Abstract
Soy foods are known to be effective for breast cancer prevention. The habitual consumption of soy isoflavones in combination with the probiotic Lactobacillus casei Shirota (LcS) was shown to decrease the risk of breast cancer occurrence in our previous population‐based case‐controlled study among Japanese women. The present study aimed to elucidate the cooperative prevention mechanism of soymilk and LcS using an animal carcinogenic model. Female Sprague–Dawley rats received a high‐fat, AIN‐76A diet containing soymilk, LcS, both soymilk and LcS, or none and were orally exposed to 2‐amino‐1‐methyl‐6‐penylimidazo[4,5‐b]pyridine at a dose of 85 mg/kg bodyweight eight times for 2 weeks. The development of palpable mammary tumors was monitored for 17 weeks. Tumor tissues were immunohistochemically examined for estrogen receptor (ER)‐α, Ki‐67 and CD34. Compared with the control group, the incidence and multiplicity of mammary tumors were reduced by soymilk alone and soymilk in combination with LcS, while tumor volume was decreased by LcS alone and LcS in combination with soymilk. An immunohistochemical analysis revealed that soymilk in combination with LcS more effectively reduced the numbers of ER‐α‐positive and Ki‐67‐positive cells in tumors than soymilk alone and that both soymilk and LcS inhibited tumor angiogenesis. These results demonstrated that soymilk prevents the development of mammary tumors and that LcS suppresses tumor growth, potentially enhancing the preventive efficacy of soymilk. The habitual consumption of LcS in combination with soymilk might be a beneficial dietary style for breast cancer prevention.
Breast cancer is the most common malignancy among women and its incidence and associated mortality rate are increasing worldwide.1 In particular, its prevalence in Asian countries, which was lower in the past, has been rapidly increasing during the past few decades.2, 3 The most likely reason is assumed to be a change in diet from traditional Asian diet to Western diet, and a decreased amount of exercise might also be a concern. A large number of epidemiological studies have shown that the daily consumption of phytochemicals can prevent breast cancer.4 In particular, soy isoflavones have been widely studied and the habitual consumption of soy foods such as soymilk has been shown to reduce the risk of breast cancer.5 The mechanism responsible for the prevention of breast cancer by soy isoflavones, especially genistein, is known to involve phytoestrogenic activity, the inhibition of oncogenic tyrosine kinase and antiangiogenic, anti‐oxidative and anti‐inflammatory actions.6, 7, 8, 9, 10 The prevention of breast cancer by soy isoflavones has also been reviewed in the guidelines for cancer prevention published by the American Cancer Society.11
Probiotics are defined as live microorganisms that are known to be beneficial to host organisms. Some probiotic strains have been reported to prevent cancer in animal models through various modes of action, such as modulation of the immune system and/or the generation of biologically active metabolites in the intestine.12, 13 Lactobacillus casei Shirota (LcS; YIT 9029), a probiotic strain, is known to prevent cancer. In animal studies, the administration of LcS retarded experimentally induced carcinogenesis in several models.14, 15, 16, 17, 18, 19 In human studies, LcS prevented bladder cancer20, 21, 22 and suppressed the development of colorectal cancer.23 One of the presumed mechanisms for cancer prevention is that LcS augments immunopotentiative activities, such as those of natural killer (NK) cells, to induce cytotoxic effects against tumor cells through the stimulation of various cytokine productions. In addition, through the modification of intestinal microflora, LcS can increase the production of substances useful for colon homeostasis and can reduce the uptake of mutagenic substances derived from ingested foods.24, 25, 26
To investigate the effect of the consumption of soy isoflavones and LcS on the occurrence of breast cancer, a population‐based case‐controlled study among Japanese women, consisting of 316 cases with breast cancer and 662 controls, was conducted.27 This study indicated that the quantity of habitually consumed soy isoflavones and LcS since adolescence was closely associated with a risk reduction for breast cancer and that the simultaneous consumption of these products was especially effective.27 The reason why the simultaneous ingestion of soy isoflavones and LcS was more effective for the prevention of breast cancer than the ingestion of each component alone was unknown.
The present study aimed to investigate the preventive effects of soymilk, LcS and the combination of both on the development and growth of mammary tumors and to elucidate the underlying mechanism using a chemically induced mammary tumor model in rats.
Materials and Methods
Preparation of soymilk and LcS powder
Soymilk, purchased from Shikokukakoki (Tokushima, Japan), was lyophilized to prepare soymilk powder. The LcS, obtained from Yakult Honsha Co., Ltd. (Tokyo, Japan), was cultured in Lactobacillus MRS broth medium (Becton Dickinson and Company, Sparks, MD, USA) and lyophilized to prepare LcS powder.
Mammary carcinogenesis experiments in rats
All in vivo experimental protocols were approved by the animal care committee of the animal facility. Four‐week‐old female Sprague–Dawley rats (CLEA Japan, Tokyo, Japan) were maintained in individual cages in an air‐conditioned room with a 12‐h light/dark cycle; the rats were allowed free access to an AIN‐76A‐based modified high‐fat diet (CLEA Japan) and sterilized water. According to the protocol of a previous study,28 after a 1‐week acclimatization period, the rats were randomized into four experimental groups of 42 rats and one vehicle group of 15 rats. Thereafter, the rats' diet was changed to an experimental diet composed of a high‐fat basal diet containing soymilk, LcS, both soymilk and LcS, or none. The composition of the diets is shown in Table 1. The amounts of soymilk and LcS powder were determined based on the amounts used in previous studies17, 28 and the nutritional composition of each experimental diet containing soymilk and/or LcS powder was isocalorically adjusted to that of the control diet. Each experimental diet was replaced once a week with fresh chow that had been stored at 4°C to ensure the consumption of active isoflavones and LcS. The stability of the dietary isoflavones under these conditions was confirmed by a liquid chromatography‐mass spectrometry analysis.29 The performance status of the rats was monitored daily and their bodyweights were recorded once a week throughout the study period.
Table 1.
Composition of each experimental diet
| Ingredient | Amount (g/kg of chow) | |||
|---|---|---|---|---|
| Basal diet | Soymilk† | LcS‡ | Soymilk + LcS†, ‡ | |
| Casein | 235.0 | 191.2 | 233.0 | 189.2 |
| DL‐Methionine | 3.5 | 3.5 | 3.5 | 3.5 |
| Corn starch | 95.0 | 95.0 | 95.0 | 95.0 |
| Sucrose | 317.0 | 284.6 | 317.0 | 284.6 |
| Cellulose | 59.0 | 59.0 | 59.0 | 59.0 |
| Corn oil | 235.2 | 211.4 | 235.2 | 211.4 |
| Salt mixture | 41.1 | 41.1 | 41.1 | 41.1 |
| Vitamin mixture | 11.8 | 11.8 | 11.8 | 11.8 |
| Choline bitartrate | 2.4 | 2.4 | 2.4 | 2.4 |
| Soymilk powder | – | 100.0 | – | 100.0 |
| LcS powder | – | – | 2.0 | 2.0 |
†Amount of total isoflavones is 335 mg/kg of chow. ‡Lactobacillus casei Shirota (LcS) is including 2 × 1011 colony forming unit/kg of chow.
One week after the start of the experimental diets (Week 1), the chemical carcinogen 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP; Nard Institute Ltd, Osaka, Japan) was dissolved in ultrapure water and administered through a gastric tube to rats at a dose of 85 mg/kg bodyweight four times a week for 2 weeks.28, 30, 31 The vehicle was similarly administered to rats in the vehicle group. The development of mammary tumors was detected by palpation to determine tumor incidence and multiplicity. The length, width and height of each tumor were measured with calipers and the tumor volume was calculated using the following formula: tumor volume = (length) × (width) × (height) × π/6.32
At Week 17, all rats were killed under ether anesthesia after having fasted for 17 h. Blood was collected from the ventral aorta into a tube containing EDTA‐2K to prepare the plasma. The concentrations of glucose, total and free cholesterol and triglyceride in the plasma were measured using the Accute TBA‐40FR system (Toshiba Medical Systems, Tochigi, Japan) according to the manufacturer's protocol. All mammary glands were then removed and immediately fixed with 10% buffered formalin and stained with hematoxylin and eosin (H&E) for histopathological examination to diagnose the mammary tumors.33
Immunohistochemical analysis
Sections of mammary tumor, which were treated with 0.3% hydrogen peroxide in methanol to block endogenous peroxidase, immunostained with anti‐estrogen receptor (ER)‐α mouse monoclonal antibody (Daco Japan, Tokyo, Japan), anti‐Ki‐67 rabbit monoclonal antibody (Thermo Fisher Scientific Inc., Rockford, IL, USA) or anti‐CD34 goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), respectively. The sections were incubated with biotinylated secondary antibody and labeled streptavidin biotinylated antibody (Histofine Simple Stain MAX PO Kit; Nichirei Bioscience, Tokyo, Japan) according to the manufacturer's protocol. The sections were visualized with diaminobenzidine, counterstained with hematoxylin and captured using a BX51 (Olympus, Tokyo, Japan). The ER‐α‐expressing and Ki‐67‐expressing cells were counted to calculate the average ratio of the cell population among 500 tumor cells (five non‐overlapping representative fields × 100 cells). Each tumor was classified according to the ratio of ER‐α‐expressing cells or the Ki‐67 labeling index (LI).34 The microvessel density (MVD) in each tumor was defined as the mean number of CD34‐expressing vessels per field.
Statistical analysis
All data were expressed as the mean ± standard error (SE), as indicated in each figure and table. The tumor incidence was analyzed using the χ2 test. Tumor multiplicity, volume and diameter of each experimental group were analyzed using the Welch t‐test relative to the control group and the multiply was adjusted using the permutation test. The average grade score of the Ki‐67 LI was analyzed using the Wilcoxon test. Other data were analyzed using a one‐way anova followed by the Dunnett test. A P value of <0.05 was regarded as statistically significant.
The time‐course changes in multiplicity and diameter compensated for by the time lag in the period for the development of individual tumors were examined using a mixed model, with the rat as the random intercept fitted to estimate the changes in tumor multiplicity and diameter. The empirical SE was used for each coefficient. The interaction terms for soymilk and LcS were conducted using a 2 × 2 factorial design and were fitted to the mixed model; however, if the interaction terms were not significant, a reexamination was conducted using a mixed model without interaction terms. These analyses were performed by a contracted research organization for statistical analysis (Statcom Co. Ltd., Tokyo, Japan).
Results
Inhibition of mammary carcinogenesis in PhIP‐exposed rats
To examine the preventive effect of the dietary administration of soymilk and LcS on mammary carcinogenesis, female rats were exposed to PhIP to induce mammary tumors and were fed a diet including soymilk and/or LcS during the study period. Palpable mammary tumors were monitored until Week 17. Time‐course changes in tumor incidence and multiplicity are shown in Figure 1(a,b), respectively. The tumor incidences in the soymilk and soymilk plus LcS groups were lower than that in the control group throughout the experimental period. The suppression of tumor incidence in the soymilk group was statistically significant at Weeks 14 (P = 0.048) and 16 (P = 0.049), respectively (Fig. 1a). Moreover, tumor multiplicities were also greatly reduced in the soymilk and soymilk plus LcS groups compared with the control group (Fig. 1b). Thus, the administration of soymilk appeared to delay the development of mammary tumors.
Figure 1.
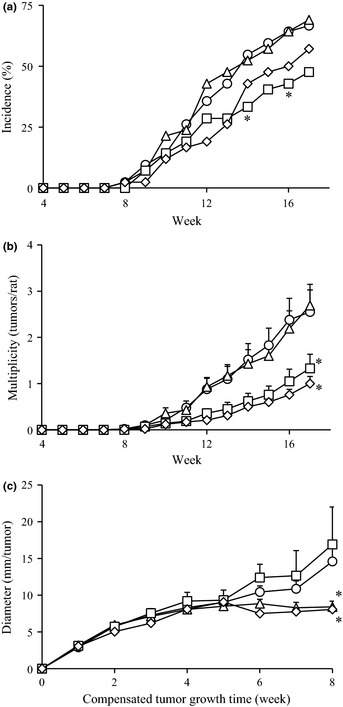
Time‐course of palpable mammary tumors in 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP)‐exposed rats. Female rats were exposed to PhIP and fed a high‐fat diet containing soymilk, LcS, both soymilk and LcS, or none. Palpable tumors were monitored for 17 weeks. (a) Tumor incidence (percentage of rats with tumors), (b) tumor multiplicity (average number of palpable tumors per rat) and (c) tumor diameter, defined as the cubic root of the tumor volume, adjusted according to the period of individual tumor development. Circle, control; square, soymilk; triangle, LcS; and rhombus, soymilk plus LcS group. In the multiplicity analysis, the asterisk is described only at Week 17. *P < 0.05.
In the histological examination of the mammary glands using H&E staining, most of the tumors were diagnosed as adenocarcinoma, except for three cases of fibroadenoma. Atypical cells were observed in most of the tissues; however, numerical differences were not detected among the groups. Based on the adenocarcinoma data, the incidence, multiplicity and volume of the mammary tumors at the end of the experiment are shown in Table 2. The incidences and multiplicities of the mammary tumors in the soymilk and soymilk plus LcS groups were lower than that of the control group. In particular, the tumor multiplicity in the soymilk plus LcS group was less than half the value in the control group (P = 0.014). Also, the tumor volumes in the LcS and soymilk plus LcS groups were lower than that of the control group. The results suggest that the administration of LcS and soymilk plus LcS may suppress tumor growth.
Table 2.
Incidence, multiplicity and volume of mammary tumors in PhIP‐exposed rats at the end of the experiment
| Group | Incidence (%)† | Multiplicity (tumors/rat)‡ | Volume (cm3/tumor)§ |
|---|---|---|---|
| Control | 73.8 (31/42) | 2.7 ± 0.5 | 1.3 ± 0.2 |
| Soymilk | 59.5 (25/42) | 1.6 ± 0.3 | 2.5 ± 1.1 |
| LcS | 73.8 (31/42) | 3.0 ± 0.5 | 0.8 ± 0.1 |
| Soymilk + LcS | 59.5 (25/42) | 1.2 ± 0.2* | 0.8 ± 0.2 |
*P < 0.05. †Number of rats with mammary tumors per effective number of rats. ‡Number of mammary tumors per rat. §Tumor volume was calculated using the following formula: tumor volume = (length) × (width) × (height) × π/6. PhIP, 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine.
Effect on development and growth of mammary tumors
One of the advantages of a chemically induced model is mimicking the alteration process of the cell from normal to cancer; however, the period until tumor formation is different in each individual tumor. Therefore, compensation for the period of individual tumor formation was performed to analyze tumor growth precisely. The time of tumor occurrence was defined as the first week using the compensated time scale and the time‐course change in tumor diameter was calculated until the eighth week, as shown in Figure 1(c). Tumor growth was significantly suppressed in the LcS and soymilk plus LcS groups after the fifth week.
To further evaluate the effect of the administration of soymilk and LcS on tumor development and growth and their interactions, the time‐course changes in tumor multiplicity and diameter were analyzed using the statistical mixed model. The time‐course changes in multiplicity were fitted into the statistical model and then the effect of soymilk and LcS on the rate of increase in the multiplicity was estimated. The intercepts and rates of change in the interaction terms between soymilk and LcS were not significant (data not shown). The rate of increase in the multiplicity was 0.135 tumors/rat per week in the soymilk group compared with 0.309 tumors/rat per week in the absence of soymilk, demonstrating that the administration of soymilk significantly inhibited tumor development (P < 0.001; Table 3). In the analysis of the time‐course changes in tumor diameter, the intercept and rates of change in the interaction term were not significant (data not shown). The rate of increase in tumor diameter was 0.957 mm/tumor per week in the LcS group compared with 1.457 mm/tumor per week in the absence of LcS, demonstrating that the administration of LcS significantly suppressed tumor growth (P = 0.003; Table 3). These findings highlight the different mode to prevent mammary tumors, that is, soymilk inhibited development and LcS suppressed growth.
Table 3.
Inhibitory effect on the development and growth of mammary tumors in a mixed model analysis
| Effect | Point estimation | SE | 95% confidence interval | P‐value |
|---|---|---|---|---|
| Tumor development | ||||
| Intercept | 0.309 | 0.047 | ||
| Soymilk | −0.174 | 0.043 | −0.258 to −0.090 | <0.001 |
| LcS | −0.027 | 0.043 | −0.112 to 0.057 | 0.524 |
| Tumor growth | ||||
| Intercept | 1.457 | 0.131 | ||
| Soymilk | 0.119 | 0.246 | −0.363 to 0.601 | 0.628 |
| LcS | −0.500 | 0.170 | −0.835 to −0.166 | 0.003 |
LcS, Lactobacillus casei Shirota; SE, standard error.
Effect on the body, liver and spleen weights in PhIP‐exposed rats
Severe toxicity was not observed in any of the experimental diets. Table 4 shows the bodyweight and relative weights of the liver and spleen per bodyweight at Week 17. The bodyweights were lower in the soymilk and soymilk + LcS groups than in the control group. However, the physical appearances, behavior, blood examination results and other activities were normal in each group, as assessed using standard veterinary criteria. The bodyweight ratio of the liver was significantly lower in the soymilk plus LcS group than in the control group, but the gross pathology at necropsy and the plasma aspartate transaminase and alanine transaminase levels were normal (data not shown). The weight of the spleen was increased by tumor formation and the bodyweight ratio of the spleen was shown as 0.136 ± 0.004% in the vehicle group (P = 0.060, compared with the control group). The ratio was lower in the LcS and soymilk plus LcS groups than in the control group, suggesting the possibility that splenomegaly associated with an increased tumor burden was restored by the administration of LcS.
Table 4.
Bodyweight and relative organ weight in PhIP‐exposed rats at Week 17
| Group | Bodyweight (g) | Organ to bodyweight (%) | |
|---|---|---|---|
| Liver | Spleen | ||
| Control | 428.5 ± 6.7 | 2.70 ± 0.03 | 0.206 ± 0.020 |
| Soymilk | 366.4 ± 6.0** | 2.62 ± 0.03 | 0.213 ± 0.018 |
| LcS | 433.8 ± 7.6 | 2.67 ± 0.02 | 0.158 ± 0.002 |
| Soymilk + LcS | 374.0 ± 6.8* | 2.51 ± 0.02** | 0.161 ± 0.002 |
*P < 0.05; **P < 0.01. PhIP, 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine.
Expression of ER‐αand Ki‐67
Although the growth of most of human breast cancers depends on hormonal stimuli such as estrogen, it is not fully investigated whether PhIP‐induced mammary tumors are associated with ER‐α. Immunohistochemical staining revealed that those tumor cells, located around the mammary duct, expressed ER‐α (Fig. 2a). The ratio of ER‐α ‐positive cells in tumor from the control group was 55.1 ± 1.7%. Therefore, this mammary tumor was confirmed to be a hormone stimuli‐responding tumor. Compared with the control group, the ratio for the soymilk plus LcS group was significantly lower (47.2 ± 2.0%; P < 0.01). To clarify the distribution of ER‐α‐positive tumors, they were classified according to the percentage of ER‐α‐positive cells per tumor. As shown in Figure 2(b), the distribution pattern of ER‐α‐positive tumors was different in each group. In particular, the frequency of tumor tissues composed of cells with a higher percentage of ER‐α was decreased in the soymilk plus LcS group when compared with the other groups. These results suggest that the ratio of ER‐α‐positive cells in the tumor were greatly reduced by the simultaneous administration of soymilk and LcS.
Figure 2.
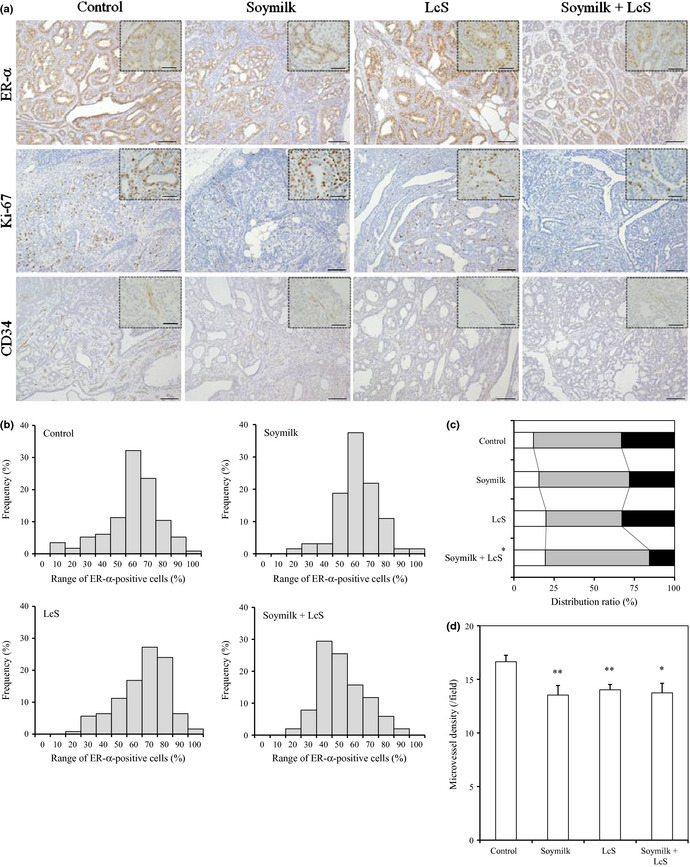
Immunohistological analysis in mammary tumors. All mammary tumors were immunostained with anti‐estrogen receptor (ER)‐α, Ki‐67 or CD34 antibody. (a) Typical images of mammary tumors immunostained for ER‐α, Ki‐67 and CD34 (bar, 100 μm). The insets show detailed images of the tumor tissues (bar, 20 μm). LcS,Lactobacillus casei Shirota. (b) Percentage of ER‐α‐positive cells in each tumor. (c) Classification of each tumor according to the Ki‐67 labeling index. White, low grade (<15%); grey, intermediate grade (15–30%); and black, high grade (>30%). (d) Microvessel density was defined as the mean number of CD34‐positive vessels per field (0.15 mm2). *P < 0.05. **P < 0.01.
To investigate the proliferation of cells in PhIP‐induced mammary tumors, immunohistochemical staining of Ki‐67, which is an important marker of cell proliferation, was performed (Fig. 2a). Compared with the control group, the ratio of Ki‐67‐positive cells in the tumors for the soymilk plus LcS group was lower (control group, 25.9 ± 1.0%; soymilk group, 24.5 ± 1.2%; LcS group, 24.5 ± 1.0%; and soymilk plus LcS group, 21.8 ± 1.4%; P = 0.056). In addition, the number of tumor cells undergoing nuclear division in the soymilk plus LcS group was lower than that in the control group. To analyze proliferation activity, the tumors were classified according to the Ki‐67 LI, as reported previously.34 The Ki‐67 LI was defined as follows: low grade, <15%; intermediate grade, 15–30%; and high grade, >30%. As shown in Figure 2(c), compared with the control group, the ratio of high‐grade population decreased slightly in the soymilk group and that of the low‐grade population was increased in the LcS group. In contrast, in the soymilk plus LcS group, both decreasing of the high‐grade population and increasing of the low‐grade population were demonstrated; the average grade of the Ki‐67 LI was significantly lower than in the control group (P = 0.019). These results suggest that the simultaneous administration of soymilk and LcS reduced the ratio of high‐grade Ki‐67‐positive cells in the tumor more effectively than the administration of each component alone.
Inhibition of angiogenesis in tumors
Tumor angiogenesis is essential for tumor growth and metastasis. To evaluate the effect of the tested products on the MVD as an angiogenesis index, CD34‐expressing vessels in the tumors were immunohistochemically examined (Fig. 2a). Compared with the control group, the MVD was significantly lower in all groups (soymilk group and LcS group, P < 0.01; soymilk plus LcS group, P = 0.017; Fig. 2d), suggesting that the administration of both soymilk and LcS inhibited tumor angiogenesis.
Discussion
The modification of dietary habits is important for the prevention of breast cancer and our previous case‐controlled study demonstrated that the habitual consumption of soy isoflavones and the probiotic strain LcS, especially their simultaneous consumption, decreases the risk of breast cancer.27 To elucidate the underlying mechanism, the present study investigated the efficacy of soymilk and LcS using PhIP‐induced breast cancer in rats fed a high‐fat diet. The administration of soymilk inhibited the development of mammary tumors, while the administration of LcS suppressed tumor growth (Table 3). Moreover, the administration of LcS in combination with soymilk showed the preventive efficacies of both LcS and soymilk (Table 2). These findings indicate that LcS and soymilk act during different phases of the carcinogenic process to help prevent breast cancer.
Obesity has been reported to be a risk factor for breast cancer and the prevention of obesity by the improvement of lipid and glucose intake might lead to a reduction in the occurrence of breast cancer.35 Obesity was likely induced in the carcinogenesis‐initiated rats by the consumption of a high‐fat diet. In the soymilk‐administered groups (soymilk group and soymilk plus LcS group), the total and free cholesterol, triglyceride and glucose levels in the plasma were significantly lower than those in the control group (Table 5), accompanied by a suppression of bodyweight gain (Table 4). Soy isoflavones and proteins reportedly have beneficial roles in improving lipometabolism and glycometabolism36 and these effects might have led to the prevention of obesity in this present study. In addition, soy isoflavones, saponin and soy peptides reportedly have the potential to increase the gene expressions of cyp1A1 and ugt1A1, the proteins that might eliminate various carcinogens such as PhIP.37 Therefore, the preventive effect of soymilk on tumor development in this present study might be due to these components, which act additively or synergistically with isoflavones.
Table 5.
Blood biochemical analysis in PhIP‐exposed rats at Week 17
| Group | Concentration in the plasma (mg/dL) | |||
|---|---|---|---|---|
| Glucose | Total cholesterol | Free cholesterol | Triglyceride | |
| Control | 182 ± 3.6 | 111.1 ± 4.8 | 29.3 ± 1.4 | 72.4 ± 7.1 |
| Soymilk | 163 ± 2.8** | 83.1 ± 3.4** | 22.3 ± 1.0* | 43.5 ± 9.8** |
| LcS | 184 ± 3.5 | 114.5 ± 4.8 | 30.7 ± 1.4 | 76.2 ± 8.9 |
| Soymilk + LcS | 161 ± 3.9** | 90.1 ± 3.9** | 23.8 ± 1.1** | 31.8 ± 3.3** |
*P < 0.05; **P < 0.01. PhIP, 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine.
It has been reported that LcS augments the immunopotentiative activity in hosts.14, 15, 16 Splenomegaly was observed in the control group but was restored in the LcS‐administered groups (LcS group and soymilk plus LcS group) (Table 4). Splenomegaly in tumor‐bearing mice is reportedly caused not only by extramedullary hematopoiesis, but also by the accumulation of myeloid‐derived suppressor cells (MDSC), inducing an immune‐suppressive status.38 In addition, the inhibition of MDSC accumulation by the administration of anticancer agents and phytochemicals is effective for cancer therapy and prevention.39, 40 In this present study, the ratio of the neutrophil band and the monocytes in the leukocyte fraction significantly increased in the LcS‐administered groups compared with the control group (data not shown), suggesting that LcS might affect the immune system. The immune‐modulation action of LcS during the carcinogenic process is also obvious based on previous reports; therefore, LcS might restore an immune‐suppressive status under the carcinogenic process, thereby suppressing tumor growth. The suppression of splenomegaly, as shown in Table 4, might reflect the above mechanism.
Similar to a previous report,41 PhIP‐induced mammary tumors were identified as ER‐α‐positive tumors by immunostaining, although our result showed a higher score (Fig. 2a). The dietary administration of soymilk alone slightly reduced the tumors that showed more than 60% of the ER‐α‐positive cells. In contrast, LcS in combination with soymilk greatly reduced the tumors that showed more than 50% of the ER‐α‐positive cells (Fig. 2b). Soy isoflavones such as daidzein, genistein and equol, which is converted from daidzein by intestinal microbes, reportedly reduce the expression of ER‐α on MCF‐7 cells, a human breast cancer cell line exhibiting estrogen‐dependent growth.42, 43 In addition, the ingestion of probiotics reportedly reduces the simultaneous excretion of soy isoflavones.44 Lactobacillus casei Shirota might enhance the antihormonal activity by stimulating the conversion from soy isoflavones to some active metabolites, such as equol, and/or the absorption of isoflavone through changes in the intestinal environment.
The classification according to the Ki‐67 LI showed that the dietary administration of LcS in combination with soymilk greatly reduced the ratio of the high‐grade population compared with that of soymilk alone (Fig. 2c). Both ER‐α‐positive cells and Ki‐67‐positive cells were cooperatively reduced by antihormonal therapy in a clinical neoadjuvant setting,45 whereas the Ki‐67 LI was not affected by the uptake of a supplement containing soy isoflavones in a phase II trial.46 These reports indicate that the reduction in the high‐grade population by the combination of LcS was caused by the enhancement of antihormonal activity induced by soymilk. In addition, the dietary administration of LcS alone increased the low‐grade population (Fig. 2c). Thus, soymilk and LcS might act on Ki‐67 expression and proliferating tumor cells in different ways. These immunohistochemical results revealed that the administration of LcS in combination with soymilk decreased the ratio of ER‐α‐positive and Ki‐67‐positive cells more effectively than that of soymilk alone (Fig. 2b,c). In addition, this combination had a similar effect on the suppression of tumor multiplicity (Table 2). These findings indicate that LcS potentially enhances the preventive efficacy of soymilk and might partly explain the underlying mechanism of the combinational effect shown in a previous epidemiological study.27
The evaluation of MVD in tumor using CD34 immunostaining showed that both soymilk and LcS inhibited tumor angiogenesis (Fig. 2d). Several reports have shown the antiangiogenesis activity of soy isoflavones in tumor carcinogenesis;9, 47 however, to the best of our knowledge, this report is the first to describe the inhibition of angiogenesis by probiotics. It has been shown that LcS has anti‐inflammatory activity through the downregulation of nuclear factor‐κB (NF‐κB).48, 49 One of the antiangiogenic mechanisms of isoflavones in tumor development is considered to be an anti‐inflammatory action via the downregulation of NF‐κB and COX‐2.50 Therefore, a similar anti‐inflammatory mechanism might be involved in the suppression of mammary tumorigenesis by LcS.
In conclusion, the dietary administration of LcS in combination with soymilk prevented mammary carcinogenesis in PhIP‐exposed rats more effectively than that of each component alone. Thus, the habitual consumption of LcS in combination with soymilk might be a beneficial dietary style for breast cancer prevention.
Disclosure Statement
Y.O. is from Statcom Co., Ltd.
Acknowledgments
We thank Drs H. Nagata and T. Ohta for their statistical and technical advice. We also thank Dr. N. Kubota, K. Shimura, M. Sahashi, M. Ikeda, A. Gomi and Y. Kamataki for preparation of the experimental articles.
(Cancer Sci 2013; 104: 1508–1514)
References
- 1. Anderson BO, Jakesz R. Breast cancer issues in developing countries: an overview of the Breast Health Global Initiative. World J Surg 2008; 32: 2578–85. [DOI] [PubMed] [Google Scholar]
- 2. Hirabayashi Y, Zhang M. Comparison of time trends in breast cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, Vols IV–IX. Jpn J Clin Oncol 2009; 39: 411–2. [DOI] [PubMed] [Google Scholar]
- 3. Shin HR, Boniol M, Joubert C et al Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci 2010; 101: 1241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donaldson MS. Nutrition and cancer: a review of the evidence for an anti‐cancer diet. Nutr J 2004; 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst 2003; 95: 906–13. [DOI] [PubMed] [Google Scholar]
- 6. Molteni A, Brizio‐Molteni L, Persky V. In vitro hormonal effects of soybean isoflavones. J Nutr 1995; 125: 751S–6. [DOI] [PubMed] [Google Scholar]
- 7. Akiyama T, Ishida J, Nakagawa S et al Genistein, a specific inhibitor of tyrosine‐specific protein kinases. J Biol Chem 1987; 262: 5592–5. [PubMed] [Google Scholar]
- 8. Su SJ, Yeh TM, Chuang WJ et al The novel targets for anti‐angiogenesis of genistein on human cancer cells. Biochem Pharmacol 2005; 69: 307–18. [DOI] [PubMed] [Google Scholar]
- 9. Zhou Y, Lee AS. Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J Natl Cancer Inst 1998; 90: 381–8. [DOI] [PubMed] [Google Scholar]
- 10. Dijsselbloem N, Vanden Berghe W, De Naeyer A, Haegeman G. Soy isoflavone phyto‐pharmaceuticals in interleukin‐6 affections. Multi‐purpose nutraceuticals at the crossroad of hormone replacement, anti‐cancer and anti‐inflammatory therapy. Biochem Pharmacol 2004; 68: 1171–85. [DOI] [PubMed] [Google Scholar]
- 11. Kushi LH, Doyle C, McCullough M et al American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012; 62: 30–67. [DOI] [PubMed] [Google Scholar]
- 12. Rowland IR, Rumney CJ, Coutts JT, Lievense LC. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen‐induced aberrant crypt foci in rats. Carcinogenesis 1998; 19: 281–5. [DOI] [PubMed] [Google Scholar]
- 13. Marotta F, Naito Y, Minelli E, Tajiri H, Bertuccelli J, Wu CC. Chemopreventive effect of a probiotic preparation on the development of preneoplastic and neoplastic colonic lesions: an experimental study. Hepatogastroenterology 2003; 50: 1914–8. [PubMed] [Google Scholar]
- 14. Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Inhibitory effect of oral administration of Lactobacillus casei on 3‐methylcholanthrene‐induced carcinogenesis in mice. Med Microbiol Immunol 1999; 188: 111–6. [DOI] [PubMed] [Google Scholar]
- 15. Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 2001; 22: 599–605. [DOI] [PubMed] [Google Scholar]
- 16. Takagi A, Ikemura H, Matsuzaki T et al Relationship between the in vitro response of dendritic cells to Lactobacillus and prevention of tumorigenesis in the mouse. J Gastroenterol 2008; 43: 661–9. [DOI] [PubMed] [Google Scholar]
- 17. Yamazaki K, Tsunoda A, Sibusawa M et al The effect of an oral administration of Lactobacillus casei strain shirota on azoxymethane‐induced colonic aberrant crypt foci and colon cancer in the rat. Oncol Rep 2000; 7: 977–82. [DOI] [PubMed] [Google Scholar]
- 18. Matsuguchi T, Takagi A, Matsuzaki T et al Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha‐inducing activities in macrophages through Toll‐like receptor 2. Clin Diagn Lab Immunol 2003; 10: 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi T, Kushiro A, Nomoto K et al Antitumor effects of the intravesical instillation of heat killed cells of the Lactobacillus casei strain Shirota on the murine orthotopic bladder tumor MBT‐2. J Urol 2001; 166: 2506–11. [PubMed] [Google Scholar]
- 20. Ohashi Y, Nakai S, Tsukamoto T et al Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int 2002; 68: 273–80. [DOI] [PubMed] [Google Scholar]
- 21. Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double‐blind trial. The BLP Study Group. Eur Urol 1995; 27: 104–9. [DOI] [PubMed] [Google Scholar]
- 22. Naito S, Koga H, Yamaguchi A et al Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J Urol 2008; 179: 485–90. [DOI] [PubMed] [Google Scholar]
- 23. Ishikawa H, Akedo I, Otani T et al Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer 2005; 116: 762–7. [DOI] [PubMed] [Google Scholar]
- 24. Hayatsu H, Hayatsu T. Suppressing effect of Lactobacillus casei administration on the urinary mutagenicity arising from ingestion of fried ground beef in the human. Cancer Lett 1993; 73: 173–9. [DOI] [PubMed] [Google Scholar]
- 25. Kato K, Mizuno S, Umesaki Y et al Randomized placebo‐controlled trial assessing the effect of bifidobacteria‐fermented milk on active ulcerative colitis. Aliment Pharmacol Ther 2004; 20: 1133–41. [DOI] [PubMed] [Google Scholar]
- 26. Scharlau D, Borowicki A, Habermann N et al Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora‐mediated fermentation of dietary fibre. Mutat Res 2009; 682: 39–53. [DOI] [PubMed] [Google Scholar]
- 27. Toi M, Hirota S, Tomotaki A et al Probiotic beverage with soy isoflavone consumption for breast cancer prevention: a case‐control study. Curr Nutr Food Sci 2013; 9: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohta T, Nakatsugi S, Watanabe K et al Inhibitory effects of Bifidobacterium‐fermented soy milk on 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine‐induced rat mammary carcinogenesis, with a partial contribution of its component isoflavones. Carcinogenesis 2000; 21: 937–1. [DOI] [PubMed] [Google Scholar]
- 29. Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr 2006; 136: 2291–6. [DOI] [PubMed] [Google Scholar]
- 30. Nakatsugi S, Ohta T, Kawamori T et al Chemoprevention by nimesulide, a selective cyclooxygenase‐2 inhibitor, of 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP)‐induced mammary gland carcinogenesis in rats. Jpn J Cancer Res 2000; 91: 886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nozawa H, Nakao W, Takata J, Arimoto‐Kobayashi S, Kondo K. Inhibition of PhIP‐induced mammary carcinogenesis in female rats by ingestion of freeze‐dried beer. Cancer Lett 2006; 235: 121–9. [DOI] [PubMed] [Google Scholar]
- 32. Tayek JA, Istfan NW, Jones CT, Hamawy KJ, Bistrian BR, Blackburn GL. Influence of the Walker 256 carcinosarcoma on muscle, tumor, and whole‐body protein synthesis and growth rate in the cancer‐bearing rat. Cancer Res 1986; 46: 5649–54. [PubMed] [Google Scholar]
- 33. Russo J, Russo IH, Rogers AE, van Zwieten MJ, Gusterson B. Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the mammary gland. IARC Sci Publ 1990; 99: 47–78. [PubMed] [Google Scholar]
- 34. Jalava P, Kuopio T, Juntti‐Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 2006; 48: 674–82. [DOI] [PubMed] [Google Scholar]
- 35. Macinnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2004; 13: 2117–25. [PubMed] [Google Scholar]
- 36. Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci 2007; 4: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satsu M, Mizutani T, Mikubo A, Shimizu K. Study of the regulation of drug metabolizing enzymes by soybean ingredients in intestinal epithelial cells. Soy Protein Res Jpn 2010; 13: 79–84. [Google Scholar]
- 38. Forghani P, Khorramizadeh MR, Waller EK. Natural suppressor cells; past, present and future. Front Biosci (Elite Ed) 2012; 4: 1237–45. [DOI] [PubMed] [Google Scholar]
- 39. Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor‐bearing mice. Int Immunopharmacol 2009; 9: 900–9. [DOI] [PubMed] [Google Scholar]
- 40. Tu SP, Jin H, Shi JD et al Curcumin induces the differentiation of myeloid‐derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012; 5: 205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qiu C, Shan L, Yu M, Snyderwine EG. Steroid hormone receptor expression and proliferation in rat mammary gland carcinomas induced by 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine. Carcinogenesis 2005; 26: 763–9. [DOI] [PubMed] [Google Scholar]
- 42. Diel P, Olff S, Schmidt S, Michna H. Molecular identification of potential selective estrogen receptor modulator (SERM) like properties of phytoestrogens in the human breast cancer cell line MCF‐7. Planta Med 2001; 67: 510–4. [DOI] [PubMed] [Google Scholar]
- 43. Sathyamoorthy N, Wang TT. Differential effects of dietary phyto‐oestrogens daidzein and equol on human breast cancer MCF‐7 cells. Eur J Cancer 1997; 33: 2384–9. [DOI] [PubMed] [Google Scholar]
- 44. Cohen LA, Crespin JS, Wolper C et al Soy isoflavone intake and estrogen excretion patterns in young women: effect of probiotic administration. In Vivo 2007; 21: 507–12. [PubMed] [Google Scholar]
- 45. Toi M, Saji S, Masuda N et al Ki67 index changes, pathological response and clinical benefits in primary breast cancer patients treated with 24 weeks of aromatase inhibition. Cancer Sci 2011; 102: 858–65. [DOI] [PubMed] [Google Scholar]
- 46. Khan SA, Chatterton RT, Michel N et al Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res 2012; 5: 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis‐correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 48. Matsumoto S, Hara T, Hori T et al Probiotic Lactobacillus‐induced improvement in murine chronic inflammatory bowel disease is associated with the down‐regulation of pro‐inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol 2005; 140: 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsumoto S, Hara T, Nagaoka M et al A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis‐associated cancer. Immunology 2009; 128: e170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davis JN, Kucuk O, Djuric Z, Sarkar FH. Soy isoflavone supplementation in healthy men prevents NF‐kappa B activation by TNF‐alpha in blood lymphocytes. Free Radic Biol Med 2001; 30: 1293–1302. [DOI] [PubMed] [Google Scholar]


