Abstract
Purpose:
This study aimed to determine the associations among physical activity, sedentary behavior, and the metabolic syndrome (MetS) in Latino and African American youth using both subjective and objective measures of activity levels.
Methods:
Cross-sectional data from 105 participants from three pediatric obesity studies that share a core set of methods and measures (Latino 74%, female 75%, mean age =13 ± 3 yr) were used. Measures included moderate-to-vigorous physical activity and sedentary behavior by accelerometry and 3-Day Physical Activity Recall (3DPAR), fat and lean tissue mass by BodPod™, fasting glucose, lipids, blood pressure, and waist circumference. Associations between physical activity, sedentary behavior, and MetS were examined using ANCOVA, Pearson correlations, partial correlations, and logistic regressions with adjustments for age, sex, ethnicity, fat and lean mass, and pubertal Tanner stage.
Results:
Accelerometry data showed that greater time engaging in moderate-to-vigorous physical activity was related to lower odds of the MetS (odds ratio = 0.49, 95% confidence interval = 0.25–0.98), independent of sedentary behavior and covariates, and inversely correlated with fasting glucose (r = −0.21, P = 0.03) and systolic blood pressure (r = −0.25, P = 0.01), adjusting for covariates. Data from the 3DPAR showed that higher levels of sedentary behavior were related to higher odds of the MetS (odds ratio = 4.44, 95% confidence interval = 1.33–14.79), independent of moderate-to-vigorous physical activity and covariates, negatively correlated with HDL-cholesterol (r = −0.21, P = 0.04) and positively correlated systolic blood pressure (r = 0.26, P = 0.009), adjusting for covariates.
Conclusions:
Future interventions aiming to improve metabolic health in youth should target both the promotion of physical activity and the reduction of sedentary behavior. Subjective and objective measures should be used in conjunction to better capture activity behaviors.
Keywords: MODERATE-TO-VIGOROUS PHYSICAL ACTIVITY, SEDENTARY ACTIVITY, METABOLIC HEALTH, LATINO AMERICAN, AFRICAN AMERICAN
The metabolic syndrome (MetS) refers to a cluster of cardiovascular and type 2 diabetes risk factors that include elevated triglycerides, low HDL-cholesterol, abdominal adiposity, hyperglycemia, and elevated blood pressure (37). On the basis of the estimates from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2002, the prevalence of MetS in youth age 12–19 yr varies widely from 2.0% to 9.4%, reaching 44.2% among obese children (7). Our group has shown that >30% of overweight Latino children in the Los Angeles area have MetS (9). This is especially important in light of the fact that other research has found that MetS in childhood predicted adult MetS and type 2 diabetes mellitus as much as 25–30 yr later (26). Thus, to reduce the increasing prevalence of such chronic diseases in adults, it is important to identify lifestyle behaviors that can be modified during childhood.
Two of the key modifiable lifestyle factors that influence metabolic health are physical activity (28) and sedentary behavior (16). Lower levels of physical activity and higher levels of sedentary behavior are known to be associated with progression toward MetS in adults (16). However, little is known regarding these relationships in youth, particularly in minority populations (11,12,28,35). In a review article on physical activity and MetS in youth (35), only one (5) of the six studies conducted focused on a minority population (Latino). More research is therefore needed to understand how physical activity and sedentary behavior affect MetS in minority youth.
Another gap in the literature on activity levels and MetS is that while there are some studies on physical activity, less is known about sedentary behavior. Although engaging in physical activity and being sedentary may seem like two sides of the same coin, research has suggested that these two behaviors should be treated as different dimensions, rather than a continuum (30). The majority of existing studies measure physical activity either solely by subjective or objective measures. Subjective measures are susceptible to memory bias and social desirability (12), whereas objective measures, such as accelerometry, suffer from issues of compliance and the accuracy is dependent on activity type (34). Using both types of measures may complement the limitations inherent in each measurement modality and hence provide more comprehensive estimations in activity levels.
Therefore, the purpose of this study was to investigate the associations between average daily physical activity, average daily sedentary behavior, and MetS in Latino and African American youth. Activity levels were measured objectively by accelerometry and subjectively by the 3-Day Physical Activity Recall (3DPAR). We hypothesized that participants with MetS would have lower levels of moderate-to-vigorous physical activity (MVPA) and higher levels of sedentary behavior than those without and that lower levels of sedentary behavior and higher levels of physical activity would be protective against odds for MetS, independent of each other and of body compositions.
METHODS
Participants
Data for this sample were taken from baseline measures in three related pediatric obesity studies that share a set of common methods and measures: TRANSITIONS (Insulin Resistance and Declining Physical Activity Levels in African American and Latina girls), SANO (Strength and Nutrition Outcomes for Latino Adolescents), and STAND (Strength Training and Nutrition Development in African American Youth). While a total of 187 participants completed a baseline visit, the final sample consisted of 105 participants (Latino 74.3%, female 75.2%, 13 ± 3 yr) who have completed responses to all measures of interests.
Participants were recruited through medical clinics, churches, community centers, local schools, and advertisements in Los Angeles County. Detailed methods for these studies have been published previously (13,40). Participants were excluded if they were using a medication or had been diagnosed with a condition known to influence body composition, fat distribution, or insulin/glucose metabolism or if they had been diagnosed with diabetes by fasting plasma glucose (≥126 mgdL−1) at the time of entry to the study. The studies were approved by the University of Southern California (USC) institutional review board. Written informed consent and assent were obtained from all parents and children before any testing procedures.
Protocol
Participants arrived in the afternoon at USC General Clinical Research Center. Weight and height were measured three times to the nearest 0.1 kg and 0.1 cm, respectively, using a beam medical scale and wall-mounted stadiometer by trained medical professionals. Body mass index (BMI) percentiles for age and sex were determined based on established Centers for Disease Control and Prevention normative curves (6). Sitting blood pressure was measured in triplicate using the right arm after the participant had rested for 5 min (27). Waist circumference was measured in triplicate at the umbilicus and recorded to the nearest 0.1 cm. Body composition (total fat mass and total lean tissue mass) was measured by air plethysmography (BodPod™), and pubertal Tanner stage was assessed by a licensed pediatric health care provider (36). Fasting blood samples were measured for triglycerides, HDL-cholesterol, and fasting glucose. Fasting lipids including triglycerides and HDL-cholesterol were assessed using Vitros Chemistry DT Slides (Johnson and Johnson Clinical Diagnostics, Inc., Rochester, NY). Glucose was assayed using a Yellow Springs Instruments 2700 analyzer (Yellow Springs Instrument, Yellow Springs, OH) that uses a membrane-bound glucose oxidase technique.
Definition of the MetS
Because no standard definition for MetS exists in pediatrics, we used a combination of pediatric definitions proposed by Cruz et al. (10) and Cook et al. (8,33),1 who applied pediatric cutoffs to the Adult Treatment Panel III definition (14). This definition has been applied in our previous studies (10,33). To be classified as having MetS, participants had to have at least three of the following features: abdominal obesity (waist circumference ≥90th percentile for age, sex, and ethnicity from Third National Health and Nutrition Examination Survey data) (15), hypertriglyceridemia (triglycerides ≥90th percentile for age, sex, and ethnicity) (19), low HDL-cholesterol (HDL-cholesterol ≤10th percentile for age, sex, and ethnicity) (19), elevated blood pressure (systolic or diastolic blood pressure ≥ 90th percentile adjusted for age, sex, and height) (28), and hyperglycemia (impaired fasting glucose ≥100 mg·dL−1) (2,8).1
Physical Activity and Sedentary Behavior
Accelerometry data.
Objective assessments of activity levels were obtained using the ActiGraph (Model GT1M; ActiGraph, LLC, Fort Walton Beach, FL). The ActiGraph is a uniaxial accelerometer that measures acceleration in the vertical plane, and it has been shown as a valid and reliable measure of physical activity for children and adolescents (31). The participants were instructed to wear the device for 7 d on the right side of the hip during waking hours, with the exception of time spent in bathing or swimming activities. Accelerometer data were processed using a SAS program developed for use with NHANES physical activity monitor data (available at: http://riskfactor.cancer.gov/tools/nhanes_pam). The raw data were processed to calculate the minutes of nonwear (defined by an interval of ≥60 consecutive minutes of zero activity intensity counts, with exceptions for up to 3 min of 0–100 counts) and the minutes of wear time.
A valid day of wear was defined as having at least 10 h of wear time, and participants with at least four valid days of data were included in the analyses (n = 105) (38) (43% of participants had at least one valid weekend day). The total number of minutes spent in MVPA and sedentary activity, was determined by summing minutes in a day where the count met the criterion for that intensity. Then, these total minutes were averaged across the number of valid days to obtain the mean minutes per day. The cut point for MVPA (≥4 METs) was age-adjusted using the Freedson criteria (16a) and sedentary cutpoints defined by Matthews et al. (23), which have both been validated in adolescents.
Self-reported physical activity data.
3DPAR was used to assess self-reported activity levels (30). 3DPAR has been validated in adolescents (29). Students identified different activities (from a list of 71 activities provided) to describe their activity in half-hour intervals during a day from 7:00 a.m. to 12:00 a.m. for 3 d and rated how much effort (intensity level) they put into each activity (light, moderate, hard, or very hard). Activity types were converted into half-hour blocks of either light, moderate, or vigorous physical activity using a combination of the intensity ratings provided by the participants and the compendium of physical activities (1). MVPA (≥4 METs) was created to be consistent to the accelerometry variable. Half-hour blocks spent watching television/movies, playing video games/surfing the internet, and talking on the telephone were coded separately as sedentary behaviors. Total time spent in MVPA and sedentary behavior was obtained by summing over the half-hour blocks for 1 day. Mean minutes per day was then obtained by averaging total minutes across 3 d.
Statistical analysis.
For the preliminary analyses, participants with complete data included in the present study were compared to those without complete data on demographic variables and distribution of MetS using independent-sample t-tests or χ2 tests. Among the analytical sample (n = 105), three sets of analyses were conducted. First, demographic characteristics and activity levels were compared between those with and without MetS. Participants were dichotomized into those with and without MetS. Independent-sample t-tests or χ2 tests were conducted to compare demographic variables between these two MetS groups. ANCOVAs were used to examine differences in MVPA and sedentary behavior between the two MetS groups. Second, associations between MVPA, sedentary behavior, and the odds of developing MetS were summarized by using odds ratios (ORs) estimated in multivariate logistic regressions. For the logistic regression models, the effects of MVPA and sedentary behavior on the odds of MetS were first examined in separate models and were then included in the same model showing the independent associations between MVPA and sedentary behavior with MetS after adjusting for each other. For model calibration, the Hosmer-Lemeshow tests (19) were performed to evaluate the goodness-of-fit. Finally, Pearson and partial correlations were used to assess the relationships of MVPA and sedentary behavior with each individual feature of MetS. To correct for multiple comparisons, Bonferroni correction was applied to evaluate correlation coefficients.
The following variables are covariates to control for the potential confounding in ANCOVA models, multivariate logistic regressions, and partial correlation: age, sex, ethnicity, pubertal Tanner stage, fat mass, and lean tissue mass. Logarithmic transformations were applied where necessary to achieve better normality where variables were skewed for analyses (i.e., fat mass, lean tissue mass, MVPA, sedentary behaviors, triglycerides, HDL-cholesterol, fasting glucose, and systolic/diastolic blood pressure). All continuous independent variables were centered on the sample’s mean to prevent collinearity. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). The significance of the findings was evaluated at the P < 0.05 level.
RESULTS
No statistical differences in MetS and sex were found between those with (n = 105, the final analytical sample) and without (n = 82) complete data. However, those with complete data were more likely to be younger (P = 0.001) and Latino (P = 0.015) (data not shown).
In this study, 16% (n = 17) participants met the criteria for MetS. Demographic characteristics by participants with and without MetS are shown in Table 1. Those with MetS were more likely to be male (P = 0.02) and have a higher Tanner stage (P = 0.02). In addition, participants with MetS had higher BMI percentiles (P < 0.001), greater fat mass (P < 0.001), greater lean tissue mass (P = 0.02), higher percent body fat (P = 0.002), and lower percent lean tissue mass (P = 0.002). Adjusted activity levels (non–log-transformed values) by MetS status are shown in Figure 1. Based on the accelerometry, participants with MetS spent 16% (5.1 fewer minutes) less time per day in MVPA (P = 0.008) than those without. Based on the 3DPAR, those with MetS spent 39% (81.7 more minutes) more time daily in sedentary behavior than those without (P = 0.008).
TABLE 1.
Demographic characteristics by MetS.
| All (n = 105) | MetS (n = 17) | Non-MetS (n = 88) | Pa | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 79 (75.24) | 9 (11.39) | 70 (88.61) | 0.02* |
| Male | 26 (24.76) | 8 (30.77) | 18 (69.23) | |
| Ethnicity, n (%) | ||||
| Latino | 78 (74.29) | 15 (19.23) | 63 (80.77) | 0.15 |
| African American | 27 (25.71) | 2 (7.41) | 25 (92.59) | |
| Age, mean ± SD (yr) | 13.05 ± 3.02 | 14.00 ± 2.37 | 12.86 ± 3.11 | 0.16 |
| Pubertal Tanner stage, n (%) | ||||
| 1 | 19 (18.10) | 0 (0.00) | 19 (21.59) | 0.02* |
| 2 | 20 (19.05) | 3 (17.65) | 17 (19.32) | |
| 3 | 4 (3.81) | 2 (11.76) | 2 (2.27) | |
| 4 | 18 (17.14) | 6 (35.29) | 12 (13.64) | |
| 5 | 44 (41.90) | 6 (35.29) | 38 (43.18) | |
| BMI percentile, mean ± SD | 90.55 ± 16.89 | 98.47 ± 1.63 | 89.02 ± 18.05 | <0.001** |
| Total fat mass, mean ± SD (kg) | 27.90 ± 17.42 | 41.80 ± 19.08 | 25.21 ± 15.83 | <0.001** |
| Percent fat mass, mean ± SD | 33.67 ± 11.11 | 41.21 ± 8.71 | 32.21 ± 10.97 | 0.002*** |
| Total lean tissue mass, mean ± SD (kg) | 47.59 ± 16.28 | 56.03 ± 14.02 | 45.96 ± 16.25 | 0.02* |
| Percent lean tissue mass, mean ± SD | 66.33 ± 11.11 | 58.79 ± 8.71 | 67.79 ± 10.97 | 0.002*** |
| MetS features, mean ± SD | ||||
| Triglycerides (mg·dL−1) | 83.69 ± 36.13 | 119.47 ± 40.49 | 76.77 ± 30.99 | <0.001** |
| HDL-cholesterol (mg·dL−1) | 39.77 ± 10.73 | 31.76 ± 3.25 | 41.32 ± 10.98 | <0.001** |
| Waist circumference (cm) | 87.54 ± 17.33 | 101.35 ± 14.38 | 84.67 ± 16.55 | <0.001** |
| Fasting glucose (mg·dL−1) | 91.61 ± 6.01 | 94.47 ± 7.87 | 91.06 ± 5.46 | 0.10 |
| Systolic blood pressure (mm Hg) | 116.27 ± 14.07 | 132.12 ± 11.97 | 113.20 ± 12.32 | <0.001** |
| Diastolic blood pressure (mm Hg) | 65.27 ± 7.19 | 70.79 ± 6.42 | 64.20 ± 6.86 | <0.001** |
P < 0.05.
P< 0.001.
P< 0.01.
χ2 tests were used for categorical variables and independent t-tests for continuous variables.
FIGURE 1—
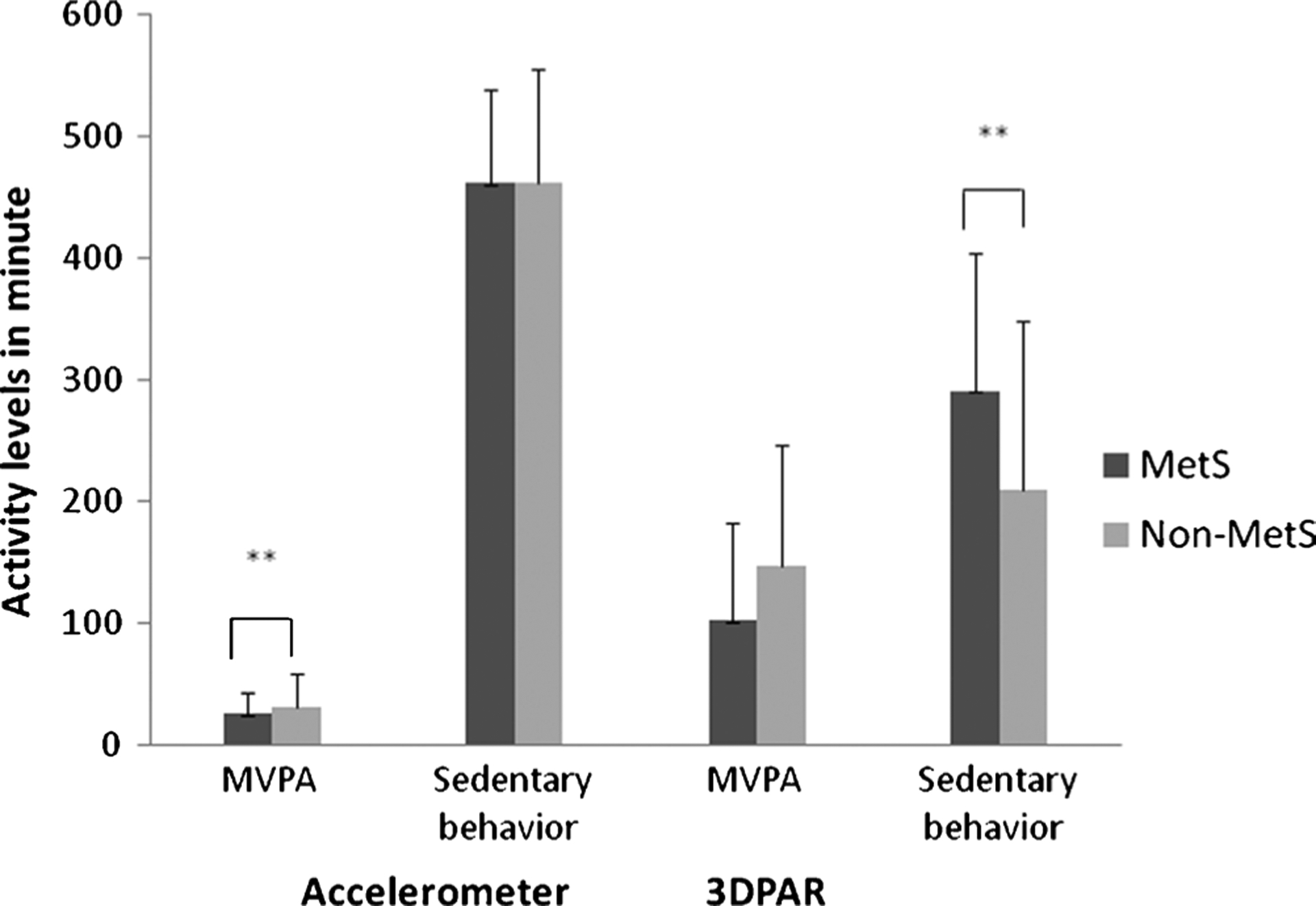
Activity levels by MetS. Non-log-transformed values are presented. These estimates were adjusted for age, sex, ethnicity, Tanner stage, fat mass, and lean tissue mass. ** P < 0.001.
Table 2 presents the results of logistic regression models demonstrating the associations between activity levels and odds of having MetS. When both MVPA and sedentary behaviors were included in the same model for each measurement modality, greater time engaging in MVPA (by accelerometry: OR = 0.49, 95% confidence interval (CI) = 0.25–0.98) was related to lower odds of MetS while greater participation in sedentary behavior was related to higher odds of MetS (by 3DPAR: OR = 4.44, 95% CI = 1.33–14.79). Although log-transformed activity levels were used for analyses, for the purpose of interpretation, the same models were run using original units of activity levels: the odds of having MetS was 5% lower for each 1-min increment of MVPA (by accelerometry: OR = 0.95), while the odds of having MetS was 1% higher for each 1-min increment of sedentary behavior (by 3DPAR: OR = 1.01). In addition, to determine whether the relationship between activity levels and odds of MetS was sensitive to measurement modality, the same relationships were assessed adjusting for the other type of measure. The relationship between activity levels and odds of MetS remained significant after including MVPA by accelerometry and sedentary behavior by 3DPAR in the same model; less time spent in MVPA as measured by accelerometry (OR = 0.34, 95% CI = 0.14–0.84) and greater time spent in sedentary behaviors by 3DPAR (OR = 6.42, 95% CI = 1.65–25.02) were related to greater odds of MetS. Based on the Hosmer-Lemeshow tests, all models presented in Table 2 demonstrated adequate model-data fit (P = 0.07–0.94).
TABLE 2.
Adjusted ORs examining the associations of activity levels with MetS.
| Adjusted OR (95% CI) for MetS (3+ or more)a | |
|---|---|
| Accelerometerb | |
| Model A | |
| MVPA | 0.49 (0.25–0.95) |
| Model B | |
| Sedentary behavior | 2.56 (0.06–119.63) |
| Model C | |
| MVPA | 0.49 (0.25–0.98) |
| Sedentary behavior | 1.01 (0.02–61.70) |
| 3DPARb | |
| Model D | |
| MVPA | 0.86 (0.58–1.26) |
| Model E | |
| Sedentary behavior | 4.44 (1.41–14.02) |
| Model F | |
| MVPA | 0.99 (0.64–1.56) |
| Sedentary behavior | 4.44 (1.33–14.79) |
| MVPA by accelerometry and sedentary behavior by 3DPARb | |
| Model G | |
| MVPA by accelerometry | 0.34 (0.14–0.84) |
| Sedentary behavior by 3DPAR | 6.42 (1.65–25.02) |
Parameters were adjusted for age, sex, ethnicity, Tanner stage, fat mass, and lean tissue mass.
Variables were not normally distributed, therefore statistical tests were run with log-transformed data.
We next examined Pearson and partial correlations between activity levels and each individual feature of MetS (Table 3). Compared with the unadjusted correlations, the number of the significant findings was reduced after adjusting for body composition and other covariates. Based on the partial correlations, there were inverse correlations between MVPA by accelerometry and fasting glucose (r = −0.21, P = 0.03) as well as systolic blood pressure (r = −0.25, P = 0.01). In addition, sedentary behavior by 3DPAR was negatively correlated with HDL-cholesterol (r = −0.21, P = 0.04) and positively correlated systolic blood pressure (r = 0.26, P = 0.009).
TABLE 3.
Pearson correlations/partial correlations between activity levels and features of MetS.
| Accelerometrya | 3DPARa | |||
|---|---|---|---|---|
| MetS Feature | MVPA | Sedentary Behavior | MVPA | Sedentary Behavior |
| Pearson correlations | ||||
| Triglyceridesa | −0.21* | 0.03 | −0.09 | 0.08 |
| HDL-cholesterola | 0.13 | −0.10 | −0.04 | −0.22* |
| Waist circumference | −0.42**,*** | 0.32***,**** | −0.17 | 0.08 |
| Fasting glucosea | −0.07 | 0.10 | 0.12 | −0.12 |
| Systolic blood pressurea | −0.33***,**** | 0.21* | −0.17 | 0.31***,**** |
| Diastolic blood pressurea | −0.27**** | 0.07 | −0.10 | 0.18 |
| Partial correlationsb | ||||
| Triglyceridesa | −0.07 | −0.19 | −0.02 | 0.05 |
| HDL-cholesteroa | 0.03 | 0.10 | −0.08 | −0.21* |
| Waist circumference | 0.01 | −0.02 | −0.10 | 0.01 |
| Fasting glucosa | −0.21* | 0.04 | 0.10 | −0.12 |
| Systolic blood pressura | −0.25* | −0.01 | −0.16 | 0.26**** |
| Diastolic blood pressura | −0.12 | −0.10 | −0.03 | 0.11 |
P < 0.05, adjusted significance with application of Bonferroni correction for multiple comparisons.
P < 0.001, adjusted significance with application of Bonferroni correction for multiple comparisons.
P < 0.002, adjusted significance with application of Bonferroni correction for multiple comparisons.
P < 0.01, adjusted significance with application of Bonferroni correction for multiple comparisons.
Variables were not normally distributed so statistical tests were run with log-transformed data.
Parameters were adjusted for age, sex, ethnicity, Tanner stage, fat mass, and lean tissue mass.
DISCUSSION
To our knowledge, this is the first study to examine the relationships between MetS and both physical activity and sedentary behavior in minority youth. The primary finding was that lower levels of physical activity and higher levels of sedentary behavior are associated with greater metabolic risk and risk for individual MetS features, after adjusting for each other and body composition. We also found that participants with MetS had lower levels of MVPA and higher levels of sedentary behavior than those without.
Inverse relationships between physical activity and metabolic risk have been found in previous studies in youth (4,21,24,25,28,32), the majority of which relied on subjective self-report activity levels (21,24,25,28). However, that studies using objective measures of physical activity (specifically accelerometry) (4,12,32) have been less conclusive. Although we examined physical activity both subjectively and objectively, significant relationships between MVPA and odds of MetS were found based on accelerometry data only. We thus compared our findings with previous work using accelerometry. In the three studies in which physical activity was measured by accelerometer, Barge et al. (4) and Ekelund et al. (12) found that increased physical activity levels were related to reduced risk for MetS in youth. However, another study (32) found that physical activity was only significantly related to MetS in adolescent girls. It should be noted that two (4,32) of these three studies did not control for body composition, which is an important confounder when examining the influence of physical activity on metabolic risk. Our findings add to the limited body of research by showing that objectively assessed physical activity is related to metabolic risks, independent of body composition.
In contrast to the growing number of studies on physical activity and the MetS, very little have examined the role of sedentary behavior on metabolic health. In adults, time spent watching television and using a computer has been shown to positively link with MetS (16). Very little research has been conducted in youth, and findings were inconclusive. One study (12) found a trend for a positive association between self-reported TV viewing and risk for MetS (P = 0.053), after adjusting for physical activity. Given that youth spend the majority of their time in sedentary behaviors and inactivity (20), more research is needed to understand how sedentary behavior is related to MetS. This study is the first to show that sedentary behavior (as measured by 3DPAR) is positively associated with metabolic risk, independent of body composition. Our results showed that increased MVPA and decreased sedentary behavior may lower all five metabolic risk factors, including low HDL-cholesterol and larger waist circumference, the two most commonly seen criteria for MetS in minority youth (9). These findings suggest that interventions to prevent MetS need to target sedentary behavior in addition to physical activity.
In this study, the significant associations between activity levels and MetS differed by measurement modality. The significant findings regarding MVPA and MetS were solely based on the accelerometer data. However, although the significant findings regarding sedentary behavior were based on the data by both accelerometer and 3DPAR, the adjusted ones were solely based on the 3DPAR data. It is worth mentioning that, when further examining the simple correlation between MVPA by accelerometer and sedentary behavior by 3DPAR, two activity variables closely related to MetS in the current study, no significant correlation was found (r = −0.10, P = 0.03). Another observation was that the derived estimates of time spent in MVPA and sedentary behavior differed by modalities (Fig. 1). For time spent in MVPA, the estimates based on 3DPAR were larger than those obtained by accelerometer. In addition, participants with and without MetS spent similar amount of time in sedentary behavior according to the accelerometers while time spent being sedentary was significantly greater in participants with MetS than those without, based the 3DPAR data. Thus, it seems that accelerometer and 3DPAR may capture different aspects of activity levels.
A better understanding of how activity is measured by each modality may help explain these differences. The accelerometer, because it assesses physical activity objectively, avoids the recall bias and social desirability issues inherent in self-report measures and hence seems to detect differences in activity levels better among participants with and without MetS. Higher estimates of MVPA by 3DPAR might be because it assesses the dominant activity for each half-hour block while accelerometers recorded actual time spent in specific intensity levels of physical activity. In addition, relatively larger variations in physical activity were observed in the 3DPAR data. This could be explained by the fact that 3DPAR is subjective and estimates are influenced by respondents’ perception of fitness levels and memory. For sedentary behavior, accelerometry captures all activities under a certain cutoff and the types of these activities were unknown. In addition, because accelerometry does not accurately measure movement of the upper body performed during low levels of activities (22), it may not accurately differentiate sedentary from light-intensity activities. On the other hand, a unique advantage of 3DPAR is its ability to provide rich contextual information on the types of sedentary activities, allowing researchers to focus on activities that have been found to have adverse effects on metabolic health. This may explain why there is a greater difference in sedentary behavior by MetS status.
Strengths and Limitations
A particular strength of this study is that it uses both subjective and objective measures of activity, which allows comparison of the relationships to MetS across measures. Another key strength is that we examined both physical activity and sedentary behavior. Recent research has suggested that the protective effect of physical activity on heath could be attenuated by sedentary behavior (12). By including both physical activity and sedentary behavior in the same model, we are able to investigate their independent effects on metabolic risk.
In addition to the aforementioned limitations for accelerometer and 3DPAR, other limitations of this study should also be considered. First, the cross-sectional study design limits the ability to establish temporal sequence of the effects and therefore no causal inferences can be made. Second, the small overall sample size and uneven sample sizes of the MetS, sex, and ethnic groups could limit the statistical power and preclude additional findings. Third, we did not adjust for potential confounders such as birth weight and dietary pattern while examining the associations between activity levels and MetS. Another limitation is that missing data may affect the validity of the results. It is important to note that this study was not designed to specifically compare the true differences between accelerometer and 3DPAR, and although both measures were usually collected at the same time, there are some instances where the data collection period for the two methods did not overlap completely. Future studies with careful designs and population-based samples are warranted to determine the differences in activity levels and their associations to metabolic-related biological markers between accelerometer and self-reported physical activity. In addition, sedentary breaks, which have been found to relate to metabolic risks in adults (3,17), were not assessed in the current study. Future research is needed to better understand the effect of sedentary breaks on MetS.
In conclusion, our results contribute to the limited data for youth regarding the beneficial effects of higher levels of physical activity on metabolic health. Furthermore, this study extends previous findings by demonstrating that higher levels of sedentary behaviors are related to greater metabolic risk. The relationships between physical activity and metabolic risk are independent of sedentary behavior and body composition and vice versa. Future interventions that aim to improve metabolic health in minority youth should target both the promotion of physical activity and the reduction of sedentary behavior. In addition, it is recommended that both objective and subjective measures of activity levels, when used in conjunction and carefully administered, may provide more complete information on the effect of activity-related behaviors on metabolic health.
Acknowledgments
This research was supported by USC Center for Transdisciplinary Research on Energetics and Cancer (National Cancer Institute, U54 CA 116848), the National Institute of Child Health and Human Development (rO1 HD/HL 33064), General Clinical Research Center National Center for Research Resources/National Institutes of Health (M01RR 00043), and Dr. Robert C. and Veronica Atkins Foundation.
The authors thank the study participants, staffs, and funders, without whom this research would not have been possible.
Footnotes
The authors have no conflicts of interest to report.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Cook et al. originally used fasting glucose ≥110 mg·dL−1 to define impaired fasting glucose. The criteria for impaired fasting glucose have been updated by the American Diabetes Association as a fasting glucose ≥100 mg·d−1 (16). The revised cut point has been used.
REFERENCES
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–516. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;34:S37–42. [DOI] [PubMed] [Google Scholar]
- 3.Bankoski A, Harris TB, McClain JJ, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barge S, Wedderkropp N, Ekelund U, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children. Diabetes Care. 2004;27: 2141–8. [DOI] [PubMed] [Google Scholar]
- 5.Butte NF, Puyau MR, Adolph AL, Vohra FA, Zakeri I. Physical activity in non overweight and overweight Hispanic children and adolescents. Med Sci Sports Exerc. 2007;39(8):1257–66. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, and National Center for Health Statistics. CDC Growth Charts: United States. [cited 2000 May 30]. Available from: http://www.cdc.gov/growthcharts/.
- 7.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. JPediatr. 2008;152:165–70. [DOI] [PubMed] [Google Scholar]
- 8.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157: 821–7. [DOI] [PubMed] [Google Scholar]
- 9.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. [DOI] [PubMed] [Google Scholar]
- 10.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89: 108–13. [DOI] [PubMed] [Google Scholar]
- 11.Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Anderssen LB, Brage S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European Youth Heart Study. Diabetologia. 2007;50:1832–40. [DOI] [PubMed] [Google Scholar]
- 12.Ekelund U, Brage S, Froberg K, et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European Youth Heart Study. PLoS Med. 2006;3:e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emken BA, Richey J, Belcher B, Hsu YW, Spruijt-Metz D. Objectively measured physical activity is negatively associated with plasma adiponectin levels in minority female youth. Int J Pediatr Endocrinol. [published online ahead of print August 11,2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Kohl HWI, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. ObesRes. 2005;13:608–14. [DOI] [PubMed] [Google Scholar]
- 16a.Freedson P, Pober D, Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc. 2005;37(11 suppl):S523–30. [DOI] [PubMed] [Google Scholar]
- 17.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008; 31:661–6. [DOI] [PubMed] [Google Scholar]
- 18.Hickman TB, Briefel BR, Carroll MD, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–90. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Hosmer T, le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- 20.Hsu Y-W, Chou C-P, Nguyen-Rodriguez ST, McClain AD, Belcher BR, Spruijt-Metz D. Influences of social support, perceived barriers, and negative meanings of physical activity on physical activity in middle school students. JPhys Act Health. 2011;8(2):2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelishadi R, Razaghi EM, Gouya MM, et al. Association of physical activity and the metabolic syndrome in children and adolescents: CASPIAN study. Horm Res. 2006;67:46–52. [DOI] [PubMed] [Google Scholar]
- 22.Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity–related energy expenditure: a validation study against whole-body indirect calorimetry. Br JNutr. 2004;91:235–43. [DOI] [PubMed] [Google Scholar]
- 23.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurray RG, Bangdiwala SI, Harrell JS, Amorim LD. Adolescents with metabolic syndrome have a history of low aerobic fitness and physical activity levels. Dyn Med. 2008;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore JB, Davis CL, Baxter SD, Lewis RD, Yin Z. Physical activity, metabolic syndrome, and overweight in rural youth. J Rural Health. 2008;24:136–42. [DOI] [PubMed] [Google Scholar]
- 26.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–5. [DOI] [PubMed] [Google Scholar]
- 27.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004; 114(4th rep suppl):555–76. [PubMed] [Google Scholar]
- 28.Pan Y, Pratt CA. Metabolic syndrome and its association with diet and physical activity in US adolescents. J Am Diet Assoc. 2008; 108:276–86; discussion 286. [DOI] [PubMed] [Google Scholar]
- 29.Pate RR, Ross R, Dowda M, Trost SG, Saland JM. Validation of a 3-day physical activity recall instrument in female youth. Pediatr Exerc Sci. 2003;15:257–65. [Google Scholar]
- 30.Physical Activity Guidelines Advisory Committee Physical Activity Guidelines Advisory Committee Report. Washington (DC): US Department of Health and Human Services; 2008. p. 1–683. [Google Scholar]
- 31.Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36(9):1625–31. [PubMed] [Google Scholar]
- 32.Rizzo NS, Ruiz JR, Hurtig-Wennlof A, Ortega FB, Sjostrom M. Relationship of physical activity, fitness and fatness with clustered metabolic risk in children and adolescents: the European Youth Heart Study. J Pediatr. 2007;150:388–94. [DOI] [PubMed] [Google Scholar]
- 33.Shaibi GQ, Goran MI. Examining metabolic syndrome definitions in overweight Hispanic youth: a focus on insulin resistance. J Pediatr. 2008;152:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spruijt-Metz D, Berrigan D, Kelly L, et al. Measures of physical activity and exercise In: Allison DB, Baskin ML, editors. Handbook of Assessment Methods for Eating Behaviors and Weight-Related Problems: Measures, Theory, and Research. Thousand Oaks (CA): Sage Publications; 2009. p. 187–254. [Google Scholar]
- 35.Steele RM, Brage S, Corder K, Wareham NJ, Ekelund U. Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol. 2008;105:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. [DOI] [PubMed] [Google Scholar]
- 37.Third Report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 38.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 39.Ventura E, Davis J, Byrd-Williams C, et al. Reduction in risk factors for type 2 diabetes mellitus in response to a low-sugar, high-fiber dietary intervention in overweight Latino adolescents. Arch Pediatr Adolesc Med. 2009;163:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


