Abstract
The development of multi‐drug resistance (MDR) represents a major obstacle in the successful treatment of cancers. However, the factors and mechanisms that lead to MDR in cholangiocarcinoma (CCA), a chemoresistant bile duct carcinoma with a poor prognosis, remain unclear. In this study, we established a human MDR CCA cell line QBC939/5‐FU. Compared with QBC939 cells, a rounder shape, a higher nuclear–cytoplasmic ratio, a shorter cell cycle, faster growth and resistance to chemotherapeutics are major characteristics of QBC939/5‐FU cells. P‐glycoprotein (P‐gp) and β‐catenin were upregulated in QBC939/5‐FU cells. Furthermore, the drug susceptibility of QBC939 cells to common chemotherapeutics was significantly decreased after Wnt3a treatment, whereas inhibition of Wnt/β‐catenin pathway by β‐catenin siRNA reversed the MDR of QBC939/5‐FU cells to chemotherapeutics. Molecular study revealed that activation of Wnt/β‐catenin pathway resulted in upregulation of P‐gp and contributed to MDR of QBC939/5‐FU cells. Extraction of Siamese Crocodile 3 (ESC‐3) bile enhanced the drug sensitivity of QBC939/5‐FU cells to 5‐FU, paralleled with downregulation of β‐catenin and P‐gp. The association of Wnt/β‐catenin pathway and P‐gp was further confirmed by the clinical data for CCA tissues. Our study represents the first implication of Wnt/β‐catenin activation in the MDR of CCA, which may be a beneficial target for the clinical treatment of CCA.
Cholangiocarcinomas (CCA) are malignant tumors originating from biliary epithelial cells.1 Surgical removal offers the unique possibility of cure, but these tumors often present at an advanced unresectable stage due to the lack of a specific tumor marker and the silent symptoms.2 Therefore, chemoradiotherapy is currently the only choice for the majority of CCA patients. However, the response to radiation therapy and currently available chemotherapeutic regimens is dismal, and, consequently, the prognosis is poor.3 The incidence of this deadly neoplasm has increased rapidly in the recent 10 years. Hence, there is an urgent need to reveal the mechanisms of multi‐drug resistance (MDR), which is important for developing novel therapeutic strategies.
Overcoming MDR is critical for developing effective therapeutic drugs for the treatment of CCA. However, the various mechanisms contributing to MDR have been only partially elucidated. One of the best‐studied mechanisms is the lowered intracellular drug concentration induced by increased drug efflux.4 The drug efflux is partially mediated by cell surface glycoproteins, which belong to the family of ATP‐binding cassette transporters.5 Several lines of evidence suggest that ABC transporters, such as P‐glycoprotein (P‐gp, encoded by the MDR1 gene) and MRP1 (MDR‐related protein), are highly expressed and are the major contributors of chemoresistance in a number of tumor cells, including CCA.6, 7, 8, 9
However, the underlying mechanisms of P‐gp overexpression are largely unknown. Downregulation of PI3K/Akt pathway is related to reversal of MDR in cancer cells.10 Furthermore, various inhibitors for PI3K/Akt or NF‐κB pathway, such as curcumin or perifosine, can suppress P‐gp expression.11, 12 Unrelated studies speculate that β‐catenin signaling may regulate P‐gp expression in rat brain endothelial cells, whereas the activation of Wnt signaling pathway may contribute to chemoresistance of human neuroblastomas through MDR1 overexpression.13, 14, 15 Although P‐gp overexpression in CCA has been confirmed, the precise mechanism is still unclear
The present study took initial steps towards elucidating the mechanisms of the P‐gp overexpression in the MDR of CCA. We found that inhibition of Wnt/β‐catenin pathway resulted in suppression of P‐gp expression, and rendered multi‐drug resistant CCA cells more sensitive to a number of chemotherapeutic agents. Hence, the Wnt/β‐catenin pathway might be an ideal chemosensitization target in the therapy of chemoresistant CCA.
Materials and Methods
Reagents
Wnt3a, 5‐fluorouracil (5‐FU), cis‐diammineplatinum(II) dichloride (CDDP), vincristine sulfate (VCR), mitogen enzyme C (MMC), MTT and DAPI were purchased from Sigma (St. Louis, MO, USA); β‐catenin siRNA (h), monoclonal antibodies against MDR1, β‐catenin, p‐IκB‐α, IκB‐α, p‐AKT1 (S473), AKT and β‐actin were provided by Santa Cruz Biotechnology (Santa Cruz, CA, USA); and an EliVision Plus Kit was provided by Maixin Bio (Fuzhou, China). PVDF membranes were obtained from Millipore (Billerica, MA, USA). Goat anti‐rabbit and anti‐mouse secondary antibodies conjugated to FITC were purchased from Tiangen Biotech (Beijing, China). Isolation of ESC‐3 from Crocodylus siamensis bile was performed as previously described.16
Patients and tumor specimens
Approval for the project was provided by the Institute for Human Investigation Committee of the First Affiliated Hospital of Xiamen University prior to commencement of the study, and the research conforms to the provisions of the Declaration of Helsinki in 1995. A cohort of 63 patients, diagnosed from 2008 to 2012, underwent complete surgical resection at diagnosis followed by chemotherapy. Metastatic tumors from other tissue origins, tumors with metastasis and interhepatic CCA were excluded from this study. None of the patients had received preoperative treatment, such as radiation or chemotherapy.
Cell culture
The human cholangiocarcinoma cell line QBC939 was kindly provided by Professor Shu‐Guang Wang from Southwest Hospital, the Third Military Medical University, Chongqing, China. Cells were cultured in RPMI‐1640 medium supplemented with 10% FBS and 100 U/mL penicillin at 37°C in an atmosphere of air containing 5% CO2.
Establishment of the multi‐drug resistance cholangiocarcinoma cell line
For the induction of MDR, different gradient concentrations of 5‐FU (including 5, 10, 15, 20, 25 and 30 μM) were selected and diluted in RPMI‐1640 medium. Higher concentrations were used when the cell growth was steady in the lower concentration for sufficient time. It took only 3–7 days for the cells to grow steadily when the concentration was lower than 20 μM, whereas more time (2 weeks to 1.5 months) was required for concentrations higher than 20 μM. After culturing for 6 months, the human MDR cholangiocarcinoma cell line QBC939/5‐FU was obtained, which could grow and be passaged steadily in 20 μM 5‐FU. Thereafter, the cells were cultured without 5‐FU for 1 month and then frozen in liquid nitrogen for 1 month before being recovered for identification of the biological characteristics and the MDR index.
Proliferation assay
Cell proliferation was analyzed by MTT assay. A total of 6 × 103 cells were seeded in 96‐well plates and MTT was added to each well every 24 h. The plates were incubated at 37°C for 4 h before addition of lysate solution (10% SDS in 0.01 M HCl). The absorbance was measured at 490 nm using a micro‐plate reader.
Real‐time RT‐PCR
Real‐time RT‐PCR was carried out using the Light Cycler 480 (Roche Molecular Biochemicals, Mannheim, Germany), as previously described.17 Human GAPDH was used as a control. The following primers were used: MDR1 forward 5′‐GACATCCCAGTGCTTCAGG‐3′, reverse 5′‐GCCACTGAACATTCAGTCG‐3′; MRP1 forward 5′‐ACGAAAGTCCGGCTGAGCGTCT‐3′, reverse 5′‐ATGGGTGACCGCAGGATGCT‐3′; MRP2 forward 5′‐CTGGGTGACTGATAAGAGG‐3′, reverse 5′‐TGAGGGATGACTTTCCAGCT‐3′; MRP3 forward 5′‐AAGGGTTCTTGGTGATGCT‐3′, reverse 5′‐AGAAGCAGTTCAGGATGCG‐3′; cyclinD forward 5′‐CCTCAACCATCCTGGCTGCG‐3′, reverse 5′‐AGGACAGACTCCGCTGTGC‐3′.
Cell cycle analysis
Cells were fixed with 70% ethanol at 4°C overnight and then resuspended in 100 μg/mL RNase A at 37°C for 30 min and subsequently stained with 50 μg/mL propidium iodide at 4°C for 30 min in the dark. The cells were analyzed by flow cytometry at 488 nm, and the data were analyzed using the cellfit software (BD Bioscience, San Jose, CA, USA).
Immunohistochemistry and immunofluorescence
Immunohistochemistry (IHC) for the expression of β‐catenin and P‐gp was performed as previously described.18 For immunofluorescence (IF), cells seeded on cover slips in 24‐well plates overnight were fixed in 4% paraformaldehyde in PBS for 10 min, washed twice with PBS, and then permeabilized with 0.1% Triton X‐100 in PBS for 10 min. Fixed cells were pre‐incubated for 30 min in PBS containing 5% BSA at room temperature. Cells were stained with primary antibody (anti‐β‐catenin monoclonal antibody, 1:300 dilution; or anti‐P‐gp monoclonal antibody, 1:200 dilution) for 1 h at room temperature followed by incubation with secondary antibody conjugated with FITC. DAPI 0.1 μg/mL) was added to the secondary antibody mixture to visualize nuclei. Fluorescence images were collected and analyzed using an inverted fluorescence microscope.
Western blot
To prepare whole cell lysates, 60‐mm dishes of cells were washed twice with PBS at room temperature and were then lysed with ice‐cold RIPA buffer (150 mM NaCl, 1% NP‐40, 0.5% DOC, 0.1% SDS, 50 mM Tris pH 8.0, 1 mM EDTA, and Halt Protease Inhibitor Cocktail) on ice for 10 min. Lysed cells were transferred into 1.5 mL tubes on ice and centrifuged at 13 500g for 20 min (4°C). The supernatants, containing total cell proteins, were collected. Aliquots containing 20 μg of total protein were electrophoresed on a 10% SDS‐PAGE gel and transferred onto PVDF membranes, which were then incubated with primary and secondary antibodies and detected by using the ECL system.
Statistical analysis
All data were collected from several independent experiments, with three replicates per experiment, and analyzed using spss 17.0 (SPSS Inc., Chicago, IL, USA). Data from cell experiments were expressed as means ± SD and analyzed using one‐way anova. Data from immunohistochemistry were analyzed using cross‐tabulation and Pearson's χ2. All statistical tests were two‐sided and significance level was set at 5% for each analysis.
Results
Establishment and characterization of multi‐drug resistance cholangiocarcinoma cell line
To investigate the mechanisms of MDR in CCA, we established a human MDR CCA cell line QBC939/5‐FU by administration of gradient concentration of 5‐FU for 6 months. Finally, the cells could grow and be passaged steadily in 20 μM of 5‐FU in appropriate medium. Microscopic observation found that QBC939/5‐FU cells were transformed into more round cells with higher nuclear–cytoplasmic ratio, arranged tightly, compared with the parent cells (Fig. 1a). The MTT results showed that the growth of QBC939/5‐FU cells was faster than QBC939 cells (Fig. 1b). Cell cycle analysis demonstrated that QBC939/5‐FU cells in G0/G1 were decreased significantly compared with QBC939 cells (Fig. 1c), indicating a shorter cell cycle of QBC939/5‐FU cells. The drug susceptibility of QBC939/5‐FU cells to common chemotherapeutics was significantly lower than that of QBC939 cells (Fig. 1d). These results suggested that QBC939/5‐FU cells acquire MDR.
Figure 1.
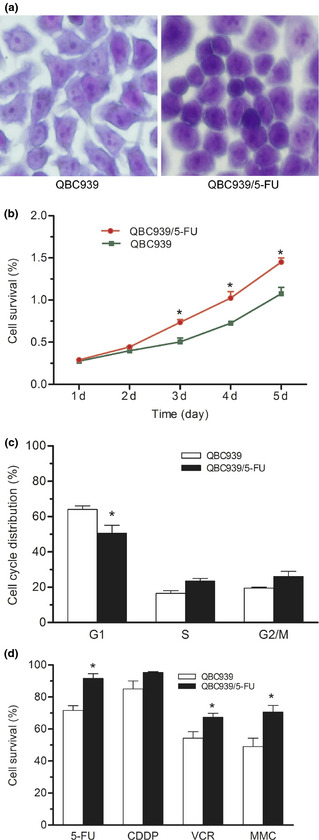
Biological characteristics of QBC939/5‐FU cells. (a) Cellular morphology dyed with 0.005% crystal violet for 30 min. Zoom: 200×. (b) Growth curve assayed by MTT. (c) Cell cycle distribution determined by flow cytometry. (d) Effects of four common chemotherapeutics (60 μM, 24 h) on the proliferation of QBC939 and QBC939/5‐FU cells assayed by MTT. *P < 0.05, **P < 0.01. 5‐FU, 5‐fluorouracil; CDDP, cis‐diammineplatinum(II) dichloride; VCR, vincristine sulfate; MMC, mitogen enzyme C.
P‐glycoprotein and β‐catenin was upregulated in QBC939/5‐FU cells
The mRNA expression of MDR‐associated genes, such as P‐gp (MDR1), MRP1 and MRP2, were markedly higher in QBC939/5‐FU cells than that in QBC939 cells (Fig. 2a), especially for P‐gp (more than 10‐fold increase). Moreover, paralleled with elevated protein expression of P‐gp, there was a remarkable increase in β‐catenin expression in whole cell lysate of QBC939/5‐FU cells, but no significant alteration was found in PI3K/AKT and NF‐κB pathway (Fig. 2b). IF staining was performed to detect the cellular localization of β‐catenin protein and revealed a nuclear and perinuclear accumulation of β‐catenin in QBC939/5‐FU cells, compared with QBC939 cells (Fig. 2c).
Figure 2.
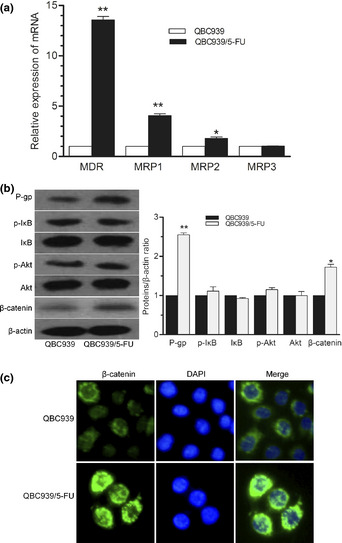
Upregulation of P‐gp and β‐catenin expression in QBC939/5‐FU cells. (a) Expression of MDR‐associated genes detected by real‐time RT‐PCR. (b) Protein expression of p‐IκB/IκB, p‐AKT/AKT, β‐catenin and P‐gp measured by western blot in QBC939 and QBC939/5‐FU cells. (c) Expression of β‐catenin and P‐gp detected by immunofluorescence staining. Minimal staining (membranous expression of β‐catenin and P‐gp) was observed in QBC939 cells, whereas QBC‐939/5‐FU cells showed high levels of fluorescent staining (nuclear and perinuclear expression of β‐catenin, and membranous expression of P‐gp), readily distinguished from background. Zoom: 200×. *P < 0.05, **P < 0.01. 5‐FU, 5‐fluorouracil; MDR, multi‐drug resistance; P‐gp, P‐glycoprotein.
Wnt/β‐catenin pathway contributes to the multi‐drug resistance of QBC939/5‐FU cells
To confirm the role of Wnt/β‐catenin pathway in the MDR of QBC939/5‐FU cells, silencing of β‐catenin mRNA with RNA interference was performed to downregulate the expression of β‐catenin. The drug susceptibility of QBC939 and QBC939/5‐FU cells to 5‐FU, CDDP, VCR and MMC was significantly enhanced by siβ‐catenin transfection, compared to the respective control (Fig. 3a). In contrast, Wnt3a treatment attenuated the susceptibility of QBC939 cells to 5‐FU and MMC, and that of QBC939/5‐FU cells to 5‐FU (Fig. 3b). The mRNA expression of cyclinD, an important target gene of β‐catenin, was remarkably elevated after Wnt3a treatment in both QBC939 and QBC939/5‐FU cells compared with the control (water served as vehicle), respectively. QBC939/5‐FU cells showed higher cyclinD mRNA expression than QBC939 cells with the same treatment. Transfection with siβ‐catenin effectively abolished the increase of cyclinD mRNA (Fig. 3c). Similar changes were seen in the protein expression of P‐gp and β‐catenin with siβ‐catenin and Wnt3a treatment (Fig. 3d).
Figure 3.
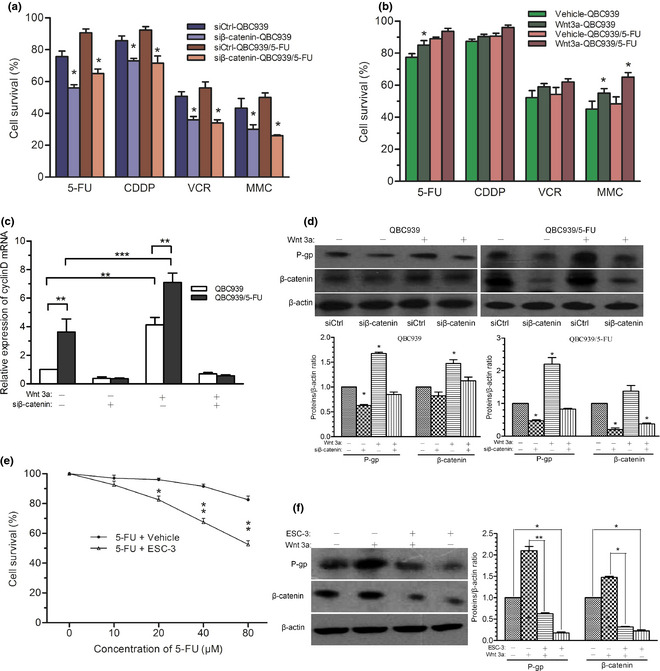
Wnt/β‐catenin pathway alters MDR of QBC‐939/5‐FU cells. (a) The drug susceptibility to chemotherapeutics (60 μM) of QBC939 and QBC939/5‐FU cells transfected with siβ‐catenin, or (b) after Wnt3a (50 nM) treatment for 6 h, compared to respective control. Equivalent amount of water served as vehicle for Wnt3a. (c) CyclinD mRNA expression in QBC939 and QBC939/5‐FU cells transfected with siCtrl or siβ‐catenin, with/without Wnt3a treatment. (d) Protein expression of β‐catenin and P‐gp in QBC939 cells after Wnt3a (50 nM) treatment for 6 h assayed by western blot. (e) Effect of ESC‐3 (10 μg/mL) on the drug susceptibility of QBC939/5‐FU cells to 5‐FU detected by MTT assays. Methanol‐0.01 M NaH2PO4 (70:30, v/v) served as vehicle. (f) Protein expression of β‐catenin and P‐gp in QBC‐939/5‐FU cells with/without ESC‐3 treatment in the absence/presence of Wnt3a (50 nM) for 24 h. *P < 0.05, **P < 0.01, ***P < 0.001. 5‐FU, 5‐fluorouracil; CDDP, cis‐diammineplatinum(II) dichloride; VCR, vincristine sulfate; MDR, multi‐drug resistance; MMC, mitogen enzyme C; P‐gp, P‐glycoprotein.
Previously, we found that extraction of Siamese Crocodile 3 (ESC‐3) bile, an active ingredient of Siamese Crocodile bile, inhibited the proliferation of CCA cells.16 In the present study, ESC‐3 enhanced the drug sensitivity of QBC939/5‐FU cells to 5‐FU compared to vehicle (Fig. 3e), paralleled with reduced protein expression of P‐gp and β‐catenin (Fig. 3f).
Association of Wnt/β‐catenin pathway with chemoresistance in cholangiocarcinoma patients
To investigate whether the activation of Wnt/β‐catenin pathway was associated with clinical chemoresistance in CCA patients, IHC was performed to evaluate the protein expression of β‐catenin and P‐gp in a series of 63 primary human CCA tissues. As a typical case shown in Figure 4, CCA tumor tissue from samples (a) and (b) with low expression of P‐gp were paralleled with low membranous expression of β‐catenin. However, the expression of P‐gp was high in samples (c) and (d), with high and nuclear accumulation (nuclear and perinuclear expression) of β‐catenin. Furthermore, Pearson's χ2 test was performed to explore the link between P‐gp expression and nuclear accumulation of β‐catenin (Table 1), and suggested a significant correlation between β‐catenin and P‐gp staining in the CCA tissues.
Figure 4.
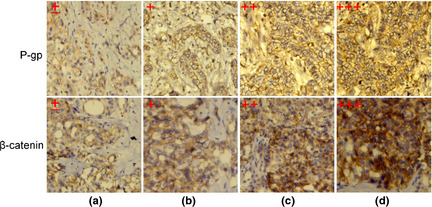
Immunohistochemistry of β‐catenin and P‐gp in cholangiocarcinoma (CCA) tissues. The staining intension of β‐catenin and P‐gp in CCA tissues were classified into four grades, ±, +, ++ and +++, according to scoring criteria. Each grade of β‐catenin and P‐gp were from the same sample. Expression levels of β‐catenin and P‐gp were categorized as low (±, +) or high (++, +++). Zoom: 200×; P‐gp, P‐glycoprotein.
Table 1.
Correlation between β‐catenin and P‐gp staining
| P‐gp staining | β‐catenin staining | χ2 | P | |
|---|---|---|---|---|
| High | Low | |||
| High | 30 | 13 | 6.808 | 0.009 |
| Low | 7 | 13 | ||
P < 0.05 is considered statistically significant.
Discussion
In the current study, we characterized the newly established MDR CCA cell line QBC939/5‐FU that exhibited drug resistance to common chemotherapeutics. Activation of Wnt/β‐catenin signaling associated with upregulation of P‐gp can induce MDR in QBC939/5‐FU cells. ESC‐3 significantly enhanced the drug susceptibility of QBC939/5‐FU cells to 5‐FU, paralleled with downregulation of β‐catenin and P‐gp. Due to the importance of P‐gp expression in MDR of several cancer types, including CCA, we speculated that P‐gp might be a key mechanism of Wnt/β‐catenin activation on induction of MDR in CCA.
Special biological characteristics of carcinoma in the bile duct system makes CCA refractory to radiotherapy and chemotherapy, resulting in a low overall 5‐year survival rate in CCA patients.3 Although great advances in studying the mechanisms of tumor MDR have been made, including overexpression of P‐gp and other related transporters, resistance to anticancer drug‐induced apoptosis, sustained proliferation by a small population of self‐renewing tumor cells and changes of tumor microenvironment,19 the complexity of the mechanisms contributing to MDR of CCA remains little known. Most published works on overcoming MDR of CCA focus on the inhibition of drug transporters. P‐gp has been considered as an important protein for the MDR formation. Previous studies show that overexpression of P‐gp contributes greatly to the MDR of tumors, including CCA.7, 9, 20 In our study, P‐gp and MRP were highly expressed in the MDR CCA cell line QBC939/5‐FU. The rate of high P‐gp expression in a set of 63 CCA tissues was 68.3%. These data suggest again that overexpression of P‐gp might contribute to the MDR of CCA.
As mentioned above, overexpression of P‐gp is broadly known to limit the efficacy of chemotherapeutic agents in anti‐cancer proliferation. However, the molecular mechanisms regulating P‐gp expression are still unclear. It is reported that several singling pathways associated with the progression of tumor play an important role in the induction of MDR, such as PI3K/Akt, NF‐κB and Wnt/β‐catenin.10, 21 In particular, activation of Wnt signaling pathway contributes to chemoresistance of human neuroblastomas or rat brain endothelial cells through upregulation of P‐gp expression.13, 14, 15 Mutations or altered expression of several components of the Wnt/β‐catenin pathway leading to its pathological activation are described to promote tumor progression and survival, including CCA.22, 23 However, the role of the Wnt/β‐catenin pathway has not yet been implicated in chemoresistance in CCA. There were no differences in the activation of PI3K/Akt or NF‐κB signaling pathways between QBC939/5‐FU and QBC939 cells, but increased total level and nuclear/perinuclear accumulation of β‐catenin were observed in QBC939/5‐FU cells. Upregulation and nuclear translocation of β‐catenin result in its interaction with T‐cell factor/lymphoid enhancer factor, and subsequently the regulation of gene expression.24, 25 This evidence suggests β‐catenin protein stabilization and Wnt/β‐catenin pathway activation in QBC939/5‐FU cells. In addition, inhibition of Wnt/β‐catenin pathway using β‐catenin siRNA restored the drug susceptibility of QBC939/5‐FU cells to chemotherapeutics, and this effect is probably via downregulation of P‐gp expression. In contrast, treatment of Wnt3a, an activator of Wnt/β‐catenin pathway, repressed the drug susceptibility of QBC939 cells paralleled with upregulated P‐gp expression. Importantly, we found a strong link between β‐catenin and P‐gp expression profile in clinical CCA tissues. These results strongly support our view that P‐gp expression regulated by Wnt/β‐catenin pathway might play a critical role in the MDR of CCA.
Taken together, the present study is the first to demonstrate an important role of the Wnt/β‐catenin pathway in P‐gp expression and mediating MDR of CCA cells. Wnt/β‐catenin signaling is a potential therapeutic target for overcoming chemoresistance in CCA in the clinic.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81101502 and 81100194) and the Excellent Youth Foundation of Fujian Province (2009D016), China.
(Cancer Sci 2013; 104: 1303–1308
References
- 1. Renshaw K. Malignant neoplasms of the extrahepatic biliary ducts. Ann Surg 1922; 76: 205–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology 2005; 128: 1655–67. [DOI] [PubMed] [Google Scholar]
- 3. Shaib Y, El‐Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004; 24: 115–25. [DOI] [PubMed] [Google Scholar]
- 4. Rocchi E, Khodjakov A, Volk EL et al The product of the ABC half‐transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem Biophys Res Commun 2000; 271: 42–6. [DOI] [PubMed] [Google Scholar]
- 5. Gillet JP, Efferth T, Remacle J. Chemotherapy‐induced resistance by ATP‐binding cassette transporter genes. Biochim Biophys Acta 2007; 1775: 237–62. [DOI] [PubMed] [Google Scholar]
- 6. Liu ZH, He YP, Zhou Y, Zhang P, Qin H. Establishment and identification of the human multi‐drug‐resistant cholangiocarcinoma cell line QBC939/ADM. Mol Biol Rep 2011; 38: 3075–82. [DOI] [PubMed] [Google Scholar]
- 7. Rau S, Autschbach F, Riedel HD et al Expression of the multidrug resistance proteins MRP2 and MRP3 in human cholangiocellular carcinomas. Eur J Clin Invest 2008; 38: 134–42. [DOI] [PubMed] [Google Scholar]
- 8. Naus PJ, Henson R, Bleeker G, Wehbe H, Meng F, Patel T. Tannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathways. J Hepatol 2007; 46: 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP‐dependent transporters. Nat Rev Cancer 2002; 2: 48–58. [DOI] [PubMed] [Google Scholar]
- 10. Kuo MT, Liu Z, Wei Y et al Induction of human MDR1 gene expression by 2‐acetylaminofluorene is mediated by effectors of the phosphoinositide 3‐kinase pathway that activate NF‐kappaB signaling. Oncogene 2002; 21: 1945–54. [DOI] [PubMed] [Google Scholar]
- 11. Lin X, Zhang X, Wang Q et al Perifosine downregulates MDR1 gene expression and reverses multidrug‐resistant phenotype by inhibiting PI3K/Akt/NF‐kappaB signaling pathway in a human breast cancer cell line. Neoplasma 2012; 59: 248–56. [DOI] [PubMed] [Google Scholar]
- 12. Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down‐regulates the multidrug‐resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett 2008; 259: 111–18. [DOI] [PubMed] [Google Scholar]
- 13. Chikazawa N, Tanaka H, Tasaka T et al Inhibition of Wnt signaling pathway decreases chemotherapy‐resistant side‐population colon cancer cells. Anticancer Res 2010; 30: 2041–8. [PubMed] [Google Scholar]
- 14. Flahaut M, Meier R, Coulon A et al The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta‐catenin pathway. Oncogene 2009; 28: 2245–56. [DOI] [PubMed] [Google Scholar]
- 15. Lim JC, Kania KD, Wijesuriya H et al Activation of beta‐catenin signalling by GSK‐3 inhibition increases p‐glycoprotein expression in brain endothelial cells. J Neurochem 2008; 106: 1855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song W, Shen DY, Kang JH et al Apoptosis of human cholangiocarcinoma cells induced by ESC‐3 from Crocodylus siamensis bile. World J Gastroenterol 2012; 18: 704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen DY, Kang JH, Song W et al Apoptosis of human cholangiocarcinoma cell lines induced by beta‐escin through mitochondrial caspase‐dependent pathway. Phytother Res 2011; 25: 1519–26. [DOI] [PubMed] [Google Scholar]
- 18. Shen DY, Zhan YH, Wang QM, Rui G, Zhang ZM. Oncogenic potential of Cyclin Kinase Subunit‐2 in cholangiocarcinoma. Liver Int 2013; 33: 137–48. [DOI] [PubMed] [Google Scholar]
- 19. Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol 2010; 46: 308–16. [DOI] [PubMed] [Google Scholar]
- 20. Liu ZH, Ma YL, He YP, Zhang P, Zhou YK, Qin H. Tamoxifen reverses the multi‐drug‐resistance of an established human cholangiocarcinoma cell line in combined chemotherapeutics. Mol Biol Rep 2011; 38: 1769–75. [DOI] [PubMed] [Google Scholar]
- 21. Zhang H, Zhang X, Wu X et al Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/beta‐catenin pathway. Cancer Lett 2012; 323: 106–13. [DOI] [PubMed] [Google Scholar]
- 22. Tokumoto N, Ikeda S, Ishizaki Y et al Immunohistochemical and mutational analyses of Wnt signaling components and target genes in intrahepatic cholangiocarcinomas. Int J Oncol 2005; 27: 973–80. [PubMed] [Google Scholar]
- 23. Sugimachi K, Taguchi K, Aishima S et al Altered expression of beta‐catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol 2001; 14: 900–5. [DOI] [PubMed] [Google Scholar]
- 24. Clevers H, Nusse R. Wnt/beta‐catenin signaling and disease. Cell 2012; 149: 1192–205. [DOI] [PubMed] [Google Scholar]
- 25. Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell 2004; 6: 497–506. [DOI] [PubMed] [Google Scholar]


