Abstract
Cancer development is often preceded by the appearance of preneoplastic lesions. In gastric carcinogenesis, chronic inflammation and histopathologic progression of the stomach epithelium lead to the development of metaplasia and eventually adenocarcinoma. The cell surface protein CD44, especially its variant isoforms (CD44v), has been implicated in metaplasia–carcinoma sequence progression in the stomach. We recently found that CD44v interacts with and stabilizes xCT, a subunit of the cystine transporter system xc(–), in cancer cells and thereby increases cystine uptake and confers resistance to various types of cellular stress in vivo. The functional relevance of CD44v and xCT in the development of preneoplastic lesions, however, has remained unknown. We have now examined the role of the CD44v‐xCT system in the development of spasmolytic polypeptide‐expressing metaplasia (SPEM) in mouse models of gastric carcinogenesis. CD44v was found to be expressed de novo in SPEM, and CD44v+ metaplastic cells manifested upregulation of xCT expression compared with CD44v− cells. Genetic ablation of CD44 or treatment with sulfasalazine, an inhibitor of xCT‐dependent cystine transport, suppressed the development of SPEM and subsequent gastric tumor growth. Therapy targeted to CD44v‐xCT could thus prove effective for prevention or attenuation of the CD44v‐dependent development of preneoplastic lesions and cancer.
Gastric cancer is strongly linked to inflammation and histopathologic progression of the stomach epithelium initiated by chronic infection with Helicobacter pylori. Gastric carcinogenesis triggered by infection with this bacterium is thus characterized by a transition from normal mucosa to gastritis, which then leads to metaplasia and eventually to adenocarcinoma.1 Spasmolytic polypeptide‐expressing metaplasia (SPEM) results from a metaplastic change in H. pylori‐infected gastric mucosa and is observed in the mucosa adjacent to gastric adenocarcinoma.2, 3, 4
We previously showed that prostaglandin E2 (PGE2) and the inflammatory cytokine tumor necrosis factor‐α play key roles in the development of SPEM in mice.5 More recently, interleukin‐1β has been shown to initiate the development of gastric metaplasia and dysplasia in transgenic mice.6 Although the emergence of metaplasia or dysplasia is a key event in inflammation‐related gastric carcinogenesis, the molecular mechanism underlying the development of such preneoplastic lesions is largely unknown.
A major adhesion molecule for the ECM, CD44, has been implicated in a wide variety of physiological processes, including leukocyte homing and activation, wound healing, and cell migration, as well as in tumor cell invasion and metastasis.7, 8, 9, 10 CD44 is expressed at a higher level in gastric epithelial cells isolated from the non‐cancerous stomach of H. pylori‐positive individuals than in those from H. pylori‐negative individuals.11 Furthermore, the abundance of CD44 mRNA has been found to be increased in gastric tissues of human atrophic gastritis and intestinal metaplasia.12, 13
We and others have shown that expression of splice variant isoforms of CD44 (CD44v) is associated with the progression of colon14 and gastric12, 15 cancers. Moreover, we recently showed that interaction of CD44v with xCT (SLC7A11), a subunit of the cystine/glutamate antiporter known as system xc(–), stabilizes the latter protein and thereby potentiates the ability of cancer cells to promote glutathione synthesis and defend themselves against reactive oxygen species (ROS).16, 17 Ablation of CD44 resulted in the loss of xCT from the cell surface and thereby impaired defense against ROS, leading to suppression of tumor growth in K19‐Wnt1/C2 mE mice, a transgenic model of gastric cancer induced by activation of Wnt and PGE2 signaling pathways.18 However, the functional relevance of the CD44v‐xCT system in the development of preneoplastic lesions was unknown.
We now show that de novo expression of CD44v in fundic glands is associated with a metaplastic change in gastric epithelial cells of K19‐Wnt1/C2 mE mice or Helicobacter felis‐infected mice. Inhibition of cystine transport by system xc(–) with sulfasalazine as well as genetic ablation of CD44 each suppressed the development of both SPEM and gastric tumors in K19‐Wnt1/C2 mE mice, indicating that CD44v and xCT play a key role in gastric carcinogenesis by promoting metaplasia of the gastric epithelium.
Materials and Methods
Transgenic mice
K19‐Wnt1/C2 mE transgenic mice and CD44 −/− K19‐Wnt1/C2 mE mice were described previously.16, 18 All animal experiments were carried out according to protocols approved by the Ethics Committee of Keio University (Tokyo, Japan).
Clinical specimens
Gastric adenocarcinoma specimens were collected, with informed consent, from individuals who underwent surgery at Kumamoto University Hospital (Kumamoto, Japan). We examined 55 representative moderately/well‐differentiated gastric adenocarcinomas and the adjacent gastric mucosa. This study was approved by the Human Ethics Review Committee of the Graduate School of Life Sciences, Kumamoto University.
Histology and immunohistochemistry
Tissue was fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. Sections were depleted of paraffin and then rehydrated in a graded series of ethanol solutions. For histology, sections were stained with H&E. For immunohistochemistry, sections were washed with TBS, subjected to antigen retrieval by heating for 10 min at 100°C in 0.01 M sodium citrate (pH 6.0), and exposed to 3% hydrogen peroxide before incubation with primary antibodies. Immune complexes were detected with the use of a Vectastain Elite Kit (Vector Laboratories, Burlingame, CA, USA) and 3,3′‐diaminobenzidine, and the sections were counterstained with hematoxylin. Rat mAbs IM7 (diluted 1:100) and CD44v (1:100) were used to detect mouse CD44, and a rat mAb specific for human CD44v9 (1:100) was used to detect human CD44.16 Mouse and human TFF2 were detected with a mouse mAb (1:5 dilution).3 Proliferating cells were detected with a rat mAb to Ki‐67 (clone Bu201, diluted 1:50; DakoCytomation, Carpinteria, CA, USA). Parietal cells were detected with a mouse monoclonal antibody to H+,K+‐ATPase (MBL, Nagoya, Japan). TFF2 or CD44v9 positive areas in five randomly selected microscopic fields (magnification, ×40) per section were measured using analysis software (BZ‐9000; Keyence, Tokyo, Japan) and the mean percentage of the positive area was calculated. Existence of TFF2 or CD44v9 positive areas per total area of SPEM as well as gastric cancer at 10% or more was defined as positive.
Helicobacter felis infection
C57BL/6 mice were orally inoculated with H. felis as described previously.19
Cell isolation and flow cytometry
Gastric tumor tissue and adjacent mucosa from 30‐week‐old K19‐Wnt1/C2 mE mice were digested for 3 h at 37°C in Ham's F12 medium supplemented with 5% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), insulin (5 μg/mL), collagenase (300 U/mL), and hyaluronidase (100 U/mL). The digested tissue was subjected to vigorous mixing, and red blood cells were then lysed by exposure to NH4Cl at room temperature. The tissue fragments were dissociated further by gentle passage through a pipette tip in the presence of 0.25% trypsin for 1–2 min and then in the presence of dispase (5 mg/mL) and DNase I (0.1 mg/mL) for 2 min. The mixture was then passed through a 40‐μm nylon mesh to obtain a single‐cell suspension. All reagents for cell isolation were obtained from Stemcell Technologies (Vancouver, BC, Canada). For flow cytometry, the cells were incubated for 30 min at 4°C with labeled antibodies. To exclude lineage marker positive cells from gastric tissues, we used antibodies against CD31 (endothelial cell marker), CD45, and TER119 (hematopoietic antigens). The FITC‐conjugated antibodies to CD31, CD45, and TER119 as well as allophycocyanin‐conjugated antibodies to CD44 were obtained from eBioscience (San Diego, CA, USA). Mouse xCT was detected with FITC‐labeled rabbit polyclonal antibodies (ab37185; Abcam, Tokyo, Japan). Apoptotic cells were excluded by elimination of cells positive for staining with propidium iodide before analysis. Flow cytometry was carried out with a FACSAria Cell Sorter (BD Biosciences, Tokyo, Japan).
Sulfasalazine treatment in vivo
K19‐Wnt1/C2 mE mice at 10 weeks of age were injected i.p. with physiological saline or sulfasalazine (10 mg/day; Sigma, St. Louis, MO, USA) 5 days a week for 20 weeks. Sulfasalazine solution was freshly prepared each day in 0.1 M NaOH and was subsequently adjusted with 1 M HCl to a pH of ~8. K19‐Wnt1/C2 mE mice treated with or without sulfasalazine were monitored daily for signs of stress. In the course of the experiment, neither severe weight loss (with loss of >20% of the initial body weight) nor abnormal behavior was observed in those mice.
Microarray analysis
Total RNAs were extracted from CD44+ and CD44− gastric tumor cells in 30‐week‐old K19‐Wnt1/C2 mE mice and subjected to microarray analysis as previously described.16 The microarray data, available through GEO accession number GSE20913, were used to determine the expression profiles of SPEM‐related genes in CD44+ and CD44− gastric tumor cells. A heat map was created using the top 100 upregulated genes in mouse SPEM cells.3
Statistical analysis
Data are presented as the mean ± SD and were analyzed with the unpaired Student's t‐test as carried out with the use of Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA). A P‐value of <0.05 was considered statistically significant.
Results
Expression of CD44 in SPEM and gastric tumors of K19‐Wnt1/C2 mE mice
To address the functional role and relevance of CD44 expression in gastric carcinogenesis, we examined K19‐Wnt1/C2 mE mice, a genetic model of gastric cancer resulting from metaplasia–carcinoma sequence progression.18 These mice develop large, well‐differentiated gastric tumors and manifest metaplasia adjacent to the tumors (Fig. 1a). Expression of CD44 and TFF2 (spasmolytic polypeptide), a marker of SPEM, was apparent at high levels in the metaplastic epithelium adjacent to the tumors of K19‐Wnt1/C2 mE mice compared with that in normal mouse gastric mucosa (Fig. 1b). Expression of CD44 was mostly absent in the normal columnar epithelium (Fig. 1b), whereas CD44 was highly expressed in SPEM as well as in gastric tumors (Fig. 1c). These results thus indicated that CD44 expression is upregulated in metaplastic gastric epithelial cells and gastric tumor cells compared with normal columnar epithelial cells.
Figure 1.
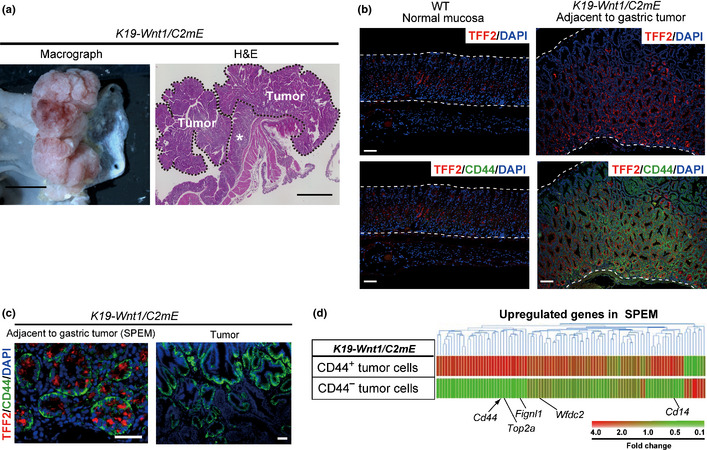
Development of gastric tumors and SPEM in K19‐Wnt1/C2 mE mice and the expression profiles of spasmolytic polypeptide‐expressing metaplasia (SPEM)‐related genes in CD44+ or CD44− tumor cells. (a) Macroscopic image and H&E staining of the stomach of K19‐Wnt1/C2 mE mice at 30 weeks of age. The dashed line indicates the tumor margin, and the asterisk indicates the SPEM region adjacent to the gastric tumor. Scale bar = 5 mm (left) and 2 mm (right). (b) Immunofluorescence analysis of TFF2 and double‐staining for TFF2 and CD44 in the normal gastric epithelium of a WT mouse and the SPEM region of a K19‐Wnt1/C2 mE mouse at 30 weeks of age. Nuclei were also stained with DAPI. The dashed lines demarcate the gastric epithelium. Scale bar = 100 μm. (c) Immunofluorescence analysis of TFF2 and CD44 in SPEM and a gastric tumor of a K19‐Wnt1/C2 mE mouse at 30 weeks of age. Scale bar = 50 μm. (d) Gene expression profiles of SPEM‐related genes3 derived from our previous microarray analysis of CD44+ or CD44− gastric tumor cells from 30‐week‐old K19‐Wnt1/C2 mE mice.16
Given that SPEM represents a premalignant change of the gastric epithelium,2, 4 our results suggested that CD44+ metaplastic cells might be candidates for the cells of origin of CD44+ tumor cells in K19‐Wnt1/C2 mE mice. To test this hypothesis, we examined whether the gene expression profile in CD44+ tumor cells is similar to that in CD44+ metaplastic cells. We previously isolated CD44+ or CD44− tumor cells from 30‐week‐old K19‐Wnt1/C2 mE mice by FACS and subjected them to DNA microarray analysis.16 Therefore, we examined these data for the expression of 98 SPEM‐related genes that are upregulated in pharmacologically induced SPEM cells3, 20 and found that the expression of SPEM‐related genes, including Top2a, Fignl1, Wfdc2, and Cd14, was markedly increased in CD44+ tumor cells compared with the CD44− cells (Fig. 1d, Table S1). These results suggested that CD44+ tumor cells in K19‐Wnt1/C2 mE mice might arise from the metaplastic cell lineage that gives rise to CD44+ SPEM.
Expression of CD44v9 is associated with the presence of SPEM adjacent to human gastric adenocarcinoma
We next investigated whether our observation in the mouse model is found in human gastric cancer specimens. Immunohistochemical analysis with specific antibody against CD44v9, a major variant isoform of CD44 associated with human gastric cancer,12 revealed that the development of CD44v9+ gastric cancer in humans was significantly associated with the presence of SPEM in the region adjacent to the tumor (Fig. S1, Table 1). These results suggested that the development of CD44v9‐expressing gastric cancer in humans is associated with a metaplastic change of the gastric epithelial cell lineage.
Table 1.
Association between CD44v9 expression in human gastric adenocarcinoma and spasmolytic polypeptide‐expressing metaplasia (SPEM)
| SPEM | CD44v9+ (n = 20) (%) | CD44v9− (n = 35) (%) | Total (n = 55) | P‐valuea |
|---|---|---|---|---|
| Present | 15 (75) | 5 (14.3) | 20 | 0.00001 |
| Absent | 5 (25) | 30 (85.7) | 35 |
The relation between CD44v9 expression in tumor cells and the presence of SPEM was analyzed with Fisher's exact test. Specimens of human gastric adenocarcinoma and adjacent tissue were subjected to immunohistochemical staining to determine whether the tumor cells expressed CD44v9 and whether the adjacent tissue expressed TFF2.
Ablation of CD44 inhibits development of SPEM as well as gastric tumors in K19‐Wnt1/C2 mE mice
We previously showed that genetic ablation of CD44 inhibits expansion of stem‐like tumor cells and thereby suppresses tumor growth in K19‐Wnt1/C2 mE mice (Fig. 2a).16 We therefore examined whether CD44 ablation affects SPEM development as well as tumor formation. Immunohistochemical analysis of 30‐week‐old CD44 +/+ or CD44 −/− K19‐Wnt1/C2 mE mice revealed that the development of SPEM was significantly attenuated by CD44 ablation (Fig. 2b,c). Furthermore, we investigated whether splice variant isoforms of CD44 (CD44v) are expressed in SPEM with the use of an antibody to mouse CD44v8–10. In the same manner as gastric tumor cells in mice21 and humans (Fig. S1), CD44v was found to be highly expressed in the metaplastic cells of SPEM in K19‐Wnt1/C2 mE mice (Fig. S2). These findings suggest that CD44v contributes not only to gastric tumor growth but also to the development of premalignant lesions in the stomach.
Figure 2.
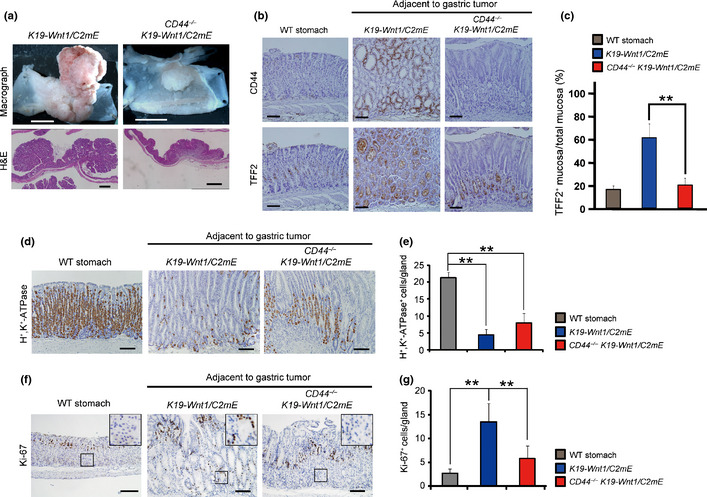
CD44 ablation inhibits gastric tumor growth and spasmolytic polypeptide‐expressing metaplasia (SPEM) development but not parietal cell loss in K19‐Wnt1/C2 mE mice. (a) Macroscopic images and H&E staining of the stomach of K19‐Wnt1/C2 mE or CD44 −/− K19‐Wnt1/C2 mE mice at 30 weeks of age. Scale bar = 5 mm (top) and 1 mm (bottom). (b) Immunohistochemical staining for CD44 and TFF2 in serial sections of the basal fundic gland region of WT,K19‐Wnt1/C2 mE, or CD44 −/− K19‐Wnt1/C2 mE mice at 30 weeks of age. Scale bar = 100 μm. (c) Area of TFF2+ gastric epithelium determined from sections similar to those in (b). Data are means ± SD for four mice of each genotype. **P < 0.01. (d) Immunohistochemical staining for H +,K +‐ATPase in the glandular stomach of WT,K19‐Wnt1/C2 mE, or CD44 −/− K19‐Wnt1/C2 mE mice at 30 weeks of age. Scale bar = 100 μm. (e) Mean number of H +,K +‐ATPase‐positive cells per gland determined from sections similar to those in (d). The numbers of H +,K +‐ATPase‐positive cells in 100 randomly selected glands in normal or SPEM regions were counted. Data are means ± SD for five mice of each genotype. **P < 0.01. (f) Immunohistochemical staining for Ki‐67 in the glandular stomach of WT,K19‐Wnt1/C2 mE, or CD44 −/− K19‐Wnt1/C2 mE mice at 30 weeks of age. The boxed regions at the base of fundic glands are shown at higher magnification in the insets. Scale bar = 100 μm. (g) Mean number of Ki‐67‐positive cells per gland determined from sections similar to those in (f). The numbers of Ki‐67+ cells in 100 randomly selected glands in normal or SPEM regions were counted. Data are means ± SD for five mice of each genotype. **P < 0.01.
Given that the loss of parietal cells and the subsequent emergence of proliferating progenitor‐like cells in the lower portion of gastric glands are the characteristic process of SPEM development,3, 22, 23, 24, 25 we next examined whether CD44v expression influences these events in 30‐week‐old K19‐Wnt1/C2 mE mice. Immunohistochemical analysis with antibodies to the α subunit of H+‐dependent and K+‐dependent ATPase, a marker for parietal cells, revealed that CD44 ablation had little effect on the number of remaining parietal cells in gastric glands of K19‐Wnt1/C2 mE mice (Fig. 2d,e), suggesting that CD44v expression is not associated with parietal cell loss during SPEM development. Immunohistochemical analysis also revealed, however, that CD44 ablation significantly reduced the number of Ki‐67+ proliferating cells in gastric glands (Fig. 2f,g). Furthermore, most Ki‐67+ cells in CD44 −/− K19‐Wnt1/C2 mE mice were localized to the midgland zone, in contrast to the localization of these cells to the lower portion of gastric glands in CD44 +/+ K19‐Wnt1/C2 mE mice (Fig. 2f). These results suggested that CD44v expression is required for the emergence of proliferating progenitor‐like cells in the lower portion of gastric glands and that it consequently promotes SPEM development in K19‐Wnt1/C2 mE mice.
Chronic H. felis infection triggers development of CD44v+ SPEM at the base of gastric glands
To examine whether the de novo expression of CD44v arises in cells at the base of gastric glands in preneoplastic lesions of the mouse stomach, we studied the H. felis‐infected C57BL/6 mouse, a model of H. pylori infection in humans.5, 26 Immunohistochemical analyses revealed that infection with H. felis for 12 weeks induced both the development of SPEM and the de novo expression of CD44v in the basal region of gastric glands (Fig. 3a). Further expansion of the CD44v+ cell population in SPEM was apparent at 33 weeks after H. felis infection (Fig. 3b). These results thus suggested that de novo CD44v expression is associated with a metaplastic change of gastric epithelial cells at the base of gastric glands and that chronic infection with Helicobacter promotes the expansion of CD44v+ cells in preneoplastic lesions.
Figure 3.
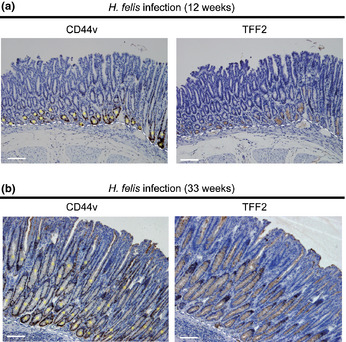
Development of spasmolytic polypeptide‐expressing metaplasia and CD44v expression at the base of gastric glands in Helicobacter felis‐infected mice. Serial sections of the fundic gland region of mice infected with H. felis for 12 weeks (a) or 33 weeks (b) were subjected to immunohistochemical staining for CD44v and TFF2. Asterisks indicate CD44v+ metaplastic glands. Dashed lines indicate the border of the gastric mucosa and submucosa. Scale bar = 100 μm.
CD44v+ SPEM and gastric tumor cells manifest a high level of xCT expression in K19‐Wnt1/C2 mE mice
Given that CD44v promotes expression of xCT at the cell surface in cancer cells,16, 27 we next examined whether expression of CD44v is associated with that of xCT at the surface of metaplastic cells in K19‐Wnt1/C2 mE mice. Flow cytometric analysis revealed that CD44v+ metaplastic cells in SPEM as well as CD44v+ gastric tumor cells express xCT at a high level at the cell surface, compared with that apparent in CD44v− cells (Fig. 4). These results suggested that CD44v enhances xCT expression at the surface of metaplastic cells and thereby allows the expansion of these cells as well as that of tumor cells by the ROS defense mechanism in these animals.
Figure 4.
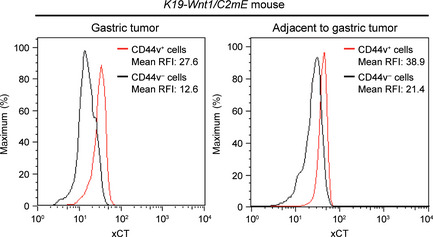
Expression of xCT, a subunit of the cystine transporter system xc(–), at the cell surface in spasmolytic polypeptide‐expressing metaplasia cells and gastric tumor cells of K19‐Wnt1/C2 mE mice. Flow cytometric analysis of CD44v and xCT expressions on lineage marker‐negative spasmolytic polypeptide‐expressing metaplasia cells and tumor cells isolated from the stomach of 30‐week‐old K19‐Wnt1/C2 mE mice. RFI, relative fluorescence intensity.
Sulfasalazine suppresses the development of SPEM and gastric tumors in K19‐Wnt1/C2 mE mice
We recently showed that pharmacological inhibition of the cystine transport by system xc(–) with the use of sulfasalazine suppressed CD44v‐dependent expansion of cancer cells.16, 27, 28 To examine whether sulfasalazine inhibits the development of SPEM as well as that of gastric tumors, we treated K19‐Wnt1/C2 mE mice with this drug by i.p. injection (Fig. 5a). Treatment with sulfasalazine for 20 weeks resulted in significant inhibition of gastric tumor growth in these animals (Fig. 5b,c), suggesting that sulfasalazine blocked expansion of CD44v+ gastric tumor cells. Immunohistochemical analysis of TFF2 expression revealed that development of SPEM was also suppressed in the stomach of sulfasalazine‐treated mice (Fig. 5d,e). These results suggest that sulfasalazine inhibited CD44v‐xCT dependent expansion of SPEM cells as well as tumor cells.
Figure 5.
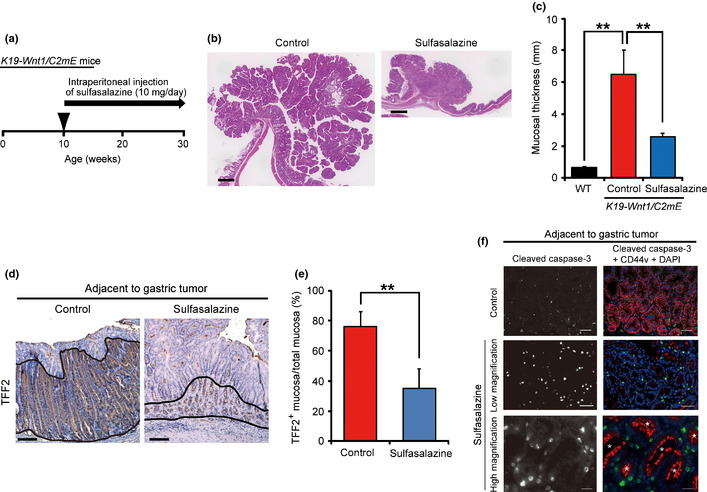
Sulfasalazine treatment suppresses the development of spasmolytic polypeptide‐expressing metaplasia (SPEM) and gastric tumors in K19‐Wnt1/C2 mE mice. (a) Protocol for sulfasalazine treatment in 10‐week‐old K19‐Wnt1/C2 mE mice. (b) H&E staining of the stomach of K19‐Wnt1/C2 mE mice at 30 weeks of age after treatment with saline (control) or sulfasalazine. Scale bar = 1 mm. (c) Gastric mucosal thickness in WT mice and K19‐Wnt1/C2 mE mice at 30 weeks of age after treatment with saline or sulfasalazine. Data are means ± SD for five mice in each group. **P < 0.01. (d) Immunohistochemical staining for TFF2 in the glandular stomach of K19‐Wnt1/C2 mE mice at 30 weeks of age after treatment with saline or sulfasalazine. Solid black lines demarcate the TFF2‐positive area of the mucosa. Scale bar = 100 μm. (e) Area of the TFF2+ gastric epithelium determined from sections similar to those in (d). Data are means ± SD for five mice of each group. **P < 0.01. (f) Immunofluorescence analysis of cleaved caspase‐3 and CD44 together with DAPI staining of nuclei (blue fluorescence), in SPEM glands of K19‐Wnt1/C2 mE mice at 30 weeks of age after treatment with saline (control) or sulfasalazine. Scale bar = 50 μm (upper and middle panels) and 20 μm (lower panels).
Given that cysteine depletion by sulfasalazine triggers apoptosis in CD44v‐expressing tumor cells,28 we next examined whether sulfasalazine induced apoptosis in SPEM cells. The SPEM cells in sulfasalazine‐treated K19‐Wnt1/C2 mE mice manifested the expression of an apoptosis marker cleaved caspase‐3 (Fig. 5f), indicating that sulfasalazine induced apoptosis in SPEM cells. Furthermore, CD44v was found to be expressed only at apical/endoluminal membrane but not basolateral membrane of SPEM cells in sulfasalazine‐treated mice (Fig. 5f). Given that the altered expression of CD44 from basolateral to apical/endoluminal membrane has been shown to be associated with disruption of cell–cell adhesion in damaged renal tubular cells,29 apical/endoluminal expression of CD44v might be one of the consequences of the damage of SPEM glands in sulfasalazine‐treated mice. Together, these results suggested that sulfasalazine is a potential drug for prevention of the CD44v‐dependent emergence of SPEM and subsequent development of gastric cancer.
Discussion
Gastric carcinogenesis in response to chronic H. pylori infection is mediated through global changes in the lineages of the stomach, including oxyntic atrophy and the emergence of metaplastic cells.1 The biosynthetic pathway for PGE2 is upregulated at the level of COX2 or microsomal PGE synthase 1 expression in metaplastic tissue as well as in esophageal adenocarcinoma,30, 31 suggesting that constitutive activation of PGE2 signaling is essential for the metaplastic transition of epithelial cells that precedes cancer development. We have now shown that CD44v is expressed de novo in SPEM of the stomach in K19‐Wnt1/C2 mE mice, in which PGE2 signaling is activated, and that CD44 ablation significantly attenuated SPEM development by suppressing the proliferation of metaplastic cells at the base of gastric glands in these mice. Given that SPEM is thought to be initiated by activation of basal cryptic progenitor‐like cells (BCPCs), possibly through the transdifferentiation of chief cells,4, 22 we propose that CD44v expression is required for the expansion of such BCPCs and consequent SPEM development.
It has been reported that the gene expression profile of tumor in K19‐Wnt1/C2 mE mouse is similar to that of human intestinal‐type gastric cancer,32 suggesting that this mouse model reproduces the process of inflammation‐mediated tumorigenesis in stomach. Therefore, the induction of CD44v expression in proliferating progenitor‐like cells is potentially a key event of inflammation‐mediated carcinogenesis in human as well and can be a therapeutic target.
We previously showed that CD44v interacts with and stabilizes xCT, a subunit of the cystine/glutamate antiporter known as system xc(–), and it thereby promotes cystine uptake for glutathione synthesis.16 The level of xCT‐dependent cystine transport is thus increased by CD44v in CD44+ stem‐like cancer cells, providing support for their proliferation and survival.27, 28 We have now shown that sulfasalazine, an inhibitor of xCT‐dependent cystine transport, suppressed CD44‐dependent development of SPEM and gastric tumor growth in K19‐Wnt1/C2 mE mice, suggesting that the CD44v‐xCT system plays a key role in the expansion of CD44v+ metaplastic cells and CD44v+ tumor cells in the stomach.
In conclusion, our present data provide evidence that de novo expression of CD44, especially CD44v, enhances xCT expression at the cell surface and promotes the survival and proliferation of BCPCs that give rise to SPEM. Therapy targeted to CD44v‐xCT may therefore inhibit the expansion of BCPCs as well as that of BCPC‐derived stem‐like cancer cells, resulting in attenuation of metaplasia–carcinoma sequence progression in the stomach.
Disclosure Statement
O.N. received a research grant from Kyowa Hakko‐Kirin Inc. H.S. received research grants from Diichi Sankyo Inc., Kyowa Hakko‐Kirin Inc., LinkGenomics Inc., and Shimadzu Corp. These do not alter the authors' adherence to all the journal's policies on sharing data and materials. The other authors declare no conflict of interest.
Supporting information
Fig. S1. Representative immunohistochemical analysis of CD44v9 and TFF2 expression in gastric mucosa adjacent to human gastric tumor tissue.
Fig. S2. Expression of CD44v in spasmolytic polypeptide‐expressing metaplasia of K19‐Wnt1/C2 mE mice.
Table S1. List of spasmolytic polypeptide‐expressing metaplasia‐related genes.
Acknowledgments
This work was supported by grants (to H.S.) from, as well as in part by, the Project for Development of Innovative Research on Cancer Therapeutics (P‐Direct) (to O.N.) of, the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank I. Ishimatsu, N. Suzuki, M. Nakata, and S. Hayashi for technical assistance as well as K. Arai for help in preparation of the manuscript.
(Cancer Sci 2013; 104: 1323–1329
References
- 1. Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2002; 2: 28–37. [DOI] [PubMed] [Google Scholar]
- 2. Nomura S, Baxter T, Yamaguchi H et al Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis‐infected mice. Gastroenterology 2004; 127: 582–94. [DOI] [PubMed] [Google Scholar]
- 3. Nozaki K, Ogawa M, Williams JA et al A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 2008; 134: 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt PH, Lee JR, Joshi V et al Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 1999; 79: 639–46. [PMC free article] [PubMed] [Google Scholar]
- 5. Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX‐2/mPGES‐1 transgenic mice. EMBO J 2004; 23: 1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tu S, Bhagat G, Cui G et al Overexpression of interleukin‐1β induces gastric inflammation and cancer and mobilizes myeloid‐derived suppressor cells in mice. Cancer Cell 2008; 14: 408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990; 61: 1303–13. [DOI] [PubMed] [Google Scholar]
- 8. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003; 4: 33–45. [DOI] [PubMed] [Google Scholar]
- 9. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci 2004; 95: 930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunthert U, Hofmann M, Rudy W et al A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991; 65: 13–24. [DOI] [PubMed] [Google Scholar]
- 11. Fan X, Long A, Goggins M, Fan X, Keeling PW, Kelleher D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut 1996; 38: 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayer B, Jauch KW, Gunthert U et al De‐novo expression of CD44 and survival in gastric cancer. Lancet 1993; 342: 1019–22. [DOI] [PubMed] [Google Scholar]
- 13. Kuniyasu H, Yasui W, Yokozaki H, Tahara E. Helicobacter pylori infection and carcinogenesis of the stomach. Langenbecks Arch Surg 2000; 385: 69–74. [DOI] [PubMed] [Google Scholar]
- 14. Tanabe KK, Ellis LM, Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet 1993; 341: 725–6. [DOI] [PubMed] [Google Scholar]
- 15. Miwa T, Watanabe A, Yamada Y et al Progression in gastric carcinoma relative to the ratio of CD44 epithelial variant transcript to CD44 hematopoietic variant transcript. Cancer 1996; 77: 25–9. [DOI] [PubMed] [Google Scholar]
- 16. Ishimoto T, Nagano O, Yae T et al CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(–) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387–400. [DOI] [PubMed] [Google Scholar]
- 17. Nagano O, Okazaki S, Saya H. Redox regulation in stem‐like cancer cells by CD44 variant isoforms. Oncogene 2013. doi: 10.1038/onc.2012.638 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 2006; 131: 1086–95. [DOI] [PubMed] [Google Scholar]
- 19. Oguma K, Oshima H, Aoki M et al Activated macrophages promote Wnt signalling through tumour necrosis factor‐alpha in gastric tumour cells. EMBO J 2008; 27: 1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weis VG, Sousa JF, Lafleur BJ et al Heterogeneity in mouse spasmolytic polypeptide‐expressing metaplasia lineages identifies markers of metaplastic progression. Gut 2013; 62: 1270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishimoto T, Oshima H, Oshima M et al CD44+ slow‐cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci 2010; 101: 673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldenring JR, Nam KT, Mills JC. The origin of pre‐neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res 2011; 317: 2759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild‐type and gastrin‐deficient mice. Am J Physiol 2005; 288: G362–75. [DOI] [PubMed] [Google Scholar]
- 24. Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR. Association of spasmolytic polypeptide‐expressing metaplasia with carcinogen administration and oxyntic atrophy in rats. Lab Invest 2002; 82: 1045–52. [DOI] [PubMed] [Google Scholar]
- 25. Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol 2006; 291: G999–1004. [DOI] [PubMed] [Google Scholar]
- 26. Fox JG, Li X, Cahill RJ et al Hypertrophic gastropathy in Helicobacter felis‐infected wild‐type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 1996; 110: 155–66. [DOI] [PubMed] [Google Scholar]
- 27. Yae T, Tsuchihashi K, Ishimoto T et al Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun 2012; 3: 883. [DOI] [PubMed] [Google Scholar]
- 28. Yoshikawa M, Tsuchihashi K, Ishimoto T et al xCT inhibition depletes CD44v‐expressing tumor cells that are resistant to EGFR‐targeted therapy in head and neck squamous cell carcinoma. Cancer Res 2013; 73: 1855–66. [DOI] [PubMed] [Google Scholar]
- 29. Asselman M, Verhulst A, Van Ballegooijen ES, Bangma CH, Verkoelen CF, De Broe ME. Hyaluronan is apically secreted and expressed by proliferating or regenerating renal tubular cells. Kidney Int 2005; 68: 71–83. [DOI] [PubMed] [Google Scholar]
- 30. Shirvani VN, Ouatu‐Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology 2000; 118: 487–96. [DOI] [PubMed] [Google Scholar]
- 31. Jang TJ, Min SK, Bae JD et al Expression of cyclooxygenase 2, microsomal prostaglandin E synthase 1, and EP receptors is increased in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut 2004; 53: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Itadani H, Oshima H, Oshima M, Kotani H. Mouse gastric tumor models with prostaglandin E2 pathway activation show similar gene expression profiles to intestinal‐type human gastric cancer. BMC Genomics 2009; 10: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative immunohistochemical analysis of CD44v9 and TFF2 expression in gastric mucosa adjacent to human gastric tumor tissue.
Fig. S2. Expression of CD44v in spasmolytic polypeptide‐expressing metaplasia of K19‐Wnt1/C2 mE mice.
Table S1. List of spasmolytic polypeptide‐expressing metaplasia‐related genes.


