Abstract
Aim:
The aim of this study was to investigate the long-term outcomes of endoscopic submucosal dissection (ESD) for superficial esophageal squamous cancer.
Methods:
A literature search was conducted using PubMed, ProQuest and Cochrane Library databases. Primary outcomes were overall survival, disease-specific survival and recurrence-free survival at 5 years. Secondary outcomes included adverse events, recurrence and metastasis. Hazard ratios were calculated based on time to events for survival analysis, and odds radios were used to compare discrete variables.
Results:
A total of 3796 patients in 21 retrospective studies, including 5 comparative studies for ESD and esophagectomy were enrolled. The invasion depth was 52.0% for M1–M2, 43.2% for M3–SM1 and 4.7% for SM2 or deeper. The 5-year survival rate was: overall survival 87.3%, disease-specific survival 97.7%, and recurrence-free survival 85.1%, respectively. Pooled local recurrence of ESD was 1.8% and metastasis was 3.3%. In terms of the comparison between ESD and esophagectomy, there was no difference in the overall survival (86.4% versus 81.8%, hazard ratio = 0.66, 95% CI = 0.39–1.11) as well as disease-specific and recurrence-free survival. In addition, ESD was associated with fewer adverse events (19.8 % versus 44.0%, odds ratio = 0.3, 95% CI = 0.23–0.39).
Conclusions:
For superficial esophageal squamous cancer, ESD may be considered as the primary treatment of for mucosal lesions, and additional treatment should be available for submucosal invasive cancers.
Keywords: Endoscopic submucosal dissection, Esophagectomy, Esophageal squamous cell cancer, Meta-analysis
Introduction
Esophageal squamous cell cancer is a worldwide health threat that accounts for up to 90% of annually diagnosed esophageal cancer.1 Although the prognosis is poor in advanced cancer, early detection and management may result in excellent outcome.2 Esophagectomy has traditionally been the gold standard for superficial esophageal squamous cell cancer (SESCC); however, it is associated with substantial morbidity and mortality.3,4 For lesions with low risk of lymph node metastasis, endoscopic resection may be curative. In such cases, endoscopic submucosal dissection (ESD) is the treatment of choice to since it may achieve en-bloc resection in experienced hands, even for large lesions.
Although ESD has been proven as a promising technique in terms of complete resection and safety,5–9 its long-term outcome in comparison to esophagectomy is not well understood, especially in lesions invading the muscularis mucosa and superficial submucosa layer, where the risk of lymph node metastasis is not negligible.
Recently, several groups from East Asia and Europe reported satisfactory overall and disease-specific survival of ESD up to 5 years,10–20 and the latest studies suggested similar oncologic outcomes compared to esophagectomy.21,22 Therefore, it is time to further explore the survival outcomes and safety with the different treatment modality to determine the optimal approach for the patients. The aim of this study is to comprehensively evaluate the long-term outcome of SESCC treated by ESD, and compared it with esophagectomy based on the up-to-date evidence.
Methods
Study design and search strategy
This study is a systematic review and meta-analysis. Two authors (JHY and CTL) independently underwent meticulous literature searches of the online database resources: PubMed, Cochrane Library and ProQuest in January 2019. The search queries and keywords were “esophageal endoscopic submucosal dissection” in all the databases, and the detail is described within the Appendix.
After retrieving the search records and excluding duplicated articles, manual reference review was performed (JHY and TCW) to extract relevant studies. All identified records were reviewed via the title, abstracts, and full-text article as necessary, for eligibility. When there was a discrepancy, the two authors would discuss with each other to reach a consensus. With any unsolved issues, the corresponding author (WLW) made the final judgement.
The inclusion criteria were cohort studies, including patients received ESD for SESCC, with at least one of the following outcomes was reported: (a) overall survival, (b) disease-specific survival, and (c) recurrence-free survival. The exclusion criteria were: (a) lack of long-term outcome (defined as follow-up period ⩾ 3 years), (b) studies with fewer than 20 cases of ESD, in order to ensure patients were treated in centers with adequate expertise (c) studies including mainly patients with adenocarcinoma, (d) identical patient group with other eligible studies, and (e) radiation or chemotherapy was performed preceding ESD.
Data extraction and assessment of outcome and validity
The following data were independently extracted by JHY and JCC: name of first author, year of publication, country of origin, number and characteristics of patients, study design and treatment modality, as well as the primary and secondary outcomes.
Primary outcomes of this study were the overall survival, disease-specific survival and recurrence-free survival in patients treated by ESD. The secondary outcomes were
(a) adverse events, (b) R0 resection, (c) recurrence and metastasis, and (d) procedure
time and hospital stay. In addition, we also compared these outcomes with those received esophagectomy via analysis of the comparative cohort studies. All data were extracted as originally stated or following appropriate calculations. When the necessary data were unavailable in a study, we would try to contact the corresponding author to request additional information.
In terms of the depth of invasion of the lesions, the following classification was used: M1 (confined to the intraepithelium), M2 (confined to the lamina propria), M3 (confined to the muscularis mucosa), SM1 (submucosal invasion < 200 μm), and SM2 and 3 (submucosal invasion ⩾ 200 μm),23 and T stage by the 8th edition of the American Joint Committee on Cancer.24
Statistical analysis
In this study, odds ratios (ORs) were used generally for discrete variables, and hazard ratios (HRs) were used for time-events variables, with the corresponding 95% confidence interval (CI) used to compare the outcomes between ESD and esophagectomy. The only exception was that the OR was used for the comparison of survival between mucosal and submucosal lesions, because the HR was not available in all studies. For comparison of baseline characteristics, standardized mean difference was used for calculation of statistical significance.
All the meta-analyses were performed by Comprehensive Meta-Analysis version 3.3.070 (Biostat, Englewood, NJ, USA, 2014). The pooled effect size was considered statistically significant if (a) p < 0.05, or (b) the range of 95% CI spares 1 for OR and HR. The I2 statistic, which indicated the percentage of total variation and inconsistency across studies caused by heterogeneity rather than chance, was used to assess heterogeneity across studies. Presence of significant statistical heterogeneity was defined as p < 0.1 by a chi-square test or I2 > 50%. In this study, a fixed-effects model was used for meta-analysis, except for the presence of significant heterogeneity when a random-effects model was used.25
Risk of bias assessment
For all the eligible studies, we used a Newcastle–Ottawa score26 to assess the quality and risk of bias by the other two authors (CWL and PJH). Publication bias was evaluated by the funnel plot, in which the natural logarithm of the effect estimates was plotted against inverse standard error for each study, and Egger’s test, in which p < 0.1 was considered to be positive.27 In this study, R0 resection rate was chosen as the variable to test publication bias.
Results
Search results and studies included
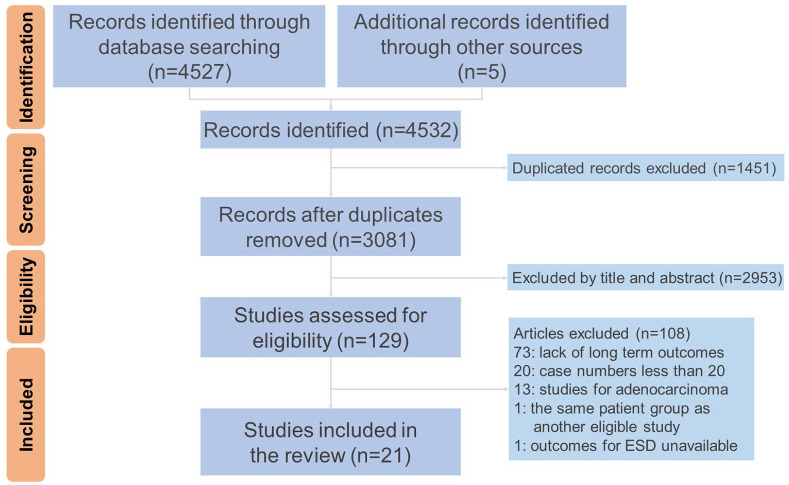
After the search process and excluding the duplicates, there were 3081 relevant records left for further analysis. Subsequent review showed that 129 articles met the inclusion criteria and 21 articles were finally eligible for this study.5–8,10–22,28–31 The most common reasons that studies were excluded were lack of long-term outcomes (67.5%) and low case numbers (18.5%). These articles consisted of 19 fully published papers and 2 academic abstracts. The review process is illustrated by a flow chart of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)32 (Figure 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow chart.
Baseline characteristics of included studies and patients
Among the enrolled studies, 16 articles only reported the outcomes of ESD and the other 5 were cohort studies that compared the outcomes of ESD and esophagectomy. Most of the eligible studies came from East Asia and there were only two conducted in Western countries (Germany and France).13,18 The baseline characteristics of all studies are summarized in Table 1 and Supplemental Table 1. A total of 3796 patients (86.0% men) with 4076 lesions were included in these studies with the weighted-average age of 68.2 years.
Table 1.
Baseline characteristics of included studies.
| Study name | Site | Article type | Design (intervention) | Patient number | Age (Mean) | Median size of lesions (mm) | Tumor location |
Invasion depth |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | U | M | L | M12 | M3–SM1 | SM2+ | |||||||
| Ono et al.5 | Japan | Poster | Case series | 84 | NA | NA | NA | NA | |||||
| Takahashi et al.6 | Japan | Published | Cohort ESD versus EMR | 300 | 67.1 | 30 | 14 | 76 | 26 | 99 | 17 | ||
| Toyonaga et al.7 | Japan | Published | Case series | 138 | 69 | 23 | NA | NA | |||||
| Joo et al.28 | Korea | Published | Case series | 27 | 64 | 13 | 4 | 7 | 17 | 23 | 4 | 1 | |
| Nakagawa et al.10 | Japan | Published | Cohort ESD versus EMR | 204 | 68.5 | NA | 7 | 157 | 78 | 207 | 35 | ||
| Ikeda et al.11 | Japan | Published | Case series | 43 | 71 | NA | 2 | 22 | 19 | 19 | 24 | ||
| Probst et al.13 | Germany | Published | Case series | 24 | 67.9 | 25 | NA | 12 | 12 | ||||
| Kim et al.12 | Korea | Published | Cohort ESD versus EMR | 129 | 67 | 15 | 2 | 70 | 36 | 92 | 16 | ||
| Tsujii et al.8 | Japan | Published | Case series | 307 | 69 | 18 | 61 | 215 | 87 | 268 | 57 | 23 | |
| Park et al.14 | Korea | Published | Case series | 225 | 65 | 18.8 | 11 | 156 | 94 | 223 | 38 | ||
| Park et al.15 | Korea | Published | Case series | 32 | 64 | 17 | 3 | 13 | 17 | 24 | 12 | ||
| Lizuka et al.16 | Japan | Published | Case series | 420 | 67.3 | 23.8 | 45 | 405 | 398† | 52† | |||
| Nagami et al.17 | Japan | Published | Case series | 83 | 68 | 20 (M1–M2) 41 (M3+) | 13 | 48 | 22 | 60 | 19 | 4 | |
| Yamauchi et al.29 | Japan | Poster | Cohort ESD versus surgery | 51 | N/A | NA | NA | NA | |||||
| Baisi et al.30 | China | Published | Cohort ESD versus surgery | 116 | 63.7 | 45.9 | 9 | 43 | 17 | 52 | 17 | ||
| Berger et al.18 | France | Published | Cohort ESD versus EMR | 68 | 65.7 | 33.7 | 37 | 42 | 26 | ||||
| Furue et al.19 | Japan | Published | Cohort ESD versus EMR | 370 | 70.2 | 20.7 | 8 | 21 | 174 | 71 | 177 | 68 | 28 |
| Min et al.21 | Korea | Published | Cohort ESD versus surgery | 240 | 63.9 | 17 | 12 | 42 | 66 | 64 | 35 | 21 | |
| Qi et al.20 | China | Published | Case series | 158 | 65 | 27 | 13 | 127 | 22 | 89 | 69 | ||
| Takeuchi et al.31 | Japan | Published | Cohort ESD versus surgery | 127 | 68 | 20 | 3 | 7 | 30 | 32 | 10 | 41 | 22 |
| Zhang et al.22 | China | Published | Cohort ESD versus surgery | 596 | 63.5 | 26 | 43 | 217 | 61 | 107 | 215 | ||
only reported tumors within the mucosa or those with submucosal invasion.
C, cervical part; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; M, middle part; L, lower part (of the esophagus); M1, intraepithelium; M2, within lamina propria; M3, within muscularis mucosa; NA, not available; SM1, within 200 µm of the submucosal layer; SM2+, beyond 200 µm of the submucosal layer; U, upper part.
Except for 488 lesions that underwent endoscopic mucosal resection and were excluded from analysis, 3039 lesions were treated by ESD and 549 lesions received esophagectomy. The median size of lesions ranged 13–45 mm with ESD and 16–42 mm with esophagectomy (p = 0.163). Among lesions that underwent ESD, 36.5% exceeded 50% of circumference, and 8.6% of lesions had more than 75% circumferential involvement. In addition, these lesions were mostly located at the middle esophagus (61.1%), followed by lower (29.0%) and upper (9.9%) parts. While there were only a few documented cases having lesions at cervical esophagus, the study by Lizuka et al.16 reported cervical lesions in one-tenth of patients and made the comparison of outcomes versus the noncervical lesions.
In terms of the lesion invasion depth and histology, there was 52.0% for M1–M2, 43.2% for M3–SM1 and 4.7% for SM2 or deeper, and only a minority (0.36%) of lesions were poorly differentiated carcinoma. On the other hand, the data from three studies showed up to 35.6% of cases had discrepancy in invasion depth between clinical and pathological stage (p = 0.002), and most of them were upstaging after pathological evaluation.11,30,31
Pathological status, immediate postoperative outcomes, and adverse events
The mean en-bloc and R0 resection rate for ESD were 97.1% and 92.0% among all studies; however, the latter became as low as 78% for lesions invading the submucosal layer.11,31 Curative resection, which had a diverse definition regarding the required invasion depth, ranged 76–99% for studies used M1–M3 as the required invasion depth,6,8,14,15 and 73–90.5% when SM1 invasion was included.7,10,12,18 In contrast, the curative resection rate was as low as 19.1% by Takeuchi et al.31 because they intentionally included patients with submucosal invasive cancer; and 45.7% by Probst et al. as only resected lesions confined at M1–M2 were considered curative.
In terms of the adverse events, the most commonly reported with ESD were perforation/mediastinal emphysema (3.4%), bleeding (2.0%), and stricture formation (9.4%).5,6,8,10,12–22,28–31 While they were exclusively managed by conservative treatment, repeated dilatation was often required for the latter. Moreover, the incidence and sessions of endoscopic balloon dilatation significantly increased with cervical lesions or lesions exceeded 75% of circumference. For post-procedure esophageal stricture, the median sessions of balloon dilatation typically ranged from 2 to 8-times, and more procedures were necessary for cervical lesions.16
Survival analysis, local recurrence and metastasis of ESD
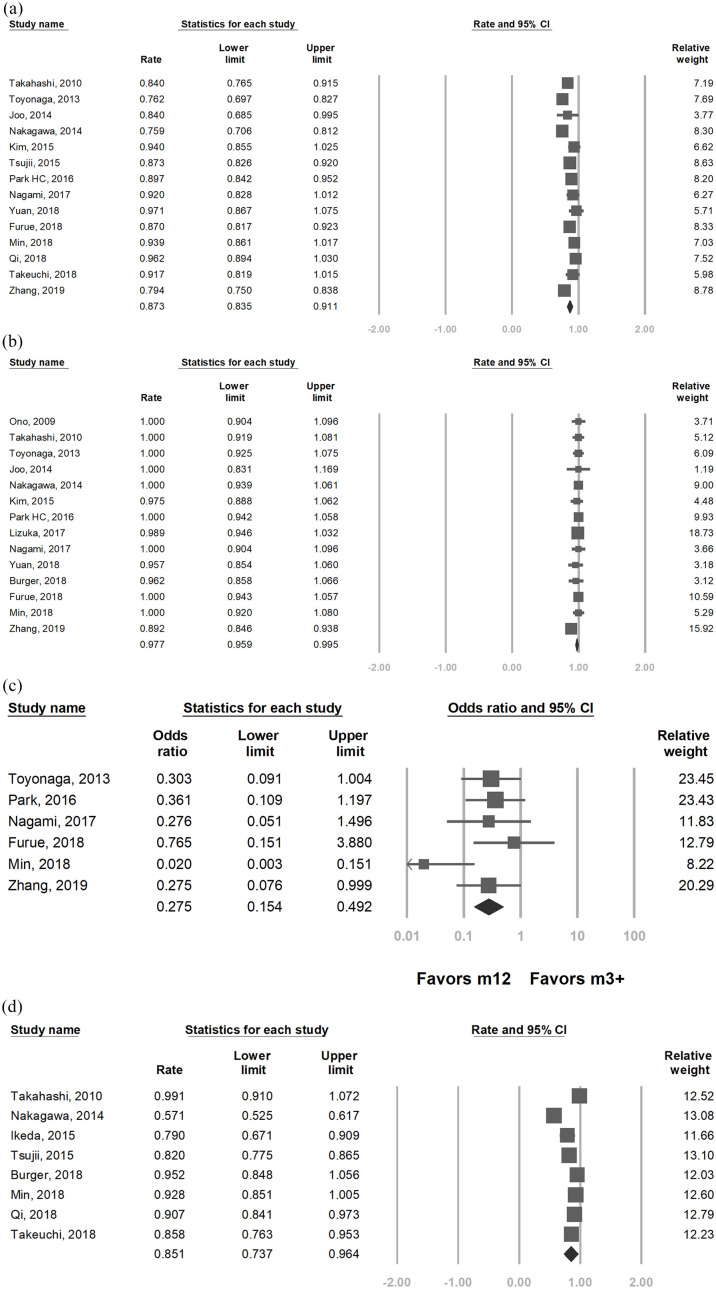
The survival, recurrence and lymph node metastasis rates are summarized in Table 2. Pooled overall survival rates for ESD was 90.5% at 3 years6,7,10–13,19,21,22,28,30 and 87.3% at 5 years [95% CI = 83.5–91.1%; Figure 2(a)].6,7,10,12–14,19–22,28,30,31 Disease-specific survival was 98.7 at 3 years13–15,17,19,21,22,28 and 97.7% at 5 years [95% CI = 95.9–99.5%; Figure 2(b)].5–7,10,12,14,16–19,21,22,28,30 In addition, the 5-year overall survival for ESD was significantly better for M1–M2 lesions compared with deeper invasions [93.5% versus 85.1%, OR = 0.27, 95% CI = 0.15–0.49; Figure 2(c)]. Patients with curative resection by ESD had excellent 5-year disease-specific survival (97.5%12 and 100%14) in two studies, whereas noncurative resection was found to have worse outcome by Tsujii et al.8 (3-year overall survival 85.9% versus 91.6%, p = 0.03).
Table 2.
Circumference of lesions, adverse events, recurrence, and survival outcomes.
| Study | Lesions >3/4 circumference (%) | R0 resection (%) | Adverse events (%) |
Recurrence (%) |
LN and distal metastasis (%) | OS |
DSS |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Perforation | Stricture | Local | MTC | 3 year | 5 year | 3 year | 5 year | ||||
| Ono et al.5 | NA | 87.90% | 4.8% | 17.9% | 1.2% | NA | 2.4% | NA | NA | NA | 100%† |
| Takahashi et al.6 | 22.4% | 97.40% | 2.6% | 17.2% | 0.9% | 9% | 0.0% | 90% | 84% | NA | 100% |
| Toyonaga et al.7 | NA | 95.70% | 0.0% | NA | 0.0% | NA | NA | 88.5% | 76.2% | NA | 100% |
| Joo et al.28 | 29.6% | 86% | 7.4% | 7.4% | 7.4% | 4% | 0.0% | 84% | 84% | 100% | 100% |
| Nakagawa et al.10 | NA | 90.50% | 0.5% | 3.9% | 2.0% | 11% | 0.0% | 80% | 75.9% | NA | 100% |
| Ikeda et al.11 | 30.2% | 79% | NA | NA | 2.3% | NA | 27.9% | 83.9% | NA | NA | NA |
| Probst et al.13 | NA | 91.70% | 0.0% | 20.8% | 0.0% | NA | 4.2% | 66.7% | NA | 95.8% | NA |
| Kim et al.12 | 5.1% | 91.70% | 12.1% | 5.1% | 3.0% | 3% | 0.0% | 94% | 94% | NA | 97.5% |
| Tsujii et al.8 | 4.2% | 84.50% | 6.2% | 8.5% | 1.6% | 10% | NA | 90.2% | 86.1% | NA | NA |
| Park et al.14 | 4.9% | 89.70% | 5.3% | 7.6% | NA | 5% | NA | NA | 89.7% | 100% | 100% |
| Park et al.15 | 18.8% | 91.70% | 6.3% | 15.6% | 0 | NA | 0.0% | NA | NA | 100% | NA |
| Lizuka et al.16 | NA | 95.4% | 1.0% | 5.5% | 0.2% | NA | NA | 96.7% | NA | NA | 98.9% |
| Nagami et al.17 | 2.4% | 90.40% | 0.0% | 18.1% | 0 | 20% | NA | NA | 92% | 100% | 100% |
| Yamauchi et al.29 | NA | NA | NA | NA | NA | NA | NA | NA‡ | NA | NA | NA |
| Baisi et al.30 | 26.1% | 92.70% | 2.9% | 17.4% | 8.7% | 3% | 1.4% | 98.6% | 97.1% | NA | 95.7% |
| Berger et al.18 | 0.3% | 91.10% | 2.9% | NA | 2.9% | NA | NA | NA | NA | NA | 96.2% |
| Furue et al.19 | NA | NA | 7.1% | 8.3% | 0.0% | NA | NA | 96.6% | 87% | 100% | 100% |
| Min et al.21 | NA | NA | 8.9% | 10.2% | NA | NA | NA | 96.5% | 93.9% | 100% | 100% |
| Qi et al.20 | 5.7% | 99.30% | 0.0% | 25.9% | 8.2% | NA | 1.3% | NA | 96.2% | NA | NA |
| Takeuchi et al.31 | 17.8% | NA | 1.4% | 9.6% | 2.7% | NA | 8.2% | NA | 91.7% | NA | NA |
| Zhang et al.22 | NA | 91.90% | 1.2% | 13.4% | 9.1% (including local and distal) | 91.0% | 79.4% | 96.1% | 89.2% | ||
survival for M1 lesions; the rate was 84.9% for lesions with invasion to M2 or beyond.
only hazard ratio of overall survival for ESD versus esophagectomy was recorded.
DSS, disease-specific survival; ESD, endoscopic submucosal dissection; LN, lymph node; MTC, metachronous; NA, not available; OS, overall survival.
Figure 2.
Pooled survival of endoscopic submucosal dissection. (a) Pooled 5-year overall survival rate of ESD among the included studies Heterogeneity: I2 = 77.1%, τ2 = 0.004, p < 0.001. (b)Pooled 5-year disease specific survival rate of ESD among the included studies Heterogeneity: I2 = 22.8%, τ2 = 0, p = 0.208. (c) Pooled odds ratio of 5-year overall survival of ESD in M1-M2 lesions compared to m3 and deeper lesions Heterogeneity: I2 = 39.0%, τ2 = 0.346, p = 0.14. (d) Pooled 5-year recurrence free survival rate of ESD among the included studies Heterogeneity: I2 = 95.2%, τ2 = 0.025, p < 0.001.
With regard to recurrence, the pooled 5-year recurrence-free survival was 85.1% [95% CI = 73.7–96.4%, Figure 2(d)] among available studies.5,8,10,11,18,20,21,31 The overall local recurrence, metachronous recurrence and nodal/distal metastasis rate of ESD among all included studies were 1.8%, 8.5%, and 3.3% respectively. Of note, Lizuka et al.16 reported similar complete resection, recurrence rate and overall survival for cervical and noncervical lesions, albeit the former had higher rate of postoperative stricture (20% versus 6.6%, p < 0.001).
For patients who were considered noncurative after ESD, 57.8% received additional therapy.8,11,13–18,20,21,31 Except for the study by Min et al., where proportions of specific treatment were not reported, 22.5% of these patients received esophagectomy and 32.6% had radiation or chemotherapy. In terms of the efficacy of additional therapy, Ikeda et al.11 demonstrated better 3-year recurrence-free survival with additional treatment versus observation for noncurative resection (88% versus 64%, p = 0.04). However, overall or recurrence-free survival was found to be similar among patients with additional surgery or chemoradiation therapy.11,31
Comparison of the outcomes between ESD and esophagectomy
Among the five comparative studies for ESD and surgery, 638 underwent ESD and 546 had primary esophagectomy. The esophagectomy procedure varied among studies that Takeuchi et al.31 performed mostly minimally invasive three-field lymphadenectomy (85%) for their patients. Min et al.21 primarily used an Ivor–Lewis or McKeown operation with limited lymphadenectomy and Zhang et al. underwent both minimally invasive (62%) and open surgery (38%)22
The baseline characteristics, stage and comorbidities were similar in patients with ESD and esophagectomy (Table 3 and Supplemental Table 2), though the surgery group had more middle-lower esophageal lesions and large circumferential lesions. Moreover, esophagectomy was associated with a higher R0 resection rate (97.0% versus 89.8%, p < 0.001). The median procedure time for ESD was <90 min in most studies6,8,12,14–16,19,22,28 except for the study by Probst et al., which reported the median as 152 min.13 On the other hand, the procedure time (median 49 versus 240 mins, p < 0.001) and hospital stay (median 3 versus 11 days, p < 0.001) was significantly shorter in ESD than esophagectomy.22
Table 3.
Baseline characteristics of patients underwent endoscopic submucosal dissection and primary surgery for superficial esophageal squamous cell cancer.
| Reference | ESD | Surgery | p-value | |
|---|---|---|---|---|
| Case number | Min et al.,21 Zhang et al.,22 Yamauchi et al.29 Baisi et al.,30 Takeuchi et al.31 | 638 | 546 | |
| Age (median) | Min et al.,21 Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 64.1 | 62.6 | 0.136 |
| Sex (male %) | Min et al.,21 Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 82.5 | 78.7 | 0.069 |
| Lesion size (median, mm) | Min et al.,21 Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 17–45 | 16–52 | 0.163 |
| Location | Min et al.,21 Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 0.842 | ||
| Upper | 74 | 42 | 0.043* | |
| Middle | 332 | 264 | 0.332 | |
| Lower | 176 | 189 | 0.687 | |
| Invasion depth | Min et al.,21 Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 0.057† | ||
| T1a (mucosa) | 425 | 207 | ||
| T1b (submucosa) | 159 | 288 | ||
| Lesions > 3/4 circumference (%) | Baisi et al.,30 Takeuchi et al.31 | 21.8 | 44.5 | <0.001* |
| Lymphovascular invasion (%) | Min et al.,21 Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 7.7 | 15.3 | 0.132† |
| Poorly differentiated (%) | Min et al.,21 Baisi et al.,30 | 2.1 | 2.3 | 0.678 |
| R0 resection (%) | Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 89.8 | 97.0 | <0.001* |
| Recurrence and metastasis (%) | Zhang et al.,22 Baisi et al.,30 Takeuchi et al.31 | 9.4 | 12.2 | 0.646† |
| Metachronous recurrence (%) | Min et al.,21 Baisi et al.,30 | 7.4 | 0 | 0.028* |
| Procedure time (min, median) | Zhang et al.22 | 53 | 240 | <0.001* |
| Hospital stay (days) | Zhang et al.,22 Baisi et al.30 | 4.3 | 12.2 | 0.02* |
p < 0.05.
random-effects model owing to significant heterogeneity.
ESD, endoscopic submucosal dissection.
Compared with esophagectomy, ESD had significantly lower overall (19.8% versus 44.0%, OR = 0.29, 95% CI = 0.19–0.43, Supplemental Table 3) and early adverse events. In addition, Min et al. also reported 21 more late adverse events for esophagectomy. However, the difference was not significant for severe adverse events, defined as Clavien–Dindo grade III–IV,33 when heterogeneity was taken into consideration (12.5% for ESD versus 20.5% for esophagectomy, p = 0.256). In addition, patients treated with esophagectomy had different patterns of adverse events, as they tended to suffer from pulmonary complications (8.0%) such as pneumonia and respiratory compromise, and fistula/leakage (13.3%).21,22,31 In this review, perioperative death was rare in both treatment modalities (0.1% versus 1.0%, p = 0.076).21,22,30,31
With regards to survival (Supplemental Table 4), the meta-analysis showed similar 5-year overall survival for all lesions that underwent ESD versus esophagectomy (86.4% versus 81.8%, HR = 0.66, 95% CI = 0.39–1.11), as well as lesions with submucosal invasion (HR = 1.24, 95% CI = 0.71–2.14, Supplemental Figure 1). Likewise, disease-specific survival (97.5% versus 94.1%, HR = 0.57, 95% CI = 0.22–1.47)21,22 and recurrence-free survival (HR = 1.52, 95% CI = 0.74–3.09)21,31 were not significantly different between the ESD and esophagectomy groups. However, metachronous recurrence rate was higher with ESD at 5 years (9.7% versus 0%, p = 0.004) as reported by Min et al.21
Sensitivity analysis and risk of bias assessment
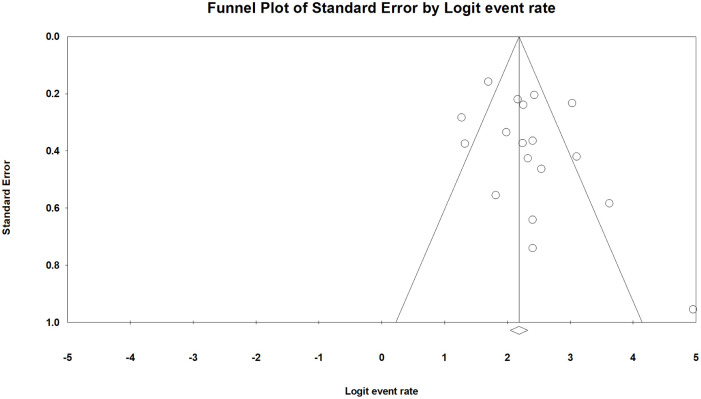
Sensitivity analysis by excluding one study at each time34 for all the meta-analyses of this study overall survival showed robustness of the pooled effect estimates. Howevery, given the high statistical heterogeneity in the meta-analysis of 5-year overall survival and recurrence-free survival, we tried to analyze both outcomes by studies without deep submucosal invasive lesions (SM2 or deeper). However, there was still significant heterogeneity in both overall and recurrence-free survival (I2 = 84.8% and 54.9%, respectively) without deep invasions. By contrast, the result was more consistent for overall survival for studies including deep lesions (I2 = 0). Publication bias, evaluated by R0 resection rate, was not evident based on the funnel plot (Figure 3) and Egger’s test (p = 0.13). The assessment for risk of bias was recorded in Supplemental Table 5. Most studies in this review were qualified based on the evaluation.
Figure 3.
Funnel plot of R0 resection rate among included studies.
Discussion
In theory, esophagectomy may have the best chance of cure for SESCC given the potential risk of lymph node metastasis. However, considerable morbidity is still noted despite the recent improvement in perioperative mortality, and the long-term life quality is usually impaired after esophagectomy.35,36 Thus, endoscopic treatment may be preferred for lesions with low rate of nodal metastasis. The current guideline recommends that endoscopic treatment is most suitable for SESCC confined to M1 and M2, and it may be considered for M3 lesions.37 Although ESD has been reported to be highly effective for SESCC,5–8,10–15,28 its long-term outcomes and comparison to esophagectomy were reported by a few retrospective studies until recently.21,22,29–31 Since a randomized trial is not realistic, the systematic review and meta-analysis is important to provide further evidence in terms of the risks and benefits as well as to guide future treatment decisions.
Our study demonstrated that ESD had excellent long-term outcomes and safety profile as the treatment of SESCC, and the prognosis was encouraging not only in Eastern Asia but also in Western countries. Furthermore, in the subgroup of comparative studies, ESD showed similar efficacy to esophagectomy with much lower perioperative adverse events. These results suggest that ESD may be the treatment of choice for SESCC with available expertise, especially for elderly and frail patients since it has minimal invasiveness and complications including stricture are frequently treatable with endoscopy. However, it should be noted that ESD alone is not adequate for deep submucosal invasive lesions, and up to one third of patients were found to have upgraded T stage by histology despite meticulous preoperative evaluation. This finding, however, highlights the importance of en-bloc resection and careful subsequent pathologic examination. For patients with noncurative resection by ESD, timely additional treatment like chemoradiation therapy or salvage esophagectomy is necessary to improve the outcomes.
Although there were only five studies directly compared ESD and esophagectomy in this review, they still provide some insights in clinical practice. The overall survival was similar despite lower R0 resection rate with ESD, which may be explained by several reasons. Firstly, the excellent en-bloc resection rate of ESD allows accurate evaluation of the invasion depth, hence timely additional treatment can be advised for high risk lesions. Secondly, the effectiveness of the additional therapies such as chemoradiation therapy and salvage esophagectomy may enhance local control and reduce nodal metastasis.38,39 Thirdly, it might need more time for esophagectomy to translate the theoretical lower recurrence/metastasis rate into survival benefit, after offsetting the potential life-threatening morbidity.22
Moreover, the adverse events, procedural time and hospital stay were also significantly lower with ESD than with esophagectomy. The complication rate of esophagectomy has been reported as high at 20–40% even among the modern series,4,40–42 which is also similar to the current study. The complications of ESD and esophagectomy differs not only in frequency but also severity. For instance, some complications of esophagectomy, such as fistula and anastomotic leakage may be life-threatening and difficult to treat. By contrast, perioperative complication of ESD, such as perforation and delayed bleeding are rare in high volume centers, and they can be readily treated by endoscopic treatment. Recently, minimally invasive surgery has been introduced to reduce the postoperative complications, but the learning curve was steep and over 100 procedures may be necessary before being qualified,43 which was even more difficult to be competent than the training of ESD. The two Western studies in our review suggested 30 cases of experience may be sufficient for a satisfactory outcome for esophageal ESD.13,18
Stricture is another complication shared by esophagectomy and ESD. Although the studies in our review did not focus on stricture formation after esophagectomy, the prevalence was as high as 9–23% and it was not always associated with an ischemic conduit.4,44,45 On the other hand, stricture of ESD is more frequent in cervical esophagus or lesions more than 75% circumference, and most of them can be prevented by various therapies.46–48 Although endoscopic balloon dilatation has been proved to be effective in both post-esophagectomy and post-ESD stricture, it is unclear whether the outcome become different with the two etiologies. We believe that future observational studies may clarify this issue.
To our knowledge, this is the first systemic review and meta-analysis to evaluate the long-term outcomes of ESD for SESCC. The strength comes from a comprehensive literature review and data collection, as well as the meta-analysis of long-term survival based on the up-to-date studies. However, there were still some limitations. First, these studies were all retrospective, and there might be some selection bias. For example, the lymph node metastasis (3.3%) was lower than expected, given nearly half of the lesions had invasion to muscularis mucosa or deeper, though the finding may be partly explained by the effect of additional treatment. Secondly, the relatively low numbers of the comparative studies make it difficult to conclude whether ESD is a better modality than esophagectomy for SESCC. Thirdly, though our analysis demonstrated the survival rate of submucosal invasive lesions was not significantly different for both modalities, the data were only derived from two cohort studies.21,22 Nevertheless, the two studies were large cohort with propensity score matching, and all the studies were published within the last 3 years. Hence, the results probably reflect the outcomes of SESCC with the current practice of ESD and esophagectomy. Fourthly, the heterogeneity in overall/recurrence-free survival was quite high despite our sensitivity analysis based on the depth of included lesions. This may probably reflect the various inclusion criteria and difference in the subsequent additional therapies. Further studies for the comparison of ESD and esophagectomy, and the relative efficacy of each additional therapy, are required to improve the treatment of SESCC.
In summary, our study showed excellent long-term outcomes and safety of ESD for SESCC. Moreover, ESD had similar survival outcomes of mucosal lesions to those of esophagectomy and fewer adverse events. Therefore, ESD may be considered as the primary treatment of choice for mucosal lesions, and additional treatment should be available for submucosal invasive cancers.
Supplemental Material
Supplemental material, Supp for Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis by Jen-Hao Yeh, Ru-Yi Huang, Ching-Tai Lee, Chih-Wen Lin, Ming-Hung Hsu, Tsung-Chin Wu, Po-Jen Hsiao and Wen-Lun Wang in Therapeutic Advances in Gastroenterology
Acknowledgments
We appreciated the help of English refinement by Wallace Academic Editing.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the EDAHP109009 project of E-Da Hospital granted to Dr. Jen-Hao Yeh. The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
ORCID iD: Wen-Lun Wang  https://orcid.org/0000-0002-4623-4385
https://orcid.org/0000-0002-4623-4385
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jen-Hao Yeh, Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Hospital, Kaohsiung, Taiwan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Dachang Hospital, Kaohsiung, Taiwan; Department of Medical technology, College of Medicine, I-Shou University, Kaohsiung, Taiwan.
Ru-Yi Huang, School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan; Department of Family Medicine, E-DA Hospital, Kaohsiung, Taiwan.
Ching-Tai Lee, Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Hospital, Kaohsiung, Taiwan; School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan.
Chih-Wen Lin, Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Hospital, Kaohsiung, Taiwan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Dachang Hospital, Kaohsiung, Taiwan.
Ming-Hung Hsu, Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Hospital, Kaohsiung, Taiwan; School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan.
Tsung-Chin Wu, Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Dachang Hospital, Kaohsiung, Taiwan; Department of Medical technology, College of Medicine, I-Shou University, Kaohsiung, Taiwan.
Po-Jen Hsiao, Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-DA Dachang Hospital, Kaohsiung, Taiwan.
Wen-Lun Wang, School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan; Department of Internal Medicine, E-Da Hospital. Kaohsiung City 82445, Taiwan.
Reference
- 1. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018; 154: 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union against Cancer Cancer Staging Manuals. Cancer 2010; 116: 3763–3773. [DOI] [PubMed] [Google Scholar]
- 3. Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg 2012; 16: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 4. Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011; 28: 29–35. [DOI] [PubMed] [Google Scholar]
- 5. Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70: 860–866. [DOI] [PubMed] [Google Scholar]
- 6. Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010; 72: 255–264, 264.e251–252. [DOI] [PubMed] [Google Scholar]
- 7. Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc 2013; 27: 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy 2015; 47: 775–783. [DOI] [PubMed] [Google Scholar]
- 9. Lee CT, Chang CY, Tai CM, et al. Endoscopic submucosal dissection for early esophageal neoplasia: a single center experience in South Taiwan. J Formos Med Assoc 2012; 111: 132–139. [DOI] [PubMed] [Google Scholar]
- 10. Nakagawa K, Koike T, Iijima K, et al. Comparison of the long-term outcomes of endoscopic resection for superficial squamous cell carcinoma and adenocarcinoma of the esophagus in Japan. Am J Gastroenterol 2014; 109: 348–356. [DOI] [PubMed] [Google Scholar]
- 11. Ikeda A, Hoshi N, Yoshizaki T, et al. Endoscopic submucosal dissection (ESD) with additional therapy for superficial esophageal cancer with submucosal invasion. Intern Med 2015; 54: 2803–2813. [DOI] [PubMed] [Google Scholar]
- 12. Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Probst A, Aust D, Markl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015; 47: 113–121. [DOI] [PubMed] [Google Scholar]
- 14. Park HC, Kim DH, Gong EJ, et al. Ten-year experience of esophageal endoscopic submucosal dissection of superficial esophageal neoplasms in a single center. Korean J Intern Med 2016; 31: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park JS, Youn YH, Park JJ, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal squamous neoplasms. Clin Endosc 2016; 49: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lizuka T, Kikuchi D, Hoteya S, et al. Efficacy and safety of endoscopic submucosal dissection for superficial cancer of the cervical esophagus. Endosc Int Open 2017; 5: E736–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagami Y, Ominami M, Shiba M, et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis 2017; 49: 427–433. [DOI] [PubMed] [Google Scholar]
- 18. Berger A, Rahmi G, Perrod G, et al. Long-term follow-up after endoscopic resection for superficial esophageal squamous cell carcinoma: a multicenter Western study. Endoscopy. Epub ahead of print 27 September 2018. DOI: 10.1055/a-0732-5317. [DOI] [PubMed] [Google Scholar]
- 19. Furue Y, Katada C, Tanabe S, et al. Effectiveness and safety of endoscopic aspiration mucosectomy and endoscopic submucosal dissection in patients with superficial esophageal squamous cell carcinoma. Surg Endosc. Epub ahead of print 27 September 2018. DOI: 10.1007/s00464-018-6418-3. [DOI] [PubMed] [Google Scholar]
- 20. Qi ZP, Chen T, Li B, et al. Endoscopic submucosal dissection for early esophageal cancer in elderly patients with relative indications for endoscopic treatment. Endoscopy 2018; 50: 839–845. [DOI] [PubMed] [Google Scholar]
- 21. Min YW, Lee H, Song BG, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointest Endosc 2018; 88: 624–633. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Ding H, Chen T, et al. Outcomes of endoscopic submucosal dissection vs esophagectomy for T1 esophageal squamous cell carcinoma in a real-world cohort. Clin Gastroenterol Hepatol 2019; 17: 73–81.e73. [DOI] [PubMed] [Google Scholar]
- 23. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019; 16: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017; 6: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 26. Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute, 2011. [Google Scholar]
- 27. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joo DC, Kim GH, Park DY, et al. Long-term outcome after endoscopic submucosal dissection in patients with superficial esophageal squamous cell carcinoma: a single-center study. Gut Liver 2014; 8: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamauchi K, Iwamuro M, Obayashi Y, et al. Long-term outcomes of endoscopic resection versus surgical resection for MM-SM1 esophagus squamous cell carcinoma. In: United European gastroenterology journal conference: 25th united European gastroenterology week, UEG 2017, Spain, 2017; 5: Abstract 361. [Google Scholar]
- 30. Baisi Y, Liu L, Huang H, et al. Comparison of the short-term and long-term outcomes of surgical treatment versus endoscopic treatment for early esophageal squamous cell neoplasia larger than 2 cm: a retrospective study. Surg Endosc 2018; 33: 2304–2312. [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi M, Suda K, Hamamoto Y, et al. Technical feasibility and oncologic safety of diagnostic endoscopic resection for superficial esophageal cancer. Gastrointestinal Endosc 2018; 88: 456–465. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 2008; 37: 1158–1160. [DOI] [PubMed] [Google Scholar]
- 35. Djarv T, Derogar M, Lagergren P. Influence of co-morbidity on long-term quality of life after oesophagectomy for cancer. Br J Surg 2014; 101: 495–501. [DOI] [PubMed] [Google Scholar]
- 36. Kim D, Min YW, Park JG, et al. Influence of esophagectomy on the gastroesophageal reflux in patients with esophageal cancer. Dis Esophagus 2017; 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 37. Ishihara R, Arima M, Iizuka T, et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc 2020; 32: 452–493. [DOI] [PubMed] [Google Scholar]
- 38. Kawaguchi G, Sasamoto R, Abe E, et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol 2015; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kudou M, Shiozaki A, Fujiwara H, et al. Efficacy of additional surgical resection after endoscopic submucosal dissection for superficial esophageal cancer. Anticancer Res 2017; 37: 5301–5307. [DOI] [PubMed] [Google Scholar]
- 40. Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010; 90: 936–942; discussion 942. [DOI] [PubMed] [Google Scholar]
- 41. Morita M, Nakanoko T, Fujinaka Y, et al. In-hospital mortality after a surgical resection for esophageal cancer: analyses of the associated factors and historical changes. Ann Surg Oncol 2011; 18: 1757–1765. [DOI] [PubMed] [Google Scholar]
- 42. Rutegard M, Lagergren P, Rouvelas I, et al. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol 2012; 38: 555–561. [DOI] [PubMed] [Google Scholar]
- 43. van Workum F, Stenstra M, Berkelmans GHK, et al. Learning curve and associated morbidity of minimally invasive esophagectomy: a retrospective multicenter study. Ann Surg 2019; 269: 88–94. [DOI] [PubMed] [Google Scholar]
- 44. Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004; 198: 536–541; discussion 541–532. [DOI] [PubMed] [Google Scholar]
- 45. Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012; 16: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 46. Sato H, Inoue H, Kobayashi Y, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc 2013; 78: 250–257. [DOI] [PubMed] [Google Scholar]
- 47. Xu M, Chu Y, Zhang C. Intralesional steroid injection combined with oral steroid administration to prevent esophageal stricture after endoscopic submucosal dissection of lesion no less than a half of circumference. In: United European gastroenterology journal conference: 24th united European gastroenterology week, UEG 2016, Austria, 2016; 4: Abstract 47. [Google Scholar]
- 48. Martinek J. How to prevent post-ESD esophageal stricture. Endosc Int Open 2019; 7: E771–E773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supp for Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis by Jen-Hao Yeh, Ru-Yi Huang, Ching-Tai Lee, Chih-Wen Lin, Ming-Hung Hsu, Tsung-Chin Wu, Po-Jen Hsiao and Wen-Lun Wang in Therapeutic Advances in Gastroenterology