Abstract
Objectives:
Age-related hearing loss (ARHL) is a prevalent condition associated with increased risk for depression and cognitive decline. This 12-week prospective, double-blind pilot randomized controlled trial (RCT) of hearing aids (HAs) for depressed older adults with ARHL evaluated the feasibility of a novel research design.
Methods/Design:
N=13 individuals aged ≥60 years with Major Depressive Disorder or Persistent Depressive Disorder and at least mild hearing loss (pure tone average ≥30dB) were randomized to receive full- (active) vs. low-amplification (sham) HAs added to psychiatric treatment as usual. Duration of HA use in hours/day, adverse events frequency, attrition rate, and maintenance of the study blinding were the primary outcome measures.
Results:
Compliance with HAs was excellent (>9 hours/day for both groups) and rates of adverse events and drop-outs did not differ between groups. Preliminary data demonstrated differential improvement for active vs. sham HAs on hearing functioning (Hearing Handicap Inventory for the Elderly [nonparametric effect size (np-ES) =.62]), depressive symptoms (Inventory for Depressive Symptomatology [np-ES=.31]), cognition (Repeatable Battery for the Assessment of Neuropsychological Status Immediate Memory [np-ES=.25]), and general functioning (World Health Organization Disability Assessment Schedule [np-ES=.53]). Significantly greater than 50% of both groups correctly guessed their treatment assignment, indicating incomplete concealment of treatment allocation.
Conclusions:
This pilot RCT for ARHL and late-life depression was feasible to execute and showed clinical promise, but improved methods of blinding the experimental treatments are needed. Larger studies should investigate whether hearing remediation may be an effective preventative and/or therapeutic strategy for late-life depression and cognitive decline.
Keywords: hearing loss, hearing aids, depression, cognitive impairment
INTRODUCTION
Age-related hearing loss (ARHL) is the third most common health condition affecting older adults after heart disease and arthritis.1 The prevalence of ARHL rises steeply with age, from 3% among adults 20-29, to 45% of adults 60-69, to above 80% in individuals over 80 years.2,3 Untreated hearing loss affects more than 38 million Americans2 and has been estimated, by using two different datasets, to result in $852 billion in increased medical costs over 10 years.4 While historically considered a benign effect of aging or exclusively a quality of life issue, ARHL is in fact associated with psychological and medical morbidity, including social isolation, frailty, and falls.5,6
Recently, ARHL has been associated with the development of neuropsychiatric dysfunction, including impaired cognitive performance, increased risk for dementia diagnosis, and late-life depression.7,8 For example, recent reviews9, and meta-analyses10, and a National Institute on Aging workshop on the topic11, linked ARHL to cognitive decline and dementia in older adults. Similarly, our group showed in multiple data sets that ARHL is associated with increased depressive symptoms as well as syndromal depression in older adults, in both cross-sectional and longitudinal analyses.12,13 ARHL may increase risk for depression and cognitive decline through both brain-based (e.g., de-afferentiation induced atrophy, compensatory neuroplastic changes) and social/behavioral mechanisms (e.g., social isolation, decreased behavioral activation, and increased loneliness).14
The obvious therapeutic implication of the above-reviewed evidence is that treatment of ARHL may help avoid these adverse outcomes, theoretically by preventing or reversing de-afferentiation induced atrophy, restoring more normative brain activation patterns, and improving social engagement. There are emerging data to suggest that restoring auditory input (with hearing aids [HAs] or cochlear implants) may improve cognitive functioning, as naturalistic assessments of neuropsychiatric status before and after hearing treatment show improvement on short- and long-term global cognition, memory tasks, and social functioning.15–18 A large ongoing randomized controlled trial will definitively test the efficacy of an open HA (vs. successful aging health education) intervention on reducing cognitive decline in older adults with ARHL.19 One of the few existing studies targeting depressive symptoms compared hearing treatment to a wait list control group and reported increased self-reported quality of life and cognitive function as well as decreased depressive symptoms post HA prescription.20 However, wait list groups are in general weak controls that may result in an overestimation of treatment effects. Positive expectancies instilled by discernible changes in hearing are likely to lead to substantial placebo effects, and sham-controlled studies are particularly important to control for expectancy-related placebo effects in studies with depression outcomes.21,22 In addition, interpretation of these data is limited by most studies’ failure to select participants based on the presence of clinical depression, comprehensively assess cognition and depression, and measure HA compliance objectively.
Thus, there is a need for rigorously designed research to determine whether hearing remediation is effective for improving depressive symptoms and cognition. The goal of this pilot study was to test the feasibility of conducting a prospective, double blind, randomized clinical trial of full-amplification (active) vs. low-amplification (sham) HAs to treat comorbid ARHL and depression in outpatient older adults. We hypothesized that participants would comply with the experimental HA intervention (>8 hours/day device usage), that differential dropout rate would be <10% between groups, and that participants would correctly guess active vs. sham HA assignment at chance. We were also interested in obtaining preliminary information on whether treating hearing loss improves cognition and reduces depressive symptoms in this population.
METHODS
Participants
The study was conducted at the Otology and Neurotology Clinical Practice at Columbia University Medical Center (CUMC) and the Late Life Depression Research Clinic (LLDRC) at New York State Psychiatric Institute (NYSPI). It was approved by the NYSPI Institutional Review Board and registered on Clinicaltrials.gov as NCT03321006. Participants were recruited from clinicians at CUMC and through advertisements (e.g. flyers, local newspapers, CUMC RecruitMe website). All participants met eligibility criteria and signed informed consent to participate in the study.
Eligible participants were men and women ≥60 who met Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5)23 criteria for major or persistent depressive disorder, had a 24-item Hamilton Depression Rating Scale (HRSD)24 score ≥16, had mild to severe hearing loss with a pure-tone average (PTA) ≥30dB (average hearing threshold at 0.5, 1, 2, and 4 kHz), no HA use within the past 6 months, and were willing to and capable of providing informed consent and complying with study procedures. Participants were excluded if they had a history of psychosis, mania, bipolar disorder, substance use disorder within the past 12 months, or had current suicidal ideation. Other exclusion criteria included severe or unstable medical illness, significant retrocochlear pathology or organic lesion responsible for hearing loss, Mini-Mental State Examination score ≤ 24, or a diagnosis of probable Alzheimer’s disease, Vascular Dementia, or Parkinson’s Disease.
Study Design and Feasibility Measures
Participants were enrolled in a 12-week clinical trial in which they were randomly assigned to receive either active or sham HAs. The study statistician performed a computer-generated randomization schedule, which was delivered to the audiologists who provided each participant their HA devices. The audiologists were not blinded to the HA assignment but conducted identical procedures for active and sham HAs. Participants, treating clinicians, and depressive and cognitive outcome assessors were blinded to the HA assignment and did not have access to the randomization schedule. Active or sham HAs were added to psychiatric treatment as usual in a naturalistic study design. Based on a discussion of the clinical options and their preference, participants could continue their antidepressant medication if they were taking one, start a new medication, or participate in the study while off medications. Because this is a pilot study, feasibility, compliance and tolerability were considered the primary outcomes. Feasibility was measured by the participant attrition rate over the 12-week study, compliance was measured by median duration of HA use in hours/day, and tolerability of the study treatment was assessed using the Treatment Emergent Side Effect Scale.
Audiologic Procedures
Prior to HA fitting, all participants obtained audiological assessment performed by an audiologist at either CUMC or at an outside facility of their choosing. All audiological assessments were performed in a double walled IAC soundproof booth. Pure tone testing was performed using insert earphones and bone conducted stimuli, and pure tone average was measured as the average hearing threshold at 0.5, 1, 2, and 4 kHz in both ears. Speech reception thresholds were obtained in each ear using standard spondee words. Word recognition was assessed in each ear using recorded consonant-vowel nucleus-consonant type word list (25 words) at 40dB sound level above the participant’s speech reception threshold.
Following randomization, Phonak Audeo B-R90 (Sonova, Stafa, Switzerland) hearing devices were fit at Week 0 by an audiologist. Active and sham HAs were identical in appearance, battery use, and data logging capability. Sham HAs were programmed to a hearing threshold of 10dB across all frequencies, which resulted in a small but noticeable volume increase without substantively improving the ability to discriminate speech. The hearing gain of the active devices was determined by the audiometric profile as per standard clinical practice. To assess compliance with HAs, usage rates (hours/day) were measured using data log technology built into the devices25–26 and participants were informed that a minimum of 8 hours/day of HA usage is required to participate in the study. Participants returned for follow-up audiology visits at Weeks 2, 6, 9, and 12, which served to verify fitting and provide counseling. As all participants were new HA users, they were counseled on their hearing loss as well as proper use of the HA in order to achieve a high level of comfort with the devices.
Study Assessments
At evaluation, participants were screened for significant medical problems with a medical history and physical examination, laboratory screening, an electrocardiogram, urinalysis, and urine toxicology. Vital signs were monitored throughout the study. Structured Clinical Interview for DSM5 Disorders27 was performed to confirm participant eligibility. Participants then returned for six psychiatric follow-up appointments at the LLRDC over the 12-week trial. At each follow-up appointment (Weeks 0, 2, 4, 6, 9, and 12), participants met with a study clinician and research assistant at LLDRC. Follow-up visits are standardized in structure and duration (45 min total). During these visits, the research assistant greeted the participant and conducted clinical assessments (25 min) which included the 24-item HRSD, Treatment Emergent Side Effect Scale, Inventory of Depressive Symptoms—Self Report (IDS-SR)28, Social Adjustment Scale Self-Report29, and Blind Assessment—Patient Version (rates participant’s guess as to the HA group). At each follow-up visit, the study clinician also met the participant to review interval events and symptom change since the previous visit, inquired about compliance and tolerance of the HAs and study medications, and provided education about study progress and procedures (20 min.).
Neuropsychological and functional evaluation measures were also performed at the Weeks 0 and 12 follow-up visits. Neuropsychological assessment included the Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Populations (RBANS-H), which assesses five cognitive domains (Immediate Memory, Delayed Memory, Language, Attention, and Visuospatial/Constructional).30–33 The RBANS-H is appropriate for use in participants with hearing loss34, as the original RBANS requires instructions and stimuli to be presented auditorily. All components of the RBANS-H are simultaneously presented orally and in written format on an external computer monitor. Coverage of executive functioning was augmented with the NIH Toolbox (Flanker Inhibitory Control and Attention Test).35 The functional evaluation included Short Physical Performance Battery36 to provide measures of gait, balance, and lower extremity strength and the 36-item self-report World Health Organization Disability Assessment Schedule 2.0 (WHODAS)37 to provide a global measure of disability. The Hearing Handicap Inventory for the Elderly Screening Version (HHIE-S)38, a 10-item questionnaire developed to assess the social and emotional effects of hearing loss, was also administered at Weeks 0 and 12. Blinding was assessed at Week 12 as the proportion of participants who correctly guessed whether they were wearing active or sham devices. All participants were eligible for travel reimbursement at up to a $25 per visit. No protocol deviations occurred during the study.
Statistical Analyses
Baseline clinical and demographic characteristics of participants were summarized as medians and interquartile ranges [IQR] for continuous variables, and proportions for categorical variables. We chose to present the medians and IQRs, as opposed to the means and SDs, as given the potential for skewed continuous variables in our small sample size. Baseline characteristics were compared between the active and sham groups using nonparametric Mann-Whitney U tests for continuous measures and Fisher Exact test tests for categorical measures, both of which are appropriate for small sample sizes.
Between-group differences in duration of HA usage per day were tested using Mann-Whitney U tests at each visit. Attrition rates and the proportion of participants experiencing adverse events were compared between groups using Fisher Exact test tests. One sample, non-parametric, proportion tests were used to determine whether the proportions of participants in each treatment group who correctly guessed their treatment assignment were significantly different from 50%. Changes (from Week 0 to Week 12) in median clinical outcome scores were compared between groups using non-parametric Mann-Whitney U tests. Mann-Whitney U and Fisher Exact tests are non-parametric hypothesis tests that are appropriate for skewed variables and/or small sample sizes.
Additionally, we estimated the observed effect sizes for the clinical outcome scores. Since the estimates of means and SDs are not always reliable with such a small sample size, we computed a nonparametric effect size (np-ES; difference in medians divided by average IQR) between both groups. A clinically meaningful effect was defined as an np-ES of ≥0.2, with an np-ES ≥0.2 for a small effect, np-ES ≥0.5 for a moderate effect, and np-ES ≥0.8 for a large effect.
All results were produced using SAS® 9.4 and all statistical tests were two-sided with pre-selected level of significance of 5%.
RESULTS
Participant Disposition and Characteristics
Thirteen subjects participated in the 12-week trial (N=7 randomized to active and N=6 randomized to sham treatment, see Table 1). Participants in the active group were significantly younger (median [IQR] age was 66.2 [63.1 - 67.5] vs. 78.2 [70.8 – 85.4] years, p = 0.005), but there were no other differences found in baseline characteristics. Severity of hearing loss did not differ between active vs. sham groups (median [IQR] PTA was 48.1 dB [33.3 – 51.9] vs. 42.5 dB [40.6 – 53.1], respectively, p=.62).
Table 1:
Baseline Characteristics for the Hearing Aid Groups
| Active | Sham | p-value | |
|---|---|---|---|
| N = 7 | N = 6 | ||
| N (%) Median (IQR) |
N (%) Median (IQR) |
||
| Gender (Male) | 4 (57%) | 3 (50%) | 1.00 |
| Age | 66.2 (63.1 – 67.5) | 78.2 (70.8 – 85.4) | 0.005 |
| Education Years | 16 (12 – 18) | 14.5 (13 – 17) | 0.83 |
| Race/Ethnicity | 0.39 | ||
| White | 4 (57%) | 5 (83.3%) | |
| Asian | 1 (14%) | 0 | |
| Black/African-American | 1 (14%) | 1 (16.7%) | |
| Hispanic/Latino | 1 (14%) | 0 | |
| Antidepressant (AD) Group | 1.00 | ||
| +AD | 5 (71%) | 4 (67%) | |
| Started New AD | 3 (43%) | 3 (50%) | |
| Remained on AD | 2 (29%) | 1 (16.7%) | |
| No AD | 2 (29%) | 2 (33%) | |
| Depression | |||
| HRSD | 20 (19-26) | 18.5 (15-27) | 0.67 |
| IDS-SR | 30 (23 – 36) | 27.5 (23 – 30) | 0.62 |
| Cognition | |||
| MMSE | 28 (28 – 29) | 27 (27 – 27) | 0.21 |
| RBANS (Total) | 100 (84 – 110) | 86.5 (85 – 102) | 0.75 |
| General Functioning | |||
| WHODAS | 51 (43 – 57) | 48.5 (43 – 62) | 1.00 |
| SAS-SR | 2.9 (1.8 – 3.0) | 2.7 (1.9 – 3.0) | 0.94 |
| Hearing | |||
| HHIE-S | 34 (30 – 40) | 34 (26 – 36) | 0.47 |
| Pure Tone Average (PTA) | 48.1 (33.3 – 51.9) | 42.5 (40.6 – 53.1) | 0.62 |
Notes: HRSD = 24-item Hamilton Rating Scale for Depression; IDS-SR = Inventory of Depressive Symptomatology Self-Report; MMSE = Mini-Mental State Examination; WHODAS = WHO Disability Assessment Schedule 2.0; SAS-SR = Social Adjustment Scale Self-Report; RBANS (Total) = Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Populations; HHIE-S = Hearing Handicap for the Elderly Screening Version.
Feasibility Outcomes
Because this was a pilot study, feasibility, compliance and tolerability were considered the primary outcomes. Feasibility was measured by the participant attrition rate and as shown in Figure 1, all 13 randomized participants completed the 12-week study, resulting in zero dropout. As shown in Table 2, the median duration of HA use was > 9 hrs/day and not different for participants randomized to active vs. sham treatment using Mann-Whitney U tests (median use 10.9 vs. 10.5 [p=.55] at Week 2; median use 10.0 vs. 10.3 [p=.79] at Week 6; median use 9.7 vs. 11.4 [p=.28] at Week 9; median use 9.3 vs. 10.7 [p=.66] at week 12). Moreover, HA utilization (hrs/day) was not different for participants taking antidepressant medications compared with those who were not (Mann-Whitney U tests for participants taking vs. not taking ADs: median use 11.1 vs. 8.0 [p=.36] at Week 2; median use 10.0 vs. 12.5 [p=.29] at Week 6; median use 11.1 vs. 9.7 [p=.99] at Week 9; median use 10.7 vs. 10.7 [p=.74] at Week 12).
Figure 1:
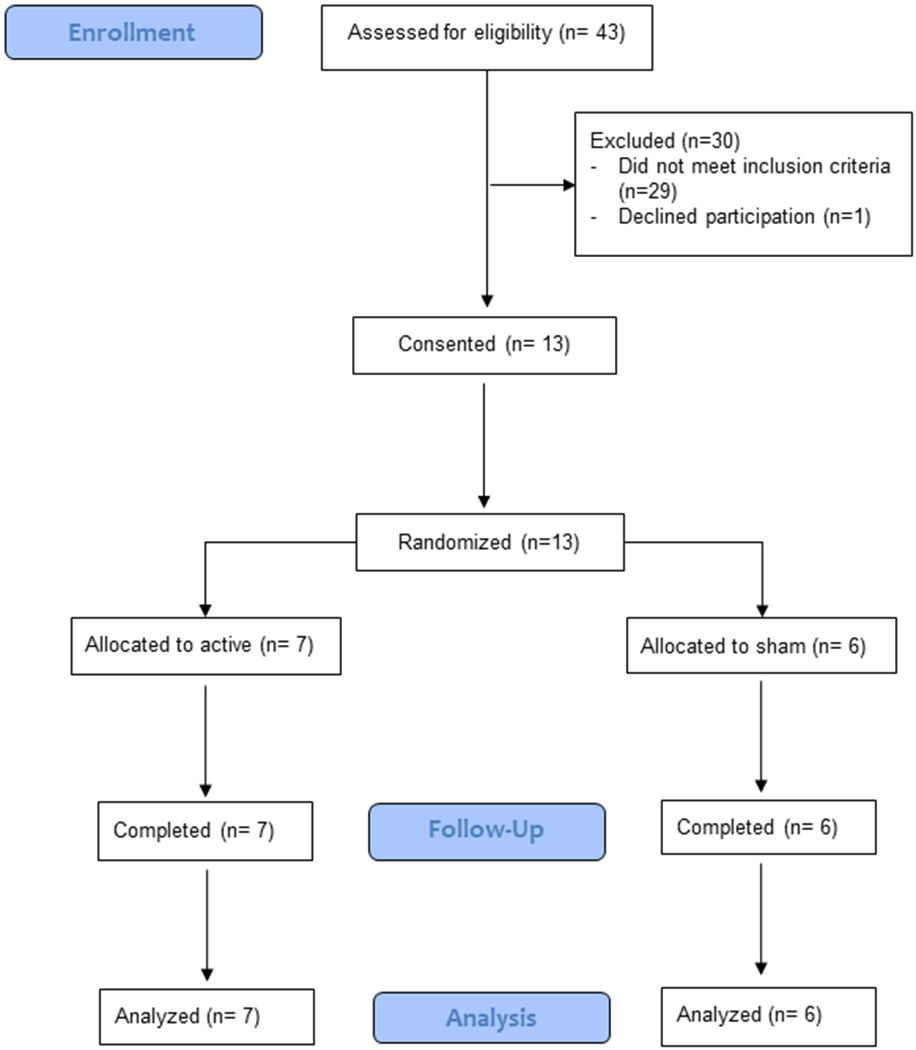
CONSORT trial flow diagram. Participant flow through each stage of the randomized controlled trial (enrollment, follow-up, and data analysis).
Table 2:
Duration of Use by Hearing Aid Group
| Week | Active Usage* | Sham Usage* | Difference |
|---|---|---|---|
| Median (IQR) Usage (N) | Median (IQR) Usage (N) | P-Value | |
| 2 | 10.9 (8.8-12.0), (5) | 10.5 (7.2 – 11.4), (5) | 0.55 |
| 6 | 10.0 (9.4 – 12.5), (6) | 10.3 (10.0-10.3), (5) | 0.79 |
| 9 | 9.7 (8.3-11.5), (7) | 11.4 (11.1-12.0), (5) | 0.28 |
| 12 | 9.3 (6.3-12.8), (7) | 10.7 (10.7-12.0), (3) | 0.66 |
Median usage as measured in hours of usage per day. N = sample size varies by week as participants may have missed audiology follow-up appointments. Difference in HA usage between groups is calculated with Mann-Whitney U test.
Blinding was evaluated as the proportion of participants who correctly guessed whether they were wearing active or sham devices at Week 12. Rather than the anticipated proportion of 50% if full blinding were in effect, 86% of the active (p=.126, Binomial Exact test) and 83% of the sham (p=.22, Binomial Exact test) HA group guessed their treatment assignment correctly at Week 12. Tolerability was measured by treatment side effects experienced by participants and were primarily related to the antidepressant medication. They included dry mouth (3 mild, 2 severe), insomnia (1 mild, 1 severe), and drowsiness (2 severe), and the number of occurrences did not differ between HA groups.
Neuropsychiatric Outcomes
While the feasibility results served as our primary outcome measures, we were also interested in obtaining preliminary information about neuropsychiatric outcomes. As shown in Table 3 and Figure 2, provision of active HAs resulted in clinically meaningful improvement in the social and emotional effects of hearing loss as measured by the HHIE-S (np-ES=.62). Clinically meaningful improvement in depressive symptoms favoring active treatment was observed on the IDS-SR (np-ES=.31). In terms of cognitive outcomes, differential numerical improvement favoring active HAs was observed on tasks testing Immediate Memory (np-ES=.25). In contrast, HRSD (np-ES=0), Delayed Memory (np-ES=.18), Language (np-ES=.06), Executive Function (Flanker task: np-ES=.33), Visuospatial/Constructional Ability (np-ES=.60) and Attention (np-ES=.16) did not demonstrate improvement. Clinically meaningful effect sizes were observed in functional improvement measured by the WHODAS (np-ES=.53), and in magnitude of social functioning measured by the SAS-SR (np-ES=.33). No clinically meaningful improvement was observed in physical functioning measured by the SPPB (np-ES=0). These improvements in hearing, depressive symptoms, immediate memory, and general functioning resulted in clinically meaningful adaptive changes.
Table 3:
Pre-Post Changes in Clinical Outcomes between Hearing Aid Groups
| Active | Sham | *np-ES | |
|---|---|---|---|
| N = 7 | N = 6 | ||
| Median (IQR) | Median (IQR) | ||
| Hearing | |||
| HHIE-S | −14.0 (−26 to −4) | −6.0 (−8 to −4) | .62 |
| Depression | |||
| IDS-SR | −8.0 (−17 to −6) | −3.5 (−16 to 2) | .31 |
| HRSD | −5.0 (−16 to −3) | −5.0 (−12 to 7) | .00 |
| Cognition | |||
| RBANS – Immediate Memory | +19.5 (12 to 26) | +16.0 (15 to 29) | .25 |
| RBANS – Delayed Memory | +7.5 (1 to 11) | +5.5 (0 to 12) | .18 |
| RBANS – Attention | +4.5 ( −9 to 9) | +1.5 (−7 to 12) | .16 |
| RBANS – Visuospatial/Constructional | −6.0 ( −19 to −2) | +6.0 (0 to 23) | .60 |
| RBANS – Language | +9.0 (−4 to 25) | +7.5 (−9 to 16) | .06 |
| Flanker – Executive Function | 0 (−1 to 0) | −0.5 (−2 to 0) | .33 |
| General Functioning | |||
| WHODAS | −25.0 (−30 to −8) | −9.5 (−42 to −6) | .53 |
| SAS-SR | −0.32 (−0.83 to −0.09) | −0.10 (−0.67 to −0.03) | .33 |
| SPPB | 1.0 (0 to 2) | 1.0 (0 to 2) | .00 |
Notes: Week 0 (pre-) to Week 12 (post-) change in median clinical outcome scores. HHIE-S = Hearing Handicap for the Elderly Screening Version; IDS-SR = Inventory of Depressive Symptomatology Self-Report; HRSD = 24-item Hamilton Rating Scale for Depression; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Populations; Flanker = Flanker Inhibitory Control and Attention Test from the NIH toolbox; WHODAS = World Health Organization Disability Assessment Schedule 2.0; SAS-SR = Social Adjustment Scale Self-Report; SPPB = Short Physical Performance Battery.
np-ES = nonparametric effect size
Figure 2:
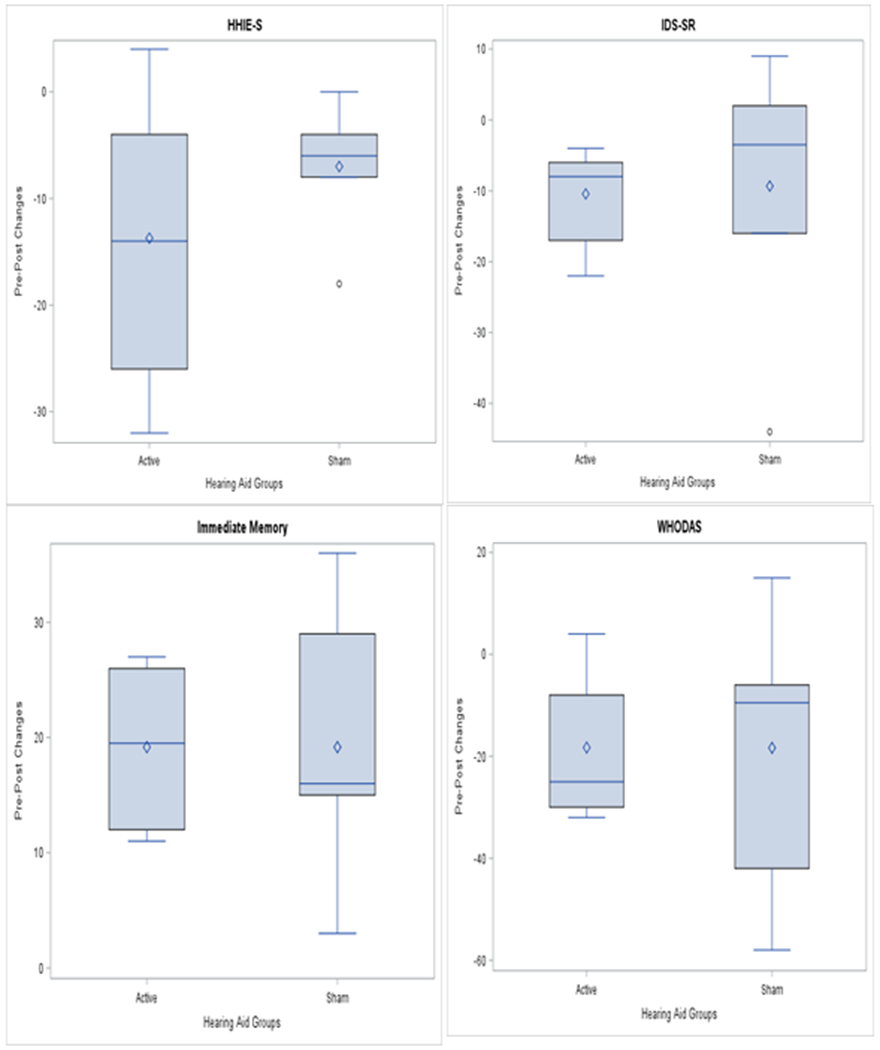
Pre-Post Changes in Clinical Outcomes between Hearing Aid Groups.
Notes: Week 0 (pre-) to Week 12 (post-) change in median clinical outcome scores. HHIE-S = Hearing Handicap for the Elderly Screening Version; IDS-SR = Inventory of Depressive Symptomatology Self-Report; WHODAS = World Health Organization Disability Assessment Schedule 2.0.
DISCUSSION
The primary finding in this study was a double-blind randomized pilot trial of HAs as a treatment for depression and cognitive decline secondary to age-related hearing loss was highly acceptable to participants. As opposed to what is typically observed in clinical treatment with HAs39, compliance in this study was exceedingly high, with a median usage rate of > 9 hours/day in each group that was maintained through the study, and there was zero dropout over 12 weeks. We observed small to moderate effect size improvements favoring active treatment over sham in our preliminary data on self-reported depressive symptoms, immediate memory, and both hearing-related as well as general functioning.
Given that the current treatments for both late-life depression and cognitive impairment are significantly limited in efficacy, ARHL merits further empirical attention as a causal and/or precipitating factor for the development of dementia and depression. Data from this study suggest that larger prospective randomized controlled studies of HAs for older adults with comorbid ARHL and late-life depression are feasible and that a signal of effect may exist. Our research group is currently undertaking a larger National Institute on Aging funded R21 trial of individuals with comorbid late-life depression and ARHL, and we are incorporating rigorous methodology such as compressive neuropsychiatric assessments, objective measures of HA compliance, and randomization. To obtain data on the mechanisms linking ARHL to neuropsychiatric outcomes, multimodal neuroimaging has been incorporated into this larger study.
Concealment of treatment allocation (to active or sham HAs) was incomplete in this study, as many individuals correctly guessed their treatment assignment. While blinding for a treatment with immediately apparent subjective effects such as HAs is challenging, controlling for expectancy-related placebo effects is important for studies with depression outcomes.21,22 It is possible that providing more amplification to the sham HA group may improve blinding, though the degree of amplification must be balanced against dilution of a signal for active vs. sham treatment. For example, the provision of even a 10dB gain provided in the sham HA in our study may provide significant benefit to participants depending on the shape of hearing loss, especially if an individual’s hearing threshold is just under “normal” for important frequencies such as that of soft consonants (i.e. 2000-4000 Hz). The magnitude and audiologic characteristics of a given individual’s hearing loss are important factors and something to consider in future studies, as individuals with mild ARHL may observe a large hearing benefit from a small volume increase that would not be noticeable to individuals with severe ARHL. To improve methods of blinding in such studies, we may consider increasing the amplification to the sham HA device (e.g. to a 30dB gain) and selectively including participants with at least moderate hearing loss (e.g. PTA ≥50dB).
The findings of our study must be interpreted in light of several limitations. The small sample size achieved in this pilot study limited our ability to calculate accurate effect sizes and yielded differences between the active and sham HA groups that were unreliable. Second, the naturalistic study design limits the specific interpretations that can be made regarding the therapeutic value of HAs for depression. Nearly half of the participants in each treatment arm started a new antidepressant treatment, and while the rates of treatment initiation were not different between groups, this may have contributed to the symptomatic and functional benefits observed. Additionally, the sham HA group was significantly older, which may have influenced the depressive and cognitive outcomes. Finally, blinding of treatment assignment failed for study participants, so differential placebo effects operative between the active and sham conditions may have contributed to the results observed.
CONCLUSION
In summary, data from this first study of its kind suggest that rigorously designed clinical trials to test the efficacy of hearing treatment for depression and cognitive decline in older adults are possible. Given the promising benefits observed with active HAs vs. sham, larger studies that will be powered to determine whether hearing remediation may be an effective therapeutic strategy for late-life depression and cognitive impairment are necessary. Should future studies prove to be successful, this suggests a novel therapeutic strategy for late-life depression and cognitive impairment and may thereby mitigate their public health burden, while also contributing to the increased recognition and treatment of ARHL more generally.
Key Points:
Age-related hearing loss is a prevalent condition that has been associated with the development of significant neuropsychiatric dysfunction, but there is a need for rigorously designed research to determine whether hearing remediation is effective for improving depressive symptoms and cognition.
We found that a double-blind sham-controlled pilot trial of hearing aids as a treatment for depression and cognitive decline was highly acceptable to participants, and we observed small to moderate improvements favoring active treatment for depressive symptoms and memory performance.
Given the promising benefits we observed in our study, should larger studies of hearing remediation prove to be successful, this suggests a novel therapeutic strategy for late-life depression and cognitive impairment.
ACKNOWLEDGEMENTS
This research was supported by Irving Institute at Columbia University as a Phase I and Phase II Collaborative and Multidisciplinary Pilot Research Planning Grant [PI Rutherford]. It was also supported by the National Institute of Mental Health (R25 MH086466 [PI Arbuckle]). Drs. Brewster, Rutherford, Brown, Kim, Roose, Pavlicova, Brickman as well as Ms. Mei Chen, Chen Chen, Stein, and Galatioto have no disclosures to report. Ms. Kuhlmey serves on the advisory board at Med El (cochlear implant company). Dr. Golub has received consulting fees and travel stipends to attend industry conferences for Advanced Bionics, Oticon Medical, and Cochlear (cochlear implant companies); Auditory Insight (a consulting company); and has received unrestricted educational grants from Storz, Stryker, Acclarent, and 3NT (medical device companies). The Phonak Audeo B-R90 hearing devices utilized by the study were donated by Phonak.
Footnotes
Data Availability Statement: The data that support the findings of this study are available on request from the corresponding author, [KKB]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
REFERENCES
- 1.Collins JG: Prevalence of selected chronic conditions: United States 1990–1992. Vital Health Statist 10 1997; 194:1–89. [PubMed] [Google Scholar]
- 2.Goman AM, Lin FR: Prevalence of Hearing Loss by Severity in the United States. Am J Public Health 2016; 106(10):1820–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper JC, Gates GA: Hearing in the elderly—the Framingham cohort, 1983–1985: Part II. Prevalence of central auditory processing disorders. Ear Hear 1991;12(5):304–11. [DOI] [PubMed] [Google Scholar]
- 4.Reed NS, Altan A, Deal JA, et al. Trends in Health Care Costs and Utilization Associated With Untreated Hearing Loss Over 10 Years. JAMA Otolaryngol Head Neck Surg 2019; 145(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamil RJ, Betz J, Powers BB, et al. Association of Hearing Impairment with Incident Frailty and Falls in Older Adults. J Aging Health 2016; 28:644–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 2014; 150:378–384. [DOI] [PubMed] [Google Scholar]
- 7.Lin FR, Yaffe K, Xia J, et al. Hearing Loss and Cognitive Decline Among Older Adults. JAMA Intern Med 2013; 173:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol 2011; 68:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panza F, Solfrizzi V, Logroscino G. Age-related hearing impairment—a risk factor and frailty marker for dementia and AD. Nat Rev Neurol 2015; 11:166–175. [DOI] [PubMed] [Google Scholar]
- 10.Taljaard DS, Olaithe M, Brennan-Jones CG, Eikelboom RH, Bucks RS. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol 2016; 41:718–729. [DOI] [PubMed] [Google Scholar]
- 11.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers & Dement 2015; 11:70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewster KK, Ciarleglio A, Brown PJ, et al. Age-Related Hearing Loss and Its Association with Depression in Later Life. Am J Geriatr Psychiatry 2018;26:788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golub JS, Brewster KK, Brickman AM, et al. Association of Audiometric Age-Related Hearing Loss With Depressive Symptoms Among Hispanic Individuals. JAMA Otolaryngol Head Neck Surg 2019; 145(2):132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutherford BR, Brewster K, Golub JS et al. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am J Psychiatry 2018;175:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein BE, Sirow LW, Moser S. Relating hearing aid use to social and emotional loneliness in older adults. Am J Audiol 2016;25:54–61. [DOI] [PubMed] [Google Scholar]
- 16.Contrera KJ, Sung YK, Betz J et al. : Change in loneliness after intervention with cochlear implants or hearing aids. Laryngoscope 2017;127(8):1885–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JS, Betz J, Li L, et al. : Association of Using Hearing Aids or Cochlear Implants With Changes in Depressive Symptoms in Older Adults. JAMA Otolaryngol Head Neck Surg 2016; 142:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acar B, Yurekli MF, Babademez MA, et al. : Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr 2011; 52:250–252. [DOI] [PubMed] [Google Scholar]
- 19.Deal JA, Albert MS, Arnold M, et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: Results from the Aging and Cognitive Health Evaluation in Elders Pilot Study. Alzheimers Dement 2 2017;3(3):410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulrow CD, Aguilar C, Endicott JE, et al. : Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med 1990; 113:188–194. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford BR, Wall MM, Brown PJ et al. Patient Expectancy as a Mediator of Placebo Effects in Antidepressant Clinical Trials. Am J Psychiatry 2017;174(2):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutherford BR, Wall MM, Glass A, Stewart JW. The role of patient expectancy in placebo and nocebo effects in antidepressant trials. J Clin Psychiatry 2014;75(10):1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th edition Washington, DC, American Psychiatric Publishing, 2013. [Google Scholar]
- 24.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez E, Edmonds BA. A systematic review of studies measuring and reporting hearing aid usage in older adults since 1999: a descriptive summary of measurement tools. PLoS One. 2012; 7:e31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle JB, Raghunathan R, Cellum I, Li G, Golub JS. Longitudinal tracking and prediction of sound exposure and usage in hearing aid wearers using objective data logs American Otological Society at COSM. April 2017. San Diego, CA. [Google Scholar]
- 27.First MB, Williams JB, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-V Disorders, Clinician Version (SCID-5-CV). Washington, DC, American Psychiatric Publishing, 2016. [Google Scholar]
- 28.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger JE, Burns CT. The Inventory of Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Res 1986; 18:65–87. [DOI] [PubMed] [Google Scholar]
- 29.Weissman MM. Social adjustment scale –self-report technical manual. Toronto, OT: Multi-Health Systems, 1999. [Google Scholar]
- 30.Randolph C RBANS Update: Repeatable Battery for the Assessment of Neuropsychological Status. Bloomington, MN: NCS Pearson, 2012. [Google Scholar]
- 31.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998; 20:310–319. [DOI] [PubMed] [Google Scholar]
- 32.Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable Battery for the Assessment of Neuropsychological Status as a Screening Test in Schizophrenia, I: Sensitivity, Reliability, and Validity. Am J Psychiatry 1999; 156:1944–1950. [DOI] [PubMed] [Google Scholar]
- 33.Garcia C, Leahy B, Corradi K, Forchetti C. Component Structure of the Repeatable Battery for the Assessment of Neuropsychological Status in dementia. Arch Clin Neuropsychol 2008; 23:63–72. [DOI] [PubMed] [Google Scholar]
- 34.Claes AJ, Mertens G, Gilles A, et al. The Repeatable Battery for the Assessment of Neuropsychological Status for Hearing-Impaired Individuals (RBANS-H) before and after Cochlear Implantation: A Protocol for a Prospective, Longitudinal Cohort Study. Front Neurosci 2016; 10:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institutes of Health Toolbox Cognition Battery (NIH Toolbox CB). Monographs Society Research Child Devel 2013; 78:1–172. [DOI] [PubMed] [Google Scholar]
- 36.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49:M85–94. [DOI] [PubMed] [Google Scholar]
- 37.Ustun TB, Kostanjsek N, Chatterji S, et al. Measuring Health and Disability: Manual for Who Disability Assessment Schedule (Whodas 2.0). Switzerland: World Health Organization, 2010. [Google Scholar]
- 38.Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA 1983; 25:37–42. [PubMed] [Google Scholar]
- 39.Salonen J, Johansson R, Karjalainen S, Vahlberg T, Jero JP, Isoaho R. Hearing aid compliance in the elderly. B-ENT 2013;9(1):23–8. [PubMed] [Google Scholar]


