Abstract
Current strategies to improve clinical outcomes in KRAS-mutant non-small cell lung cancer (NSCLC) patients include MEK inhibitor and PD-1 / PD-L1 immune checkpoint blockade (ICB) combinations. Experience from melanoma suggests that anti-CTLA-4 and anti-PD-1 / PD-L1 combinations improve outcomes, but similar benefits remain to be seen in NSCLC. This report describes a single center, investigator-initiated Phase I/II clinical trial comparing two combination schedules of intermittent or continuous selumetinib (AZD6244, ARRY-142886), tremelimumab (anti-CTLA-4) and durvalumab (anti-PD-L1) with historical controls in patients with previously treated, unresectable NSCLC. Forty patients will be accrued at the University of Texas MD Anderson Cancer Center. Primary objectives include maximum tolerated dose (MTD; dose-escalation phase) and Progression-Free Survival (PFS; dose expansion phase). Secondary objectives include response rate by RECIST 1.1, disease control rate, overall survival, safety and duration of response. Exploratory objectives will assess biomarkers of response and resistance based on pre-and on-treatment biopsies and peripheral blood using immune profiling, transcriptome and protein readouts.
Micro Abstract
This investigator-initiated trial was designed to provide novel data regarding: 1) the safety of durvalumab, tremelimumab and selumetinib combination therapy; 2) whether this combination can improve PFS in metastatic NSCLC; 3) potential biomarkers of response and resistance based on pre-and on-treatment biopsies and peripheral blood.
Keywords: Non-Small Cell Lung Cancer (NSCLC), Anti-PD-L1, Anti-CTLA-4, MEK inhibitor, Immunotherapy
Study rationssssale
Lung cancer is the leading cause of cancer-related death in the United States, and clinical outcomes have remained poor for patients with advanced, previously treated Non-Small Cell Lung Cancer (NSCLC) [1]. KRAS-mutant NSCLC represents the most common molecularly-defined subset, with 32.2% of lung adenocarcinomas harboring this activating mutation according to The Cancer Genome Atlas [2]. Despite therapeutic progress in other subsets of lung cancer (e.g., mutant EGFR or ALK), little progress has been made for KRAS-mutant NSCLC patients. Direct KRAS inhibitors (i.e., AMG 510 and MRTX849) have shown promising preclinical activity [3] and have recently entered clinical trials (e.g., NCT03600883 and NCT03785249), but the activity of these inhibitors is limited to KRAS G12C mutations. Agents targeting downstream elements of the Mitogen-Activated Protein Kinase (MAPK) signaling pathway have also been developed, such as MEK inhibitors [5]. However, pre-clinical and clinical evidence have not demonstrated substantial benefits due to the development of drug resistance [6, 7]. Current clinical trials are exploring intermittent vs continuous dosing of the MEK inhibitor selumetinib [8, 9]. Pre-clinical evidence suggests that pulsatile MEK inhibition may improve anti-tumor immunity [10]. Although final results from different clinical trials have not been reported yet, the TATTON Phase 1B clinical trial combining osimertinib and selumetinib selected the intermittent selumetinib dosing for Part B since no drug limiting toxicities (DLTs) were observed with the intermittent schedule, whereas DLTs occured in six patients for the continuous selumetinib schedule [8].
Recent randomized trials using immune checkpoint blockade (ICB) involving the PD-1 / PD-L1 axis have produced survival benefits as well as improvements in safety over docetaxel monotherapy [11]. However, even when patients with 50% or higher positivity for PD-L1 expression are selected, overall response rates still do not exceed 31% [11, 12]. Given the high percentage of primary resistance to ICB, different combinatorial treatments have been proposed [13]. Current strategies in the clinic strive to improve the results in the KRAS-mutant NSCLC population by combining MEK inhibitors and PD-1 / PD-L1 ICB. One example of such effort is the IM-BATTLE-2 Program -A Biomarker-Integrated Targeted Therapy in Non-Small Cell Lung Cancer (NSCLC), a Phase IB clinical trial combining MEK inhibitor trametinib and anti-PD-1 monoclonal antibody pembrolizumab (NCT03225664). Indeed, pre-clinical evidence suggests that the combination of a MEK inhibitor and anti-PD-L1 ICB can increase survival in TP53 and KRAS-mutated animal models by increasing tumor-infiltrating CD8+ and CD4+ T cells as well as by decreasing Myeloid-Derived Suppressor Cells [14]. Benefits were also found using ex vivo human NSCLC spheroids, where MEK and PD-L1 inhibition improved outcomes through the result of direct toxicity produced by the MEK inhibitor, but also its immune-stimulatory effect on cytokine production [15].
Although combined anti-CTLA-4 and anti-PD-1 / PD-L1 ICB has demonstrated improved response rates in melanoma [16], mixed results have been seen in the context of NSCLC. The CheckMate 227 Phase III clinical trial recently reported that overall survival (OS) was significantly longer with nivolumab plus ipilimumab compared to standard of care chemotherapy, irrespective of PD-L1 expression levels. Patients treated with nivolumab plus ipilimumab had a median OS of 17.1 months, as compared to 13.9 months in the chemotherapy group (p = 0.007) [17]. However, MYSTIC trial results failed to show a PFS advantage over standard of care for either anti-PD-L1 monotherapy or anti-PD-L1 / CTLA-4 combination blockade [18].
Objectives
This study was designed to evaluate the efficacy and safety of combined modality treatment of durvalumab, tremelimumab with continuous or intermittent MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with pretreated, advanced or metastatic NSCLC, and to determine whether further investigation is warranted. Primary objectives include: 1) the maximum tolerated dose (MTD; dose-escalation phase) and; 2) PFS (dose expansion phase). Secondary objectives include: 1) response rate by RECIST criteria (version 1.1); 2) disease control rate (complete response + partial response + stable disease); 3) OS; 4) safety (i.e., incidence of adverse events) and; 5) duration of response. Exploratory objectives will assess biomarkers of response and resistance in pre-and on-treatment biopsies as well as peripheral blood using immune profiling, transcriptome and protein readouts.
Study design
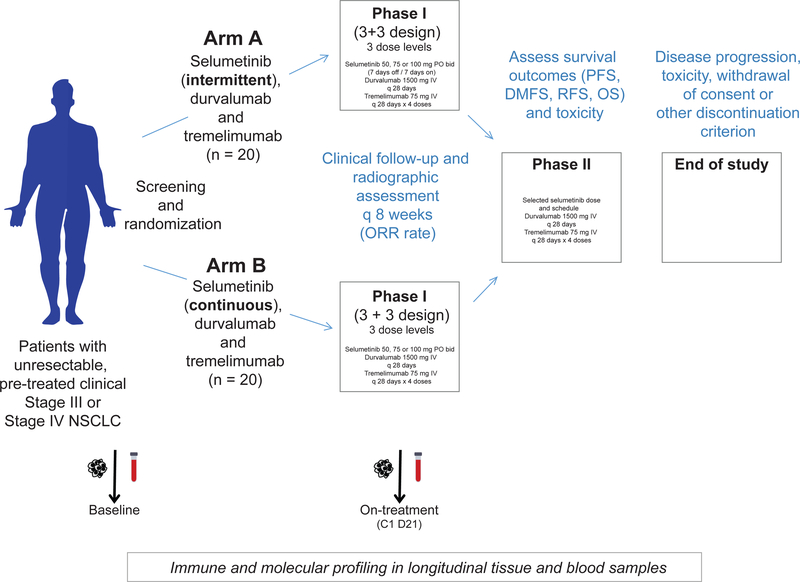
This is a single center, investigator-initiated Phase I/II study comparing two combination schedules of selumetinib, tremelimumab and durvalumab with historical controls in patients with previously treated, unresectable NSCLC. A maximum of 40 patients will be accrued at the University of Texas MD Anderson Cancer Center. Figure 1 provides an overview of the trial design and related correlative studies.
Figure 1.
Overview of the Phase I/II Trial of Immunotherapy with Durvalumab and Tremelimumab With Continuous or Intermittent MEK Inhibitor Selumetinib in NSCLC.
Study population
Patients enrolled have unresectable stage III or IV Non-Small Cell Lung Cancer (NSCLC), ineligible for concomitant radio-chemotherapy, who have experienced disease progression on or after platinum-containing systemic therapy. All patients who meet the inclusion and exclusion criteria will be eligible for screening; these criteria are listed in Table 1.
Table 1.
Patient Eligibility.
|
Inclusion criteria |
| • Written informed consent |
| • Histologically or cytologically confirmed recurrent NSCLC not amenable to curative intent therapy or stage IV NSCLC |
| • Known KRAS mutation status by CLIA certified test |
| • Documented progression following at least one line of chemotherapy or immunotherapy for metastatic or recurrent disease, or progression within 6 months of receiving adjuvant chemotherapy or concurrent chemotherapy for early stage or locally advanced disease |
| • Biopsy accessible disease and willingness to undergo tumor biopsy |
| • Measurable disease by RECIST 1.1 criteria |
| • Age ≥18 years at time of study entry |
| • Total body weight > 30 kg |
| • ECOG performance status 0 or 1 |
| • Ability to take pills by mouth |
| • Normal organ and marrow function as defined below: leukocytes ≥3,000/mcL; absolute neutrophil count ≥1,500/mcL; platelets≥100,000/mcL; haemoglobin ≥ 9.0 g/dL; total bilirubin ≤1.5 × upper limit of normal (ULN) (higher is allowed if in the setting of known Gilbert’s disease); AST(SGOT)/ALT(SGpT) ≤ 2.5 × institutional upper limit of normal or ≤5 × ULN if liver metastases are present; Alkaline phosphatase ≤ 3.5 × institutional upper limit of normal or <6 × ULN if liver metastases are present; Measured creatinine clearance (CL) >40 mL/min or Calculated creatinine clearance CL>40 mL/min by the Cockcroft-Gault formula (Cockcroft and Gault 1976) or by 24-hour urine collection for determination of creatinine clearance. |
| • Brain metastases are allowed, as long as they are stable and do not require treatment with anticonvulsants or escalating doses of steroids |
| • Evidence of post-menopausal status or negative urinary or serum pregnancy test for female pre-menopausal patients |
| • Life expectancy of at least 12 weeks |
|
Exclusion criteria |
| • Investigational medicinal product (IMP) or other systemic anticancer treatment within 4 weeks prior to the first dose of study treatment, or within a period during which the IMP or systemic anticancer treatment has not been cleared from the body. |
| • Prior randomisation or treatment in a previous durvalumab and/or tremelimumab clinical study regardless of treatment arm assignment |
| • Current or prior use of immunosuppressive medication within 14 days before the first dose of durvalumab or tremelimumab (some exceptions apply) |
| • Any concurrent chemotherapy, immunotherapy, biologic, or hormonal therapy for cancer treatment (some exceptions apply) |
| • Radiation therapy to more than 30% of the bone marrow or with a wide field of radiation within 4 weeks prior to starting study treatment. Limited field of radiation for palliation at any time prior to the start of study treatment is acceptable if the lung is not in the radiation field or if the irradiated lesions are not used as target lesions. |
| • Receipt of live attenuated vaccine within 30 days prior to the first dose |
| • Prior treatment with a MEK, RAS, or RAF inhibitor |
| • Patients who have received prior anti-PD-1, anti-PD-L1 or anti-CTLA-4: 1) must not have experienced a toxicity that led to permanent discontinuation of prior immunotherapy; 2) all AEs while receiving prior immunotherapy must have completely resolved or resolved to baseline prior to screening for this study; 3) must not have experienced a > Grade 3 immune related AE or an immune related neurologic or ocular AE of any grade while receiving prior immunotherapy |
| • Patients who are receiving any other investigational agents |
| • Any unresolved toxicity NCI CTCAE Grade >2 from previous anticancer therapy (some exceptions apply) |
| • Known hypersensitivity to selumetinib, durvalumab, tremelimumab or any compounds of similar chemical or biologic composition |
| • Active or prior documented autoimmune or inflammatory disorders (some exceptions apply) |
| • Known history of previous clinical diagnosis of tuberculosis, or active infection including tuberculosis, hepatitis B, hepatitis C, or human immunodeficiency virus |
| • History of leptomeningeal carcinomatosis |
| • History of primary immunodeficiency or allogenic organ transplantation |
| • Cardiac conditions including QTcF ≥470ms; uncontrolled hypertension; acute coronary syndrome within 6 months prior to starting treatment; uncontrolled angina; symptomatic heart failure NYHA Class II-IV; severe valvular heart disease; prior or current cardiomyopathy |
| • Current or past history of retinal pigment epithelial detachment /central serous retinopathy or retinal vein occlusion; Intraocular pressure (IOP) > 21 mmHg or uncontrolled glaucoma (irrespective of IOP) |
| • Any gastrointestinal disorder expected to limit absorption of selumetinib |
| • History of another primary malignancy within 5 years prior to starting study treatment (some exceptions apply) |
| • Major surgical procedure within 28 days prior to the first dose |
| • Uncontrolled intercurrent illness |
| • Receiving or have received systemic anti-cancer therapy within 4 weeks prior to starting study treatment (6 weeks for nitrosoureas, mitomycin, and suramin), or any anticancer therapy which has not been cleared from the body by the time of starting study treatment |
| • Any other significant clinical disorder or laboratory finding that, as judged by the investigator, makes it undesirable for the patient to participate in the study |
| • Female patients who are pregnant or breastfeeding or male / female patients of reproductive potential who are not willing to employ effective birth control from screening to 90 days after the last dose of durvalumab and selumetinib combination or 180 days after the last dose of durvalumab + tremelimumab combination therapy |
Intervention
The two arms (i.e., combination schedules) of the study include: 1) an intermittent selumetinib schedule (i.e., one week on and one week off) and; 2) a continuous selumetinib schedule. Each arm will first go through a dose escalation phase, for a total of three dose levels of selumetinib (i.e., 50, 75 and 100mg PO BID). The starting dose has been determined from the safety data of a currently ongoing clinical trial, and the standard 3+3 design will be applied to determine the MTD among the three pre-defined dose levels. The best tolerated regimen will be selected for the dose expansion phase.
In the first arm, participants receive selumetinib 50mg PO BID on days 1–7 and 15–21 and durvalumab 1500mg intravenously (IV) over 60 minutes on day 1. Participants also receive tremelimumab 75mg IV over 60 minutes on day 1 for courses 1–4. Courses repeat every 28 days in the absence of disease progression or unacceptable toxicity. The fixed dosing schedules of durvalumab and tremelimumab are based on simulation results demonstrating that body weight-based and fixed dosing regimens yield similar median steady-state exposures and associated variability. Moreover, a fixed dosing approach is preferred by the prescribing community, as it is easier to use and reduces dosing errors.
In the second arm, participants receive selumetinib 50 mg PO BID on days 1–28 and durvalumab 1500mg IV over 60 minutes on day 1. Participants also receive tremelimumab 75mg IV over 60 minutes on day 1 for courses 1–4. Courses also repeat every 28 days in the absence of disease progression or unacceptable toxicity.
Statistical analyses
A total of 40 patients will be accrued with an accrual rate of 2 patients/month, assigned with random permuted blocks into the two schedules of the dose escalation (9 to 18 patients in each of the two arms), followed by another 4 to 22 patients treated in the expansion cohort (and followed for an additional 12 months). The 6 patients treated at MTD of the selected schedule during the dose escalation phase will be included in the dose expansion phase. The sample size has been determined based on feasibility to obtain an initial estimate of the treatments’ toxicity and efficacy profiles. Given a total of 28 patients (22 patients + 6 patients treated at the MTD), we will have 83% power for testing the improvement of the median PFS from 6 months (two-drug combination therapy with selumetinib from historical data) to 10 months (three-drug combination therapy), with a one-sided 10% type I error rate (STPLAN version 4.5).
Data from variables of interest including patients’ demographic and clinical characteristics will be summarized using standard descriptive statistics such as mean, standard deviation, median, and range for continuous variables, frequency and proportion for categorical variables. Correlations will be assessed among continuous variables using Pearson or Spearman correlation coefficient, whichever is appropriate. Associations between categorical variables will be examined by Chi-Squared test or Fisher’s exact test when appropriate. Wilcoxon-Mann-Whitney test will be used to examine the difference on continuous variables between or among patient’s characteristic groups. OS and PFS will be estimated using the Kaplan-Meier method and the comparison between or among patient’s characteristic groups will be evaluated by the log-rank test. The Cox regression model may be applied to assess the effect of covariates of interest on OS and PFS. The disease control rate will be estimated with 95% confidence intervals, and toxicity data will be summarized by frequency tables.
Ethical considerations
Written informed consent must be obtained from the patient before any screening or inclusion procedure. This study is conducted in compliance with the principles of the Declaration of Helsinky, and the protocol was approved by the institutional review board of the University of Texas MD Anderson Cancer Center (protocol number: 2017–0888).
Trial and data management
Clinical data capture called DMI (Data Management Initiative) is the electronic database used for this study’s electronic case report forms. The MD Anderson Data Safety Monitoring Board/DSMB will be monitoring this study.
Active enrollment
Participant accrual for this trial (NCT03581487) started on April 1st, 2019. The trial is actively screening and enrolling patients, and the estimated study completion is scheduled for April 2021.
Discussion and conclusion
Clinical outcomes using MEK inhibitors in KRAS-mutant NSCLC have been disappointing so far [7]. However, pre-clinical results highlighting synergistic activity of MEK inhibitors combined anti-PD-1 / PD-L1 ICB are promising [14, 15]. Ongoing trials (e.g., BATTLE-2) will determine if this combination is effective in the clinical setting, but the question about whether the addition of anti-CTLA-4 ICB can be safely tolerated and lead to improved outcomes remains unresolved. Thus, this investigator-initiated trial was designed in the hope that it could provide novel data regarding: 1) the most tolerated treatment schedule for durvalumab, tremelimumab and selumetinib combination therapy; 2) whether this triple combination can improve PFS in metastatic NSCLC, as compared to two-drug combination therapy with selumetinib from historical data; 3) potential biomarkers of response and resistance based on pre-and on-treatment biopsies, as well as peripheral blood using immune profiling, transcriptome and protein readouts.
Acknowledgements
Funding for this investigator-initiated trial is provided by AstraZeneca, with funding provided by the NCI for correlative analyses of the tumor biopsies, R37CA214609 to DLG. POG is supported by the Fonds de Recherche Quebec-Santes (FRQS) Resident Physician Health Research Career Training Program program (32667).
Conflicts of interest
J.V. Heymach has received research support from AstraZeneca, Bayer, GlaxoSmithKline, and Spectrum; participated in advisory committees for AstraZeneca, Boehringer Ingelheim, Exelixis, Genentech, GlaxoSmithKline, Guardant Health, Hengrui, Lilly, Novartis, Specrtum, EMD Serono, and Synta; and received royalties and/or licensing fees from Spectrum.
D.L. Gibbons has received research funding from AstraZeneca, Janssen, Ribon Therapeutics and Takeda and has participated in advisory boards for AstraZeneca and Sanofi.
Abbreviation list
- DLT
Drug Limiting Toxicity
- DMI
Data Management Initiative
- ICB
Immune Checkpoint Blockade
- MAPK
Mitogen-Activated Protein Kinase
- MTD
Maximum Tolerated Dose
- NSCLC
Non-Small Cell Lung Cancer
- OS
Overall Survival
- PFS
Progression-Free Survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2019. CA Cancer J Clin, 2019. 69(1): p. 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research, N., Comprehensive molecular profiling of lung adenocarcinoma. Nature, 2014. 511(7511): p. 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seton-Rogers S, KRAS-G12Cin the crosshairs. Nat Rev Cancer, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Cox AD, et al. , Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov, 2014. 13(11): p. 828–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lito P, et al. , Disruption of CRAF-mediatedMEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell, 2014. 25(5): p. 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenschein GR Jr., et al. , A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)dagger. Ann Oncol, 2015. 26(5): p. 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janne PA, et al. , Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial. JAMA, 2017. 317(18): p. 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramalingam SS, et al. , Abstract CT034: Osimertinib plus selumetinib for patients (pts) with EGFR-mutant NSCLC following disease progression on an EGFR-TKI: Results from the Phase Ib TATTONstudy. Cancer Research, 2019. 79(13 Supplement): p. CT034-CT034. [Google Scholar]
- 9.Komatsubara KM, et al. , Multi-center phase Ib study of intermittent dosing of the MEK inhibitor, selumetinib, in patients with advanced uveal melanoma not previously treated with a MEK inhibitor. Journal of Clinical Oncology, 2017. 35(15_suppl): p. TPS9597-TPS9597. [Google Scholar]
- 10.Choi H, et al. , Pulsatile MEK Inhibition Improves Anti-tumor Immunity and T Cell Function in Murine Kras Mutant Lung Cancer. Cell Rep, 2019. 27(3): p. 806–819 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittmeyer A, et al. , Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet, 2017. 389(10066): p. 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, et al. , Pembrolizumab versus docetaxel for previously treated, PD-Ll-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet, 2016. 387(10027): p. 1540–50. [DOI] [PubMed] [Google Scholar]
- 13.Meng X, et al. , PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: New development and challenges. Cancer Lett, 2017. 405: p. 29–37. [DOI] [PubMed] [Google Scholar]
- 14.Lee JW, et al. , The Combination of MEK Inhibitor With Immunomodulatory Antibodies Targeting Programmed Death 1 and Programmed Death Ligand 1 Results in Prolonged Survival in Kras/p53-Driven Lung Cancer. J Thorac Oncol, 2019. 14(6): p. 1046–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Della Corte CM, et al. , Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J Exp Clin Cancer Res, 2019. 38(1): p. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolchok JD, et al. , Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med, 2013. 369(2): p. 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann MD, et al. , Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med, 2019. 381(21): p. 2020–2031. [DOI] [PubMed] [Google Scholar]
- 18.Astra Zeneca, AstraZeneca reports initial results from the ongoing MYSTIC trial in Stage IVlung cancer. 2017. [Google Scholar]
- 19.West JH Combining Immunotherapy Agents in NSCLC: Is MYSTIC a Misstep? 2017. August 16th, 2017 [cited 2017 October 10th 2017]. [Google Scholar]
- 20.Hellmann MD, et al. , Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med, 2018. 378(22): p. 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]