Abstract
Amyloid precursor protein (APP) is a transmembrane protein that is highly expressed in brain tissue. Recently, APP has been implicated in some human malignancies, and its regulation by androgens has also been demonstrated. Such findings suggest the importance of APP in hormone‐dependent breast carcinoma, but APP has not yet been examined in breast carcinoma tissues. Therefore, in this study, we examined the biological and clinical significance of APP in breast carcinoma using immunohistochemistry and in vitro studies. APP immunoreactivity was detected in 57 out of 117 (49%) breast carcinoma tissues examined, and it was positively associated with androgen receptor (AR) expression. APP immunoreactivity was also significantly associated with Ki‐67 LI and increased risk of recurrence in the estrogen receptor (ER)‐positive cases, and was an independent prognostic factor in these patients. Subsequent in vitro experiments demonstrated that APP mRNA expression was significantly induced by biologically active androgen dihydrotestosterone in both a dose‐dependent and a time‐dependent manner in MCF‐7 breast carcinoma cells, which was potently suppressed by an AR blocker hydroxyflutamide. Moreover, cell proliferation activity of MCF‐7 and MDA‐MB‐231 cells was significantly associated with their APP expression level. These findings suggest that APP is an androgen‐induced gene that promotes proliferation activity of breast carcinoma cells. Moreover, APP immunohistochemical status is considered a potent prognostic factor in ER‐positive breast cancer patients.
Breast carcinoma is known as a hormone‐dependent neoplasm, and estrogens play crucial roles in the development and/or progression of breast carcinoma. In addition, androgen receptor (AR) is expressed in a great majority of breast carcinoma tissues1, 2 and bioactive androgen dihydrotestosterone (DHT) is locally produced in the carcinoma,3 suggesting the importance of androgens in breast carcinoma. Androgens are in general considered to suppress breast carcinoma cell proliferation,4, 5 but some divergent findings have been reported.6, 7 Therefore, it is very important to examine molecular functions of androgens, including exploration of the androgen‐regulated genes, in breast carcinoma.
Amyloid precursor protein (APP) is a type I transmembrane protein, processed by α‐, β‐ and γ‐secretase. One of the processed APP products, β‐amyloid, is a major component of amyloid plaque, which is frequently detected in the brain tissues with Alzheimer's disease.8 APP is also expressed in various nonneural tissues, and it is suggested to be involved in the growth of these cells.9 APP has been implicated in several human malignancies, including lung, colon, pancreas, parathyroid, thyroid and prostate carcinomas,10, 11, 12, 13, 14, 15 and the soluble N‐terminal ectodomain fragment (sAPP) is reported to be responsible for the pro‐proliferative effects of APP on carcinoma cells.16 In addition, Takayama et al.15 report that APP is an androgen‐induced gene in prostate carcinoma and functions as an important mediator of the androgen actions. These findings suggest possible roles of APP in human breast carcinoma associated with androgen actions and/or cell proliferation. However, details of APP have not yet been studied in breast carcinoma, and its significance has remained largely unclear. Therefore, in the present study, we examine APP in breast carcinoma using immunohistochemistry and in vitro studies to explore its clinical significance and biological functions.
Materials and Methods
Patients and tissues
A total of 117 specimens of invasive ductal carcinoma of human breast were obtained from female Japanese patients who underwent surgical treatment from 1990 to 1999 in the Department of Surgery, Tohoku University Hospital, Sendai, Japan. None of the patients received chemotherapy, irradiation or hormonal therapy before the surgery. Review of the charts revealed that 85 patients received adjuvant endocrine therapy, 52 patients received adjuvant chemotherapy and 26 patients received radiation therapy following surgery. The clinical outcome of the patients was evaluated by disease‐free and breast cancer‐specific survival in our present study. Disease‐free survival was defined as the time from surgery to the date of the first locoregional recurrence or first distant metastasis within the follow‐up time after surgery, and we used stage I to III breast carcinoma patients (n = 108) included in a previous report.17 Breast cancer‐specific survival was defined as the time from surgery to death from the breast cancer, and we examined the stage I to IV patients (n = 117). The mean age was 56 years (range 31–81 years), and the mean follow‐up time was 100 months (range 1–175 months). All the specimens had been fixed in 10% formalin and embedded in paraffin wax.
Research protocols for this study were approved by the Ethics Committee of the Tohoku University School of Medicine.
Immunohistochemistry
Characteristics of the primary antibody for APP are summarized in a previous report.15 Mouse monoclonal antibodies for estrogen receptor (ER; ER1D5), progesterone receptor (PR; MAB429), androgen receptor (AR; AR441) and Ki‐67 (MIB1) were purchased from Immunotech (Marseille, France), Chemicon (Temecula, California, USA) and DAKO (Carpinteria, CA, USA), respectively. Rabbit polyclonal antibody for HER2 (A0485) was purchased from DAKO. A Histofine Kit (Nichirei, Tokyo, Japan), which employs the streptavidin–biotin amplification method, was used. The antigen–antibody complex was visualized with 3,3‐diaminobenzidine and counterstained with hematoxylin. As a positive control for APP immunostaining, human cerebral tissue from a patient with Alzheimer's disease was used in this study,18 which was retrieved from the autopsy files of the Department of Pathology, Tohoku University Hospital, Sendai, Japan.
Scoring of immunoreactivity and subgroup definition of the breast carcinoma
Immunoreactivity of APP was detected in the cytoplasm, and cases that had more than 10% positive carcinoma cells were considered positive in this study.15 ER, PR, AR and Ki‐67 immunoreactivity were detected in the nucleus of breast carcinoma cells. The immunoreactivity was evaluated in more than 1000 carcinoma cells for each case, and, subsequently, the percentage of immunoreactivity (i.e. labeling index [LI]), was determined. Cases with ER LI, PR LI or AR LI of more than 10% were considered ER‐, PR‐ or AR‐positive breast carcinomas in this study.19 HER2 immunoreactivity was evaluated according to a grading system proposed in HercepTest (DAKO), and strongly circumscribed membrane staining of HER2 in more than 10% of carcinoma cells was considered positive in this study.
Intrinsic subtypes of the breast carcinoma was defined according to the 2011 St Gallen surrogate definition20 as follows: luminal A (ER and/or PR positive, HER2 negative, Ki‐67 LI < 14%), luminal B (ER and/or PR positive, HER2 negative, Ki‐67 LI ≧ 14% [HER2 negative], or ER and/or PR positive, HER2 positive [HER2 positive]), HER2 positive (ER and PR negative, HER2 positive) and triple negative (ER, PR, HER2 negative).
Cell line and chemicals
Human breast carcinoma cell line MCF‐7 and MDA‐MB‐231 were provided by the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan), and cultured in RPMI 1640 (Sigma‐Aldrich, St. Louis, MO, USA) with 10% FBS (Biological Industries, Beit‐Haemek, Israel). MCF‐7 cells were cultured in phenol‐red‐free RPMI 1640 with 10% dextran‐coated charcoal‐stripped FBS for 3 days before treatment with sex steroids. DHT and an AR antagonist hydroxyflutamide were purchased from Wako Pure Chemical Industries (Osaka, Japan) and Toronto Research Chemicals (Downsview, ON, Canada), respectively.
Real‐time PCR
Total RNA was extracted using TRIzol reagent (Carlsbad, CA, USA), and cDNA was synthesized using a QuantiTect reverse transcription kit (Qiagen, Hilden, Germany). Real‐time PCR was carried out using the LightCycler System and FastStart DNA Master SYBR Green I (Roche Diagnostics, Mannheim, Germany). The PCR primer sequence of APP and ribosomal protein L13A (RPL13A) used in this study was as follows. APP: forward 5′‐TCTCCCTGCTCTACAACGTG‐3′ and reverse 5′‐TTT CCGTAACTGATCCTTGG‐3′; and RPL13A: forward 5′‐CCTGGAGGAGAAGAGGAAAGAGA‐3′ and reverse 5′‐TTGAGGACCTCTGTGTATTTGTCAA‐3′. PCR products were purified and subjected to direct sequencing to verify amplification of the correct sequences. APP mRNA levels were summarized as the ratio of the RPL13A mRNA level (%).
siRNA transfection
Three siRNA oligonucleotides for APP (siAPP) and negative control (siCTRL) were obtained from RNAi (Tokyo, Japan). siRNA sense sequences were as follows: siAPP‐1: 5′‐GUUCCUGACAAGUGCAAAUUC‐3′, siAPP‐2: 5′‐GAUCCAUCAGGGACCAAAACC‐3′, and siCTRL: 5′‐GUACCGCACGUCAUUCGUAUC‐3′. The siRNA was transiently transfected in breast carcinoma cells using Lipofectamine 2000 (Life technologies, Carlsbad, CA, USA).
Immunoblotting
The cell protein from MCF‐7 and MDA‐MB‐231 cells was extracted using M‐PER mammalian protein extraction reagent (Thermo Fisher Scientific Pierce Biotechnology, Rockford, IL, USA). A total of 10 μg of the protein was subjected to SDS‐PAGE (10% acrylamide gel). Following SDS‐PAGE, proteins were transferred onto Hybond polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, UK). The primary antibodies used were anti‐APP antibody for immunohistochemistry and anti‐β‐actin antibody (AC‐15; Sigma‐Aldrich) as an internal control. Antibody–protein complexes on the blots were detected using ECL‐Prime Western Blotting detection reagents (GE Healthcare), and the protein bands were visualized with an LAS‐4000 image analyzer (Fuji Photo Film, Tokyo, Japan).
Cell proliferation assay and apoptosis analysis
MCF‐7 and MDA‐MB‐231 cells were transfected with siAPP‐1, siAPP‐2 or siCTRL in a 96‐well culture plate. The cell proliferation was measured using a 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (a tetrazole) assay kit (Nacalai Tesque, Kyoto, Japan) 1, 2 and 4 days after transfection.
For apoptosis analysis, cells were stained with Annexin V‐FITC 2 days after the transfection using the Tali Apoptosis Kit (Life Technologies) according to the manufacturer's protocol. Cells were subsequently analyzed using a Tali Image Based Cytometer (Life technologies).21, 22
Statistical analysis
An association between APP immunoreactivity and clinicopathological factors was evaluated using Student's t‐test or a cross‐table using the χ2‐test. Disease‐free and breast cancer‐specific survival curves were generated according to the Kaplan–Meier method, and statistical significance was calculated using the log‐rank test. Univariate and multivariate analyses were evaluated using the proportional hazard model (Cox). Dunnett's test was used in the in vitro experiments. P‐values <0.05 were considered significant in this study.
Results
Amyloid precursor protein immunolocalization in human breast carcinoma
Amyloid precursor protein immunoreactivity was detected in the cytoplasm of breast carcinoma cells (Fig. 1a), while it was negative in the morphologically normal glands or stroma (Fig. 1b). In the positive control, APP was immunolocalized in the cytoplasm of nerve cells in the cerebral cortex of Alzheimer's disease (Fig. 1c).
Figure 1.
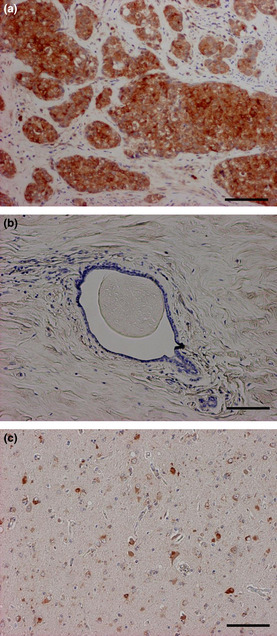
Immunolocalization of amyloid precursor protein (APP) in human breast carcinoma. (a, b) APP immunoreactivity was detected in the cytoplasm of carcinoma cells (a), while negative in the morphologically normal mammary grand or stroma (b). (c) Positive control of APP immunostaining (human cerebral tissue from a patient with Alzheimer's disease). Bar = 100 μm, respectively.
Associations between APP immunoreactivity and various clinicopathological parameters in the breast carcinoma cases are summarized in Table 1. In this study, the number of APP‐positive cases was 57 out of 117 (49%). APP immunoreactivity was significantly associated with AR status (P = 0.02) and AR LI (P < 0.0001), and it was frequently negative in the triple negative breast carcinomas (P = 0.047). In contrast, no significant association was detected in patients' age, menopausal status, clinical stage, pathological tumor factor (pT), lymph node metastasis, distant metastasis, histological grade, ER status, PR status, HER2 status and Ki‐67 LI. As shown in Tables 2 and 3, APP status was significantly associated with AR LI regardless of ER status of the cases (P = 0.002 in ER‐positive group, and P = 0.01 in ER‐negative group). Significant association (P = 0.002) was also detected between APP status and Ki‐67 LI in the ER‐positive breast carcinomas (Table 2).
Table 1.
Association between APP immunohistochemical status and clinicopathological parameters in 117 breast carcinomas
| APP status | P‐value | ||
|---|---|---|---|
| + (n = 57) | − (n = 60) | ||
| Age† (years) | 55.3 ± 1.4 | 56.9 ± 1.6 | 0.46 |
| Menopausal status | |||
| Premenopausal | 19 (16%) | 21 (18%) | 0.85 |
| Postmenopausal | 38 (32%) | 39 (33%) | |
| Stage | |||
| I | 15 (13%) | 22 (19%) | 0.63 |
| II | 28 (24%) | 25 (21%) | |
| III | 10 (9%) | 8 (7%) | |
| IV | 4 (3%) | 5 (4%) | |
| Pathological T factor (pT) | |||
| pT1 | 22 (19%) | 26 (22%) | 0.60 |
| pT2–4 | 35 (30%) | 34 (29%) | |
| Lymph node metastasis | |||
| Positive | 29 (25%) | 24 (21%) | 0.24 |
| Negative | 28 (24%) | 36 (31%) | |
| Distant metastasis | |||
| Positive | 4 (3%) | 5 (4%) | 0.79 |
| Negative | 53 (45%) | 55 (47%) | |
| Histological grade | |||
| 1 (well) | 11 (9%) | 14 (12%) | 0.60 |
| 2 (moderate) | 26 (22%) | 30 (26%) | |
| 3 (poor) | 20 (17%) | 16 (14%) | |
| ER status | |||
| Positive | 45 (38%) | 39 (33%) | 0.09 |
| Negative | 12 (10%) | 21 (18%) | |
| PR status | |||
| Positive | 33 (28%) | 30 (26%) | 0.39 |
| Negative | 24 (21%) | 30 (26%) | |
| AR status | |||
| Positive | 47 (40%) | 38 (32%) | 0.02* |
| Negative | 10 (9%) | 22 (19%) | |
| AR LI† (%) | 39.3 ± 3.8 | 20.3 ± 2.4 | <0.0001* |
| HER2 status | |||
| Positive | 14 (12%) | 10 (9%) | 0.29 |
| Negative | 43 (37%) | 50 (43%) | |
| Ki‐67 LI† (%) | 21.8 ± 2.2 | 18.6 ± 2.1 | 0.29 |
| Intrinsic subtype‡ | |||
| Luminal A | 25 (21%) | 31 (26%) | 0.047* |
| Luminal B | 20 (17%) | 10 (9%) | |
| HER2 positive | 8 (7%) | 7 (6%) | |
| Triple negative | 4 (3%) | 12 (10%) | |
*P‐values less than 0.05 were considered significant. †Data are presented as mean ± SEM. All other values represent the number of cases and percentage. ‡Intrinsic subtype was defined according to the 2011 St Gallen surrogate definition.20 APP, amyloid precursor protein; AR, Androgen receptor; ER, estrogen receptor; PR, progesterone receptor.
Table 2.
Association between APP status and clinicopathological parameters in 84 ER‐positive breast carcinomas
| APP status | P‐value | ||
|---|---|---|---|
| + (n = 45) | − (n = 39) | ||
| Age† (years) | 54.6 ± 3.4 | 57.7 ± 4.0 | 0.24 |
| Menopausal status | |||
| Premenopausal | 17 (20%) | 13 (15%) | 0.67 |
| Postmenopausal | 28 (33%) | 26 (31%) | |
| Stage | |||
| I | 12 (14%) | 19 (22%) | 0.17 |
| II | 23 (28%) | 15 (19%) | |
| III | 6 (12%) | 4 (11%) | |
| IV | 4 (9.3%) | 1 (2.6%) | |
| Pathological T factor (pT) | |||
| pT1 | 17 (20%) | 23 (27%) | 0.05 |
| pT2–4 | 28 (33%) | 16 (19%) | |
| Lymph node metastasis | |||
| Positive | 22 (26%) | 12 (14%) | 0.09 |
| Negative | 23 (27%) | 27 (32%) | |
| Distant metastasis | |||
| Positive | 4 (4.8%) | 1 (1.2%) | 0.22 |
| Negative | 41 (49%) | 38 (45%) | |
| Histological grade | |||
| 1 (well) | 11 (13%) | 13 (15%) | 0.07 |
| 2 (moderate) | 22 (26%) | 23 (27%) | |
| 3 (poor) | 12 (14%) | 3 (3.6%) | |
| PR status | |||
| Positive | 33 (39%) | 28 (33%) | 0.87 |
| Negative | 12 (14%) | 11 (13%) | |
| AR status | |||
| Positive | 36 (43%) | 28 (33%) | 0.38 |
| Negative | 9 (11%) | 11 (13%) | |
| AR LI† (%) | 39.8 ± 8.7 | 22.6 ± 5.8 | 0.002* |
| HER2 status | |||
| Positive | 6 (7.1%) | 2 (2.4%) | 0.29 |
| Negative | 39 (46%) | 37 (44%) | |
| Ki‐67 LI† (%) | 20.6 ± 5.2 | 11.0 ± 2.7 | 0.002* |
*P‐values less than 0.05 were considered significant. †Data are presented as mean ± SEM. All other values represent the number of cases and percentage. APP, amyloid precursor protein; AR, Androgen receptor; ER, estrogen receptor; PR, progesterone receptor.
Table 3.
Association between APP status and clinicopathological parameters in 33 ER‐negative breast carcinomas
| APP status | P‐value | ||
|---|---|---|---|
| + (n = 12) | − (n = 21) | ||
| Age† (years) | 57.8 ± 5.8 | 55.5 ± 5.6 | 0.56 |
| Menopausal status | |||
| Premenopausal | 2 (6.1%) | 8 (24%) | 0.20 |
| Postmenopausal | 10 (30%) | 13 (39%) | |
| Stage | |||
| I | 3 (9.1%) | 3 (9.1%) | 0.32 |
| II | 5 (15%) | 10 (30%) | |
| III | 4 (12%) | 4 (12%) | |
| IV | 0 (0%) | 4 (19%) | |
| Pathological T factor (pT) | |||
| pT1 | 5 (15%) | 3 (9.1%) | 0.08 |
| pT2–4 | 7 (21%) | 18 (55%) | |
| Lymph node metastasis | |||
| Positive | 7 (21%) | 12 (36%) | 0.95 |
| Negative | 5 (15%) | 9 (27%) | |
| Distant metastasis | |||
| Positive | 0 (0%) | 4 (12%) | 0.11 |
| Negative | 12 (36%) | 17 (52%) | |
| Histological grade | |||
| 1 (well) | 0 (0%) | 1 (3%) | 0.74 |
| 2 (moderate) | 4 (12%) | 7 (21%) | |
| 3 (poor) | 8 (24%) | 13 (39%) | |
| PR status | |||
| Positive | 0 (0%) | 2 (6.1%) | 0.27 |
| Negative | 12 (36%) | 19 (58%) | |
| AR status | |||
| Positive | 11 (33%) | 10 (30%) | 0.01* |
| Negative | 1 (3.0%) | 11 (33%) | |
| AR LI† (%) | 37.7 ± 17.3 | 16.2 ± 8.9 | 0.01* |
| HER2 status | |||
| Positive | 8 (24%) | 8 (24%) | 0.20 |
| Negative | 4 (12%) | 13 (39%) | |
| Ki‐67 LI† (%) | 26.4 ± 8.5 | 32.8 ± 8.2 | 0.30 |
*P‐values less than 0.05 were considered significant. †Data are presented as mean ± SEM. All other values represent the number of cases and percentage. APP, amyloid precursor protein; AR, Androgen receptor; ER, estrogen receptor; PR, progesterone receptor.
Association between amyloid precursor protein status and clinical outcome of the patients
Immunohistochemical APP status was not significantly (P = 0.24) associated with recurrence in the stage I–III breast carcinoma patients (n = 108) (Fig. 2a), but it was significantly (P = 0.01) correlated with an increased risk of recurrence in the ER‐positive cases (n = 79) (Fig. 2b). No significant association (P = 0.13) was detected between APP status and recurrence in the ER‐negative cases examined (n = 29) (data not shown). In the ER‐positive group, results of the univariate analyses revealed that lymph node metastasis (P = 0.004), APP immunoreactivity (P = 0.02), pT (P = 0.03) and HER2 status (P = 0.048) were significant prognostic factors for disease‐free survival, and following multivariate analysis demonstrated that lymph node metastasis (P = 0.01) and APP immunoreactivity (P = 0.045) were independent prognostic factors for disease‐free survival with relative risks over 1.0 (Table 4).
Figure 2.
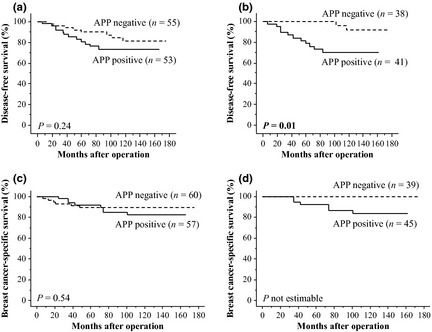
Disease‐free survival (a, b) and breast cancer‐specific survival (c, d) of breast carcinoma patients according to amyloid precursor protein (APP) status studied using the Kaplan–Meier method. (a) Stage I–III cases (n = 108), (b) estrogen receptor (ER)‐positive stage I–III cases (n = 79), (c) stage I–IV cases (n = 117) and (d) ER‐positive stage I–IV cases (n = 84). Solid line, APP positive cases; dashed line, APP negative cases. P‐value is not estimable for Figure 2d, because no patients had died in the APP negative group.
Table 4.
Univariate and multivariate analyses of disease‐free survival in stages ER‐positive stages I–III breast carcinoma patients examined (n = 79)
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| P‐value | P‐value | Relative risk (95% CI) | |
| Lymph node metastasis (positive/negative) | 0.004a | 0.01a | 6.3 (1.7–23.5) |
| APP status (positive/negative) | 0.02a | 0.045a | 4.9 (1.03–22.8) |
| pT (pT2–4/pT1) | 0.03a | 0.11 | |
| HER2 status (positive/negative) | 0.048a | 0.10 | |
| Histological grade (3/1,2) | 0.42 | ||
| AR status (negative/positive) | 0.57 | ||
| Ki‐67 LI (82–0%) | 0.66 | ||
Data considered significant (P < 0.05) in the univariate analyses were examined in the following multivariate analysis. APP, amyloid precursor protein; AR, Androgen receptor; CI, confidence interval; ER, estrogen receptor.
Breast cancer‐specific survival curves of the patients according to APP status are summarized in Figure 2c,d. APP status was not significantly (P = 0.54) associated with the clinical outcome of the stage I–IV patients examined (n = 117) (Fig. 2c), but was associated with a worse prognosis in the ER‐positive cases (n = 84), although the P‐value was not estimable because no patients died in the APP‐negative group (Fig. 2d). In contrast, no significant correlation (P = 0.36) between APP status and breast cancer‐specific survival was detected in the ER‐negative cases (n = 33) (data not shown).
Amyloid precursor protein as an androgen‐responsive gene in the breast carcinoma
The results of our immunohistochemical study demonstrated significant correlation between APP status and AR LI in breast carcinoma, suggesting possible regulation of APP expression by androgens in breast carcinoma tissues. Therefore, we examined in vitro experiments using MCF‐7 cells that expressed AR.23
As shown in Figure 3a, APP mRNA expression was significantly induced by DHT in a dose‐dependent manner in MCF‐7 cells, and it became significant at 10 nM (1.9‐fold, P < 0.01). The DHT‐mediated induction of APP mRNA expression was potently suppressed by an AR blocker hydroxyflutamide (0.35‐fold and P < 0.01, compared to a group treated by DHT alone), whereas hydroxyflutamide alone did not significantly alter APP mRNA level in MCF‐7 cells (0.79‐fold, P = 0.69) (Fig. 3b). DHT (10 nM) also induced APP mRNA expression in a time‐dependent manner, and it became significant from 2 days (1.6‐fold, P < 0.01, Fig. 3c).
Figure 3.
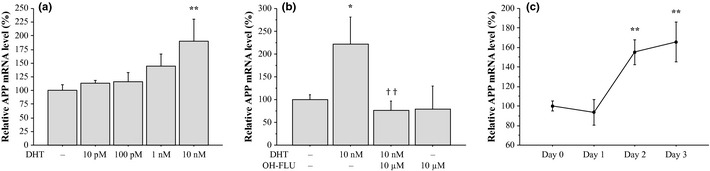
Induction of amyloid precursor protein (APP) mRNA expression in MCF‐7 cells. Effects of bioactive androgen dihydrotestosterone (DHT) on APP mRNA expression was examined by real‐time PCR analysis. MCF‐7 cells were treated with indicated concentration of DHT and/or androgen receptor (AR) blocker hydroxyflutamide (OH‐FLU; 10 μM) (a, b) for 3 days, or treated with DHT (10 nM) for the indicated period (c). APP mRNA level was evaluated as a ratio of RPL13A mRNA level, and subsequently relative APP mRNA level was summarized as a ratio (%) compared with the basal level (nontreatment). Data were presented as the mean ± SD (n = 3), respectively. *P < 0.05 and **P < 0.01 compared to nontreatment (left column); ††P < 0.01 compared to a group treated by DHT alone.
Effects of amyloid precursor protein on breast carcinoma cell proliferation
Results of our immunohistochemical study also revealed that APP status was associated with Ki‐67 LI and worse prognosis in ER‐positive breast carcinoma cases. Ki‐67 LI represents proliferative activity of breast carcinoma tissues,24 and it is a known prognostic factor in breast carcinoma patients.25 Therefore, we examined the effects of APP on proliferation activity in breast carcinoma cells.
As shown in Figure 4a,b, APP mRNA levels were significantly decreased both in ER‐positive MCF‐7 (Fig. 4a, upper panel) and ER‐negative MDA‐MB‐231 (Fig. 4b, upper panel) cells transfected with specific APP siRNA (i.e. siAPP‐1 and siAPP‐2) at 2 days after transfection compared to cells transfected with negative control siRNA (siCTRL) (P < 0.001, respectively). The ratios of the APP mRNA level compared to the control were: MCF‐7, 0.21‐fold (siAPP‐1) and 0.19‐fold (siAPP‐2); and MDA‐MB‐231, 0.23‐fold (siAPP‐1) and 0.22‐fold (siAPP‐2). APP protein levels were also markedly decreased in these cells transfected with APP siRNA under the same conditions (Fig. 4a,b, lower panels).
Figure 4.
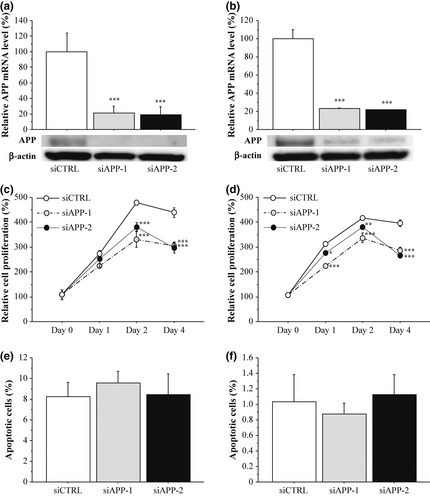
Effects of amyloid precursor protein (APP) on cell proliferation and apoptosis in breast carcinoma cells. (a, b) Expression of APP in MCF‐7 (a) and MDA‐MB‐231 (b) cells transfected with APP‐specific siRNA (siAPP‐1 and siAPP‐2) or negative control siRNA (siCTRL) at mRNA level evaluated by real‐time PCR (upper panels) and protein level by immunoblotting (lower panels). For the immunoblotting, 10 μg of protein was loaded in each lane, and β‐actin immunoreactivity is shown as the internal control. (c, d) Proliferation activity of MCF‐7 (c) and MDA‐MB‐231 (d) cells transfected with siRNA summarized as a ratio (%) compared to that 0 days after treatment. (e, f) Percentage of apoptotic cells in the MCF‐7 (e) and MDA‐MB‐231 (f). Open bar/circle: siCTRL, gray bar/circle: siAPP‐1; and closed bar/circle: siAPP‐2. Data were presented as the mean ± SD (n = 3), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with siCTRL groups.
Cell proliferation activity was significantly suppressed in MCF‐7 cells transfected with APP siRNA from 2 to 4 days after the transfection (Day 2, 0.69‐fold and P < 0.001 [siAPP‐1] and 0.80‐fold and P < 0.001 [siAPP‐2]; and Day 4, 0.70‐fold and P < 0.001 [siAPP‐1] and 0.68‐fold and P < 0.001 [siAPP‐2]). Similar results were obtained when MDA‐MB‐231 cells were examined under the same conditions (Fig. 4d). In contrast, the percentages of apoptotic cells in MCF‐7 and MDA‐MB‐231 cells transfected with siRNA against APP at 2 days after transfection were at similar levels to their controls in our study (Fig. 4e,f, respectively).
Discussion
This is the first study to demonstrate immunolocalization of APP in human breast carcinoma tissues. In the present study, APP immunoreactivity was detected in 49% of breast carcinomas, while it was negligible in morphologically normal mammary glands. APP immunolocalization has been reported in several human carcinomas. For instance, Ko et al.26 report that APP immunoreactivity was detected in 100% of oral squamous cell carcinomas, and the patients exhibiting increased APP mRNA expression had significantly lower survival rates compared to the patients exhibiting the opposite status. In Takayama et al.15 demonstrate that APP immunoreactivity was detected in 56% of prostate carcinomas, and was associated with adverse clinical outcomes of the patients. Yang et al.27 report that APP immunoreactivity was detected in 40% of papillary thyroid carcinomas, and was a potential marker of the malignancy and prognosis. Our present results suggest that APP expression is increased in breast carcinomas compared to normal breast tissues, and the relatively wide distribution of APP immunoreactivity also suggests biological importance of APP in human breast carcinomas.
In the present study, APP immunoreactivity was positively associated with Ki‐67 LI in the ER‐positive breast carcinomas, and it was an independent prognostic factor for recurrence in these patients. Subsequent in vitro experiments demonstrated that APP significantly promoted cell proliferation of ER‐positive MCF‐7 breast carcinoma cells, while it did not significantly change the apoptotic status. Previous studies indicate that APP functions as a local mediator of cell proliferation in several malignant tumors, such as lung,10 colon,11 pancreas,12 parathyroid,13 thyroid14 and prostate15 carcinomas, which is concurs with the present results for breast carcinoma. In addition, Jaffe et al.28 report that estrogen increased accumulation of sAPP in the conditioned medium of ER‐positive ZR‐75‐1 breast carcinoma cells. Therefore, it is suggested that APP plays an important role in the cell proliferation of ER‐positive breast carcinomas, possibly through interacting with ER functions.
However, we could not find significant association between APP status and Ki‐67 LI in the ER‐negative breast carcinoma cases. Nevertheless APP significantly increased the proliferation activity of ER‐negative MDA‐MB‐231 cells. This may be partly because the number of ER‐negative cases was limited (n = 33) in our immunohistochemical study, or it may be that other factors play more important roles than APP in the growth of ER‐negative breast carcinomas. Further studies with a larger sample set are necessary to clarify the significance of APP in ER‐negative breast carcinoma.
In the present study, APP status was significantly associated with AR LI in breast carcinoma regardless of the ER status. Results of our real‐time PCR analyses also indicated that the expression level of APP mRNA was significantly induced by DHT in MCF‐7 cells in a dose‐dependent and time‐dependent manner, which was significantly suppressed by the addition of hydroxyflutamide. Takayama et al. (2009) identified androgen‐responsive elements in the 3′‐downstream and intron 1 of APP using ChIP‐chip technique, and reported that APP mRNA and protein were significantly induced by R1881, synthetic androgens in LNCaP prostate carcinoma cells. Results of our present study were in good agreement with these findings, and suggest that APP expression was upregulated by androgens in breast carcinoma.15
Androgens are generally considered to exert anti‐proliferative effects in breast carcinomas, but it is also true that some divergent findings have been reported. For instances, MDA‐MB‐453 breast carcinoma cells have been shown a cell proliferative response to androgens in an ER‐independent manner, which was reversed by treatment of an anti‐androgen flutamide,29 and Naderi and Hughes‐Davies30 report functional crosstalks between AR and HER2 signaling. Moreover, Takagi et al.31 report that androgen induced KLF5 expression, which is known to promote breast carcinoma cell proliferation. Considering that androgen functions are determined by expression profiles of the target genes, pro‐proliferating effects of androgens may be, at least in part, mediated by APP in breast carcinoma. Further examination is required to clarify the molecular mechanisms and/or clinical significance of androgen actions in human breast carcinoma.
In summary, APP immunoreactivity was detected in 49% of breast carcinoma tissues, and the APP status was significantly associated with AR LI in these cases. Moreover, APP status was significantly associated with Ki‐67 LI in ER‐positive breast carcinomas, and it was an independent prognostic factor for disease‐free survival of these patients. Subsequent in vitro experiments demonstrated that APP mRNA expression was significantly induced by DHT in MCF‐7 cells, and APP significantly increased the proliferation activity of MCF‐7 and MDA‐MB‐231 cells. These results suggest that APP is an androgen‐induced gene in breast carcinoma that plays an important role in the cell proliferation. In particular, APP plays an important role in the proliferation and/or progression of ER‐positive breast carcinomas, and it is a potent prognostic factor in breast carcinoma patients.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We appreciate the skillful technical assistance of Mr Katsuhiko Ono and Mr Yoshiaki Onodera (Department of Anatomic Pathology, Tohoku University Graduate School of Medicine).
(Cancer Sci 2013; 104: 1532–1538)
References
- 1. Søreide JA, Lea OA, Varhaug JE, Skarstein A, Kvinnsland S. Androgen receptors in operable breast cancer: relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur J Surg Oncol 1992; 18: 112–8. [PubMed] [Google Scholar]
- 2. Isola JJ. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol 1993; 170: 31–5. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki T, Darnel AD, Akahira JI et al 5alpha‐reductases in human breast carcinoma: possible modulator of in situ androgenic actions. J Clin Endocrinol Metab 2001; 86: 2250–7. [DOI] [PubMed] [Google Scholar]
- 4. Poulin R, Baker D, Labrie F. Androgens inhibit basal and estrogen‐induced cell proliferation in the ZR‐75‐1 human breast cancer cell line. Breast Cancer Res Treat 1988; 12: 213–25. [DOI] [PubMed] [Google Scholar]
- 5. de Launoit Y, Dauvois S, Dufour M, Simard J, Labrie F. Inhibition of cell cycle kinetics and proliferation by the androgen 5 alpha‐dihydrotestosterone and antiestrogen N, n‐butyl‐N‐methyl‐11‐[16′ alpha‐chloro‐3′,17 beta‐dihydroxy‐estra‐1′,3′,5′‐(10′)triene‐7′ alpha‐yl] undecanamide in human breast cancer ZR‐75‐1 cells. Cancer Res 1991; 51: 2797–802. [PubMed] [Google Scholar]
- 6. Birrell SN, Bentel JM, Hickey TE et al Androgens induce divergent proliferative responses in human breast cancer cell lines. J Steroid Biochem Mol Biol 1995; 52: 459–67. [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Sun Y, Liu Y, Sun Y, Liao DJ. Synergistic effects of androgen and estrogen on the mouse uterus and mammary gland. Oncol Rep 2004; 12: 709–16. [PubMed] [Google Scholar]
- 8. LaFerla FM, Green KN, Oddo S. Intracellular amyloid‐beta in Alzheimer's disease. Nat Rev Neurosci 2007; 8: 499–509. [DOI] [PubMed] [Google Scholar]
- 9. Di Luca M, Colciaghi F, Pastorino L, Borroni B, Padovani A, Cattabeni F. Platelets as a peripheral district where to study pathogenetic mechanisms of alzheimer disease: the case of amyloid precursor protein. Eur J Pharmacol 2000; 405: 277–83. [DOI] [PubMed] [Google Scholar]
- 10. Itoh H, Kataoka H, Koita H et al Establishment of a new human cancer cell line secreting protease nexin‐II/amyloid beta protein precursor derived from squamous‐cell carcinoma of lung. Int J Cancer 1991; 49: 436–43. [DOI] [PubMed] [Google Scholar]
- 11. Meng JY, Kataoka H, Itoh H, Koono M. Amyloid beta protein precursor is involved in the growth of human colon carcinoma cell in vitro and in vivo. Int J Cancer 2001; 92: 31–9. [PubMed] [Google Scholar]
- 12. Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ, Maitra A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res 2003; 63: 7032–7. [PubMed] [Google Scholar]
- 13. Haven CJ, Howell VM, Eilers PH et al Gene expression of parathyroid tumors: molecular subclassification and identification of the potential malignant phenotype. Cancer Res 2004; 64: 7405–11. [DOI] [PubMed] [Google Scholar]
- 14. Krause K, Karger S, Sheu SY et al Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J Endocrinol 2008; 198: 291–9. [DOI] [PubMed] [Google Scholar]
- 15. Takayama K, Tsutsumi S, Suzuki T et al Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res 2009; 69: 137–42. [DOI] [PubMed] [Google Scholar]
- 16. Rossjohn J, Cappai R, Feil SC et al Crystal structure of the N‐terminal, growth factor‐like domain of Alzheimer amyloid precursor protein. Nat Struct Biol 1999; 6: 327–31. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki S, Takagi K, Miki Y et al Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci 2012; 103: 136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gearing M, Wilson RW, Unger ER et al Amyloid precursor protein (APP) in the striatum in Alzheimer's disease: an immunohistochemical study. J Neuropathol Exp Neurol 1993; 52: 22–30. [DOI] [PubMed] [Google Scholar]
- 19. Takagi K, Miki Y, Nagasaki S et al Increased intratumoral androgens in human breast carcinoma following aromatase inhibitor exemestane treatment. Endocr Relat Cancer 2010; 17: 415–30. [DOI] [PubMed] [Google Scholar]
- 20. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22: 1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicolaou KA, Liapis V, Evdokiou A et al Induction of discrete apoptotic pathways by bromo‐substituted indirubin derivatives in invasive breast cancer cells. Biochem Biophys Res Commun 2012; 42: 76–82. [DOI] [PubMed] [Google Scholar]
- 22. Fukaya Y, Kuroda M, Aoyagi Y et al Platelet‐rich plasma inhibits the apoptosis of highly adipogenic homogeneous preadipocytes in an in vitro culture system. Exp Mol Med 2012; 44: 330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horwitz KB, Costlow ME, McGuire WL. MCF‐7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids 1975; 26: 785–95. [DOI] [PubMed] [Google Scholar]
- 24. van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol 2004; 57: 675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Azambuja E, Cardoso F, de Castro G Jr et al Ki‐67 as prognostic marker in early breast cancer: a meta‐analysis of published studies involving 12,155 patients. Br J Cancer 2007; 96: 1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ko SY, Lin SC, Chang KW et al Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer 2004; 111: 727–32. [DOI] [PubMed] [Google Scholar]
- 27. Yang Z, Fan Y, Deng Z, Wu B, Zheng Q. Amyloid precursor protein as a potential marker of malignancy and prognosis in papillary thyroid carcinoma. Oncol Lett 2012; 3: 1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jaffe AB, Toran‐Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem 1994; 269: 13065–8. [PubMed] [Google Scholar]
- 29. Doane AS, Danso M, Lal P et al An estrogen receptor‐negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006; 25: 3994–4008. [DOI] [PubMed] [Google Scholar]
- 30. Naderi A, Hughes‐Davies L. A functionally significant cross‐talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia 2008; 10: 542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takagi K, Miki Y, Onodera Y et al Kruppel‐like factor 5 in human breast carcinoma: a potent prognostic factor induced by androgens. Endocr Relat Cancer 2012; 19: 741–50. [DOI] [PubMed] [Google Scholar]


