Abstract
Insulin‐like growth factor 1 receptor (IGF‐1R) is critical for cancer cell proliferation; however, recent clinical anti‐IGF‐1R trials did not show clear clinical benefit in cancer therapy. We hypothesized that IGF‐1R signaling‐mediated proliferative response is heterogeneous in neuroblastoma (NB) cells, and analyzed the cell growth of 31 NB cell lines cultured in three different media, including Hybridoma‐SFM medium (with insulin) and RPMI1640 with/without 10% FBS. Three growth patterns were found. In response to IGF and insulin, cell proliferation and Akt phosphorylation were upregulated in 13 cell lines, and suppressed by MK2206 (Akt inhibitor) and picropodophyllin (IGF‐1R inhibitor). Interestingly, 3 of these 13 cell lines showed Akt self‐phosphorylation and cell proliferation in RPMI1640; their proliferation was downregulated by anti‐IGF‐1 or anti‐IGF‐2 neutralizing antibody, suggesting the existence of an autocrine loop in the IGF‐1R/Akt pathway. Eighteen NB cell lines did not proliferate in RPMI1640, even though Akt phosphorylation was upregulated by IGF and insulin. Based on the heterogeneous response of the IGF‐1R/Akt pathway, the 31 NB cell lines could be classified into group 1 (autocrine IGF‐mediated), group 2 (exogenous IGF‐mediated) and group 3 (partially exogenous IGF‐mediated) NB cell lines. In addition, group 3 NB cell lines were different from group 1 and 2, in terms of serum starvation‐induced caspase 3 cleavage and picropodophyllin‐induced G2/M arrest. These results indicate that the response of the IGF‐1R/Akt pathway is an important determinant of the sensitivity to IGF‐1R antagonists in NB. To our knowledge, this is the first report describing heterogeneity in the IGF‐1R/Akt‐mediated proliferation of NB cells.
Neuroblastoma (NB), a malignant tumor that originates from the sympathetic nervous system, is one of the most frequent pediatric solid tumors.1 NB is characterized by heterogeneous clinical behaviors, tumor invasiveness being different according to age and anatomic stage at diagnosis. The tumor is sometimes completely curable and may even regress spontaneously, especially in younger children.2 Heterogeneity of the tumors depends on the degree of morphological differentiation and on histopathology.3
Insulin and insulin‐like growth factors (IGF, including IGF‐1 and 2) belong to a family of mitogenic growth factors. IGF, insulin, and their receptors are involved in normal growth and differentiation of most tissues. The biological actions of both IGF and insulin can be mediated by the IGF‐1 receptor (IGF‐1R), a transmembrane heterotetramer, which is involved in mitogenic, anti‐apoptotic and oncogenic transforming responses.4, 5 The functional IGF‐1R contains two extracellular α‐subunits and two intracellular β‐subunits that form a heterotetrameric complex. The structural homology of IGF‐1R and insulin receptor (IR) allows formation of hybrid receptors (hybrid‐R) in which an IGF‐1R chain is connected to an IR chain.6 Ligand interaction with α‐subunits triggers the auto‐phosphorylation of tyrosine kinase domains within the β‐subunit.7, 8, 9 The tyrosine kinase domains are coupled to several intracellular pathways, including the phosphatidylinositol‐3‐kinase‐Akt (PI3K/Akt)10, 11 and the MAPK.12 Dysregulation of the IGF‐1R pathway is involved in promoting oncogenic transformation, cell proliferation, metastasis, angiogenesis and resistance in numerous malignant diseases, such as multiple myeloma,13 carcinomas14 and NB.15 IGF‐1R is also known to translocate to the nucleus to modulate gene expression.16, 17, 18
The IGF‐1R inhibitors, including IGF‐1R neutralizing antibodies, IGF‐1 mimetics and IGF‐1R anti‐sense/siRNA, have been shown to block cancer cell proliferation.19 Another target for treatment is the receptor tyrosine kinase (RTK).20, 21, 22, 23, 24 The inhibitory effect of picropodophyllin (PPP) appears to be promising, because it has selectivity for the IGF‐1R and, thus, lacks inhibitory activity on tyrosine phosphorylation of insulin RTK and other receptors, like fibroblast growth factor receptor, platelet‐derived growth factor receptor and epidermal growth factor receptor.25 Inhibition of the IGF‐1 RTK with PPP is noncompetitive in relation to ATP, suggesting interference of the IGF‐1R at substrate level.23 It is reported that PPP specifically blocks phosphorylation of the Tyr1136 residue in the activation loop of IGF‐1R kinase.26 Inhibition of IGF‐1R with PPP has been demonstrated in multiple myeloma,23 breast cancer,27 melanoma28 and glioblastoma cells.29
Although IGF‐1R and the stimulatory ligands (IGF and insulin) are important for cancer proliferation, anti‐IGF‐1R therapy has not shown enough clinical benefits in randomized phase III trials.30 The mediation of IGF‐1R signaling in cancer cell is still unclear.30 We hypothesized that these unfavorable clinical results might be due to heterogeneity of IGF‐1R signaling in cancer cells. The aim of the present study was to clarify the heterogeneous mediation of IGF‐1R signaling in NB cell lines; for this purpose, we evaluated the cell proliferation patterns of 31 human NB cell lines by using three different media, stimulatory ligands and an IGF‐1R inhibitor (PPP). The 31 NB cell lines were classified into three groups based on their differential response to the stimulatory ligands: group 1 (autocrine IGF‐mediated), group 2 (exogenous IGF‐mediated) and group 3 (partially exogenous IGF‐mediated) NB cell lines. In addition, group 3 NB cell lines were different from groups 1 and 2 in terms of serum starvation‐induced caspase 3 cleavage and PPP‐induced G2/M arrest. These results indicate that NB cell lines are heterogeneous in their IGF‐1R‐mediated signaling. The pattern of IGF‐1R/Akt pathway‐mediated proliferation is an important determinant of the response to IGF‐1R antagonistic therapy in human NB. These observations suggest that IGF‐1R/Akt pathway inhibitors, such as PPP and MK2206, may be used in NB clinical therapies.
Materials and Methods
Cell lines and cell culture
The following 31 human NB cell lines were used and evaluated in this study: SK‐N‐SH, INDEN, NB‐19; IMR‐32, KP‐N‐SI, LAN‐1, SHEP, KP‐N‐SIFA, SK‐N‐DZ, SJ‐N‐SD, SMS‐KCNR, NH‐12, SCMC‐N2; SMS‐KAN, TGW, GOTO, OZAWA, KP‐N‐RT, SMS‐KCN, NB‐69, NB‐1, SJ‐N‐KP, KP‐N‐YN, LAN‐2, LAN‐5, NB‐MASS, SCMC‐N4, SJ‐N‐JF, SJ‐N‐KS, KP‐N‐AY and SUZUKI. All these 31 cell lines were cultured in RPMI1640 (R8758, Sigma, St. Louis, MO, USA) medium supplemented with 10% FBS (GIBCO, Grand Island, NY, USA). The cells were incubated in a humidified atmosphere at 37°C with 5% CO2. Thirteen of these cell lines could be cultured in SFM medium (Hybridoma‐SFM medium, 12045‐84; GIBCO), which contains low protein (20 μg/mL protein as insulin and transferrin).
Antibodies and reagents
The following antibodies and reagents were used in the present study: (i) rabbit monoclonal antibody: anti‐Akt (#9272; Cell Signaling, Boston, MA, USA), anti‐phospho‐Akt (Ser473) (#4058; Cell Signaling); (ii) rabbit polyclonal antibody: anti‐cleaved caspase 3 (Asp175) (#9661; Cell Signaling), anti‐p44/42 MAPK (Erk1/2) (#9102; Cell Signaling), anti‐IGF‐1R β (#3027; Cell Signaling); (iii) mouse monoclonal antibody: anti‐phospho‐p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (#9106; Cell Signaling), anti‐cleaved PARP (Asp214) (#9546; Cell Signaling), anti‐IR β (C‐4) (sc‐373975; Santa Cruz; Santa Cruz Biotechnology, Santa Cruz, CA, USA); and (iv) neutralizing antibody: rabbit polyclonal IGF‐1 neutralizing antibody (MAB791, R&B, Minneapolis, MN, USA), mouse monoclonal IGF‐2 neutralizing antibody (MAB292, R&B).
Picropodophyllin (sc‐204008) was purchased from Santa Cruz Biotechnology. MK2206 (Akt inhibitor, A10003) was purchased from Selleckchem (Houston, TX, USA). U0126 (MEK inhibitor, 70970‐5) was purchased from Cayman Chemical (Ann Arbor, MI, USA).
Cell counting
WST‐8 (Cell Counting Kit‐8) cell counting reagent was obtained from Dojindo Molecular Technologies (Osaka, Japan). Cells (5 × 103) were seeded in 100 μL medium in 96‐well plates and pre‐incubated for 6 h before the addition of stimulatory ligands, inhibitors or neutralizing antibodies. WST‐8 (10 μL) was added into each well at a 1:10 ratio in cell culture medium. After 2.5 h incubation in a humidified atmosphere at 37°C with 5% CO2, the absorbance at 450 nm was measured using multi‐spectrophotometer (Viento, Dainippon, Japan). The optical density was then used to extrapolate the cell number from a standard curve. The standard curves were drawn for each cell line for each type of medium. The results are expressed as means ± SD from three independent experiments.
Western blotting
Cytoplasmic extracts were obtained as previously reported.31 The proteins (40 μg/lane) were run on 7.5, 10 or 15% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis followed by semi‐dry transfer to PVDF membrane (Invitrogen, Carlsbad, CA, USA). Transferred PVDF blots were pretreated with 5% non‐fat dry milk in TBST containing 0.1% Tween‐20 and incubated with primary antibody (1:1000–3000) at 4°C overnight. The membrane was then washed three times with TBST and incubated with horseradish peroxidase‐conjugated secondary antibody (1:1000–3000) for 1 h at room temperature. For phosphorylated target protein, transferred PVDF blots were pretreated with PVDF Blocking Reagent (TOYOBO, Osaka, Japan) for 1 h, and incubated with primary and then with secondary antibody (1:3000–6000), which were diluted with Can Get Signal Immunoreaction Enhancer Solution (TOYOBO) at room temperature for 1 h. After washing three times again, antibodies bound to protein blots were detected by using Western Lightening Chemiluminescence Reagent Plus (Perkin Elmer Life Science, Boston, MA, USA), visualized on LAS‐3000 mini (FUJIFILM, Tokyo, Japan). The blots were quantitated and cropped using Multi Gauge Ver3.0 (FUJIFILM).
Cell cycle analysis
Cell cycle analysis was performed after treatment with/without PPP for 12 h. Cells (2 × 106) were harvested and fixed in 99.5% ethanol over night at −20°C, followed by incubation with 500 μL propidium iodide Triton X‐100 solution containing RNase A at room temperature for 30 min in darkness, then the DNA content was analyzed immediately with a FACScan flow cytometer, using ModFitLT software (Verity Software House, Topsham, ME, USA).
Statistical analyses
A two‐sided paired t‐test was used to determine statistical significance. A P < 0.05 was considered as statistically significant.
Results
Sensitivity to culture conditions of neuroblastoma cell line groups
We hypothesized that NB cells are heterogeneous in their IGF‐1R signaling‐mediated cell proliferation. To test this hypothesis, 31 NB cell lines were cultured in three different media and screened using a cellular proliferation assay. Based on the response patterns we subdivided the 31 cell lines into three groups. Group 1, which included SK‐N‐SH, INDEN and NB‐19, proliferated for more than 3 days in SFM (insulin containing), RPMI1640 (serum starvation medium) and RPMI1640 with 10% FBS (serum containing medium). Group 2 included IMR‐32, KP‐N‐SI, LAN‐1, SHEP, KP‐N‐SIFA, SK‐N‐DZ, SJ‐N‐SD, SMS‐KCNR, NH‐12 and SCMC‐N2 cell lines; these cell lines could proliferate in SFM and RPMI1640 with 10% FBS but not in RPMI1640. Group 3 could proliferate only in RPMI1640 with 10% FBS. The growth curves of the three typical cell lines (SK‐N‐SH, LAN‐1 and NB‐69) in three different media are depicted in Figure 1(a). These results show that the regulation of cell proliferation induced by insulin is different among NB cell lines. Because the signal transduction of insulin is similar to IGF, these results indicate that signaling induced by IGF‐1R stimulatory ligands may be different among NB cell lines.
Figure 1.
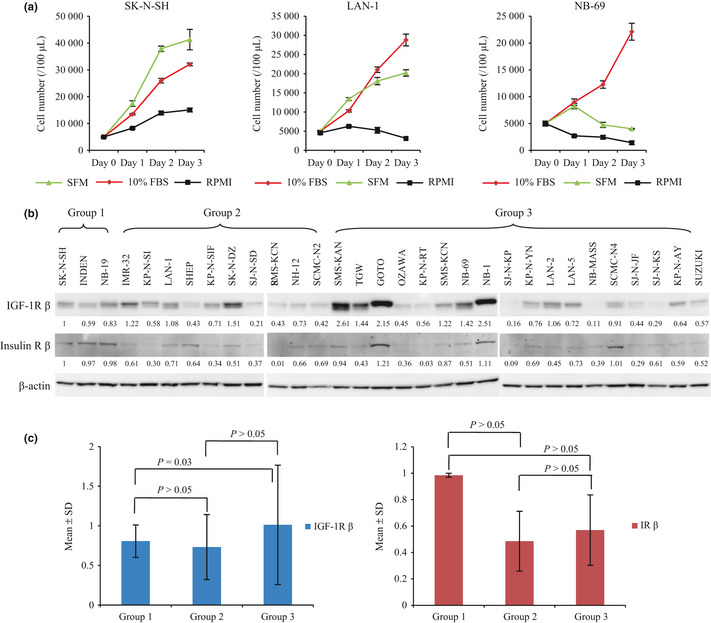
The growth phenotype of the neuroblastoma (NB) cell lines in SFM and RPMI1640 with/without 10% FBS, and the expressions of insulin‐like growth factor 1 receptor (IGF‐1R) and insulin receptor (IR). (a) Three different growth patterns were shown by SK‐N‐SH, LAN‐1 and NB‐69 cell line in SFM and RPMI1640 with/without 10% FBS. (b) Expressions of IGF‐1R and IR in 31 cell lines. Cell lines were cultured in RPMI1640 with 10% FBS, and lysed according to a previous report.31 Target proteins were detected by anti‐IGF‐1R β antibody and anti‐IR β antibody, with β‐actin loaded as a control. Densitometric quantitation of IGF‐1R β and IR β to β‐actin ratio was standardized by the value of the first lane (SK‐N‐SH). (c) Bar graph shows densitometric analysis of the IGF‐1R β and IR β subunit expression displayed according to the NB cell groups. Data are expressed as the mean (±SD) of the values showed in Figure 1b. Statistical analysis was performed using a two‐sided t‐test.
Expressions of insulin‐like growth factor 1 receptor and insulin receptor
Insulin‐like growth factor 1 receptor is the common receptor of IGF and insulin.6 Binding of IGF and insulin to the α‐subunits of IGF‐1R induces auto‐phosphorylation of tyrosine kinase domain in β‐subunit and phosphorylation of downstream signaling molecules. Expression of IGF‐1R β‐subunit and IR β‐subunit were confirmed by western blotting using anti‐IGF‐1R β antibody and anti‐IR β antibody. Both IGF‐1R β‐subunit and IR β‐subunit were expressed in all 31 NB cell lines (Fig. 1b). Although the amounts of expressed β‐subunits were not the same among NB cell lines (Fig. 1b), there was no significant statistical relationship between the receptor β subunit and the different groups of NB cell lines (Fig. 1c).
Neuroblastoma cell proliferation induced by exogenous insulin‐like growth factor 1 receptor (IGF‐1, IGF‐2) and insulin
To confirm that NB cell proliferation is induced by stimulatory ligands, we evaluated NB cell proliferation in RPMI1640 in the presence of exogenous IGF (IGF‐1, IGF‐2) and insulin. These IGF and insulin accelerated cell proliferation in SK‐N‐SH (group 1) and LAN‐1 (group 2) but not in NB‐69 (group 3) (Fig. 2). These results suggest that IGF and insulin are important for cell proliferation of groups 1 and 2 NB cell lines, but not for that of group 3. SK‐N‐SH (group 1) was able to proliferate in RPMI1640 in the absence of exogenous IGF and insulin (Fig. 2). The other NB cell lines, group 1 (INDEN and NB‐19), group 2 (SHEP, SMS‐KCNR and KP‐N‐SI) and group 3 (OZAWA, KP‐N‐RT and SMS‐KCN), showed the same response patterns of SK‐N‐SH (group 1), LAN‐1 (group 2) and NB‐69 (group 3), respectively.
Figure 2.
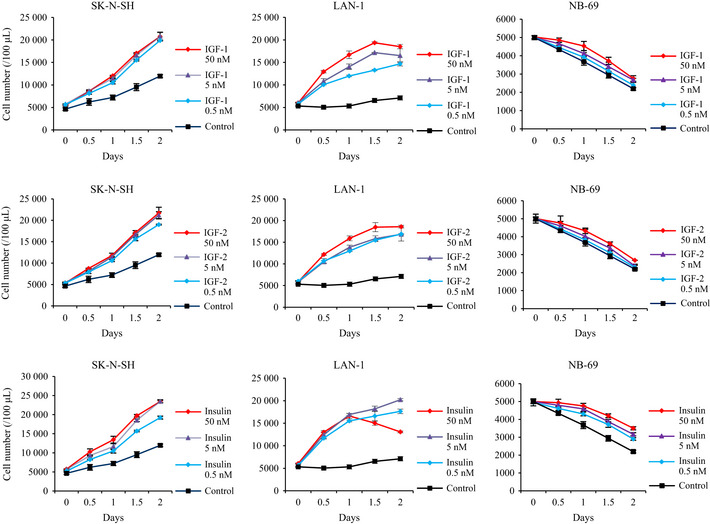
The effect of exogenous stimulatory ligands on growth of neuroblastoma (NB) cell lines. SK‐N‐SH (group 1), LAN‐1 (group 2) and NB‐69 (group 3) cell lines were incubated in RPMI1640 with/without stimulatory ligands such as IGF‐1, IGF‐2 and insulin at the indicated concentrations. Proliferation of cell lines was evaluated as cell numbers at the indicated time points, and it was repeated three times. Data are expressed as the mean (±SD).
Activation of insulin‐like growth factor 1 receptor/Akt pathway induced by exogenous insulin‐like growth factor 1 receptor (IGF‐1, IGF‐2) and insulin
Exogenous IGF and insulin induced Akt phosphorylation in SK‐N‐SH (group 1), LAN‐1 (group 2) and NB‐69 (group 3) cell lines (Fig. 3a). The Akt inhibitor, MK2206 (2.5 μM), completely inhibited Akt phosphorylation and cell proliferation induced by exogenous IGF and insulin in all 31 NB cell lines (data not shown). MK2206 strongly impaired the cell proliferation induced by exogenous IGF and insulin in SK‐N‐SH and LAN‐1 cells (Fig. 3b). Interestingly, the IGF‐1R inhibitor, PPP, inhibited Akt activation in SK‐N‐SH (group 1) and LAN‐1 (group 2) cell lines, but less in NB‐69 (group 3) (Fig. 3a). PPP suppressed the cell proliferation of SK‐N‐SH and LAN‐1 (Fig. 3c).
Figure 3.
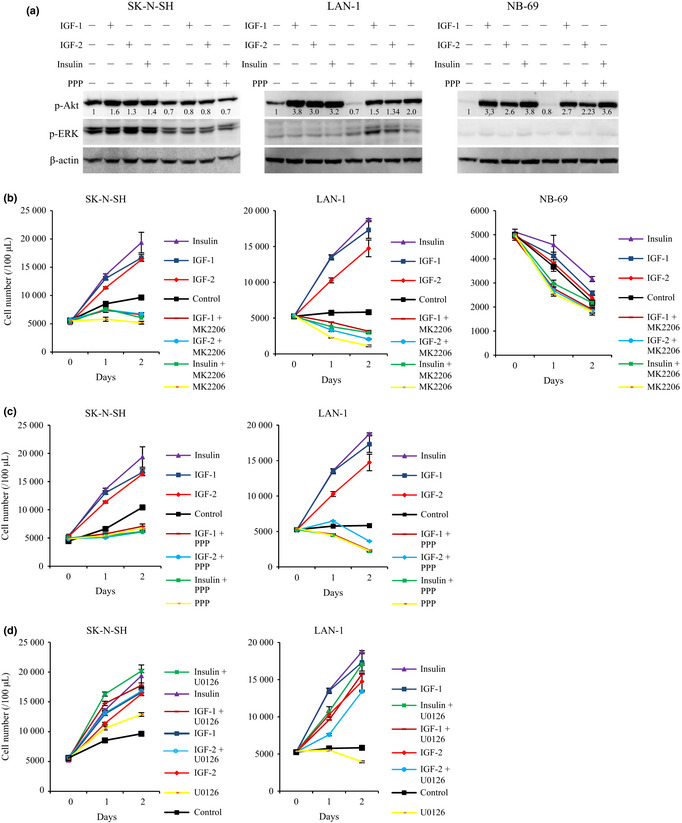
Stimulatory ligands‐induced phosphorylation of Akt and cell proliferation in RPMI1640. (a) Phosphorylation of Akt was induced by stimulatory ligands (IGF1, IGF‐2, and insulin). The cell lines (SK‐N‐SH, LAN‐1, NB‐69) were pre‐incubated with/without picropodophyllin (PPP) (2.5 μM) in RPMI1640 for 12 h, before 1 h stimulation with IGF‐1 (5 nM), IGF‐2 (5 nM), and insulin (5 nM). Phosphorylation of Akt was tested by WB, with the ratio of phospho‐Akt compared with β‐actin, and phospho‐ERK. (b) MK2206 suppressed proliferation of neuroblastoma (NB) cell lines (SK‐N‐SH, LAN‐1 and NB‐69) incubated with stimulatory ligands. IGF‐1 (5 nM), IGF‐2 (5 nM), and insulin (5 nM) were co‐incubated with cell lines with/without MK2206 (2.5 μM). (c) PPP suppressed NB (SK‐N‐SH and LAN‐1) cell proliferation induced by stimulatory ligands. IGF‐1 (5 nM), IGF‐2 (5 nM) and insulin (5 nM) were co‐incubated with cell lines with/without PPP (2.5 μM). (d) U0126 did not suppress NB cell proliferation induced by stimulatory ligands. IGF‐1 (5 nM), IGF‐2 (5 nM) and insulin (5 nM) were co‐incubated with cell lines with/without U0126 (2.5 μM). Proliferation of the cell lines was evaluated as cell numbers at the indicated time points, and it was repeated three times.
Elevation of ERK phosphorylation was only observed in SK‐N‐SH (Fig. 3a). U0126, a MEK inhibitor, effectively suppressed ERK phosphorylation in NB cell lines (data not shown). However, U0126 (2.5 μM) did not suppress SK‐N‐SH and LAN‐1 cell proliferation induced by IGF (IGF‐1, IGF‐2) or insulin (Fig. 3d). These results suggest that IGF‐1R/Akt pathway is critical for cell proliferation in group 1 and 2 NB cell lines. In group 3 NB cell line (NB‐69), IGF and insulin activated IGF‐1R/Akt pathway, but did not induce increased cell proliferation in RPMI1640, suggesting that the activation of IGF‐1R/Akt pathway is insufficient for cell proliferation. The other NB cell lines, group 1 (INDEN and NB‐19), group 2 (SHEP, SMS‐KCNR, and KP‐N‐SI) and group 3 (OZAWA, KP‐N‐RT, and SMS‐KCN), showed the same patterns of SK‐N‐SH (group 1), LAN‐1 (group 2) and NB‐69 (group 3), respectively.
Autocrine insulin‐like growth factors in group 1 neuroblastoma cell lines
In group 1 NB cell lines (SK‐N‐SH, INDEN and NB‐19), cell proliferation (Figs 1a,2) and Akt self‐phosphorylation (Fig. 3a) were observed in RPMI1640 medium without exogenous IGF or insulin. The Akt self‐phosphorylation was suppressed by PPP (Fig. 3a). This indirect evidence suggests the presence of an autocrine growth loop. Therefore, group 1 cell lines (SK‐N‐SH, INDEN and NB‐19) were cultured in the presence of anti‐IGF‐1 neutralizing antibody and anti‐IGF‐2 neutralizing antibody. Both anti‐IGF‐1 and 2 neutralizing antibody impaired cell proliferation of group 1 cell lines in RPMI1640 medium (Fig. 4).
Figure 4.
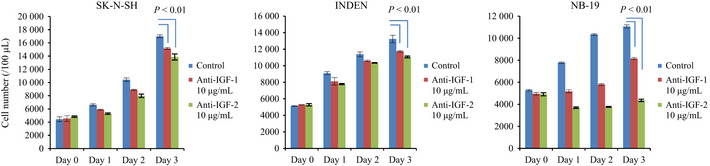
Growth inhibitions of group 1 neuroblastoma (NB) cell lines induced by anti‐IGF‐1 or 2 neutralizing antibody. Group 1 NB cell lines (SK‐N‐SH, INDEN and NB‐19) were cultured in the presence of anti‐IGF‐1 or IGF‐2 neutralizing antibody (10 μg/mL) in RPMI1640. Proliferation of the cell lines was evaluated as cell numbers at the indicated time points, and it was repeated three times. Statistical analysis was performed using a two‐sided t‐test, P < 0.01, significant different.
Apoptosis induced by serum starvation and insulin‐like growth factor 1 receptor inhibitor
Insulin‐like growth factors regulate apoptosis32 and cell cycle progression.33 Cleavages of caspase 3 and PARP were examined in NB cell lines. In group 1 (SK‐N‐SH), caspase 3 and PARP were not cleaved in RPMI1640 (Fig. 5). In group 2 (LAN‐1), caspase 3 and PARP were cleaved in RPMI1640, and the cleavage of caspase 3 was suppressed by IGF and insulin (Fig. 5). Interestingly, in group 3 (NB‐69), cleavages of caspase 3 and PARP were also observed in RPMI1640 (Fig. 5). However, they were not suppressed by IGF or insulin (Fig. 5), even though phosphorylated Akt was upregulated by IGF and insulin (Fig. 3a). In addition, in these NB cell lines, cleavages of caspase 3 and PARP were observed after treatment with PPP, regardless of the presence of exogenous IGF and insulin (Fig. 5).
Figure 5.
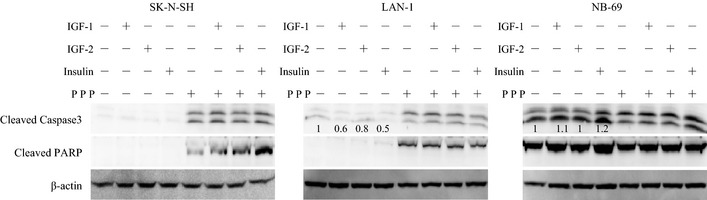
Apoptosis of neuroblastoma (NB) cells induced by serum starvation or picropodophyllin (PPP). The indicated cell lines were pre‐incubated in RPMI1640 with/without PPP (2.5 μM) for 12 h, before 1 h co‐incubation with IGF‐1 (5 nM), IGF‐2 (5 nM) and insulin (5 nM). Cleavages of caspase 3 and PARP were analyzed by WB, and the ratio cleaved caspase 3 to β‐actin was calculated.
Cell proliferation in group 3 neuroblastoma cell lines in the presence of insulin‐like growth factor in RPMI1640 with FBS
Because group 3 NB cell lines died rapidly in a serum‐deprived environment despite exogenous IGF or insulin (Figs 1a,2), Akt activation by IGF is insufficient to support cell proliferation and the proliferative stimulation may be induced by other growth factors present in FBS. Therefore, the effects of IGF, insulin, PPP, MK2206 and U0126 were evaluated on NB‐69 incubated in RPMI1640 with 10% FBS. Cell proliferation of NB‐69 was significantly accelerated by exogenous IGF‐1 (50 nM) and insulin (5 nM) after 48 h (Fig. 6a). The cell proliferation was suppressed by MK2206 (10 μM) and PPP (2.5 μM), but not by U0126 (10 μM) (Fig. 6b). Cleavages of caspase 3 and PARP were suppressed in RPMI1640 with 10% FBS (Fig. 6c), and induced by the addition of PPP (Fig. 6c). These data suggest that the proliferation of group 3 cell lines depends on IGF‐1R/Akt and on other not‐yet‐identified pathways.
Figure 6.
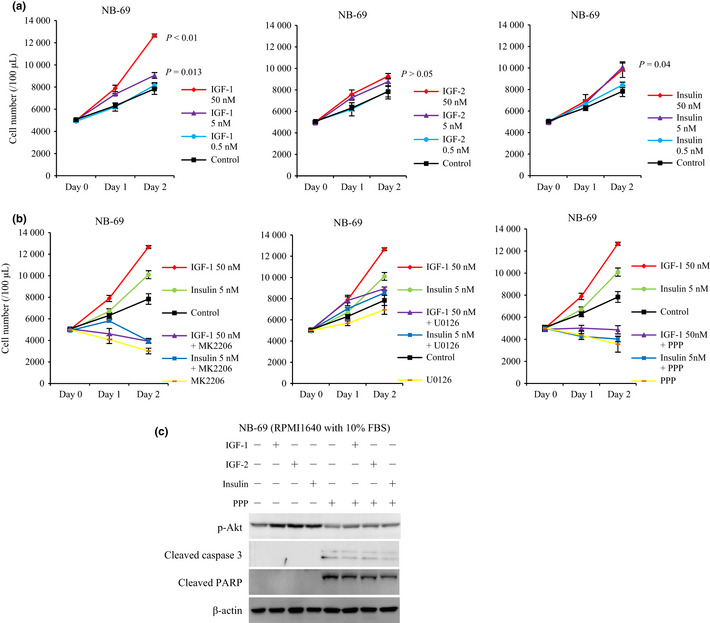
The effect of exogenous IGF and insulin on NB‐69 cell line in RPMI1640 with 10% FBS. (a) NB‐69 cell line was incubated in RPMI1640 with 10% FBS with/without IGF‐1, IGF‐2, and insulin at the indicated concentrations. Proliferation of the cells was evaluated as cell numbers at the indicated time points. The experiment was repeated three times, and statistical analysis was performed using a two‐sided t‐test, P < 0.01, significant different. (b) NB‐69 cell line subjected to MK2206 (10 μM), U0126 (10 μM) and picropodophyllin (PPP) (2.5 μM) with/without stimulatory ligands: IGF‐1 (50 nM) and insulin (5 nM) in RPMI1640 with 10% FBS. (c) NB‐69 cell line was pre‐incubated with/without PPP (2.5 μM) in RPMI1640 with 10% FBS for 12 h, before 1 h stimulation of IGF‐1 (5 nM), IGF‐2 (5 nM) and insulin (5 nM). Akt phosphorylation, cleaved caspase 3, and cleaved PARP were evaluated by WB. β‐actin was used as a control.
G2/M‐phase accumulation induced by insulin‐like growth factor 1 inhibitor (picropodophyllin)
We also analyzed cell cycle phase distribution in NB cells cultured in the presence of PPP, because PPP induced G2/M arrest and apoptosis by inhibiting IGF‐1R.23, 34 In our experiment, exposure to 2.5 μM PPP for 12 h increased the G2/M fraction of SK‐N‐SH and LAN‐1 cell lines, and shifted cell cycle profile from G0‐G1 dominant to G2/M dominant (Fig. 7). However, in the same condition, NB‐69 did not show G2/M arrest, NB‐69 only showed an elevation of G2/M from 27.1 to 37.6%, and the G0/G1 fraction was not affected (Fig. 7).
Figure 7.
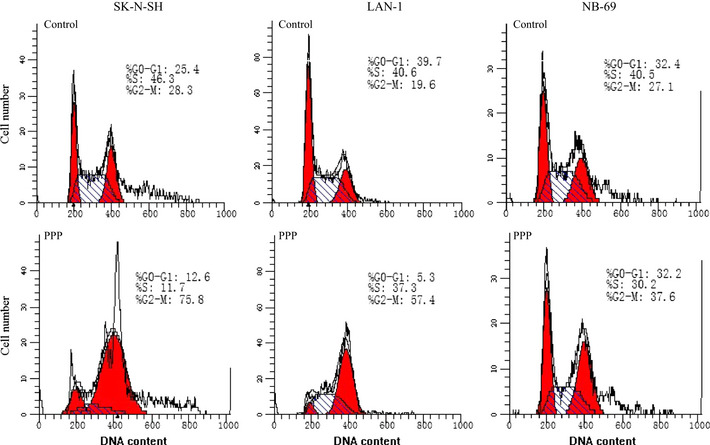
The effect of picropodophyllin (PPP) on cell cycle phase distribution. The cell lines were treated with/without PPP (2.5 μM) for 12 h followed by analysis of cell cycle phase distribution in RPMI1640 with 10% FBS.53 Cells were stained with propidium iodide for 30 min followed by FACScan flow cytometer.
In a parallel experiment, we also found that, in SK‐N‐SH and LAN‐1, cyclin B1, a marker protein of G2/M phase of cell cycle, was upregulated, and when the typical G2/M arrest occurred, cyclin D1, a marker protein of G0/G1, synchronously declined. In NB‐69 (group 3), accumulation of cyclin B1 was duplicated by PPP, but cyclin D1 was not affected (data not shown). This may be explained by the insensitiveness of group 3 cell lines to PPP‐induced G2/M arrest. The other NB cell lines, group 1 (INDEN and NB‐19), group 2 (SHEP, SMS‐KCNR, and KP‐N‐SI) and group 3 (OZAWA, KP‐N‐RT, and SMS‐KCN), showed the same patterns of SK‐N‐SH (group 1), LAN‐1 (group 2) and NB‐69 (group 3), respectively.
Discussion
In this study, we demonstrated that NB cell lines are heterogeneous in terms of their IGF‐1R/Akt pathway‐mediated cell proliferation. The cells can be categorized into three different groups (Fig. 8). Group 1 cell lines (autocrine IGF‐mediated cell lines) produce endogenous IGF; IGF‐1R/Akt pathway is activated by both exogenous and autocrine IGF; these cell lines can proliferate for more than 3 days in RPMI1640 with 10% FBS, insulin containing SFM and even in RPMI1640. Group 2 cell lines (exogenous IGF‐mediated cell lines) need the stimulation of exogenous stimulatory ligands, including IGF and insulin for cell proliferation; these cell lines can proliferate in SFM and RPMI1640 with 10% FBS, but not in RPMI1640. Group 3 cell lines (partially exogenous IGF‐mediated cell lines) can proliferate in RPMI1640 with 10% FBS, and their proliferation is accelerated by IGF and insulin only in the presence of FBS. These observations suggest that group 3 NB cell proliferation is mediated by both IGF and not‐yet‐identified growth factor presence in FBS. To our knowledge, this is the first study describing heterogeneity in the IGF‐1R/Akt pathway in NB cell lines. Subdivision of NB cell lines by their heterogeneity will help to further clarify the mechanism of IGF‐1R mediation.
Figure 8.
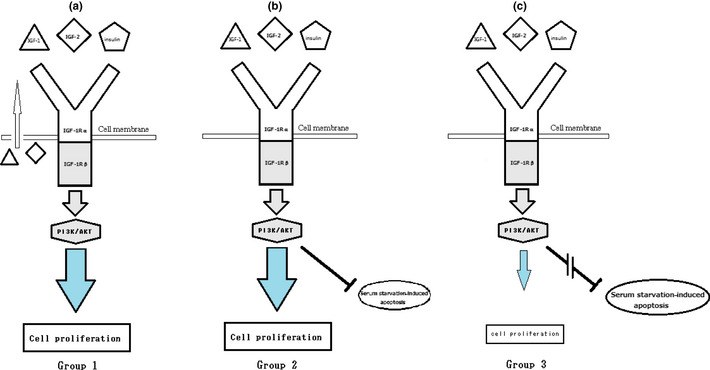
Schematic pathways of insulin‐like growth factor 1 receptor (IGF‐1R)/Akt‐mediated neuroblastoma (NB) cell proliferation in RPMI1640. (a) Proliferation of group 1 NB cell lines is mediated by IGF‐1R/Akt pathway. IGF‐1R/Akt pathway can be activated by IGF (autocrine or exogenous IGF) and insulin. (b) Proliferation of group 2 NB cell lines is mediated by IGF‐1R/Akt pathway. Stimulation of IGF‐1R/Akt pathway can be induced by exogenous stimulatory ligands such as IGF and insulin. Activated IGF‐1R/Akt pathway suppresses serum starvation‐induced apoptosis. (c) Activated IGF‐1R/Akt pathway can hardly accelerate the cell proliferation of group 3 NB cell lines. Activated IGF‐1R/Akt pathway cannot suppress serum starvation‐induced apoptosis.
Insulin‐like growth factor 1 receptor and insulin induced cell proliferation through Akt activation; blockage of IGF‐1R suppressed Akt activation only in group 1 and 2 cell lines. However, the blockage of IGF‐1R did not markedly affect Akt activation in group 3 cell lines cultured in RPMI1640. In contrast, the activation of Akt could not suppress serum starvation‐induced apoptosis in the partially exogenous IGF‐mediated NB cell lines (group 3). These findings may prompt reconsideration of cancer therapy targeting IGF‐1R and IGF.30 The anti‐IGF‐1R antibodies used in clinical trials, known as A12,35 h7C10,36 EM16437 and CP‐751, 871,38 affect IGF‐1R/Akt pathway, then cell apoptosis and cell proliferation. However, further in vivo experiments are required to support these observations.
Picropodophyllin, the IGF‐1R inhibitor, was able to effectively inhibit the exogenous IGF‐induced Akt phosphorylation in groups 1 and 2, but not in group 3. PPP blocks phosphorylation of tyrosine cluster Y1136 in IGF‐1R,26 which is the main binding site of insulin receptor substrate 1 (IRS‐1).39 IR substrate 2 (IRS‐2) interacts with IGF‐1R via multiple binding motifs,40 independent of tyrosine cluster Y1136 in IGF‐1R. Difference in IRS binding specificity may explain these observations. Our data also showed that NVP‐AEW541 (2.5 μM), a nonspecific IGF‐1R RTK inhibitor, suppressed the IGF‐1R/Akt pathway in all three groups (data not shown). These results indicate that the signal transduction from IGF‐1R to PI3K/Akt complex may be different in NB cell line groups. Both IRS‐1 and 2 are able to stimulate PI3K‐Akt signal pathway.41 IRS‐1 is important for mitogenesis, cell proliferation and survival, whereas IRS‐2 is important for adhesion, migration and metastasis.6, 42 We speculate that differences in binding of IRS to IGF‐1R may lead to different IGF‐1R/Akt‐mediated signals. Selective knockdown or overexpression of IRS‐1 or 2 are necessary to verify the relationship between IRS and IGF‐1R stimulation.
Picropodophyllin interrupts DNA synthesis by inhibition of IGF‐1R and inducing G2/M arrest.43 G2/M‐phase accumulation induced by PPP is due to interference with the CDK1/cyclin B1 complex.23 Cyclin B1 protein begins to increase during G2, peaks in mitosis, and is rapidly degraded before the cell cycle is completed; it is a specific marker of G2/M.44 Cyclin D1 is a G1‐specific cyclin that associates with CDK4 or CDK6, and promotes restriction point progression during G1 phase.45 Expression and accumulation of cyclin D1 occur at multiple levels, including increased transcription, translation and protein stability, and are affected by growth factors,46 or signal pathways including PI3K‐Akt pathway.47 Ectopic expression of cyclin D1 induces G2/M arrest.48 In response to PPP and MK2206, cyclin B1 accumulated in all three groups, but cyclin D1 was declined only in groups 1 and 2 NB cell lines, and not in group 3 (data not shown). These results indicate that IGF‐1R/Akt pathway exerts less effect on cyclin D1 in group 3 NB cell lines, and this may explain why group 3 NB cell lines are insensitive to PPP‐induced G2/M arrest.
Apoptosis and G2/M arrest induced by PPP were demonstrated in multiple myeloma cells23 and in other cancer cells. Alternative signaling of IGF‐1 and 2 may occur through other insulin‐related receptors, like the IR49 or heterodimerization of IGF‐1R with other receptors such as the epidermal growth factor receptor.50 These observations may explain the lack of efficacy of therapeutic antibodies since they exert no inhibitory activity on other receptors, as is the case for other receptor tyrosine kinase inhibitors.51, 52 Moreover, PPP has been shown to be well tolerated in vivo after oral administration.25
In summary, we showed in this study heterogeneity of the IGF‐1R/Akt pathway in several NB cells lines. NB cell lines can be categorized into three groups by the patterns of IGF‐1R/Akt pathway response. So far, the clinical and biological correlations of IGF‐1R response in tumors are poorly understood.6 Biomarkers are needed to predict clinical responses to IGF‐1R antagonists, and to select patients who can be benefited from IGF‐1R‐targeted therapy. We speculate that comparative analysis of the response between different groups with/without stimulatory ligands by using microarray analysis may be helpful to find novel biomarkers. The mechanism of this heterogeneous response could be the topic of future studies.
Disclosure Statement
The authors have no conflict of interest.
(Cancer Sci, doi: 10.1111/cas.12204, 2013)
References
- 1. Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Curr Opin Oncol 1998; 10: 43–51. [DOI] [PubMed] [Google Scholar]
- 2. Davidoff AM. Neuroblastoma. Semin Pediatr Surg 2012; 21: 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jögi A, Vaapil M, Johansson M, Påhlman S. Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors. Ups J Med Sci 2012; 17: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bentov I, Werner H. IGF, IGF receptor and overgrowth syndromes. Pediatr Endocrinol Rev 2004; 1: 352–60. [PubMed] [Google Scholar]
- 5. Werner H, Bruchim I. The insulin‐like growth factor‐1 receptor as an onogene. Arch Physiol Biochem 2009; 115: 58–71. [DOI] [PubMed] [Google Scholar]
- 6. Hartog H, Wesseling J, Boezen HM, van der Graaf WT. The insulin‐like growth factor 1 receptor in cancer: old focus, new future. Eur J Cancer 2007; 43: 1895–904. [DOI] [PubMed] [Google Scholar]
- 7. Gronborg M, Wulff BS, Rasmussen JS, Kjeldsen T, Gammeltoft S. Structure‐function relationship of the insulin‐like growth factor‐I receptor tyrosine kinase. J Biol Chem 1993; 268: 23435–40. [PubMed] [Google Scholar]
- 8. Brodt P, Samani A, Navab R. Inhibition of the type I insulin‐like growth factor receptor expression and signaling: novel strategies for antimetastatic therapy. Biochem Pharmacol 2000; 60: 1101–7. [DOI] [PubMed] [Google Scholar]
- 9. Laviola L, Natalicchio A, Giorgino F. The IGF‐I signaling pathway. Curr Pharm Des 2007; 13: 663–9. [DOI] [PubMed] [Google Scholar]
- 10. Pollak M. Insulin and insulin‐like growth factor signalling in neoplasia. Nat Rev Cancer 2008; 8: 915–28. [DOI] [PubMed] [Google Scholar]
- 11. Sartelet H, Oligny LL, Vassal G. Akt pathway in neuroblastoma and its therapeutic implication. Expert Rev Anticancer Ther 2008; 8: 757–69. [DOI] [PubMed] [Google Scholar]
- 12. Zinn RL, Gardner EE, Marchionni L et al ERK phosphorylation is predictive of resistance to IGF‐1R inhibition in small cell lung cancer. Mol Cancer Ther 2013; 12: 1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahindra A, Cirstea D, Raje N. Novel therapeutic targets for multiple myeloma. Future Oncol 2010; 6: 407–18. [DOI] [PubMed] [Google Scholar]
- 14. Barlaskar FM, Spalding AC, Heaton JH et al Preclinicaltargeting of the type I insulin‐like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 2009; 94: 204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zumkeller W, Schwab M. Insulin‐like growth factor system in neuroblastoma tumorigenesis and apoptosis: potential diagnostic and therapeutic perspectives. Horm Metab Res 1999; 31: 138–41. [DOI] [PubMed] [Google Scholar]
- 16. Deng H, Lin Y, Badin M et al Over‐accumulation of nuclear IGF‐1 receptor in tumor cells requires elevated expression of the receptor and the SUMO‐conjugating enzyme Ubc9. Biochem Biophys Res Commun 2011; 404: 667–71. [DOI] [PubMed] [Google Scholar]
- 17. Sehat B, Tofigh A, Lin Y et al SUMOylation mediates the nuclear translocation and signaling of the IGF‐1 receptor. Sci Signal 2010; 3: 10. [DOI] [PubMed] [Google Scholar]
- 18. Aleksic T, Chitnis MM, Perestenko OV et al Type 1 insulin‐like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res 2010; 70: 6412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin‐like growth factor I receptor. Oncogene 2003; 22: 6589–97. [DOI] [PubMed] [Google Scholar]
- 20. Mitsiades CS, Mitsiades NS, McMullan CJ et al Inhibition of the insulin‐like growth factor receptor‐1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004; 5: 221–30. [DOI] [PubMed] [Google Scholar]
- 21. Scotlandi K, Manara MC, Nicoletti G et al Antitumor activity of the insulin‐like growth factor‐I receptor kinase inhibitor NVPAEW541 in musculoskeletal tumors. Cancer Res 2005; 65: 3868–76. [DOI] [PubMed] [Google Scholar]
- 22. Warshamana‐Greene GS, Litz J, Buchdunger E et al The insulin‐like growth factor‐I receptor kinase inhibitor, NVP‐ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clinical Cancer Res 2005; 11: 1563–71. [DOI] [PubMed] [Google Scholar]
- 23. Stromberg T, Ekman S, Girnita L et al IGF‐1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M‐phase accumulation and apoptosis in multiple myeloma cells. Blood 2006; 107: 669–78. [DOI] [PubMed] [Google Scholar]
- 24. Tazzari PL, Tabellini G, Bortul R et al The insulin‐like growth factor‐I receptor kinase inhibitor NVP‐AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin‐like growth factor‐I secretion. Leukemia 2007; 21: 886–96. [DOI] [PubMed] [Google Scholar]
- 25. Girnita A, Girnita L, del Prete F et al Cyclolignans as inhibitors of the insulin‐like growth factor‐1 receptor and malignant cell growth. Cancer Res 2004; 64: 236–42. [DOI] [PubMed] [Google Scholar]
- 26. Vasilcanu D, Girnita A, Girnita L et al The cyclolignan PPP induces activation loop‐specific inhibition of tyrosine phosphorylation of the insulin‐like growth factor‐1 receptor. Link to the phosphatidyl inositol‐3 kinase/Akt apoptotic pathway. Oncogene 2004; 23: 7854–62. [DOI] [PubMed] [Google Scholar]
- 27. Klinakis A, Szabolcs M, Chen G et al Igf1r as a therapeutic target in a mouse model of basal‐like breast cancer. Proc Natl Acad Sci US 2009; 106: 2359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karasic TB, Hei TK, Ivanov VN. Disruption of IGF‐1R signaling increases TRAIL‐induced apoptosis: a new potential therapy for the treatment of melanoma. Exp Cell Res 2010; 316: 1994–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yin S, Girnita A, Strömberg T et al Targeting the insulin‐like growth factor‐1 receptor by picropodophyllin as a treatment option for glioblastoma. Neuro Oncol 2010; 12: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douglas Y. Insulin‐like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst 2012; 104: 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Dida F, Iwao A et al Cell cycle dependency of caspase activation in Fas‐induced apoptosis in leukemia cells. Cancer Sci 2007; 98: 1174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Varela‐Nieto I, Hartl M, Gorospe I, León Y. Anti‐apoptotic actions of insulin‐like growth factors: lessons from development and implications in neoplastic cell transformation. Curr Pharm Des 2007; 13: 687–703. [DOI] [PubMed] [Google Scholar]
- 33. Dupont J, Pierre A, Froment P, Moreau C. The insulin‐like growth factor axis in cell cycle progression. Horm Metab Res 2003; 35: 740–50. [DOI] [PubMed] [Google Scholar]
- 34. Doghman M, Axelson M, Lalli E. Potent inhibitory effect of the cyclolignanpicropodophyllin (PPP) on human adrenocortical carcinoma cells proliferation. Am J Cancer Res 2011; 1: 356–61. [PMC free article] [PubMed] [Google Scholar]
- 35. Yeh J, Litz J, Hauck P, Ludwig DL, Krystal GW. Selective inhibition of SCLC growth by the A12 anti‐IGF‐1R monoclonal antibody correlates with inhibition of Akt. Lung Cancer 2008; 60: 166–74. [DOI] [PubMed] [Google Scholar]
- 36. Mayeenuddin LH, Yu Y, Kang Z, Helman LJ, Cao L. Insulin‐like growth factor 1 receptor antibody induces rhabdomyosarcoma cell death via a process involving Akt and Bcl‐x(L). Oncogene 2010; 29: 6367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geoerger B, Brasme JF, Daudigeos‐Dubus E et al Anti‐insulin‐like growth factor 1 receptor antibody EM164 (murine AVE1642) exhibits anti‐tumour activity alone and in combination with temozolomide against neuroblastoma. Eur J Cancer 2010; 46: 3251–62. [DOI] [PubMed] [Google Scholar]
- 38. Kurmasheva RT, Dudkin L, Billups C et al The insulin‐like growth factor‐1 receptor‐targeting antibody, CP‐751,871, suppresses tumor‐derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res 2009; 69: 7662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tartare‐Deckert S, Sawka‐Verhelle D, Murdaca J, Van Obberghen E. Evidence for a differential interaction of SHC and the insulin receptor substrate‐1 (IRS‐1) with the insulin‐like growth factor‐I (IGF‐I) receptor in the yeast two‐hybrid system. J Biol Chem 1995; 270: 23456–60. [DOI] [PubMed] [Google Scholar]
- 40. He W, Craparo A, Zhu Y et al Interaction of insulin receptor substrate‐2 (IRS‐2) with the insulin and insulin‐like growth factor I receptors. Evidence for two distinct phosphotyrosine‐dependent interaction domains within IRS‐2. J Biol Chem 1996; 271: 11641–5. [DOI] [PubMed] [Google Scholar]
- 41. Stöhr O, Hahn J, Moll L et al Insulin receptor substrate‐1 and ‐2 mediate resistance to glucose‐induced caspase‐3 activation in human neuroblastoma cells. Biochim Biophys Acta 2011; 1812: 573–80. [DOI] [PubMed] [Google Scholar]
- 42. Chan BT, Lee AV. Insulin Receptor Substrates (IRSs) and breast tumorigenesis. J Mammary Gland Biol Neoplasia 2008; 13: 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ozkan EE. Plasma and tissue insulin‐like growth factor‐I receptor (IGF‐IR) as a prognostic marker for prostate cancer and anti‐IGF‐IR agents as novel therapeutic strategy for refractory cases: a review. Mol Cell Endocrinol 2011; 344: 1–24. [DOI] [PubMed] [Google Scholar]
- 44. Hwang A, Maity A, McKenna WG, Muschel RJ. Cell cycle‐dependent regulation of the cyclin B1 promoter. J Biol Chem 1995; 270: 28419–24. [DOI] [PubMed] [Google Scholar]
- 45. Sherr CJ. Mammalian G1 cyclins. Cell 1993; 73: 1059–65. [DOI] [PubMed] [Google Scholar]
- 46. Koziczak M, Hynes NE. Cooperation between fibroblast growth factor receptor‐4 and ErbB2 in regulation of cyclin D1 translation. J Biol Chem 2004; 279: 50004–11. [DOI] [PubMed] [Google Scholar]
- 47. Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl‐1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol 2013; 42: 1113–9. [DOI] [PubMed] [Google Scholar]
- 48. Loponen H, Ylikoski J, Albrecht JH, Pirvola U. Restrictions in cell cycle progression of adult vestibular supporting cells in response to ectopic cyclin D1 expression. PLoS ONE 2011; 6: e27360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sprynski AC, Hose D, Kassambara A, Vincent L et al Insulin is a potent myeloma cell growth factor through insulin/IGF‐1 hybrid. Leukemia 2010; 24: 1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riedemann J, Takiguchi M, Sohail M, Macaulay VM. The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun 2007; 355: 707–14. [DOI] [PubMed] [Google Scholar]
- 51. Haluska P, Carboni JM, Loegering DA et al In vitro and in vivo antitumor effects of the dual insulin‐like growth factor‐I/insulin receptor inhibitor, BMS‐554417. Cancer Res 2006; 66: 362–71. [DOI] [PubMed] [Google Scholar]
- 52. Huang F, Greer A, Hurlburt W et al The mechanisms of differential sensitivity to an insulin‐like growth factor‐1 receptor inhibitor (BMS‐536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res 2009; 69: 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vindelov LL. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions: a new method for rapid isolation and staining of nuclei. Virchows Arch B Cell Pathol 1977; 24: 227–42. [PubMed] [Google Scholar]


