Abstract
Early and specific diagnosis is critical for treatment of cholangiocarcinoma (CCA). In this study, a carbohydrate antigen‐S27 (CA‐S27) monoclonal antibody (mAb) was established using pooled CCA tissue‐extract as immunogen. The epitope recognized by CA‐S27‐mAb was a new Lewis‐a (Lea) associated modification of MUC5AC mucin. A Soybean agglutinin/CA‐S27‐mAb sandwich ELISA to determine CA‐S27 in serum was successfully developed. High level of CA‐S27 was detected in serum of CCA patients and could differentiate CCA patients from those of gastro‐intestinal cancers, hepatomas, benign hepatobiliary diseases and healthy subjects with high sensitivity (87.5%) and high negative predictive value (90.4%). The level of serum CA‐S27 was dramatically reduced after tumor removal, indicating tumor origin of CA‐S27. Patients with high serum CA‐S27 had significantly shorter survivals than those with low serum CA‐S27 regardless of serum MUC5AC levels. Fucosyltransferase‐III (FUT3) was shown to be a regulator of CA‐S27 expression. Suppression of CA‐S27 expression with siRNA‐FUT3 or neutralization with CA‐S27 mAb significantly reduced growth, adhesion, invasion and migration potentials of CCA cells in vitro. In summary, we demonstrate that serum CA‐S27, a novel carbohydrate antigen, has potential as diagnostic and prognostic markers for CCA patients. CA‐S27 involves in promoting cell growth, adhesion, migration and invasion of CCA cells.
Alteration of cellular glycosylation profiles during carcinogenesis and the correlation of glycosylation profiles with tumor progression have been shown in many studies.1, 2 These proteins are mostly found extracellularly on the plasma membrane and appear as secreted proteins in body fluid, which are readily accessible for diagnostic and therapeutic purposes.
Cholangiocarcinoma (CCA), the second most common liver cancer, has the highest global incidence in the northeast of Thailand.3 While CCA was rare worldwide in the past, the mortality‐incidence of CCA increased globally in the recent two to three decades.4, 5 CCA is a slow growing tumor with no early warning symptoms, resulting in a late diagnosis,6 where curative surgery is seldom possible.7 As therapeutic options increase, determining the malignant potential of a CCA may become important to match the appropriate therapy to the patient. Also, early detection of CCA could possibly allow for surgery before the tumor has metastasized. Hence, there is a great clinical need to improve the biomarkers for the detection and prediction of CCA.
In this study, we have established a carbohydrate antigen‐S27 (CA‐S27) monoclonal antibody (mAb) which recognizes a new Lewis‐a (Lea) associated epitope and evaluated the possibility of using CA‐S27 as diagnostic and prognostic markers for CCA. In addition, the functional role of CA‐S27 in tumor progression of CCA is first reported.
Materials and Methods
Patient samples and cell lines
Cholangiocarcinoma cell lines, sera and paraffin embedded tissues from histological proven intrahepatic CCA patients were obtained from the Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Thailand. Informed consent was obtained from each subject and the study protocol was approved by the Ethics Committee for Human Research, Khon Kaen University.
Human CCA cell lines were established from primary CCA tumor tissues with different histological types as described previously8, 9 (adenosquamous carcinoma, KKU‐M139; moderately differentiated, KKU‐M156 and KKU‐M214; adenosquamous with mix‐histological types, KKU‐M213; poorly differentiated type, and KKU100). An immortalized cholangiocyte cell line (MMNK1), a representative of normal cell, was a gift from Dr Kobayashi N.10 All cell lines were cultured in DMEM with 10% FBS, at 37°C and 5% CO2.
CA‐S27 mAb and identification of specific antigen recognized by CA‐S27 mAb
The establishment of CA‐S27 mAb and the characteristic of specific epitope of CA‐S27 mAb were identified as described previously.2 The CA‐S27 specific sugar was analyzed using a glycocojugate microarray.11
Immmunohistochemistry
CA‐S27 and MUC5AC in paraffin‐embedded tissue sections was performed according to the standard protocol. The sections were incubated with 5 μg/mL of CA‐S27‐mAb and 1:500 HRP conjugated goat anti‐mouse IgM (Southern Biotechnology, Birmingham, AL, USA). MUC5AC was determined using anti‐MUC5AC antibody.12 The immunoreactivity was developed and scored.2
Sandwich ELISA
Level of serum CA‐S27 was quantitated using soybean agglutinin (SBA) captured CA‐S27 mAb ELISA2 with 1 μg/mL CA‐S27 mAb and 1:4000 HRP‐conjugated goat anti‐mouse‐IgM. Serum MUC5AC was determined using MUC5AC‐SBA sandwich ELISA and considered as high level when OD > 0.074.13 The sandwich ELISA using anti‐MUC5AC mAb (clone 22C5) coupled with CA‐S27‐mAb was performed as described previously.2
Cell ELISA
Cells were fixed with 4% paraformaldehyde and blocked with 3% BSA in PBS‐Tween. After being incubated with CA‐S27 mAb (1 μg/mL) for 1 h, 1:2000 of HRP‐conjugated goat anti‐mouse IgM was added. The reactivity was detected using TMB substrate.
RNA extraction and PCR
Total RNA from cultured cells was extracted using RNeasy kit and converted to cDNA using SuperScript™ II Reverse Transciptase Kit according to the manufacturer's instructions. Expression of fucosyltransferase III (FUT3; GenBank ID: NM000149.3, NM001097639.1, NM001097640.1) was estimated by semi‐quantitative PCR and Western blotting. GAPDH and β‐actin were used as an internal control. All primers are listed in Table S1.
Glycosyltransferases profiling
The expression profiles of 186 glycosyltransferases of KKU‐M213 and MMNK1 were determined using quantitative RT‐PCR.14
CA‐S27 Immunocytofluorescent staining
Cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton‐×100 in PBS. After blocking, 2 μg/mL of CA‐S27 mAb was added followed by 1:500 of Alexa Fluor 488‐conjugated goat anti mouse‐IgM (Invitrogen, Carlsbad, CA, USA) and 1:2000 of Hoechst‐33342 (Molecular Probes, Eugene, OR, USA).
Transient knockdown of FUT3
KKU‐M139 (5 × 104 cells) were transfected with 100 pM of siFUT3 or siControl (Silencer Negative Control siRNA#1, Ambion, Austin, TX, USA) using Lipofectamin‐2000 (Invitrogen). The sequences of siRNA specific for FUT3 (siFUT3) are listed in Table S1.
Surface CA‐S27 neutralization
CA‐S27 epitopes on the cell surfaces were neutralized by incubating cells (2 × 104 cells) with 10 μg CA‐S27‐mAb in Opti‐MEM (Gibco), at 37°C for 2 h. Mouse IgM (Sigma, St. Louis, MO, USA) was used as non‐reactive IgM control.
Invasion and migration assays
The assays were performed according to the manufacturer's instruction using BioCoat Matrigel Invasion Chambers and Falcon Cell culture inserts (8 μm pore‐size, BD Biosciences). After 72 h of siRNA treatment or 2 h of antibody neutralization, 4 × 104 treated cells in Opti‐MEM were subjected to the chambers and allowed to migrate or invade for 20 h or 24 h, respectively. The migrated or invaded cells were fixed with 25% methanol, stained with 0.5% crystal violet and count under a microscope.
Adhesion assay
A 96‐well plate was pre‐coated with 50 μL of 50 μg/mL Matrigel or 10 μg/mL fibronectin in Opti‐MEM. After blocking, 2 × 104 cells were seeded and allowed to adhere for 2 h. The adhered cells were fixed with 30% methanol, stained with 0.5% crystal violet and count under a microscope.
Cell proliferation assay
Cells (3000 cells) were seeded in a 96‐well plate for 24 h, then the culture medium was replaced with 1% FBS in DMEM containing 1 μg of CA‐S27 mAb or IgM control. The cell number was measured at various times using CellTiter 96 AQueous Non‐Radioactive Cell Proliferation Assay (MTS; Promega).
Western blot analysis
Cells were lyzed with lysis buffer (8 M Urea, 2% CHAPS in 30 mM Tris‐HCl pH 8.5) and 5 μg cell lysate was subjected to a 12% SDS‐polyacrylamide gel according to Laemmli.15 The proteins were transferred onto a PVDF membrane, blocked by 3% BSA in PBS and probed by FUT3‐mAb16 at 4°C overnight followed by 1:1000 HRP‐conjugated goat anti mouse IgG (Invitrogen). The images of ECL signals were developed with ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA) and taken with ImageQuant 400 image analyzer and ImageQuant TL analysis software (GE Healthcare).
Statistical analysis
Statistical analysis was performed using spss 16.0 software (SPSS Inc., Chicago, IL, USA) and sigmastat 3.1 (Systat Software Inc., San Jose, CA, USA) as described previously.2 A P‐value <0.05 was considered statistically significant.
Results
CA‐S27 mAb recognizes CA‐S27 in tumor tissues and serum of CCA patients
We have established mAbs using pooled tumor tissues from CCA patients as immunogen.2 SBA‐captured ELISA and cell‐ELISA were used for hybridoma screening with serum glycoproteins and cell surface molecules, respectively. A CA‐S27 mAb was selected as it exhibited higher reactivity with pooled CCA sera than with healthy subjects' sera (Fig. 1a) and showed stronger reaction with CCA cells (KKU‐M213) than normal bile duct epithelium cells (MMNK1) as shown by cell ELISA (Fig. 1a) and CA‐S27‐immunocytochemical fluorescent staining (Fig. 1b).
Figure 1.

CA‐S27 mAb recognizes carbohydrate antigen related to Lea. (a) Using SBA‐CA‐S27 sandwich ELISA and cell ELISA, CA‐S27 mAb exhibited higher reactivity with pooled cholangiocarcinoma (CCA) serum than healthy sera (HE); and with CCA cell line (KKU‐M213) than normal biliary cells (MMNK1). (b) Immunofluorescent staining using CA‐S27‐mAb showed positive staining of KKU‐M213 but not MMNK1 cells. (c) Pre‐treated samples with NaIO 4 prior reacted with CA‐S27‐mAb diminished CA‐S27 reactivity observed in CCA serum and tissues. (d) Only Lea epitope was recognized by CA‐S27‐mAb in the glycoconjugate microarray. (e) LC/MS/MS analysis revealed that CA‐S27 associated glycoprotein contained a peptide mapped on MUC5AC sequence with a MASCOT score of 35.
To characterize the CA‐S27 epitope, tumor tissues and serum from CCA patients were pre‐treated with NaIO4 or protease enzymes prior to react with CA‐S27 mAb. CA‐S27 mAb failed to react with the NaIO4‐treated serum and tissue while the protease treated samples remained actively reacted with CA‐S27 mAb (Fig. 1c). These results indicated that CA‐S27 mAb recognized the glycan as the antigen epitope. Using glycoconjugate‐microarray, the glycan epitope was analyzed to be Lea associated glycan (Fig. 1d) without cross reactivity to any members of Lewis antigens‐sLea, Leb, etc.
MUC5AC mucin was the major source of CA‐S27 detected in serum
To determine the core protein of CA‐S27 found in serum, pooled CCA sera was subjected to a sepharose‐6B chromatography column, SDS‐polyacrylamide gel electrophoresis and western blotting. Results obtained from the sepharose‐6B column and western blot (Fig. S1A,B) indicated a high molecular weight of CA‐S27 associated glycoprotein. LC/MS/MS analysis of the bound fraction from CA‐S27‐affinity chromatography column demonstrated MUC5AC mucin as the CA‐S27 associated glycoprotein found in serum (Fig. 1e). The result was confirmed by the positive reactivity of serum proteins from sandwich ELISA using CA‐S27‐mAb coupled with anti‐MUC5AC (Fig. S1C). The correlation between serum CA‐S27 and serum MUC5AC levels was indicated by a scatter plot (r = 0.623; P < 0.001) (Fig. S1D).
CCA tissues exhibited high reactivity of CA‐S27
To investigate whether CA‐S27 was the bile duct epithelium origin, we performed immunohistochemistry (IHC) of CCA patient tissues using CA‐S27‐mAb as primary antibody. No CA‐S27 reactivity was found in hepatocytes but the high signal was detected in the bile duct epithelia of normal liver tissue and CCA cells (Fig. 2a), suggesting the biliary epithelium origin of the CA‐S27 found in serum. As IHC of 45 histologically proven CCA cases revealed that almost all CCA tissues (43 of 45, 95.5%) expressed high reactivity of CA‐S27, hence no analysis of the relationship between CA‐S27 expression and clinico‐pathological findings of the patients can be assessed.
Figure 2.

Immunohistochemistry of CA‐S27 in cholangiocarcinoma (CCA) tissues indicated biliary epithelium origin of CA‐S27. (a) High signal of CA‐S27 was detected in normal bile duct epithelia (arrow), hyperplasia (HP)/dysplasia (DP) and CCA. The serial sections of z tissues were also positive for MUC5AC mucin. (b) Serial sections of liver tissues from cadaveric donors, fetus and hepatoma patients (HCC) were positive with CA‐S27 but not MUC5AC. H, hepatocytes.
As MUC5AC mucin is rarely expressed in normal biliary epithelia, we questioned whether CA‐S27 detected in normal biliary cells was conjugated to MUC5AC mucin. The IHC using CA‐S27 mAb and MUC5AC antibody performed on the serial sections of normal liver tissues obtained from cadaveric donors, fetus, CCA and hepatoma patients showed that all normal biliary cells of the tested samples were positive for CA‐S27 but not MUC5AC mucin (Fig. 2b). These results implied that the positive signal of CA‐S27 found in normal bile duct epithelia was unlikely to be conjugated on MUC5AC mucin.
Serum CA‐S27 as a diagnostic indicator for CCA
The level of CA‐S27 in serum was determined using SBA/CA‐S27 mAb sandwich ELISA in 96 intrahepatic CCA patients and 190 controls, including 52 patients with active Opisthorchis viverrini infection, 39 with benign biliary diseases (adenoma = 1, cholangitis = 21, cholestasis = 6, cholecystitis = 6, cysdenoma = 1, hemangioma = 2, granuloma = 1, reactive hyperplasia = 1), 48 with gastro‐intestinal carcinoma (hepatoma = 10, pancreatic cancer = 7, colon cancer = 13, stomach cancer = 13, and cancer of ampula of Vater = 3) and 51 healthy subjects. Serum CA‐S27 of CCA patients (mean ± SD = 0.206 ± 0.234) was significantly higher than those of control groups (0.029 ± 0.029) (P < 0.001; Fig. 3a). Receiver operating characteristic (ROC) analysis revealed the significance of serum CA‐S27 level in distinguishing CCA patients from the control groups (P < 0.001) with the area under the curve of 0.822 (Fig. 3b). The cut‐off value obtained from the ROC curve was 0.0268, which provided 87.5% (84/96) sensitivity, 58.8% (113/192) specificity, 51.5% and 90.4% of positive and negative predictive values. In addition, serum CA‐S27 levels of post‐operative sera from CCA patients were significantly decreased after tumor removal (P < 0.05; Fig 3c), supporting the tumor origin of CA‐S27 found in serum.
Figure 3.
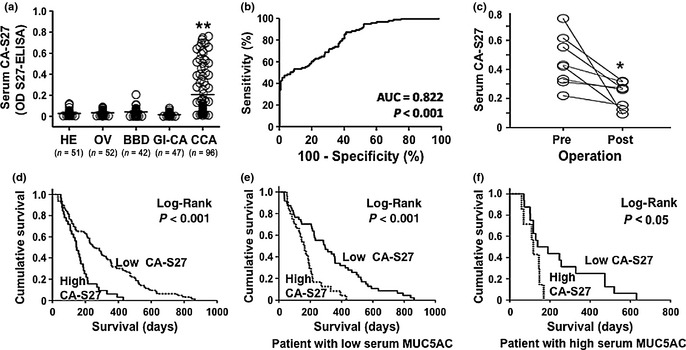
Serum CA‐S27 as diagnostic and prognostic indicators for cholangiocarcinoma (CCA). (a) Level of serum CA‐S27 was significantly higher in CCA patients than healthy people (HE), patients of Opisthorchis viverrini (OV)‐infection, benign biliary diseases (BBD) and gastro‐intestinal cancers (GI‐CA). (b) Receiver operating characteristic (ROC) analysis of serum CA‐S27 revealed the significant distinction between CCA patients and control (P < 0.001). (c) Serum CA‐S27 of post‐operative serum were significantly decreased compared with those of the corresponded pre‐operative sera (n = 8). (d) Survival analysis revealed that CCA patients with high serum CA‐S27 had significantly worse survival than those with low serum CA‐S27. (e–f) Regardless of the level of serum MUC5AC, CCA patients with high serum CA‐S27 had worse survival compared with the patients with low serum CA‐S27. *P < 0.05; **P < 0.001 (t‐test).
High level of serum CA‐S27 indicated poor survival of patients
To signify the prognostic relevance of serum CA‐S27, CCA patients were categorized into low (n = 64) and high (n = 32) CA‐S27 according to the mean value of CCA group (OD450 nm = 0.206). Survival analysis using Kaplan–Meier plot and Log‐Rank test revealed that CCA patients with low serum CA‐S27 had a better survival than those with high serum CA‐S27 (P < 0.001; Fig. 3d). The median survival time of patients with low versus high serum CA‐S27 were 256 days (95% confidence interval [CI]; 185–326 days) versus 145 days (95%CI; 115–174 days), respectively. In order to investigate the influence of CA‐S27 on the overall survival of patients in relation to MUC5AC, the survival rates of patients between low and high CA‐S27 were compared in the low MUC5AC (Fig. 3e) and high MUC5AC groups (Fig. 3f). Regardless of serum MUC5AC level, patients with high serum CA‐S27 had a significantly shorter survival than those with low serum CA‐S27.
The univariate analysis indicated that age, gender, histopathology type, tumor staging and bilirubin level had no influence on the level of serum CA‐S27. Only serum MUC5AC was found to be significantly correlated with level of serum CA‐S27 (P < 0.001, Table S2). In addition, serum CA‐S27 together with age, histopathology and staging of tumor were independent indicators for poor survival of CCA patients as shown by multivariate analysis using Cox‐proportional hazard (P < 0.001; Table 1).
Table 1.
Multivariate analysis of serum CA‐S27 in cholangiocarcinoma (CCA) patients
| Variable | n | Adjusted HR | 95% CI | P‐value |
|---|---|---|---|---|
| Serum CA‐S27 | ||||
| Low (OD ≤ 0.206) | 64 | 1 | 1.229–4.419 | 0.009 |
| High (OD > 0.206) | 32 | 2.271 | ||
| Age | ||||
| ≤56 years | 52 | 1 | 1.072–2.567 | 0.023 |
| >56 years | 44 | 1.659 | ||
| Gender | ||||
| Male | 69 | 1 | 0.496–1.251 | NS |
| Female | 27 | 0.788 | ||
| Histopathology | ||||
| Papillary type | 21 | 1 | 1.477–4.709 | 0.001 |
| Non‐papillary type | 75 | 2.638 | ||
| Tumor stage | ||||
| I–III | 14 | 1 | 1.287–4.515 | 0.006 |
| IVA and IVB | 82 | 2.411 | ||
CI, confidence interval; HR, hazard ratio; OD, optical density.
CA‐S27 expression was associated with FUT3 expression
To evaluate the functional role of CA‐S27, the glycosyltransferase profiles in KKU‐M213 and MMNK1 were quantified using RT‐PCR array.14 Of 98 glycosyltransferases tested, FUT3 (the key enzyme for Le synthesis) was highly expressed in KKU‐M213 with 461 times higher than that of the MMNK1 (Table S3). The expressions of the four top glycosyltransferase genes, namely FUT1, FUT2, FUT3 and ST3Gal1, in CCA cell lines were quantified by quantitative (q)RT‐PCR. It was obvious that FUT3 was the major glycosyltransferase found in the CA‐S27 positive CCA cell lines (M139, M213 and M214) but was undetectable in CA‐S27 negative CCA cells, KKU100 (Fig. S2).
The expression levels of FUT3 and CA‐S27 of CCA cell lines were determined by RT‐PCR and Western blotting. FUT3 was differentially expressed in the order of KKU‐M156 > KKU‐M139 and KKU‐M214 but was weakly expressed in KKU100 (Fig. 4a). The immunocytofluorescent staining of CA‐S27 indicated the corresponding positive CA‐S27 staining and the level of FUT3 expression in CCA cell lines.
Figure 4.

Expression of CA‐S27 related to FUT3 activity and metastatic potential of cholangiocarcinoma (CCA) cell lines. (a) CCA cell lines differentially expressed FUT3 as determined by semi‐quantitative PCR (qPCR), and western blotting; the CA‐S27 immunocytofluorescent staining of CCA cell lines are corresponded to FUT3 levels. (b) siFUT3 treatment markedly decreased FUT3 mRNA and protein, and reduced CA‐S27 immunocytofluorescent staining of KKU‐M139 cells. (c) The ability of KKU‐M139 in invasion, migration, and adhesion to extracellular matrix (ECM) and fibronectin (FN) were significantly decreased after FUT3 knocked down. Compared with the IgM‐treated cells, CCA cell lines treated with CA‐S27‐mAb significantly decreased (d) cell invasion, migration; (e) cell adhesion; and (f) cell proliferation of KKU‐M139, KKU‐M156, KKU‐M214 but not KKU‐100. Cell proliferation rates of CA‐S27 positive cells (KKU‐M139, KKU‐M156, KKU‐M214) were significantly higher than that of negative cells (KKU‐100). (*P < 0.05, t‐test) The assays were performed in triplicate and repeated at least twice in separated experiments. (IgM treated, dotted lines and white bar; CA‐S27 mAb treated, solid lines and black bar).
Suppression CA‐S27 significantly decreased the metastatic potential of CCA cell lines
To address the functional importance of CA‐S27, we used RNAi to deplete the expression of FUT3 in KKU‐M139. GAPDH was detected to assess the specificity of siFUT3 and used as an internal control. The efficiency of siFUT3 was demonstrated by RT‐PCR and Western blotting. siFUT3 significantly suppressed FUT3 expression of KKU‐M139 to 45% of the siControl cells and almost all positive signals of CA‐S27 disappeared as demonstrated by CA‐S27‐immunocytofluorescent cell staining (Fig. 4b). Suppression of CA‐S27 expression by siFUT3 consequently decreased as compared to the controls in the number of cell invaded and migrated (Fig. 4c). Given control as 100%, the relative numbers of invaded (33 ± 18%) and migrated cells (61 ± 20%) in the siFUT3 treated group were lower than those of the controls (P < 0.05). A similar observation was made from the adhesion assay. The number of adhered cells for siFUT3 treated cells were 40 ± 18% for ECM and 59 ± 20% for FN adhesion assay as compared to the siControl (P < 0.05).
The roles of CA‐S27 on cell invasion, migration and adhesion were further addressed using four CCA cell lines which differentially expressed CA‐S27. Neutralizing of surface CA‐S27 with CA‐S27 mAb significantly reduced the numbers of cells invaded, migrated (Fig. 4d) and adhered (Fig. 4e) of KKU‐M139, KKU‐M156 and KKU‐M214 to approximately 40% of the isotype‐matched (IgM) controls. In contrast, the similar treatment did not alter the above functions of KKU‐100 that had low CA‐S27 expression.
Suppression of CA‐S27 significantly decreased cell proliferation
The role of CA‐S27 on cell proliferation of CCA cell lines was evaluated. The growths of CCA cell lines with high and low expression of CA‐S27 were compared. Cell lines with high expression of CA‐S27‐namely KKU‐M139, KKU‐M156 and KKU‐M214 had significantly higher growth rates than KKU‐100 with low expression of CA‐S27 (P < 0.05; Fig. 4f). The proliferation of CA‐S27 expressing cells was suppressed 2–6‐fold when cells were treated with CA‐S27 mAb as compared with the cells treated with IgM control (P < 0.05; Fig. 4f). However, the similar treatment did not suppress growth of KKU100 with low CA‐S27 expression. The growth suppression observed was not due to apoptosis since the flow cytometry and acrydine orange staining assay indicated that the number of apoptotic cells in CA‐S27‐mAb treated cells did not differ from those of IgM treated control (Fig. S3).
Discussion
In the present study, a CA‐S27 mAb recognizing CA‐S27 with Lea associated epitope, was established. We demonstrated for the first time that CA‐S27 is a novel CCA associated marker. First, high levels of CA‐S27 were detected in serum from CCA patients as compared to the controls, suggesting the diagnostic value of serum CA‐S27 for CCA. Second, serum CA‐S27 was shown to be of tumor origin and associated with poor patient outcome which underscored the prognostic value of serum CA‐S27. Third, CA‐S27 significantly contributed to the metastatic activity and growth of CCA cells.
In the current study, CA‐S27 mAb was established and the epitope was demonstrated to be Lea associated glycan on serum MUC5AC mucin. The molecular features of CA‐S27 core protein in serum were characterized by gel filtration, SDS‐PAGE/western blotting, affinity chromatography‐LC/MS/MS analyses, and MUC5AC captured CA‐S27 mAb ELISA. All of these findings indicated MUC5AC mucin as a source of CA‐S27 detected in serum. CA‐S27 detected in serum of CCA patients was shown to be the tumor origin, as CA‐S27 was detected in CCA tissues and its level in serum was dramatically reduced after tumor removal. This finding was supported by the fact that positive signals of CA‐S27 in CCA tissues and the level of CA‐S27 in serum were associated with the expression of MUC5AC in tissues and serum.
Aberrantly high levels of MUC5AC mucin in tissues and serum from CCA patients has been reported.2, 17 We have shown previously that such expression was not related to tumor size.12 Therefore the level of serum CA‐S27 may not be directly related to the size of the tumor per se but rather to the level of MUC5AC mucin originating from the tumor. This speculation may support the observation in this study that serum CA‐S27 did not correlate with histopathology type and tumor staging. In addition, in patients with high levels of serum MUC5AC mucin, one would therefore expect to observe a reduction of serum CA‐S27 level when the tumor was removed. This expectation was confirmed by our demonstration that the level of serum CA‐S27 decreased significantly after tumor tissue removal (Fig. 3c). Normal biliary epithelia also expressed CA‐S27 but not MUC5AC mucin. The CA‐S27 detected in normal biliary cells is probably attached to different core glycoproteins other than MUC5AC mucin. This notion is supported by the fact that normal biliary epithelia of liver tissues from CCA patients and non CCA related subjects were positive for CA‐S27 but not for MUC5AC mucin.
In this study, we successfully developed SBA/CA‐S27 mAb sandwich ELISA to determine CA‐S27 in serum. SBA, a GalNAc‐α3Gal binding lectin, was previously shown to bind specifically to glycoproteins in the serum of patients with CCA and benign biliary diseases but not those of healthy persons and non‐biliary disease subjects,18 and was used effectively to capture CCA‐associated serum glycoproteins for the MUC5AC mucin sandwich ELISA system.13 In this study, SBA/CA‐S27 mAb sandwich ELISA was developed to detect two distinct glycans; GalNAc‐α3Gal and Lea associated glycan on MUC5AC mucin. The ELISA system provided a high sensitivity and specificity in distinguishing sera of CCA patients from the non‐CCA controls The levels of serum CA‐S27 of CCA patients were significantly higher than those of the control groups, allowing one to effectively differentiate CCA patients from the patients with gastro‐intestinal cancers, hepatomas, benign hepatobiliary diseases and healthy subjects. Our analysis had high sensitivity (87.5%) and high negative predictive value (90.4%). Unlike CA19‐9,19, 20 serum CA‐S27 is not associated with cholestasis or serum bilirubin level, which raised the advantage of CA‐S27 than CA19‐9 in being the marker for CCA. However, it should be noted that CA‐S27 may limit the liver fluke associated CCA as the vast majority of CCA cases in this study are secondary to parasitic infection and may not fully reflect CCA with other predisposing risk factors such as sclerosing cholangitis.
The level of serum CA‐S27 was significantly associated with survival of CCA patients. Since high level of serum MUC5AC has been shown to be associated with poor survival of CCA patients13, 17 and CA‐S27 detected in serum from CCA patients was identified to be conjugated to MUC5AC mucin, therefore we further investigated whether CA‐S27 or MUC5AC mucin had more influence on the survival of CCA patients. It is shown that patients with high serum CA‐S27 had a shorter survival than those with low serum CA‐S27 regardless of serum MUC5AC levels (Fig. 3e,f). Moreover, the level of serum CA‐S27 was found to be an independent prognostic factor that influenced the patients' survival. Patients with high serum CA‐S27 had 2.3 times higher risk of death than those with low serum CA‐S27 (Table 1).
Although a functional role for MUC5AC mucin has never been demonstrated in CCA, the potential roles of MUC5AC on cell adhesion and invasion were clearly shown in pancreatic cancer cells (SW1990 and BxPC3) in vitro and in vivo.21 Therefore, a significant role for MUC5AC mucin in metastasis of CCA cannot be excluded. Conversely, as shown in the current study, significant reduction of cell migration, invasion, adhesion and proliferation were observed after suppression of CA‐S27 expression by FUT3‐siRNA without alteration of MUC5AC mucin expression. Based on this observation, CA‐S27 may have an additional metastatic role to MUC5AC mucin and hence patients with high levels of serum CA‐S27 exhibited a worse prognosis in spite of MUC5AC levels (Fig. 3d–f).
Using a glycoconjugate‐microarray, the glycan epitope of CA‐S27 was found to be an Lea‐associated glycan (Fig. 1d) without cross reactivity with other Lewis antigens‐sLea, Leb, etc. We hypothesized that CA‐S27 mAb may recognize a structurally complex Lea polymer rather than the monomer form based on the following evidence. (i) Lea polymers not Lea monomers were immobilized on the glycoconjugate‐microarray; (ii) CA‐S27 mAb failed to react with the Lea positive blood cells (data not shown). As a result, CA‐S27 mAb is more likely to react with a complex 3‐dimensional structure or the topology of Lea polymer rather than the Lea monomer. Lea is a trisaccharide of Gal‐β1,3(Fuc‐α1,4)‐GlcNAc, which is expressed in epithelia of several gastro‐intestinal organs. Our study demonstrated, for the first time, the clinical significance of CA‐S27, a Lea associated epitope. Silencing of FUT3 expression using siRNA targeting to FUT3 significantly suppressed the expression of CA‐S27 in KKU‐M139 and resulted in the significant decrease in cell proliferation, invasion, migration, and adhesion of KKU‐M139.
Suppression of FUT3 not only affects the expression of Lea but also other Lewis antigens22, 23 such as sLea and sLex, therefore neutralizing CA‐S27 with mAb specifically to CA‐S27 is a more precise approach to determine the possible role of CA‐S27. The significant role of CA‐S27 in tumor progression was demonstrated in four CCA cell lines with various degrees of CA‐S27 expression. Neutralization of CA‐S27 expression using CA‐S27 mAb significantly reduced cell proliferation, invasion, migration and adhesion of KKU‐M139, KKU‐M156 and KKU‐M214 but not those of KKU‐100, which had low CA‐S27 expression. The mechanism by which CA‐S27 involved in tumor progression is not yet clear and more experiments are needed to address this point. The functional role of CA‐S27 in promoting cancer progression suggests a function for CA‐S27 in cellular and molecular signaling which possibly underlined the patients' poor outcome.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. MUC5AC mucin was the major source of CA‐S27 detected in serum.
Fig. S2. FUT3 was the major glycosyltransferase of cholangiocarcinoma (CCA) cell lines.
Fig. S3. S27‐mAb decreased cholangiocarcinoma (CCA) cell proliferation by cell growth suppression but not apoptosis induction.
Table S1. List of Primers and siRNA.
Table S2. Correlation of serum CA‐S27 and clinical data of cholangiocarcinoma (CCA) patients.
Table S3. Glycosyltransferase expression profile of KKU‐M213 and MMNK1.
Acknowledgments
This work was supported by the NRU Project of Thailand through SHeP‐GMS, Khon Kaen University; and the Research Team Strengthening Grant, National Genetic Engineering and Biotechnology Center, NSTDA, Thailand (to SW). We are grateful to JASSO (to AS), the Ministry of Health Labour and Welfare of Japan (to NA); a Grant‐in‐Aid for Science Research in a Priority Area from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Advanced Education Program for Integrated Clinical, Basic and Social Medicine, Kumamoto University and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases (to NS); the Adaptable and Seamless Technology Transfer Program through Target‐driven R&D to K.K., Heiwa Nakajima Foundation (to SO, SW, KV, and KK). We thank Professor G. J. Riggins, Johns Hopkins University, for assistance with the English‐language presentation; and A. Romphruk, Faculty of Medicine, Khon Kaen University for determination of Lea blood group on the referent red cells.
(Cancer Sci 2013; 104: 1278–1284
References
- 1. Hakomori S. Aberrant glycosylation in tumors and tumor‐associated carbohydrate antigens. Adv Cancer Res 1989; 52: 257–331. [DOI] [PubMed] [Google Scholar]
- 2. Silsirivanit A, Araki N, Wongkham C et al A novel serum carbohydrate marker on mucin 5AC: values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer 2011; 117: 3393–403. [DOI] [PubMed] [Google Scholar]
- 3. IARC . Opisthorchis viverrini and Clonorchis sinensis. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens. 100. Lyon, France: International Agency for Research on Cancer; 2011; 347–76. [Google Scholar]
- 4. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001; 33: 1353–7. [DOI] [PubMed] [Google Scholar]
- 5. Shaib YH, Davila JA, McGlynn K, El‐Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004; 40: 472–7. [DOI] [PubMed] [Google Scholar]
- 6. Morise Z, Sugioka A, Tokoro T et al Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World J Hepatol 2010; 2: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guglielmi A, Ruzzenente A, Campagnaro T et al Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009; 33: 1247–54. [DOI] [PubMed] [Google Scholar]
- 8. Sripa B, Leungwattanawanit S, Nitta T et al Establishment and characterization of an opisthorchiasis‐associated cholangiocarcinoma cell line (KKU‐100). World J Gastroenterol 2005; 11: 3392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obchoei S, Weakley SM, Wongkham S et al Cyclophilin A enhances cell proliferation and tumor growth of liver fluke‐associated cholangiocarcinoma. Mol Cancer 2011; 10: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maruyama M, Kobayashi N, Westerman KA et al Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation 2004; 77: 446–51. [DOI] [PubMed] [Google Scholar]
- 11. Tateno H, Mori A, Uchiyama N et al Glycoconjugate microarray based on an evanescent‐field fluorescence‐assisted detection principle for investigation of glycan‐binding proteins. Glycobiology 2008; 18: 789–98. [DOI] [PubMed] [Google Scholar]
- 12. Boonla C, Sripa B, Thuwajit P et al MUC1 and MUC5AC mucin expression in liver fluke‐associated intrahepatic cholangiocarcinoma. World J Gastroenterol 2005; 11: 4939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bamrungphon W, Prempracha N, Bunchu N et al A new mucin antibody/enzyme‐linked lectin‐sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett 2007; 247: 301–8. [DOI] [PubMed] [Google Scholar]
- 14. Ito H, Kuno A, Sawaki H et al Strategy for glycoproteomics: identification of glyco‐alteration using multiple glycan profiling tools. J Proteome Res 2009; 8: 1358–67. [DOI] [PubMed] [Google Scholar]
- 15. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–5. [DOI] [PubMed] [Google Scholar]
- 16. Kimura H, Kudo T, Nishihara S et al Murine monoclonal antibody recognizing human alpha(1,3/1,4)fucosyltransferase. Glycoconj J 1995; 12: 802–12. [DOI] [PubMed] [Google Scholar]
- 17. Boonla C, Wongkham S, Sheehan JK et al Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer 2003; 98: 1438–43. [DOI] [PubMed] [Google Scholar]
- 18. Luengpailin S, Wongkham S, Wongkham C et al Demonstration of a biliary‐associated glycoprotein in human serum. Clin Chim Acta 1996; 244: 237–40. [DOI] [PubMed] [Google Scholar]
- 19. Dogan UB, Gumurdulu Y, Golge N, Kara B. Relationship of CA 19‐9 with choledocholithiasis and cholangitis. Turk J Gastroenterol 2011; 22: 171–7. [DOI] [PubMed] [Google Scholar]
- 20. Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19‐9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol 2011; 9: 434–9. [DOI] [PubMed] [Google Scholar]
- 21. Yamazoe S, Tanaka H, Sawada T et al RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res 2010; 29: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aubert M, Panicot‐Dubois L, Crotte C et al Peritoneal colonization by human pancreatic cancer cells is inhibited by antisense FUT3 sequence. Int J Cancer 2000; 88: 558–65. [DOI] [PubMed] [Google Scholar]
- 23. Yin X, Rana K, Ponmudi V, King MR. Knockdown of fucosyltransferase III disrupts the adhesion of circulating cancer cells to E‐selectin without affecting hematopoietic cell adhesion. Carbohydr Res 2010; 345: 2334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. MUC5AC mucin was the major source of CA‐S27 detected in serum.
Fig. S2. FUT3 was the major glycosyltransferase of cholangiocarcinoma (CCA) cell lines.
Fig. S3. S27‐mAb decreased cholangiocarcinoma (CCA) cell proliferation by cell growth suppression but not apoptosis induction.
Table S1. List of Primers and siRNA.
Table S2. Correlation of serum CA‐S27 and clinical data of cholangiocarcinoma (CCA) patients.
Table S3. Glycosyltransferase expression profile of KKU‐M213 and MMNK1.


