Abstract
Community-acquired pneumonia is one of the commonest and deadliest of the infectious diseases, yet our understanding of it remains relatively poor. The recently published American Thoracic Society and Infectious Diseases Society of America Community-acquired pneumonia guidelines acknowledged that most of what we accept as standard of care is supported only by low quality evidence, highlighting persistent uncertainty and deficiencies in our knowledge. However, progress in diagnostics, translational research, and epidemiology has changed our concept of pneumonia, contributing to a gradual improvement in prevention, diagnosis, treatment, and outcomes for our patients. The emergence of considerable evidence about adverse long-term health outcomes in pneumonia survivors has also challenged our concept of pneumonia as an acute disease and what treatment end points are important. This review focuses on advances in the research and care of community-acquired pneumonia in the past two decades. We summarize the evidence around our understanding of pathogenesis and diagnosis, discuss key contentious management issues including the role of procalcitonin and the use or non-use of corticosteroids, and explore the relationships between pneumonia and long-term outcomes including cardiovascular and cognitive health.
Keywords: bacterial, diagnosis, pneumonia, treatment
Advances in diagnosis: what is pneumonia?
Although it is the leading infectious cause of death in the United States and most western countries,1 pneumonia continues to escape a simple clinical definition. The clinical presentation and findings – symptoms of cough, fever, dyspnea, rales (or crackles) on exam, and infiltrate on chest imaging – are non-specific, with several alternative diagnoses. Because we only observe its manifestations, one might say that we never actually see pneumonia, but only have access to indirect views through perspectives offered by our diagnostic tools.
One changing perspective is imaging, with the widespread adoption of chest computed tomography (CT) and advancement of ultrasound technologies. Chest CT provides a more accurate view of lung parenchyma than X-rays, which have demonstrated both low sensitivity and positive predictive value when compared to chest CT.2,3 The use of CT has dramatically increased in the past two decades,4 which raises the question as to whether previous studies that employed chest radiographs as a gold standard carry the same meaning forward, or whether patients diagnosed by CT may be sufficiently different to indicate different treatment. One surveillance study of hospitalized patients with a pneumonia diagnosis compared 66 hospitalizations with CT-confirmed, radiograph-negative pneumonia to 2185 radiograph-positive cases, and found similar rates of pathogen detection, Intensive Care Unit (ICU) admission, and length of stay,5 suggesting that we may be able to apply similar diagnosis and treatment approaches to newer populations. However, larger studies could examine these populations more thoroughly.
Ultrasound is also an emerging technology that has expanded in its availability of point-of-care tools with increasingly high quality. Several studies have suggested that lung ultrasound demonstrating airspace consolidation or focal distribution of B lines may have closer alignment with the clinician diagnosis of pneumonia6 or CT findings7 than chest radiographs. However, the quality of chest ultrasound is dependent on both the skill of the technician and interpretation of images, both of which are less standardized in current practice than radiographic imaging. Further, no studies yet exist that examine whether lung ultrasound improves diagnosis or outcomes. The perceived relative advantage over chest radiographs, degree of adoption, and consistency with this technique will influence the role of this new technology in the future.
Advances in knowledge: what causes pneumonia?
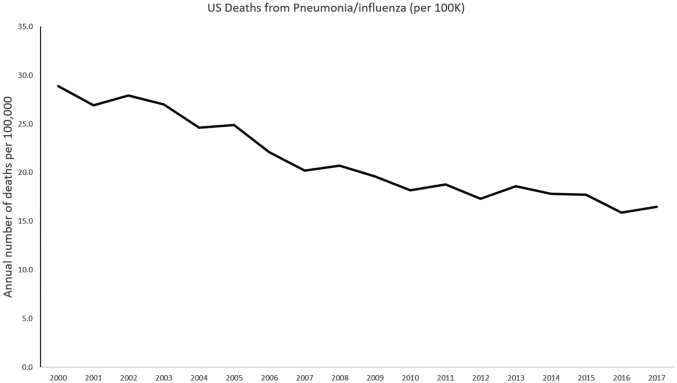
Our perspective on the pathogenesis of pneumonia is also changing due to advances in microbial detection and clinical epidemiology (Figure 1). Previously thought to be a sterile space, the lung is now recognized as a complex ecosystem of microbes, with equally complex relationships to their host and each other, analogous to an ‘adaptive island’ with dynamic interactions that drive changes in species prevalence within a host.8 Within this model, the theory of lung infection has changed from pathogen invasion of a sterile space to disruption of balance in existing microbes, with or without the introduction of a new pathogen. The characteristics of the host, and the interaction between the host and pathogen, are additional factors that influence the ultimate consequences of this disruption.9
Figure 1.
Timeline of annual number of US deaths from pneumonia by year, 2000–2017. Markers indicate milestones in treatment (blue) and research (red).
The widespread availability of rapid molecular diagnostic testing has provided a new insight into etiologies of pneumonia that challenges paradigms set by earlier studies from microbiology cultures. One example is a large population-based prospective observational study of adults hospitalized with radiographically confirmed pneumonia,10 which employed aggressive diagnostic testing including rapid molecular diagnostic testing and found a predominance of viruses, with rhinovirus and influenza overshadowing Streptococcus pneumoniae as the most commonly detected pathogens. Human metapneumovirus, respiratory syncytial virus, parainfluenza, coronaviruses, and adenoviruses were also identified, and multiple pathogens were detected in 13% of all cases in which a cause was identified. These findings were similar to other smaller studies,11,12 with viruses being commonly identified with S. pneumoniae. Uncertainties remain as to the meaning of these findings: whether pathogens detected in the nasopharynx or sputa represent infection versus colonization versus co-infections with other undetected microbes remains unclear, and the lack of a gold standard against which to measure the accuracy of new testing technologies continues to obscure our understanding. Further, only 38–63% of patients studied yielded a pathogen at all, which raises the question as to whether failure to detect pathogens in pneumonia reflects continued shortcomings of our diagnostic capabilities, novel pathogens yet to be discovered, or misclassification of non-infectious syndromes that mimic lung infection. Biomarkers, including C-reactive peptide, procalcitonin, and newer diagnostic technologies that combine microbial detection with inflammatory patterns12 are a promising path to elucidate our understanding further and refine our paradigm of lung infection, but to date have yet to deliver meaningful clinical interventions.
The increasing detection of multiple potential pathogens in patients with Community-acquired pneumonia (CAP) is also a challenge to our classic model of lung infection. Whether these cases represent true co-infection, sequential infection or acute infection with one pathogen but chronic carriage of one or more other pathogens that are not responsible for the pneumonia remains to be seen, and only serial quantitative assays on lower respiratory tract specimens are likely to resolve this question.
Advances in treatment: antimicrobials for pneumonia
Empiric therapy
Comparing the 2019 CAP guidelines published by American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA)1 to the first published by these societies in 1993,13 relatively little has changed in medical treatment for pneumonia over 25 years. Antibiotic therapy remains empiric because diagnostic techniques have still not progressed to a point where the pathogen can accurately be identified in a clinically useful period of time. Respiratory fluoroquinolones were introduced as an alternative to combination beta-lactam and macrolide therapy in the guidelines in 200114 based on randomized, controlled trials showing efficacy, and fear of increasing pneumococcal antibiotic resistance, although this remains a contentious recommendation particularly in the arena of antimicrobial stewardship. In some countries respiratory fluoroquinolones remain limited to patients with significant beta-lactam allergy due to concern about the over-use of these broader-spectrum antibiotics, but they are widely used in the United States and many European countries.
The largest change in the 2019 CAP guidelines1 is the move away from the concept of healthcare-associated pneumonia (HCAP) in an attempt to limit the overuse of broad-spectrum antipseudomonal and anti-methicillin-resistant Staphylococcus aureus (MRSA) antibiotics. Introduced in the 2005 hospital-acquired pneumonia guidelines,15 HCAP was an attempt to recognize that in some centers there was a much larger number of patients presenting with pneumonia and organisms not covered by standard empiric therapy, typically Pseudomonas aeruginosa and MRSA. Fortunately, subsequent research showed that not only was this phenomena probably limited to relatively few centers,16 the introduction of HCAP resulted in a massive increase in broad-spectrum antibiotic use17 that almost certainly harmed more patients than it benefited.17,18 The new guidelines recommend that P. aeruginosa and MRSA are only covered if risk factors are present AND local data have confirmed that these pathogens are problematic. Acknowledging that many centers may not have local data currently, the guidelines further recommend that sputum and blood cultures be taken whenever broad-spectrum antibiotic coverage is used to generate this information. The adoption of the rapid polymerase chain reaction nasal swab test for MRSA was also incorporated into the most recent guidelines, which recommend discontinuing or withholding anti-MRSA therapy based on a negative test due to its high negative predictive value.
Two new antibiotics, ceftaroline and lefamulin, have been approved by the United States Food and Drug Administration in the past few years. As both ceftaroline and lefamulin have very broad antimicrobial coverage including MRSA, under basic antimicrobial stewardship principles their current role is in patients with confirmed MRSA pneumonia.
Reflected in the recent ATS-IDSA CAP guidelines,1 accumulated evidence over the past two decades is that the addition of a macrolide to a beta-lactam is associated with better patient outcomes, particularly in patients with severe disease.19 Most of the macrolide data are however observational and retrospective. Two randomized controlled trials have attempted to address the issue. Postma et al.20 did not find a benefit of macrolides but that study is highly problematic with 25% of patients not having radiologically confirmed pneumonia, 40% of patients with supposed monotherapy being given macrolides and a huge discrepancy between macrolide use in the combination group (mostly erythromycin) and the macrolides used in the ‘monotherapy’ group who also got a macrolide (no erythromycin). Essentially Postma et al.20 does not help inform the management of CAP. Garin and colleagues21 took a different approach attempting to show that monotherapy was not inferior to the combination therapy of a beta-lactam and macrolide in a standard prospective, randomized, controlled trial of patients with CAP. As monotherapy did not reach statistical significance for non-inferiority, combination therapy remains the standard of care.
Biomarkers
Clinical judgment is needed in managing CAP patients, including the selection of appropriate antibiotics and assignment of the appropriate location of care. Thus biomarkers that might objectively determine appropriate choices, either by distinguishing bacterial versus viral infection or determining illness severity, would have significant appeal. The medical literature contains an enormous number of studies comparing biomarkers in CAP, either against each other or against traditional scoring systems like the pneumonia severity index of the CURB-65. The majority of biomarkers studied are acute phase reactants, which rise during a patient’s inflammatory response. The best studied biomarker to date is procalcitonin, a peptide precursor of the hormone calcitonin that appears to rise disproportionately during responses to bacterial infection. Multiple studies have assessed its sensitivity and specificity for the presence of bacterial infection in a variety of lower respiratory tract infections.
At a threshold of 1.0 ng/mL, procalcitonin has a reasonably high predictive value for typical bacterial infection.22,23 However, the use of a low procalcitonin to withhold antibiotic therapy on the presumption of a viral infection has significant limitations and is not recommended.1 In the setting of Legionella and Mycoplasma, two common CAP pathogens, procalcitonin is often not elevated,24,25 although reports suggest in severe Legionella it may be.26,27 Several studies have also raised concern that procalcitonin has a poor sensitivity in the presence of mixed bacterial and viral infection.24,25,28,29 A single interventional trial in the setting of CAP in adults attempted to withhold antibiotics on the basis of a low procalcitonin level.30 In that study, 22 of 43 patients with procalcitonin concentrations below 0.25 ng/mL had antibiotics withheld, although five subsequently had antibiotics started because of a higher reading at 6 hours. No adverse effects of withholding antibiotics were observed. Given that the study was very small, has not been replicated, and clinicians ignored the procalcitonin-guided advice to withhold antibiotics in nearly 50%, this approach cannot be recommended.
Procalcitonin has also been explored as a tool to reduce the duration of antibiotic therapy. Several randomized studies that used serial procalcitonin measurement to determine the duration of antibiotic treatment in patients with CAP have shown a reduced length of therapy, but in all cases the standard therapy arm had durations well beyond 7 days,30–33 much longer than recommended in current guidelines. Therefore, physicians should only use procalcitonin if their standard duration of antibiotic therapy exceeds that recommended in the guidelines.
Corticosteroids
A subgroup of patients with pneumonia progress to septic shock or acute respiratory distress syndrome despite appropriate antimicrobial therapy, outcomes which are considered to be driven by the patient’s immune response. The desire to mitigate this response has led to the consideration of immune-modulating agents such as corticosteroids. A series of meta-analyses based on small trials with significant flaws has led to a significant increase in interest in corticosteroids in CAP,34,35 with evidence of large scale overuse of these potentially toxic agents.36 A more detailed discussion of the primary papers used by the meta-analyses has been published.37 Of significance, Torres et al.38 used an entry criteria of a C-reactive protein greater than 150 mg/dL, but found no difference in mortality or length of hospital stay in 120 patients. A composite endpoint of treatment success, driven mostly by improved radiology, was reported as improved by steroids (p = 0.02), but in the absence of any difference in mortality or organ failure it is difficult to know what this result means. In contrast, Lloyd et al.,39 in a randomized, placebo controlled study published after both the meta-analyses and the new CAP guidelines, found no benefit of steroids in 816 patients with CAP, but did find a higher rate of gastrointestinal bleeding in the steroid group. As further studies have questioned the safety of even short doses of corticosteroids,40 and there is significant concern over the potential for increased mortality in influenza,41 the CAP guidelines recommend against steroids in patients with CAP. While it remains possible that there may be a subgroup of patients who may benefit, this group has yet to be defined.
Cardioprotection
There is now a large volume of data demonstrating that CAP is associated with both a high rate of acute cardiac complications including myocardial infarction and arrhythmia,42–44 as well as there being a substantially increased risk in survivors of myocardial infarction, stroke and heart failure for some years after the acute event.45 At present there are no confirmed therapies to prevent either the acute or long-term adverse cardiovascular impacts of pneumonia. A small randomized controlled trial of 100 mg of aspirin in patients with CAP did not reduce the cardiovascular event rate;42 however, an even smaller study of 300 mg of aspirin did significantly reduce the incidence of myocardial infarction.46 One study observed that ticagrelor was associated with fewer cardiovascular events in the setting of pneumonia than clopidogrel,47 but this has not been tested in a randomized, placebo controlled trial. Retrospective studies have suggested that statin therapy may be associated with better outcomes in CAP,48–50 but this has not been a universal finding.51 A small pilot randomized study of simvastatin suggested some benefit of therapy,52 although larger studies are needed. A variety of other potential cardioprotective drugs have been studied without any consistent trends. Moving forward, cardioprotection in the setting of CAP remains a major area requiring research.
Implementation of best practice through bundled interventions
As the vast majority of pneumonia instances is likely to be cared for by non-pulmonary specialists,53,54 healthcare systems have the opportunity and obligation to support generalist providers with up-to-date information and tools to ensure optimal and equitable care for patients presenting to all settings. Treatment bundles, a small, straightforward set of evidence-based practices that can be executed consistently,55 take a systems and behavioral economics approach to clinical performance and quality improvement.56,57 The most common elements included in bundled interventions for CAP include: timely, first-line antibiotics with appropriate de-escalation and duration; recognition and resuscitation of sepsis; use of validated severity assessment tools to guide site-of-care and other treatment decisions; consistent diagnostic work-up for microbial etiology and other diagnoses; and early mobilization. Bundled interventions have been suggested to improve outcomes dramatically for sepsis,58,59 respiratory failure requiring mechanical ventilation,60 and CAP,61–63 and the widespread availability of electronic health record systems capable of decision support has further facilitated the adoption of bundled interventions in the form of informatics tools.64 However, with the exception of one randomized controlled trial,63 the conclusions that can be drawn from most studies examining bundled interventions for CAP are unclear due to their observational pre–post designs. In addition, it is sometimes unclear which components are effective.39 For care to be the most effective, clinicians must find a balance between bundled care, diagnostic uncertainty, and individualization of care to their patients’ needs.
Trends in incidence and short-term outcomes
Observational studies examining trends in outcomes have suggested steady overall declines in short-term mortality attributed to pneumonia over the past 20 years,65,66 although some studies suggest the trend is not consistent for all settings.67 Several mechanisms have been proposed, including changes in care processes,68,69 changes in practice models such as staffing and performance measures, or mitigation of disease burden due to the adoption of childhood pneumococcal vaccines70 and more comprehensive influenza vaccination programs. An additional contributing factor could be in the shift of the diagnostic labels surrounding pulmonary infection, as population studies often rely on diagnostic coding to identify cases, and increased attention to performance measures results in a shift from labelling of pneumonia as a primary or principal diagnosis to sepsis and respiratory failure for patients.71 Due to the lack of clinical data for the majority of the studies examining population-level trends, it is unclear how much of the reduced mortality we have seen in pneumonia is driven by changes in populations, illness presentation, or management.
Long-term outcomes from CAP
Another advancement in pneumonia is our understanding of its consequences on long-term health. While it is often conceptualized as an acute, completely reversible disease, numerous recent studies have documented that CAP survivors continue to have significantly greater mortality than expected over the following 2 to 5 years,72–74 with some studies suggesting even longer-term adverse impacts75,76 and reduced quality of life.77 A significant proportion of this excess mortality may be due to cardiovascular disease, either myocardial infarction or stroke, and heart failure.78,79 The mechanism(s) driving this excess of cardiovascular and cardiac disease has not been definitively determined, but accelerated atherosclerosis and direct cardiac damage during acute pneumonia are both strong hypotheses.45 Prospective observational studies have also demonstrated a greater burden of long-term cognitive impairment, functional impairment, and depressive symptoms after pneumonia or sepsis.80–82 The mechanism of this burden is also unclear and probably has many factors, but one hypothesis includes a similar pathway of endovascular inflammation. It is critically important that moving forward, pneumonia studies focus more on these longer-term outcomes which have significantly more impact on overall mortality, morbidity and healthcare utilization.
What outcome are we most interested in?
Both epidemiological and experimental studies comparing treatments for CAP have historically used endpoints that are easy to measure, including short-term mortality, or resolution of clinical symptoms or microbiological clearance if they study milder disease (as is common for pharmaceutical trials). However, these outcomes may represent only the extreme measures for pneumonia and may be poor surrogates for other meaningful outcomes, without clear causal relationships to the interventions that we are studying. Death is arguably a meaningful outcome for many patients. However, mortality in pneumonia is often driven by unmodifiable factors including advanced age, comorbidity, and patient preferences toward aggressive care. Many patients with pneumonia have goals of care restrictions such as ‘not for resuscitation’ or ‘not for intensive care’.83 Equally, some studies suggest that a large proportion of deaths in the first 30 days after a diagnosis of pneumonia occur after discharge from hospital,84,85 rendering much of inpatient mortality neither preventable nor meaningful for measurement,86 and not reflective of the quality of care provided. However, return to independence, baseline physical and cognitive function, social engagement, patient experience, and quality of life are all meaningful, potentially modifiable outcomes to patients of all ages that thus far have been understudied.
A clinically far more valuable but more difficult concept is preventable mortality and the prevention of other key outcomes (Table 1).87 Moving from our long-held standard of all-cause mortality to a different paradigm of identifying patients in whom a specific intervention might modify outcomes that are clinically meaningful to individual patients will be challenging, requiring more elegant approaches to both observational and experimental study design. However, it is necessary if we are to move forward and truly improve outcomes. A starting point will be abandoning pneumonia researchers’ long-standing fascination with classifying patients based on a static forecast of 30-day death, which does very little to guide effective interventions, primarily because most mortality is in patients of advanced age and/or comorbidity when there is either no intent to avoid death or no prospect of doing so.87 With respect to pharmaceutical trials in CAP, we can no longer be satisfied with clinical and/or microbiological improvement as the primary outcome. The significant evidence discussed in this review on the longer-term outcomes of CAP, and especially the cardiovascular impact need to be properly considered. Presently, we do not know if acute therapy of CAP makes any difference to longer-term outcomes but we clearly need to know. We must move beyond measuring only what is easy to measuring what is meaningful.
Table 1.
Proposed outcomes for community-acquired pneumonia.
| Mechanism | Quantitative measures | Limitations | Solutions | |
|---|---|---|---|---|
| Longer-term outcomes beyond 30 days | Cardiovascular, cognitive impairment, debilitation from acute diseases, dysbiosis, confounding comborbidities | 90-Day, 180-day, 365-day mortality | Direct attribution to pneumonia may be difficult | Large populations, longitudinal |
| Cardiovascular events | ||||
| Modifiable mortality | Care processes | OBS: Propensity matched/weighted risk differences | Causal inference/confounding, dynamic/time-varying exposures | Large populations, granular data, prospective pragmatic trials/SMART |
| Dx, Site of care, abx, resp/hemodynamic support | Trials: cluster-RCTs with bundled interventions? | |||
| Cardiovascular impairment | Endothelial inflammation, dysbiosis, stress axis | ACS events | Confounding | |
| Heart failure new diagnoses | ||||
| Neurologic impairment | Endothelial inflammation, dysbiosis, delirium/post-ICU syndrome, hypoxemia/hypoperfusion | CVA events | Causal inference/confounding | Concurrent matched control population |
| New diagnoses dementia | Ascertainment/ | |||
| Recall bias | ||||
| Functional impairment | Debilitation/immobility, endothelial inflammation, post-ICU syndrome | Return to work, loss of independence, job loss, homelessness, separation/divorce | Recall bias | Concurrent control population |
| Patient experience | Care processes, organization factors, patient factors | Survey | Influenced by patient factors | Longitudinal pre/post data |
| Healthcare engagement | ||||
| Misdiagnosis | Patient complexity, provider/organizational factors | Diagnostic discordance | ||
| Re-admission | ||||
| ?Lung cancer dx | ||||
| Surrogate endpoints: | ||||
| CRP | Patient immune response | |||
| Procalcitonin | Patient immune response, pathogen (bacterial versus viral) | |||
| Clinical stability | Patient immune response, pathogen |
abx, antibiotics; ACS, acute coronary syndrome; CRP, c-reactive protein; CVA, cerebrovascular accident; dx, diagnosis; ICU, intensive care unit; RCT, randomised controlled trial; SMART, Sequential, multiple assignment, randomized trials.
There remains much work to be done to translate the recent advances in our understanding of lung infection, technological advances in diagnostic tools, and leveraging of clinical data into real improvements in management options and outcomes for patients with pneumonia. By challenging existing definitions, advancing technology, and adopting more elegant research designs that accommodate the complexity of our patients and the host–pathogen interaction, we hope for substantial changes in diagnosis and treatment options in pneumonia in the next two decades.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Grant Waterer  https://orcid.org/0000-0002-7222-8018
https://orcid.org/0000-0002-7222-8018
Contributor Information
Barbara Jones, Division of Pulmonary and Critical Care, University of Utah and Salt Lake City VA Healthcare System, Salt Lake City, UT, USA.
Grant Waterer, University of Western Australia, Royal Perth Hospital, Perth, WA 6009, Australia.
References
- 1. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Self WH, Courtney DM, McNaughton CD, et al. High discordance of chest X-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 2013; 31: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015; 192: 974–982. [DOI] [PubMed] [Google Scholar]
- 4. Smith-Bindman R, Kwan ML, Marlow EC, et al. Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA 2019; 322: 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Upchurch CP, Grijalva CG, Wunderink RG, et al. Community-acquired pneumonia visualized on CT scans but not chest radiographs: pathogens, severity, and clinical outcomes. Chest 2018; 153: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest X-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med 2014; 32: 115–118. [DOI] [PubMed] [Google Scholar]
- 7. Cortellaro F, Colombo S, Coen D, et al. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J 2012; 29: 19–23. [DOI] [PubMed] [Google Scholar]
- 8. Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med 2014; 2: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dela Cruz CS, Wunderink RG, Christiani DC, et al. Future research directions in pneumonia. NHLBI Working Group Report. Am J Respir Crit Care Med 2018; 198: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holter JC, Muller F, Bjorang O, et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC Infect Dis. 2015; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caglayan Serin D, Pullukcu H, Cicek C, et al. Bacterial and viral etiology in hospitalized community acquired pneumonia with molecular methods and clinical evaluation. J Infect Dev Ctries 2014; 8: 510–518. [DOI] [PubMed] [Google Scholar]
- 13. Niederman MS, Bass JB, Jr, Campbell GD, et al. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. American Thoracic Society, Medical Section of the American Lung Association. Am Rev Respir Dis 1993; 148: 1418–1426. [DOI] [PubMed] [Google Scholar]
- 14. Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163: 1730–1754. [DOI] [PubMed] [Google Scholar]
- 15. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 16. Chalmers JD, Rother C, Salih W, et al. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014; 58: 330–339. [DOI] [PubMed] [Google Scholar]
- 17. Jones BE, Ying J, Stevens V, et al. Empirical anti-MRSA vs standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA Intern Med 2020; 180: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Webb BJ, Sorensen J, Jephson A, et al. Broad-spectrum antibiotic use and poor outcomes in community-onset pneumonia: a cohort study. Eur Respir J 2019; 54(1):1900057. [DOI] [PubMed] [Google Scholar]
- 19. Wunderink RG, Waterer G. Advances in the causes and management of community acquired pneumonia in adults. BMJ 2017; 358: j2471. [DOI] [PubMed] [Google Scholar]
- 20. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med 2015; 372: 1312–1323. [DOI] [PubMed] [Google Scholar]
- 21. Garin N, Genne D, Carballo S, et al. β-Lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med 2014; 174: 1894–1901. [DOI] [PubMed] [Google Scholar]
- 22. Pfister R, Kochanek M, Leygeber T, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care 2014; 18: R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu MH, Lin CC, Huang SL, et al. Can procalcitonin tests aid in identifying bacterial infections associated with influenza pneumonia? A systematic review and meta-analysis. Influenza Other Respir Viruses 2013; 7: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Musher DM, Bebko SP, Roig IL. Serum procalcitonin level, viral polymerase chain reaction analysis, and lower respiratory tract infection. J Infect Dis 2014; 209: 631–633. [DOI] [PubMed] [Google Scholar]
- 25. Kruger S, Ewig S, Papassotiriou J, et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respir Res 2009; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franzin L, Cabodi D. Legionella pneumonia and serum procalcitonin. Curr Microbiol 2005; 50: 43–46. [DOI] [PubMed] [Google Scholar]
- 27. de Jager CP, de Wit NC, Weers-Pothoff G, et al. Procalcitonin kinetics in Legionella pneumophila pneumonia. Clin Microbiol Infect 2009; 15: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 28. Bello S, Minchole E, Fandos S, et al. Inflammatory response in mixed viral-bacterial community-acquired pneumonia. BMC Pulm Med 2014; 14: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellmann-Weiler R, Ausserwinkler M, Kurz K, et al. Clinical potential of C-reactive protein and procalcitonin serum concentrations to guide differential diagnosis and clinical management of pneumococcal and Legionella pneumonia. J Clin Microbiol 2010; 48: 1915–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006; 174: 84–93. [DOI] [PubMed] [Google Scholar]
- 31. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 32. Akagi T, Nagata N, Wakamatsu K, et al. Procalcitonin-guided antibiotic discontinuation might shorten the duration of antibiotic treatment without increasing pneumonia recurrence. Am J Med Sci 2019; 358: 33–44. [DOI] [PubMed] [Google Scholar]
- 33. Ito A, Ishida T, Tokumasu H, et al. Impact of procalcitonin-guided therapy for hospitalized community-acquired pneumonia on reducing antibiotic consumption and costs in Japan. J Infect Chemother 2017; 23: 142–147. [DOI] [PubMed] [Google Scholar]
- 34. Wu WF, Fang Q, He GJ. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: a meta-analysis. Am J Emerg Med 2018; 36: 179–184. [DOI] [PubMed] [Google Scholar]
- 35. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med 2015; 163: 519–528. [DOI] [PubMed] [Google Scholar]
- 36. Lin KJ, Dvorin E, Kesselheim AS. Prescribing systemic steroids for acute respiratory tract infections in United States outpatient settings: a nationwide population-based cohort study. PLoS Med 2020; 17: e1003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waterer G, Metersky ML. Corticosteroids for community-acquired pneumonia: overstated benefits and understated risks. Chest 2019; 156: 1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015; 313: 677–686. [DOI] [PubMed] [Google Scholar]
- 39. Lloyd M, Karahalios A, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019; 179: 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017; 357: j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2016; 3: Cd010406. [DOI] [PubMed] [Google Scholar]
- 42. Cangemi R, Casciaro M, Rossi E, et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J Am Coll Cardiol 2014; 64: 1917–1925. [DOI] [PubMed] [Google Scholar]
- 43. Corrales-Medina VF, Serpa J, Rueda AM, et al. Acute bacterial pneumonia is associated with the occurrence of acute coronary syndromes. Medicine 2009; 88: 154–159. [DOI] [PubMed] [Google Scholar]
- 44. Perry TW, Pugh MJ, Waterer GW, et al. Incidence of cardiovascular events after hospital admission for pneumonia. Am J Med 2011; 124: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bartlett B, Ludewick HP, Lee S, et al. Cardiovascular complications following pneumonia: focus on pneumococcus and heart failure. Curr Opin Cardiol 2019; 34: 233–239. [DOI] [PubMed] [Google Scholar]
- 46. Oz F, Gul S, Kaya MG, et al. Does aspirin use prevent acute coronary syndrome in patients with pneumonia: multicenter prospective randomized trial. Coron Artery Dis 2013; 24: 231–237. [DOI] [PubMed] [Google Scholar]
- 47. Storey RF, James SK, Siegbahn A, et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 2014; 25: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grudzinska FS, Dosanjh DP, Parekh D, et al. Statin therapy in patients with community-acquired pneumonia. Clin Med 2017; 17: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chung SD, Tsai MC, Lin HC, et al. Statin use and clinical outcomes among pneumonia patients. Clin Microbiol Infect 2014; 20: 879–885. [DOI] [PubMed] [Google Scholar]
- 50. Chalmers JD, Singanayagam A, Murray MP, et al. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med 2008; 121: 1002–1007.e1. [DOI] [PubMed] [Google Scholar]
- 51. Havers F, Bramley AM, Finelli L, et al. Statin use and hospital length of stay among adults hospitalized with community-acquired pneumonia. Clin Infect Dis 2016; 62: 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sapey E, Patel JM, Greenwood H, et al. Simvastatin improves neutrophil function and clinical outcomes in pneumonia. A pilot randomized controlled clinical trial. Am J Respir Crit Care Med 2019; 200: 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marrie TJ, Carriere KC, Jin Y, et al. Mortality during hospitalisation for pneumonia in Alberta, Canada, is associated with physician volume. Eur Respir J 2003; 22: 148–155. [DOI] [PubMed] [Google Scholar]
- 54. Horwood CM, Hakendorf P, Thompson CH. Comparison of specialist and generalist care. Aust Health Rev 2018; 42: 579–583. [DOI] [PubMed] [Google Scholar]
- 55. Resar R, Griffin F, Haraden C, et al. Using care bundles to improve health care quality. Cambridge, MA: Institute for Healthcare Improvement, 2012. [Google Scholar]
- 56. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. Rev. and expanded. New York: Penguin Books, 2009, viii, 312 pp. [Google Scholar]
- 57. Reason J. Human error: models and management. BMJ 2000; 320: 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 2008; 299: 2294–2303. [DOI] [PubMed] [Google Scholar]
- 59. Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015; 43: 3–12. [DOI] [PubMed] [Google Scholar]
- 60. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lim WS, Rodrigo C, Turner AM, et al. ; British Thoracic Society. British Thoracic Society community-acquired pneumonia care bundle: results of a national implementation project. Thorax 2016; 71: 288–290. [DOI] [PubMed] [Google Scholar]
- 62. Hortmann M, Heppner HJ, Popp S, et al. Reduction of mortality in community-acquired pneumonia after implementing standardized care bundles in the emergency department. Eur J Emerg Med 2014; 21: 429–435. [DOI] [PubMed] [Google Scholar]
- 63. Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med 2005; 143: 881–894. [DOI] [PubMed] [Google Scholar]
- 64. Dean NC, Jones BE, Jones JP, et al. Impact of an electronic clinical decision support tool for emergency department patients with pneumonia. Ann Emerg Med 2015; 66: 511–520. [DOI] [PubMed] [Google Scholar]
- 65. Marshall DC, Goodson RJ, Xu Y, et al. Trends in mortality from pneumonia in the European Union: a temporal analysis of the European detailed mortality database between 2001 and 2014. Respir Res 2018; 19: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chang DH, Bednarczyk RA, Becker ER, et al. Trends in U.S. hospitalizations and inpatient deaths from pneumonia and influenza, 1996–2011. Vaccine 2016; 34: 486–494. [DOI] [PubMed] [Google Scholar]
- 67. de Miguel-Diez J, Jimenez-Garcia R, Hernandez-Barrera V, et al. Trends in hospitalizations for community-acquired pneumonia in Spain: 2004 to 2013. Eur J Intern Med 2017; 40: 64–71. [DOI] [PubMed] [Google Scholar]
- 68. Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 69. ARISE; ANZICS APD Management Committee. The outcome of patients with sepsis and septic shock presenting to emergency departments in Australia and New Zealand. Crit Care Resusc 2007; 9: 8–18. [PubMed] [Google Scholar]
- 70. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lindenauer PK, Lagu T, Shieh MS, et al. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012; 307: 1405–1413. [DOI] [PubMed] [Google Scholar]
- 72. Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med 2004; 169: 910–914. [DOI] [PubMed] [Google Scholar]
- 73. Mortensen EM, Kapoor WN, Chang CC, et al. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis 2003; 37: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 74. Johnstone J, Eurich DT, Majumdar SR, et al. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine 2008; 87: 329–334. [DOI] [PubMed] [Google Scholar]
- 75. Koivula I, Sten M, Makela PH. Prognosis after community-acquired pneumonia in the elderly: a population-based 12-year follow-up study. Arch Intern Med 1999; 159: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 76. Cecere LM, Rubenfeld GD, Park DR, et al. Long-term survival after hospitalization for community-acquired and healthcare-associated pneumonia. Respiration 2010; 79: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mangen MJ, Huijts SM, Bonten MJ, et al. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis 2017; 17: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Corrales-Medina VF, Taljaard M, Yende S, et al. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia in elderly adults. Am Heart J 2015; 170: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davydow DS, Hough CL, Levine DA, et al. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med 2013; 126: 615–624.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Girard TD, Self WH, Edwards KM, et al. Long-term cognitive impairment after hospitalization for community-acquired pneumonia: a prospective cohort study. J Gen Intern Med 2018; 33: 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Walkey AJ, Weinberg J, Wiener RS, et al. Association of do-not-resuscitate orders and hospital mortality rate among patients with pneumonia. JAMA Intern Med 2016; 176: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Metersky ML, Waterer G, Nsa W, et al. Predictors of in-hospital vs postdischarge mortality in pneumonia. Chest 2012; 142: 476–481. [DOI] [PubMed] [Google Scholar]
- 85. Borzecki AM, Christiansen CL, Chew P, et al. Comparison of in-hospital versus 30-day mortality assessments for selected medical conditions. Med Care 2010; 48: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 86. Waterer GW, Self WH, Courtney DM, et al. In-hospital deaths among adults with community-acquired pneumonia. Chest 2018; 154: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Waterer G. Severity scores and community-acquired pneumonia. Time to move forward. Am J Respir Crit Care Med 2017; 196: 1236–1238. [DOI] [PubMed] [Google Scholar]