specific NHBI changes such as bilateral occipital/occipito parietal insult [21], [22], [23].
2.6. Etiological classification:
Depending on case record review, neuroimaging and genetic results, patients were categorized into etiological groups using the International League Against Epilepsy 2017 classification [24]. Etiological groups were- i) Structural, which was further subdivided into structural-acquired, structural-genetic, and structural unknown); ii) genetic; iii) infectious; iv) immunologic, v) metabolic; and vi) unknown. The structural-unknown group included patients with cortical malformation and a normal comprehensive genetic evaluation.
2.7. Infantile spasm treatment protocol:
All IS patients with Tuberous Sclerosis (TS) are given vigabatrin at our center according to the United Kingdom Infantile Spasm Study (UKISS) protocol [25]. Non-TS patients are treated with oral steroids (40 milligram/day). Intramuscular corticosteroid administration for TS cortis not used at our center. If clinical spasms continue after 14 days of treatment, vigabatrin is added, as per our protocol [25]. Follow up EEGs are not routinely performed if there is clinical cessation of IS.
2.8. Treatment initiation and response classification:
Case notes were reviewed for the time gap between onset of spasms and initiation according to above treatment protocol. Lead-time to initial treatment for infantile spasms was accessed from clinic notes. Lead-time refers to the delay between clinical onset of spasms and initiation of treatment [13]. The lead time was categorized into four time periods (≤7 days, 8–14 days, 15 days to 2 months, and > 2 months). The effect of lead time on treatment responsiveness was analyzed. The treatment response was noted from follow-up clinic notes. Spasm cessation for at least 3 months without relapse or progression to other seizure types was considered as complete clinical response (responders). This favorable outcome was studied in relation to lead time in starting treatment. The patients were classified as incomplete clinical responders (non-responders) if they continued to have IS despite of use of steroids and vigabatrin up to a 3-month follow-up appointment, or had progressed to other seizure types or had relapsed within this period. Only patients with at least three months’ follow up after initiation of treatment according to UKISS protocol2, were included in the final analysis for treatment response.
2.9. Neuro developmental follow up:
All patients with IS are routinely reviewed for their neurodevelopmental progress and co-morbidities by the attending pediatric neurologist in the follow up clinic visits.
Clinical notes from follow up visits of all recruited cases to the clinic were reviewed and their neurodevelopment status was recorded.
The impact of treatment delay on the neurodevelopment was not assessed in this study.
2.10. Statistics
Data was compiled and collated on Microsoft excel spreadsheets. Central tendency (mean/median), standard deviation, Mann-Whitney-U test, χ2 and Fisher’s exact test were applied on data using SPSS Software. P values < 0.05 were considered significant.
2.11. Ethics
The study was approved by the local institutional ethics committee (IEC/2018/14). Informed consent was taken from families wherever genetic testing was performed.
3. Results
From a screened total of 124 patients, 113 were eligible; in 11, adequate information was not available from the case records, hence excluded from the study. Mean (±SD) IS onset age was 6.86 months (±4.25). Male to female ratio was 3.3:1.
Brain MRI results were available for all but 5 patients. All 5 had a history of documented birth asphyxia with low APGAR scores and a neonatal clinical course consistent with hypoxic ischemic encephalopathy and later static encephalopathy. Brain MRIs’ were abnormal in 82 (76%) of the remaining 108 patients. Metabolic screen was normal in all patients, wherever it was performed.
There were 36 patients with a presumed genetic etiology, including 5 patients with clinical features of TS (Fig. 1). One patient who satisfied clinical criteria for a diagnosis of TS did not receive genetic testing due to family reluctance. In the remaining patients, a genetic etiology could be identified in 46% (16/35) patients (Fig. 2). This included 12/31 (39%) of non-TS patients (9/29 on trio WES; 3/22 on array aCGH) and 4 with TS.
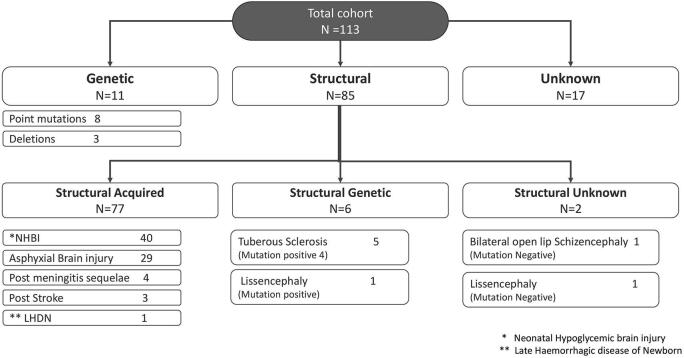
Fig. 2.
Etiological classification and specific etiology of all infantile spasm patients.
The 113 patients after clinical, radiological and genetic evaluation could be subdivided into 3 etiological groups- structural 85; genetic 11; and unknown 17 (Fig. 2). No patient could be classified into infectious, immunologic or metabolic groups.
Etiology was confirmed in 94/113 (83%) patients (Fig. 2). This included 83/85 patients in the structural group (structural-acquired 77; structural-genetic 6), and 11 in genetic group. Cause could not be determined in 2 patients within the structural-unknown and 17 in unknown group.
3.1. Structural group
From a total of 85 patients, 77 had structural-acquired, 6 structural-genetic, and 2 had structural-unknown etiology (Fig. 2e).
3.1.1. Structural acquired
The structural-acquired group (77/113, 69%) was the largest etiological subgroup. The most common cause in this group was NHBI followed by neonatal asphyxiation brain injury.
Neonatal hypoglycemic brain injury (40/113, 35.3%) was the most common acquired cause of IS in the whole cohort. Low blood sugar levels (mean blood sugar level- 21.4 mg% ±3.83 [±SD]) in the postnatal period were documented in 30/40 patients. In the remaining 10/40, a diagnosis was made based on postnatal history of decreased feeding, lethargy and neuroimaging demonstrating NHBI specific changes.
Nearly all of NHBI patients were male (38/40, 95%) and the majority (29/40, 72%) were born at term. Only 10/40 (25%) were small for birth date. All except two patients (38/40, 95%) had global developmental delay. Cortical visual impairment (35/40, 87.5%), static encephalopathy (19/40, 47.5%) and social-communication disorder (12/40, 30%) were other common co-morbidities.
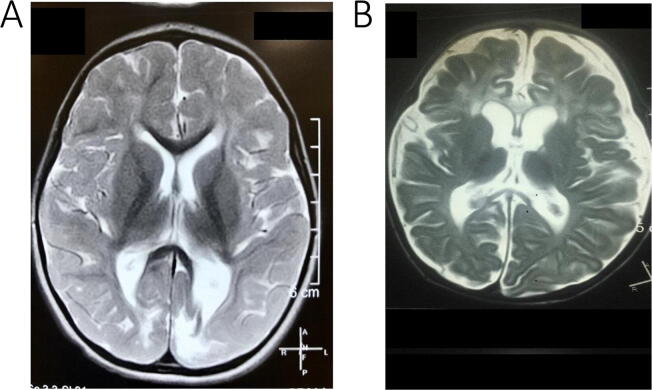
MRI’s were accessible and found to be abnormal in all NHBI patients. The majority (36/40, 90%) had bilateral occipital involvement (Fig. 3A) including 11 with associated parietal lobe pathology. Five also had periventricular leukomalacia. Four patients, all with documented postnatal hypoglycemia, had unilateral changes –2 hemispheric atrophy; 1 each had unilateral occipital and parieto- occipital gliosis.
Fig. 3.
(A) Axial T2 MRI image of a 30-month-old baby with history of neonatal hypoglycemia and later infantile spasms- Shows asymmetric bilateral occipital T2 hyper intensity suggestive of occipital gliosis; (B) Axial T2 MRI image of an 18-month-old baby with history of perinatal asphyxia and later infantile spasms- Shows diffuse cortical atrophy especially in the peri-rolandic region with a paucity of white matter and bilateral frontal T2 hyper intensity.
Neonatal asphyxiationbrain injury was seen in a total of 29/113 (25.7%) patients) (Fig 2); 21/29 (72%) were males. All patients (29/29; 100%) had global developmental delay and static encephalopathy. A significant number also had cortical visual impairment (16/29, 55%) and one had social-communication disorder.
MRIs performed in 24/29 patients, were abnormal in all. The majority showed predominant cortical involvement (Fig. 3B) in the form of diffuse bilateral or frontal encephalomalacia (12/24), parasagittal (5/24) or peri rolandic gliosis (3/24), while some also had associated basal ganglia (5/24) and/or thalamic (3/24) involvement.
The remaining 8/113 (7%) in the structural-acquired group included 4 with meningitis sequelae (2 tubercular meningitis; 2 neonatal meningitis), 3 perinatal stroke and 1 late hemorrhagic disease of newborn.
3.1.2. Structural genetic
There were 6 patients in the structural-genetic group (TS 5; lissencephaly 1). Mutations in the TSC2 gene were detected in 4 of the 5 children diagnosed with TS (one family refused genetic testing; Supplementary data 1-Table S1). Trio exome analysis detected a de novo mutation (Arg309His) in the DYN1C1HI gene (Table 1, case 7) in the child with lissencephaly.
Table 1.
List of significant variants (Pathogenic/Likely Pathogenic) detected in non-Tuberous Sclerosis cohort.
| S.No. | Gender/IS Onset age (Months) | Seizure types (as appeared) | Development | Neuroimaging (MRI) | Gene | Variant Details | Amino acid change | Zygosity and Segregation/ inheritance | Significance ACMG 201518 | Literature |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | F/9 | Erratic Myoclonus, IS, Tonic | GDD, Social communication disorder | Cerebellar atrophy | SCN2A |
NM_001040142.1 c.638 T > G |
p.Val213Gly* | Heterozygous De novo | Likely Pathogenic | This study PMID : 23935176 [Different variant affecting the same codon V213D] |
| 2a | M/6 | Focal Clonic, IS | GDD, Social communication disorder | Normal | STXBP1 |
NM_003165.3 c.1610 T > C |
p.Leu537Pro* | Heterozygous De novo | Likely Pathogenic | This study |
| 3a | M/8 | Myoclonus, tonic, IS | GDD | Normal | STXBP1 |
NM_003165.3 c.1439C > T |
p.Pro480Leu* | Heterozygous De novo | Pathogenic | PMID:21770924 |
| 4a | M/8 | Tonic, IS | GDD, Social communication disorder | Normal | GRIN1 |
NM_001185090.1 c.2039G > A |
p.Arg680Gln* | Heterozygous De novo | Likely Pathogenic | This study |
| 5a | M/6 | IS | GDD, Social communication disorder | Normal | SIN3A |
NM_001145358.1 c.1888del |
p.Ile630SerfsTer55* | Heterozygous De novo | Likely Pathogenic | This study |
| 6a | F/6 | Tonic Seizures, IS, Focal | GDD, Social communication disorder | Normal | CYFIP2 |
NM_001037332.2 c.259C > T |
p.Arg87Cys* | Heterozygous De novo | Likely Pathogenic | PMID:29534297 |
| 7a | F/5 | IS, Focal Clonic | GDD | Lissencephaly | DYNC1H1 |
NM_001376.4 c.926G > A |
p.Arg309His* | Heterozygous De novo | Likely Pathogenic | Clinvar:RCV000191045.1 |
| 8a | M/10 | IS, Focal Clonic | GDD | Normal | TUBB2A |
NM_001069.2 c.484C > T |
p.Arg162Cys* | Heterozygous De novo | Likely Pathogenic | This study |
| 9a | F/10 | IS, Tonic | GDD | Normal | PRUNE |
NM_021222.1 c.46C > T/ c.316G > A |
p.Arg16Ter/ p.Asp106Asn** | Compound heterozygous variants | Pathogenic | Asp106Asn: PMID:26539891 Arg16Ter: This study |
| 10b | F/5 | IS | GDD, Social communication disorder, dyskinesia | Normal | NA | arr[hg19] 2q22.1q22.3(139,135,404–144,963,991)x1 | NA | Heterozygous Likely De novo$ | Likely Pathogenic | Heterozygous deletion involving chromosome 2 (5.8 Mb) indicating monosomy for this region. The genes in this region include SPOPL, NXPH2, YY1P2, LRP1B, KYNU, ARHGAP15 and GTDC1 |
| 11b | M/5 | IS | GDD | Corpus callosaldysgenesis | NA | arr[GRCh37] 5q14.3(87489736_88841231)x1 | NA | Heterozygous Likely De novo$ | Pathogenic | Heterozygous deletion of 1.4 Mb on chromosome 5 at q14.3 region, indicating monosomy for this region. This region contains the 3 OMIM genes; LINC00461 (616611), MIR9-2 (611187), MEF2C (600662). |
| 12b | F/5 | IS, Tonic | GDD, Social communication disorder | Normal | NA | arr[GRCh37] Xp22.11p11.23(23309293_47698937)x1 [0.76] | NA | Heterozygous Likely De novo$ | Likely Pathogenic | Mosaic loss (76%) on chromosome X within cytoregion p22.11p11.23 (24.3 Mb), indicating mosaic monosomy for this region. This mosaic region contains 76 OMIM genes and overlaps chromosome Xp21 microdeletion syndrome. |
Abbreviations: a – Trio exome analysis results; b – Microarray results; IS – Infantile spasms; GDD – Global developmental delay; * – Heterozygous De Novo variant; ** – Compound heterozygous variants (Arg16Ter inherited from mother; Asp106Asn inherited from father); $-parental testing not available. PMID- PubMed reference number, The Annotations are based on (Grch37/hg19). The nucleotide numbering reflects cDNA numbering; the initiation codon is codon 1.
Four of the five TS patients had global developmental delay. Two of them also had social communication disorder.
3.1.3. Structural unknown
There were 2 patients with antenatal cortical malformation (lissencephaly 1; bilateral open lip schizencephaly 1). On further analysis no significant variants were detected on WES and array aCGH; both patients had global developmental delay. The child with schizencephaly also had social- communication disorder.
3.2. Genetic group
Eleven patients (M: F ratio 1.1:1) had a mutation identified on genetic evaluation (Table 1). All of them had a normal metabolic work up. Neuroimaging (MRI) was available for all and was abnormal in two (cerebellar atrophy 1; corpus callosum dysgenesis 1).
Three patients (2 were dysmorphic) had microdeletions on array aCGH. Eight had point mutations on trio WES in CYFIP2, GRIN1, PRUNE, SCN2A, SIN3A, STXBP1 (two patients), TUBB2A genes (all except one were de novo).
All eleven patients had global developmental delay and a majority (8/11, 73%) also had social-communication disorder.
3.3. Unknown group
In 17/113 (15%) patients no etiology could be established, despite extensive radiological, metabolic and genetic evaluation. All had a normal brain MRI.
In this group a smaller proportion of patients had developmental delay (5/17, 29.4%) and social-communication disorder (5/17, 29.4%) as compared to the genetic group.
3.4. Lead time to treatment
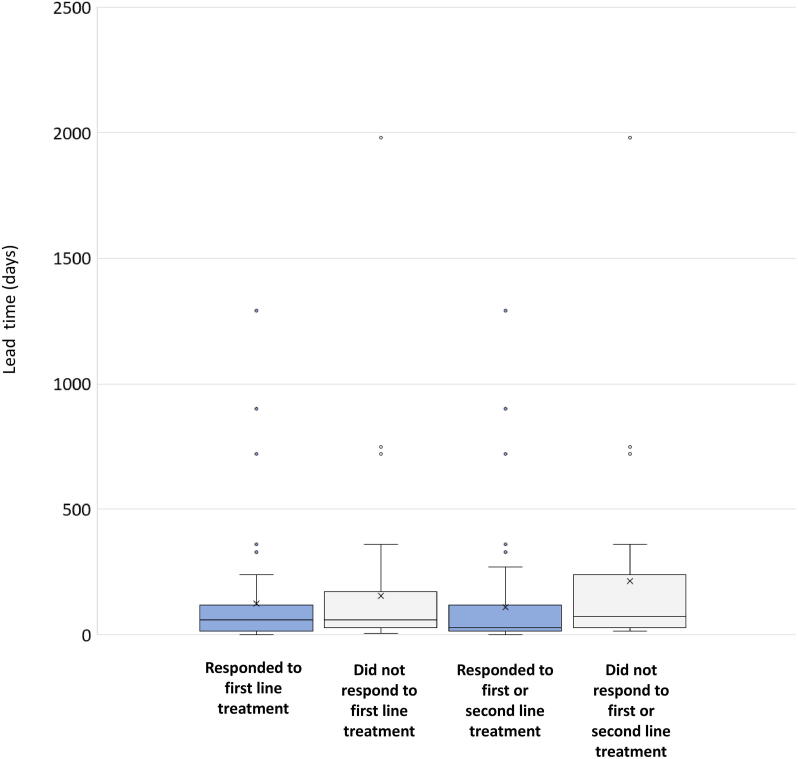
The median age at which a child was seen by a pediatric neurologist was 8 months (Range 2–72 months). The median lead time to specific spasm treatment for IS was 60 days (0–66 Months). There was a statistically significant difference (p value- 0.00193) in median lead time to treatment between responders (median- 30 days [Range- 0 days to 43 months]) and non-responders (median- 90 days [Range- 14 days to 66 months]). The distribution of lead time among responders and non-responders is depicted in supplementary figure (Fig. S1).
3.5. Treatment response
All 113 patients initially received treatment for IS. The follow up period ranged between 1–26 months (Median – 14 months). Two patients were lost to follow-up, and 1 not treated according to UKISS protocol, so these 3 were excluded from the final analysis of 110 patients. First line treatment was oral steroids in the 105 patients without TS, and vigabatrin in the 5 with TS. All 5 patients with TS had a complete response to vigabatrin.
Overall, 61/110 patients (56%) responded to first-line therapy (Table 2). A further 18 patients (17%) responded to second-line therapy, leaving 31/110 (28%) patients with drug-resistant spasms. There was no difference in age at presentation between those with resistant spasms and those responding to first or second-line therapy. Patients in the structural group were less likely to have resistant spasms than those in the genetic or unknown groups (p = 0.013 (OR 0.30 [0.12–0.76]). We investigated for whether underlying specific etiology was associated with treatment response. In the genetic group more than half (55%) (Table 2) had drug resistant spasms.
Table 2.
Relation of age at presentation and etiology to treatment response.
| N (%) responding to first line therapy | Cumulative N (%) responding to second line therapy | N (%) with resistant spasms at 3 months | P value, Fishers Exact test. (Odds ratio [95% CI]) for resistant spasms at 3 months | |
|---|---|---|---|---|
| All patients¶ | 61/110 (56%) | 79/110 (72%) | 31/110 (28%) | NA |
| Age at presentation with spasms¶¶ | ||||
| ≤ 6 months | 40/70 (57%) | 53/70 (76%) | 17/70 (24%) | > 0.05 |
| > 6 months | 19/36 (53%) | 24/36 (66%) | 12/36 (37%) | |
| Etiological Groups | ||||
| Structural | 48/82 (59%) | 64/82 (79%) | 18/82 (21%) | 0.013 (OR 0.30 [0.12–0.76]) |
| Genetic | 5/11 (45%) | 5/11 (45%) | 6/11 (55%) | > 0.05 |
| Unknown | 8/17 (47%) | 10/17 (59%) | 7/17 (41%) | > 0.05 |
| Main Etiologies | ||||
| NHBI | 24/38 (60%) | 29/38 (73%) | 8/38 (24%) | > 0.05 |
| Neonatal asphyxia | 11/27 (38%) | 19/27 (62%) | 10/28 (35%) | > 0.05 |
| Tuberous Sclerosis | 5/5 (100%) | 5/5 (100%) | 0/5 (0%) | > 0.05 |
| Other single gene disorder or microdeletion | 5/11 (45%) | 5/11 (45%) | 6/11 (55%) | 0.044 (OR 3.70 [1.03–13.21]) |
| Unknown | 8/17 (47%) | 10/17 (59%) | 7/17 (41%) | > 0.05 |
NHBI= Neonatal Hypoglycemic brain injury; NA= not applicable.
¶3/113 patients excluded; ¶¶Information not available in 7/113 patients.
There was a linear relationship between lead time to treatment and the presence of resistant spasms at 3 months (χ2 for trend = 10.0, p = 0.0015) (Table 3).
Table 3.
Lead time to treatment and treatment response.
| Lead time | N (%) responding to first line therapy | Cumulative N (%) responding to second line therapy | N (%) with resistant spasms at 3 months | χ2 test for trend (resistant spasms at 3 months) | N with missing information of treatment response |
|---|---|---|---|---|---|
| ≤7 days | 10/11 (91%) | 11/11 (100%) | 0/11 (0%) | (χ 2 = 10.0 (1 d.f.), p = 0.0015) | 1 |
| 8-14 days | 9/16 (56%) | 15/16 (94%) | 1/16 (6%) | 0 | |
| 15 days to 2 months | 23/44 (52%) | 30/44 (68%) | 14/44 (32%) | 2 | |
| > 2 months | 19/38 (50%) | 23/38 (61%) | 15/38 (39%) | 0 |
4. Discussion
This study is a comprehensive etiological evaluation of IS patients from a tertiary care hospital in North India. Our hospital serves as both a secondary and tertiary care center for the local population, and for patients from neighboring states. We believe our data authentically reflects the current circumstances of clinicians dealing with IS in India.
In each case the perinatal history was reviewed and confirmed by the same experienced pediatric neurologist. Two radiologists independently reviewed neuroimaging. A comprehensive genetic evaluation was performed, where the perinatal history and investigations were unable to reveal the cause (Fig. 1). Hence we were able to establish etiology in the majority (83%) of our patients. There have been few similar studies with a detailed clinical and genetic evaluation, with an etiological yield of 61–64% [2], [3]. These studies though were done in better equipped health care setups, than ours [2], [3]. While we had a higher proportion of patients with identified acquired causes compared to prenatal causes, the opposite was true in previous studies [2], [3]. All our patients with a presumed genetic cause also had a more comprehensive genetic evaluation which included trio WES and array aCGH. Both these factors could have contributed to the better yield in our study.
In our study, as reported by other authors from India [4], [5], [6], the most common acquired cause of IS was due to a perinatal insult. In previous works from India, neonatal asphyxiation brain injury was the commonest antecedent cause [4], [5], [6]. In contrast, NHBI was the most common cause of IS in our cohort, representing more than a third of all patients.
The babies with NHBI usually become unwell in first 72 hours of life [26]. These babies can be mistaken for having perinatal asphyxia, since both conditions may present similarly, and blood sugars are often not documented or given due importance [9]. This could be one of the reasons for only a few patients with neonatal hypoglycemia who subsequently developed IS, having been reported in the past [4], [5], [6], [11]. The reliability of neuroimaging in diagnosing etiologies of epilepsy syndromes is well established, and can be used to circumvent this problem of limited record keeping in resource limited settings [20], [22]. NHBI most commonly results in occipital gliosis, in contrast to perinatal asphyxia which causes more peri rolandic and basal ganglia insult [9], [20], [22], [23]. If blood sugar levels were not available, neuroimaging was used as an important objective parameter in determining the most likely etiology, in our study. Udani et al. [9] had also used MRI brain as an objective marker for identifying etiologies in their patients with symptomatic infantile epilepsy.
The higher incidence of neonatal hypoglycemia in developing countries is due to emphasis on exclusive breast feeding, and lack of support for mothers to help establish lactation [10], [26]. These babies are at significant risk to develop hypoglycemia even without other associated risk factors [23]. This is reflected in majority of our NHBI babies being term babies with normal birth weight; this observation was also made in a recent study from India on spectrum of epilepsies associated with NHBI [10].
There appeared to be a significant gender bias (87 males; 77% of total patients) in our study. A similar overwhelming bias has also been noted in previous studies on IS from India, as a male child is usually brought to medical attention earlier in Indian culture [4], [5], [6], [10].
Using advanced genetic testing (Fig. 1) we could achieve a molecular diagnosis in nearly half (45.7%; 16/35) of patients tested. This included 31% (9/29) yield of trio WES in our study as a first line genetic investigation in non-TS and non-dysmorphic IS patients, results comparable to other similar studies [3], [7], [27].
Despite the high number tested only 3/22 (13.6%) had significant copy number variants on chromosomal array aCGH, of whom 2 were also dysmorphic. In a similar study of 44 patients with unexplained IS, array aCGH revealed a de novo variant in only 3/44 (7%) of patients [7]. Contrary to what has been suggested [3], we would recommend applying chromosomal microarray first only to patients who along with epilepsy and developmental disability are also dysmorphic, though cost differences between array aCGH and WES must be considered.
In spite of exhaustive genetic investigations, we were still not able to determine an etiology in 19 patients (17 unknown & 2 structural-unknown; Fig. 2). It is possible that including mitochondrial gene sequencing in standard genetic testing protocol [28] and identification of novel genes could potentially unravel the etiology in some of them. Similarly, better neuroimaging techniques in the future might be able to identify small dysplastic lesions in some of the remaining patients [29].
Our study revealed a significant lead time to seeing a pediatric neurologist and initiating appropriate treatment, a delay contributing to resistant spasms later. A significant lead time from spasm onset to first presentation to a pediatric neurologist is not unusual in both a resource limited setting and even in a better equipped health care set ups [4], [5], [15]. The baby is often misdiagnosed to have infantile colic or an excessive startle response at first [15], [30]. In developing countries, even after an epilepsy diagnosis, these patients are often managed with inappropriate antiseizure medications [4]. Access to good quality standard neurodiagnostic and neurophysiological testing is also limited and concentrated to single centers, as was also common in our region [31]. All these factors could contribute to the significant delay in diagnosis and appropriate early treatment of infantile spasm.
It has been suggested that response to treatment and subsequent developmental outcomes are better if treatment is initiated earlier [13], [14]. There are no clear criteria stating what constitutes as early or late treatment. Previous studies have demonstrated that early initiation of specific treatment for spasms is associated with both earlier control of seizures [32] and more favorable neurodevelopmental outcome [33], [34]. In our study we have demonstrated an association between lead time to treatment of IS and treatment success is also observed in a lower middle-income country setting. There was a significant statistical association between lead time and treatment response in our cohort with IS (Table 3).
We found a high rate of drug resistant spasms (55%) in patients with a genetic etiology (not including those with a structural genetic etiology). A recent study also confirmed the unfavorable outcome in IS patients with an underlying genetic etiology or an unknown cause [16]. Our number of patients (Table 2) with a non-TS genetic etiology (N = 11) was small and should be validated with a larger cohort. As follow up video-EEG telemetry was not performed, it is also possible that we could have missed subtle spasms on clinical enquiry, in all etiological groups. The facility to allow a prolonged (6–24 hour) inpatient video EEG to capture subtle spasms, is unfortunately not available at our center. This also reflects the realistic situation in most of the centers in resource limited settings. This resource limitation was taken into consideration when a consensus statement for case definitions and outcome measures in studies of IS and West syndrome was proposed which agreed that studies which report only primary clinical outcome can also provide important useful information [35].
There were several limitations to our study. This is not a population-based study, hence bias is inevitable, seen by the significantly greater number of males seen. As this was a retrospective cohort group, some patients (N = 11) with inadequate information could not be recruited. Also due to the retrospective design, it is possible that some information in the perinatal period might be unavailable. This also precluded us from excluding culture negative neonatal sepsis and undocumented birth asphyxia as co-morbidities in patients with neonatal hypoglycemia. However neuroimaging in all NHBI patients had features to suggest primarily a hypoglycemic brain injury.
5. Conclusions
This study highlights the contribution of preventable perinatal insults, particularly neonatal hypoglycemia as an important cause of IS in a resource limited setting. There is an urgent need to develop guidelines for early recognition and optimal management of neonates at risk of developing hypoglycemia. We could also confirm that there was a significant delay in diagnosing and appropriately treating IS patients, leading to increased risk of drug resistant spasms. Hence it is vital for pediatricians, family practitioners, and the general public to be educated about early identification and appropriate treatment of neonates with IS.
Ethical statement
Funding: None
Consent: The study was approved by the local institutional ethics committee (IEC/2018/14). Informed consent was taken from families wherever genetic testing was performed.
CRediT authorship contribution statement
Priyanka Surana: Conceptualization, Methodology, Writing - original draft. Joseph D. Symonds: Software, Validation, Writing - review & editing. Prabhar Srivastava: Methodology, Software, Visualization. Thenral S. Geetha: Software, Validation, Writing - review & editing. Romit Jain: Visualization, Software. Ramprasad Vedant: Writing - review & editing. Sakthivel Murugan: Data curation, Formal analysis, Reviewing. Subathra Mahalingam: Software, Methodology. Vivek Bhargava: Data curation, Writing - review & editing. Pradeep Goyal: Investigation, Data curation, Reviewing. Sameer M. Zuberi: Supervision, Writing - review & editing. Vivek Jain: Writing - original draft, Writing - review & editing, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2020.100397.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. S1.
References
- 1.West W.J. On a peculiar form of infantile convulsions. The Lancet. 1841;35(911):724–725. doi: 10.1016/S0140-6736(00)40184-4. [DOI] [Google Scholar]
- 2.Osborne J.P., Lux A.L., Edwards S.W. The underlying etiology of infantile spasms (West syndrome): Information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–2174. doi: 10.1111/j.1528-1167.2010.02695.x. [DOI] [PubMed] [Google Scholar]
- 3.Wirrell E.C., Shellhaas R.A., Joshi C., Keator C., Kumar S., Mitchell W.G. How should children with West syndrome be efficiently and accurately investigated? Results from the National Infantile Spasms Consortium. Epilepsia. 2015;56(4):617–625. doi: 10.1111/epi.12951. [DOI] [PubMed] [Google Scholar]
- 4.Kaushik J.S., Patra B., Sharma S., Yadav D., Aneja S. Clinical spectrum and treatment outcome of West Syndrome in children from Northern India. Seizure. 2013;22(8):617–621. doi: 10.1016/j.seizure.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Gulati S., Jain P., Kannan L., Sehgal R., Chakrabarty B. The clinical characteristics and treatment response in children with west syndrome in a developing country: a retrospective case record analysis. J Child Neurol. 2015;30(11):1440–1447. doi: 10.1177/0883073815569304. [DOI] [PubMed] [Google Scholar]
- 6.Gupta J., Sharma S., Mukherjee S.B., Jain P., Aneja S. Neuro-developmental and epilepsy outcomes of children with west syndrome: A cross sectional study from North India. Ann Indian Acad Neurol. 2020;23:177–181. doi: 10.4103/aian.AIAN_503_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaud J.L., Lachance M., Hamdan F.F., Carmant L., Lortie A., Diadori P., Philippe M. The genetic landscape of infantile spasms. Human Molecular Genetics. 2014;23:4846–4858. doi: 10.1093/hmg/ddu199. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Du X., Bin R., Yu S., Xia Z., Zheng G. Genetic variants identified from epilepsy of unknown etiology in Chinese children by targeted exome sequencing. Sci Rep. 2017;7:40319. doi: 10.1038/srep40319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udani V., Munot P., Ursekar M., Gupta S. Neonatal hypoglycemic brain-injury, a common cause of infantile onset remote symptomatic epilepsy. Indian Pediatr. 2009;46:127–132. [PubMed] [Google Scholar]
- 10.Kapoor D., Sidharth, Sharma S., Patra B., Mukherjee S.B., Pemde H.K. Electroclinical spectrum of childhood epilepsy secondary to neonatal hypoglycemic brain injury in a low resource setting: a 10-year experience. Seizure. 2020;79:90–94. doi: 10.1016/j.seizure.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Zou LP, Wang J, Shi X, Tian S, Yang X, et al. Neonatal hypoglycemic brain injury is a cause of infantile spasms. Exp Ther Med 2016; 11:2066–70. [DOI] [PMC free article] [PubMed]
- 12.Mitta N., Menon R.N., McTague A., Radhakrishnan A., Sundaram S., Cherian A. Genotype-phenotype correlates of infantile onset Developmental & epileptic encephalopathy syndromes in South India: a single centre experience. Epilepsy Res. 2020;166 doi: 10.1016/j.eplepsyres.2020.106398. [DOI] [PubMed] [Google Scholar]
- 13.O’Callaghan F.J., Lux A.L., Darke K., Edwards S.W., Hancock E., Johnson A.L. The effect of lead time to treatment and age of onset on development outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52:1359–1364. doi: 10.1111/j.1528-1167.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- 14.Riikonen R.S. Favourable prognostic factors with infantile spasms. Eur J Paediatric Neurol. 2010;14(1):13–18. doi: 10.1016/j.ejpn.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Hussain S.A., Lay J., Cheng E., Weng J., Sankar R., Baca C.B. Recognition of infantile spasms is often delayed: the ASSIST study. J Pediatrics. 2017;190:215–221.e1. doi: 10.1016/j.jpeds.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Yuskaitis C.J., Ruzhnikov M.R.Z., Howell K.B., Allen I.E., Kapur K., Dlugos D.J., Scheffer I.E., Poduri A., Sherr E.H. Infantile spasms of unknown cause: predictors of outcome and genotype-phenotype correlation. Pediatr Neurol. 2018;87:48–56. doi: 10.1016/j.pediatrneurol.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hrachovy R.A., Frost J.D., Jr, Kellaway P. Hypsarrythmia: variations on the theme. Epilepsia. 1984;25:317–325. doi: 10.1111/j.1528-1157.1984.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 18.Richard S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J. Standards and Guidelines for the interpretation of sequence variants: a joint consensus recommendation of American College of Medical Genetics and Genomics and the association of Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe J.J. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol. 2012;72(2):156–166. doi: 10.1002/ana.23647. [DOI] [PubMed] [Google Scholar]
- 20.Gururaj A., Sztriha L., Dawodu A., Nath K.R., Varady E., Nork M. CT and MRI patterns of hypoxic-ischemic brain damage following perinatal asphyxia. J Trop Pediatr. 2002;48:5–9. doi: 10.1093/tropej/48.1.5. [DOI] [PubMed] [Google Scholar]
- 21.Lucas A., Morley R., Cole T.J. Adverse neurodevelopmental outcome of moderate neonatal hypoglycemia. BMJ. 1988;297:1304–1308. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkowich A.J., Ali F.A., Rowley H.A., Bass N. Imaging patterns of neonatal hypoglycemia. Am J Neuroradiol. 1998;19:523–528. [PMC free article] [PubMed] [Google Scholar]
- 23.Gu M.H., Amanda F., Yuan T.M. Brain injury in neonatal hypoglycemia: a hospital-based cohort study. Clinic Med Insights: Pediatr. 2019;13:1–6. doi: 10.1177/1179556519867953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., Nordli D.R., Perucca E., Tomson T., Wiebe S., Zhang Y.-H., Zuberi S.M. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lux A.L., Edwards S.W., Hancock E., Johnson A.L., Kennedy C.R., Newton R.W. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomized controlled trial. Lancet. 2004;364:1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- 26.J Ahmad H, Chisti AL. Neonatal hypoglycemia, an underreported entity in high risk neonates. Pak Pediatr J 2000; 24:9-11.
- 27.Dimassi S., Labalme A., Ville D., Calender A., Mignot C., Boutry‐Kryza N., de Bellescize J., Rivier‐Ringenbach C., Bourel‐Ponchel E., Cheillan D., Simonet T., Maincent K., Rossi M., Till M., Mougou‐Zerelli S., Edery P., Saad A., Heron D., des Portes V., Sanlaville D., Lesca G. Whole‐exome sequencing improves the diagnosis yield in sporadic infantile spasm syndrome. Clin Genet. 2016;89(2):198–204. doi: 10.1111/cge.12636. [DOI] [PubMed] [Google Scholar]
- 28.Shah N.S., Mitchell W.G., Boles R.G. Mitochondrial disorders: a potentially under-recognized etiology of infantile spasms. J Child Neurol. 2002;17:369–372. doi: 10.1177/088307380201700511. [DOI] [PubMed] [Google Scholar]
- 29.Chugani H.T., Shields W.D., Shewmon D.A., Olson D.M., Phelps M.E., Peacock W.J. Infantile spasms: 1. PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol. 1990;27:406–413. doi: 10.1002/ana.410270408. [DOI] [PubMed] [Google Scholar]
- 30.Napuri S., Gall E., Dulac O., Chaperon J., Riou F. Factors associated with treatment lag in Infantile spasms. Dev Med Child Neurol. 2010;52:1164–1166. doi: 10.1111/j.1469-8749.2010.03811.x. [DOI] [PubMed] [Google Scholar]
- 31.Bearden D. Pediatric neurology in resource-limited settings: a systematic review. Curr Pediatr Rep. 2018;6(1):34–39. doi: 10.1007/s40124-018-0155-x. [DOI] [Google Scholar]
- 32.O’Callaghan F.J., Edwards S.W., Alber F.D., Hancock E., Johnson A.L., Kennedy C.R. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomized, multicentre, open label trial. Lancet Neurol. 2017;16:33–42. doi: 10.1016/S1474-4422(16)30294-0. [DOI] [PubMed] [Google Scholar]
- 33.Kivity S., Lerman P., Ariel R., Danziger Y., Mimouni M., Sinnar S. Long term cognitive outcome of a cohort of children with infantile spasms treated with high dose adrenocorticotropic hormone. Epilepsia. 2004;45:255–262. doi: 10.1111/j.0013-9580.2004.30503.x. [DOI] [PubMed] [Google Scholar]
- 34.O’Callaghan F.J., Edwards S.W., Alber F.D., Borja M.C., Hancock E., Johnson A.L. Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomized controlled trial. Lancet Child Adolesc Health. 2018;2:715–725. doi: 10.1016/S2352-4642(18)30244-X. [DOI] [PubMed] [Google Scholar]
- 35.Lux A.L., Osborne J.P. A proposal for case definitions and outcome measures in studies of infantile spasms and west syndrome: consensus statement of the West Delphi Group. Epilepsia. 2004;45(11):1416–1428. doi: 10.1111/j.0013-9580.2004.02404.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.