Key Points
Admission plasma levels of Sdc-1 and thrombomodulin predict in-hospital mortality of iTTP.
Increases in both plasma Sdc-1 and thrombomodulin at clinical response/remission are significantly associated with iTTP exacerbation.
Abstract
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a potentially fatal blood disorder resulting from acquired deficiency of plasma ADAMTS13 activity. Despite recent advances in early diagnosis and novel therapeutics, the mortality rate of acute iTTP remains as high as 10% to 20%. Moreover, a reliable clinical and laboratory parameter that predicts disease severity and outcomes is lacking. We show in the present study that plasma levels of syndecan-1 (Sdc-1) and soluble thrombomodulin (sTM) on admission were dramatically increased in patients with acute iTTP and remained substantially elevated in a subset of patients compared with healthy controls. The elevated admission plasma levels of Sdc-1 and sTM were associated with abnormal Glasgow coma scale scores, low estimated glomerular filtration rates, the need for intensive care, and in-hospital mortality rates. Moreover, a further simultaneous increase in plasma Sdc-1 and sTM levels at the time of clinical response/remission (eg, when normalization of platelet counts and substantial reduction of serum lactate dehydrogenase activity were achieved) was highly predictive of iTTP recurrence. These results demonstrate that endothelial injury, resulting from disseminated microvascular thromboses, is severe and persistent in patients with acute iTTP. Plasma levels of Sdc-1 and sTM on admission and in remission are predictive of in-hospital mortality and recurrence of acute iTTP, respectively. Thus, an incorporation of such novel plasma biomarkers into the risk assessment in acute iTTP may help implement a more vigorous and intensive therapeutic strategy for these patients.
Visual Abstract
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a potentially life-threatening blood disorder, occurring in 3 to 10 cases per million per year.1,2 Most patients are caused by acquired immunoglobulin G (IgG) autoantibodies against ADAMTS13, a plasma metalloprotease that cleaves von Willebrand factor (VWF).3,4 This type of TTP referred to as immune-mediated TTP (iTTP).5,6 Rarely, TTP may be caused by hereditary deficiency of plasma ADAMTS13 activity due to inherited mutations in ADAMTS13, referred to as hereditary or congenital TTP.5,7 Clinical presentations of TTP vary dramatically, but the diagnosis relies on the presence of thrombocytopenia and microangiopathic hemolytic anemia, as well as a positive ADAMTS13 test result.8-11 Some patients may present with signs and symptoms of end-organ damage, including ischemic cerebral infarction or stroke,12,13 renal insufficiency,9,10 and myocardial ischemia,2,14 at various stages of the disease course.
Therapeutic plasma exchange (TPE) remains the standard of care, which has significantly reduced the mortality and morbidity rates of iTTP.6,15 This life-saving procedure aims to remove autoantibodies against ADAMTS13, ultra large VWF, and inflammatory cytokines while replenishing the missing or inhibited ADAMTS13 enzyme. International Society on Thrombosis and Haemostasis TTP guidelines conditionally recommend the early use of caplacizumab11,16,17 on top of TPE, corticosteroids, and rituximab in patients with a high probability of iTTP based on clinical assessment with access to ADAMTS13 testing.11,17 However, such a practice may not be readily adopted by all clinicians worldwide. The variability in practice and the disparity in resource utilization result in a high mortality rate (eg, 10% to 20%) of acute iTTP.2,18-20 Therefore, early identification of risk factors causing death may allow for the implementation of a more aggressive treatment strategy. Previous studies have demonstrated that old age,21 high lactate dehydrogenase (LDH) activity,21 elevated troponin levels,2,14,22 neurological involvement,21 lower ADAMTS13 antigen levels,23,24 and high anti-ADAMTS13 IgG23 levels at presentation are associated with poor outcomes of TTP. More recently, we have shown that elevated plasma levels of soluble terminal attacking complexes (sC5b-9) are predictive of iTTP mortality.2
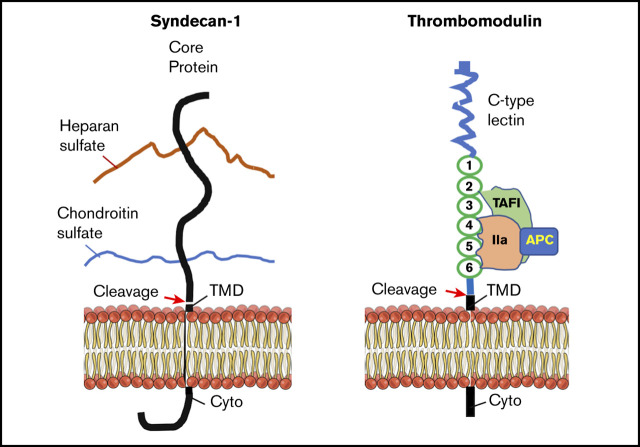
Syndecan-1 (Sdc-1)25,26 and thrombomodulin (TM)27 are the major components of the endothelial glycocalyx, which is a layer of membrane-bound macromolecules on the luminal surface of the vascular endothelium.28 Sdc-1, a 33-kDa single-pass type I transmembrane proteoglycan, comprises a core protein and ≥1 glycosaminoglycan chain covalently attached to the protein. The ectodomain of the Sdc-1 protein core bears 3 heparan sulfate chains and 2 chondroitin sulfate chains.25 TM, a 557-amino-acid type I transmembrane protein on the endothelium, binds exosite I on thrombin to alter thrombin specificity toward protein C.29 The thrombin-TM complex activates protein C and thrombin-activatable fibrinolysis inhibitor,30 thus inhibiting thrombus formation31 and complement activation.32
Under certain pathologic conditions, such as acute inflammation,33,34 hyperglycemia,35,36 endotoxemia,37 ischemia-reperfusion injury,33,38 and bypass surgery,39,40 or stimulation with tumor necrosis factor α41,42 and atrial natriuretic peptide,43 the ectodomains of Sdc-1 and TM are cleaved by leukocyte-derived proteases, metalloproteinases, and heparinase. Therefore, increased plasma levels of Sdc-1 have been observed in patients with inflammation,40 sepsis,44 traumatic brain injury,38 hemorrhagic shock,45 disseminated intravascular coagulation (DIC),46 and respiratory failure.40,44 High plasma levels of Sdc-1 in septic patients are associated with a higher likelihood of being intubated.47 Similarly, plasma or serum levels of soluble TM (sTM) are dramatically increased in patients with trauma, DIC, pulmonary thromboembolism, acute respiratory failure, chronic renal failure, and acute hepatic failure.48,49 Serum sTM levels appear to be correlated with the clinical course of DIC, multiorgan dysfunction syndrome, and mortality in patients with sepsis.50,51 In the present study, we test the hypothesis that plasma Sdc-1 and sTM may serve as sensitive biomarkers for endothelial injury and organ damage, thus predicting adverse outcome in critically ill patients such as iTTP.
Methods
Patients
Ninety-one unique iTTP patients with 107 acute episodes from April 2006 to July 2019 were enrolled into the study. Institutional review boards at University of Kansas Medical Center and University of Alabama at Birmingham approved the study protocol. Demographic information, routine laboratory results, and treatments were collected from the electronic medical records. Additional follow-ups were conducted through outpatient visits, telephone interviews, and interviews at the annual TTP fairs. Diagnostic criteria for iTTP were the same as those previously described and included2,6 (1) severe thrombocytopenia, (2) microangiopathic hemolytic anemia with or without end-organ damage, and (3) plasma ADAMTS13 activity <10 IU/dL with a detectable ADAMTS13 inhibitor or anti-ADAMTS13 IgG. Myocardial involvement was defined as troponin above the normal range (<0.025 ng/dL) on admission and neurological involvement was defined using a reduced Glasgow coma scale (GCS) as described previously.52
Blood collection
Whole-blood samples were collected from informed and consented patients prior to the initiation of TPE and anticoagulated with 0.39% sodium citrate. Serial blood samples were collected from 46 patients until clinical remission. The blood samples were centrifuged at 1500g for 15 minutes, and platelet-poor plasma was aspirated and stored in aliquots in a −80°C freezer until assays.
Assay for plasma ADAMTS13 activity
Plasma ADAMTS13 activity was determined using an in-house FRETSVWF73 assay, as described previously.53,54 The results with the in-house assay were comparable to that performed in the reference laboratory (Versiti, Milwaukee, WI). Pooled normal human plasma was used for the calibration, defined as having 100 U/dL (or 100%) of ADAMTS13 activity.
Assay for plasma ADAMTS13 inhibitor
Plasma ADAMTS13 inhibitor titers on admission were determined in the reference laboratory (Versiti).
Assay for plasma ADAMTS13 antigen
Plasma levels of ADAMTS13 antigen were determined by using a human ADAMTS13 Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Assay for plasma anti-ADAMTS13 IgG
Plasma levels of anti-ADAMTS13 IgG were determined by using Technozym ADAMTS-13 inhibitor ELISA kit (DiaPharma, West Chester Township, OH) according to the manufacturer’s instructions.
Assay for plasma levels of Sdc-1
Plasma levels of Sdc-1 were determined using a human sydecan-1 DuoSet ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Assay for plasma sTM
Plasma levels of sTM were determined using a human thrombomodulin/BDCA-3 Quantikine ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed with the Prism 8 software. All data were assumed to be in nonnormal distribution due to the rarity of iTTP, and values are expressed as the median and interquartile range (IQR). A Mann-Whitney U test (2 tailed and nonparametric) was used to analyze the data. A Kruskal-Wallis with Dunn’s multiple comparison test was used for data with ≥3 groups. The Wilcoxon test was used for paired data that were not normally distributed. The Fisher’s exact test was performed for categorical variables. The Spearman test was used to determine the correlation coefficients of the data that were not normally distributed. Furthermore, the log-rank test was used to compare the survival probability or recurrence-free probability between 2 groups. Values of P < .05 and P < .01 were considered statistically significant and highly statistically different, respectively.
Results
Patient characteristics
Of 107 acute iTTP episodes (91 unique patients), the median age was 47 years (IQR, 34-55 years). Of 91 unique patients, 53 patients (58.2%) were female and 73 (80.2%) were African American. Of 107 acute patient episodes, 63 (58.9%) were initial episodes and 44 (41.1%) were recurrent ones. Most patients (69.2%) presented with comorbidities, including hypertension (55.1%), diabetes mellitus (22.4%), systemic lupus erythematosus (13.1%), and HIV infection (9.3%). The median body mass index of these patients was 32.3 with IQR of 26.0-39.4. More than half of patients (56.1%) presented with signs and symptoms that involved the central nervous system (eg, confusion, disorientation, coma, and seizure), 43.9% had renal dysfunction (eg, estimated glomerular filtration rate [eGFR] <60), 30.1% had abdominal pain, 15.0% had fever, and 12.1% reported chest pain. Of 88 patients who reported their social history, 55.7% were smokers, 45.5% were drinkers, and 18.2% used illicit drugs (Table 1).
Table 1.
Demographic and clinical features of 107 iTTP patient episodes
| Parameters | Values |
|---|---|
| Age, median (IQR), y | 47 (34-55) |
| Sex, n (%) (n = 91) | |
| Female | 53 (58.2) |
| Male | 38 (41.8) |
| Race, n (%) (n = 91) | |
| African American | 73 (80.2) |
| White | 18 (19.8) |
| Disease status (n = 107) | |
| Initial, n (%) | 63 (58.9) |
| Exacerbation/relapse, n (%) | 44 (41.1) |
| BMI (n = 104), median (IQR) | 32.3 (26.0-39.4) |
| Comorbidities, n (%) (n = 107) | |
| Hypertension | 59 (55.1) |
| Diabetes mellitus | 24 (22.4) |
| SLE, n (%) (n = 57) | |
| Positive | 14 (24.6) |
| Negative | 43 (75.4) |
| HIV, n (%) (n = 88) | |
| Positive | 10 (11.4) |
| Negative | 78 (88.6) |
| Symptoms/signs, n (%) (n = 107) | |
| CNS symptoms | 60 (56.1) |
| GFR calc <60 | 47 (43.9) |
| Abdominal pain | 33 (30.1) |
| Fever | 16 (15.0) |
| Chest pain | 13 (12.1) |
| Social history, n (%) (n = 88) | |
| Smoking | 49 (55.7) |
| Drinking | 40 (45.5) |
| Illicit drug use | 16 (18.2) |
BMI, body mass index; CNS, central nervous system; n, number of episodes or cases; SLE, systemic lupus erythematosus.
Overall, the mortality rate was 10.3% (11/107 patients), with a median time of death of 10 days (IQR, 2-14 days) from admission. There was no significant difference in demographic parameters, blood groups, comorbidities, clinical presentation, and social history except for GCS scores between the patients who died (median, 15; IQR, 14-15) and those who survived (median, 12; IQR, 3-15) (P = .003) (supplemental Table 1).
Plasma levels of Sdc-1 and sTM in iTTP patients and healthy controls
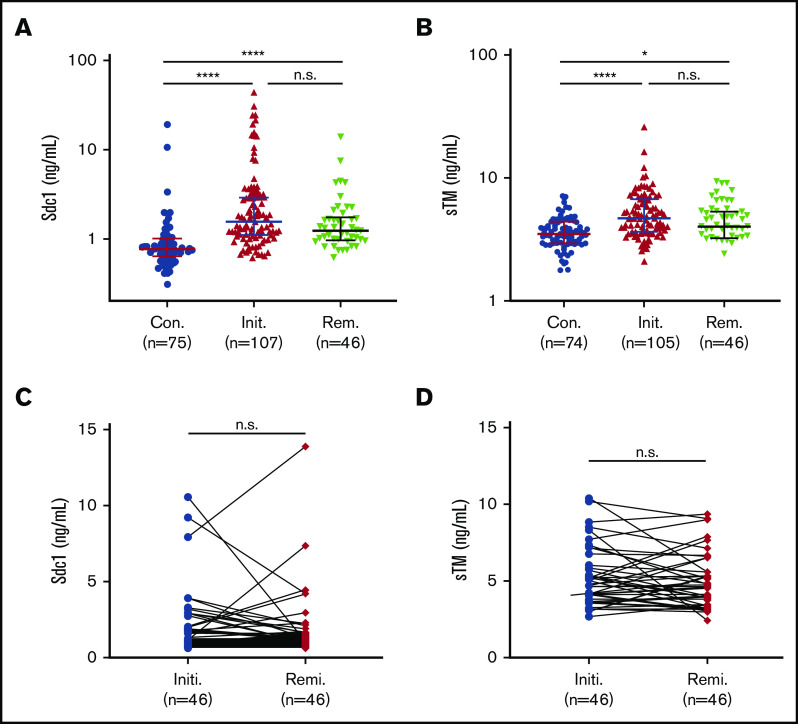
Sdc-1 and sTM are the major components of the endothelial glycocalyx, a layer of membrane-bound macromolecules on the luminal surface of vascular endothelium.55 As shown, plasma levels of Sdc-1 (Figure 1A) and sTM (Figure 1B) on admission in patients with acute iTTP were dramatically increased compared with healthy controls. Median plasma levels of Sdc-1 and sTM in patients with acute iTTP prior to TPE were 1.5 ng/mL (IQR, 1.1-2.9) and 4.7 ng/mL (IQR, 3.7-6.6), respectively. Moreover, plasma levels of Sdc-1 (median, 1.2 ng/mL; IQR, 1.0-1.7 ng/mL) (Figure 1A) (P < .0001) and sTM (median, 4.5 ng/mL; IQR, 3.5-5.6) (P < .05) (Figure 1B) remained substantially higher in patients during clinical remission, when platelet counts were essentially normalized, than in healthy controls. When comparing paired samples, plasma levels of Sdc-1 (Figure 1C) and sTM (Figure 1D) during remission were not statistically different from those on admission (P > .05). These results indicate that substantial and persistent endotheliopathy occurs in patients who have recovered from an acute iTTP episode, a condition that was not previously recognized.
Figure 1.
Plasma Sdc-1 and sTM in patients on admission and in clinical remission. Plasma levels of Sdc-1 (A) and sTM (B) in patients prior to TPE (Init.) and in clinical remission (Rem.), compared with healthy controls (Con.). Each dot represents an individual patient or control value, and horizontal bars within the dots represent the median and IQR. Kruskal-Wallis test corrected with Dunn’s multiple comparisons was performed to determine statistical significance. Longitudinal changes in plasma levels of Sdc-1 (C) and sTM (D) in paired individual patients prior to TPE (Initi., blue dots) and in clinical response/remission (Remi., red dots). A Wilcoxon matched-pairs signal test (2 tailed and nonparametric) was used to determine statistical significance. P > .05 (n.s., not significant); *P < .05; ****P < .0001.
Plasma levels of Sdc-1 and sTM are associated with disease severity in patients with acute iTTP
On admission, higher median plasma levels of Sdc-1 (P = .0049) (Figure 2A) or sTM (P = .0016) (Figure 2B) were found in patients with abnormal GCS scores. Higher median plasma levels of sTM (P < .0001) (Figure 2D), but not Sdc-1 (P = .1684) (Figure 2C), were found in patients with abnormal eGFR compared with those with normal eGFR. Patients who had systemic lupus erythematosus (SLE) also exhibited higher median plasma levels of Sdc-1 (P = .0014) (Figure 2E), but not sTM (P = .6115) (Figure 2F), than those without SLE. Moreover, patients who were admitted to the intensive care unit (ICU) had dramatically increased median plasma levels of Sdc-1 (P = .0095) (Figure 2G) or sTM (P = .003) (Figure 2H) than those without ICU admission. Similar results were obtained for Sdc-1 (Figure 2I) (P = .01) and sTM (Figure 2J) (P = .003) from patients who were or were not intubated. No statistically significant differences in plasma levels of Sdc-1 or sTM were found between patients with an initial iTTP episode and those with recurrent disease (data not shown). These results suggest that plasma levels of Sdc-1 and/or sTM are associated with disease severity in patients with acute iTTP.
Figure 2.

Plasma levels of Sdc-1 and sTM in patients with or without an adverse condition. Plasma levels of Sdc-1 (A) and sTM (B) in patients with normal (n = 70) and abnormal (n = 37) GCS scores; plasma levels of Sdc-1 (C) and sTM (D) in patients with a normal (n = 60) and abnormal (n = 47) eGFR; plasma levels of Sdc-1 (E) and sTM (F) in patients without SLE (w/o SLE) (n = 43) or with SLE (w/SLE) (n = 14); plasma levels of Sdc-1 (G) and sTM (H) in patients without (no ICU) (n = 69) or with (ICU) ICU (n = 38) admission; and plasma levels of Sdc-1 (I) and sTM (J) in patients who were not intubated (no intub., n = 86) or were intubated (Intub., n = 21). A Mann-Whitney U test (2 tailed and nonparametric) was performed to determine the statistical significance. Each dot represents 1 individual patient. The horizontal bars within the dots present the median and IQR. P > .05 (n.s., not significant); **P < .01; ****P < .0001.
Plasma levels of Sdc-1 and sTM correlate with another routine laboratory marker indicative of organ damage
To determine the relationship between plasma Sdc-1 or sTM and another routine laboratory parameter, such as troponin, LDH, and creatine, indicative of organ damage or dysfunction, Spearman correlation coefficients demonstrated that plasma Sdc-1 or sTM was modestly but positively correlated with plasma troponin-I (Figure 3A-B), serum LDH (Figure 3C-D), and creatine (Figure 3E-F). Additionally, plasma Sdc-1 or sTM was positively correlated with prothrombin time (Figure 4A-B), activated thromboplastin time (Figure 4C-D), fibrinogen (Figure 4E-F), and D-dimers (Figure 4G-H), the critical parameters for coagulopathy.
Figure 3.

Correlation between plasma levels of Sdc-1 (or sTM) and each of the routine laboratory parameters. Spearman correlation coefficients and statistical analyses were determined between plasma levels of Sdc-1 or sTM and serum levels of troponin (A-B), LDH (C-D), and creatinine (E-F). Values of P < .05 and P < .01 are considered to be statistically significant and highly significant, respectively.
Figure 4.
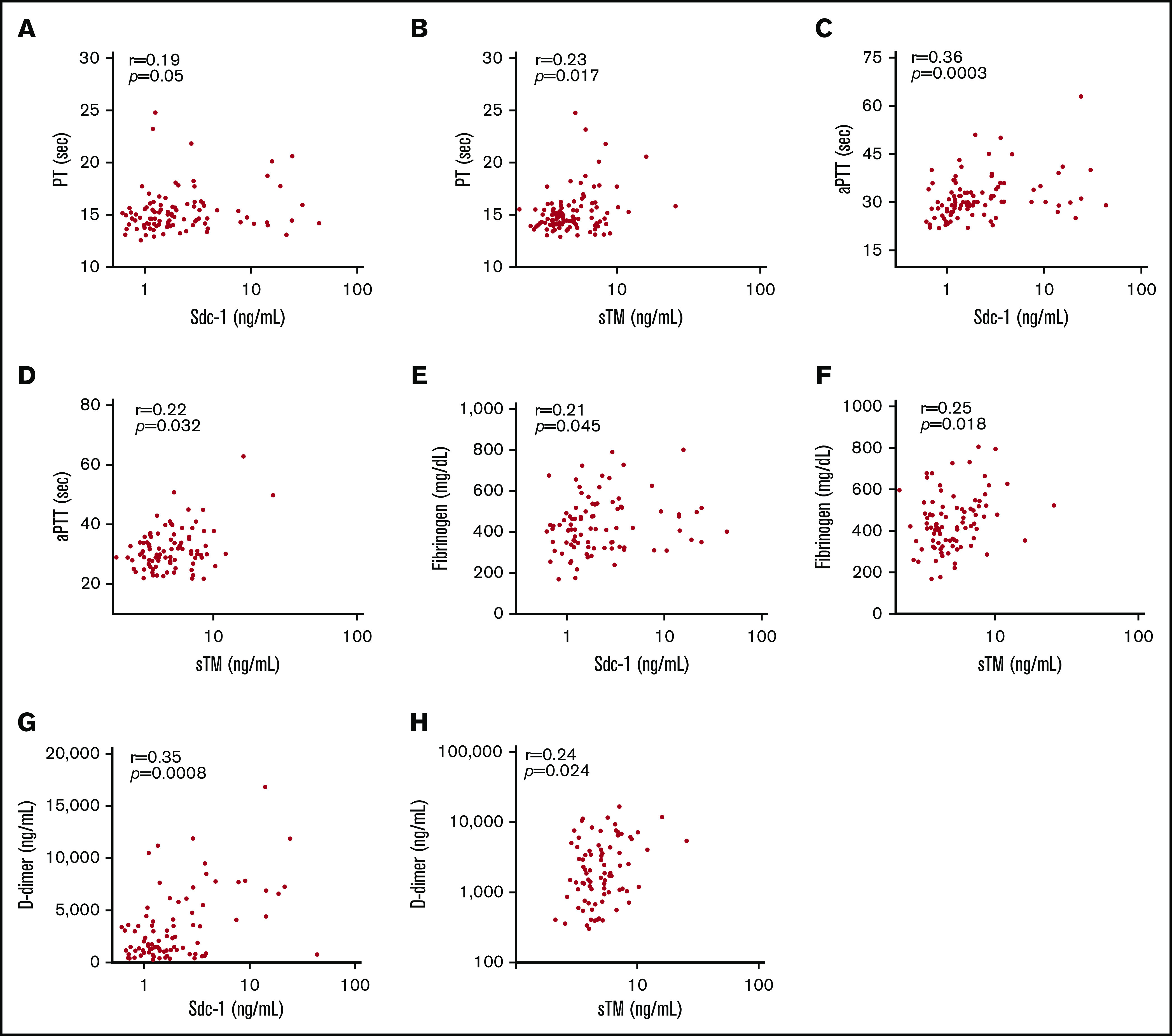
Correlation between plasma levels of Sdc-1 or sTM and each marker of coagulopathy. Spearman correlation coefficients and statistical analyses were determined between plasma levels of Sdc-1 or sTM and prothrombin time (PT) (A-B), activated thromboplastin time (aPTT) (C-D), plasma fibrinogen (E-F), and D-dimers (G-H). Values of P < .05 and P < .01 are considered to be statistically significant and highly significant, respectively.
Increased plasma levels of Sdc-1 or sTM on admission are associated with acute iTTP mortality rates
Patients who died within 60 days after admission had much higher median plasma levels of Sdc-1, but not sTM, on admission than those who survived an acute episode. The median plasma level of Sdc-1 was 15.8 ng/mL (IQR, 1.9-24.5) in patients who died compared with 1.4 ng/mL (IQR, 1.1-2.7) in those who survived (P < .0001) (Figure 5A). However, there was no statistically significant difference in plasma levels of sTM on admission between patients who died (median, 5.7 ng/mL; IQR, 4.6-7.6) and those who survived (median, 4.5 ng/mL; IQR, 3.6-6.1) (P = .0612) (Figure 5B).
Figure 5.
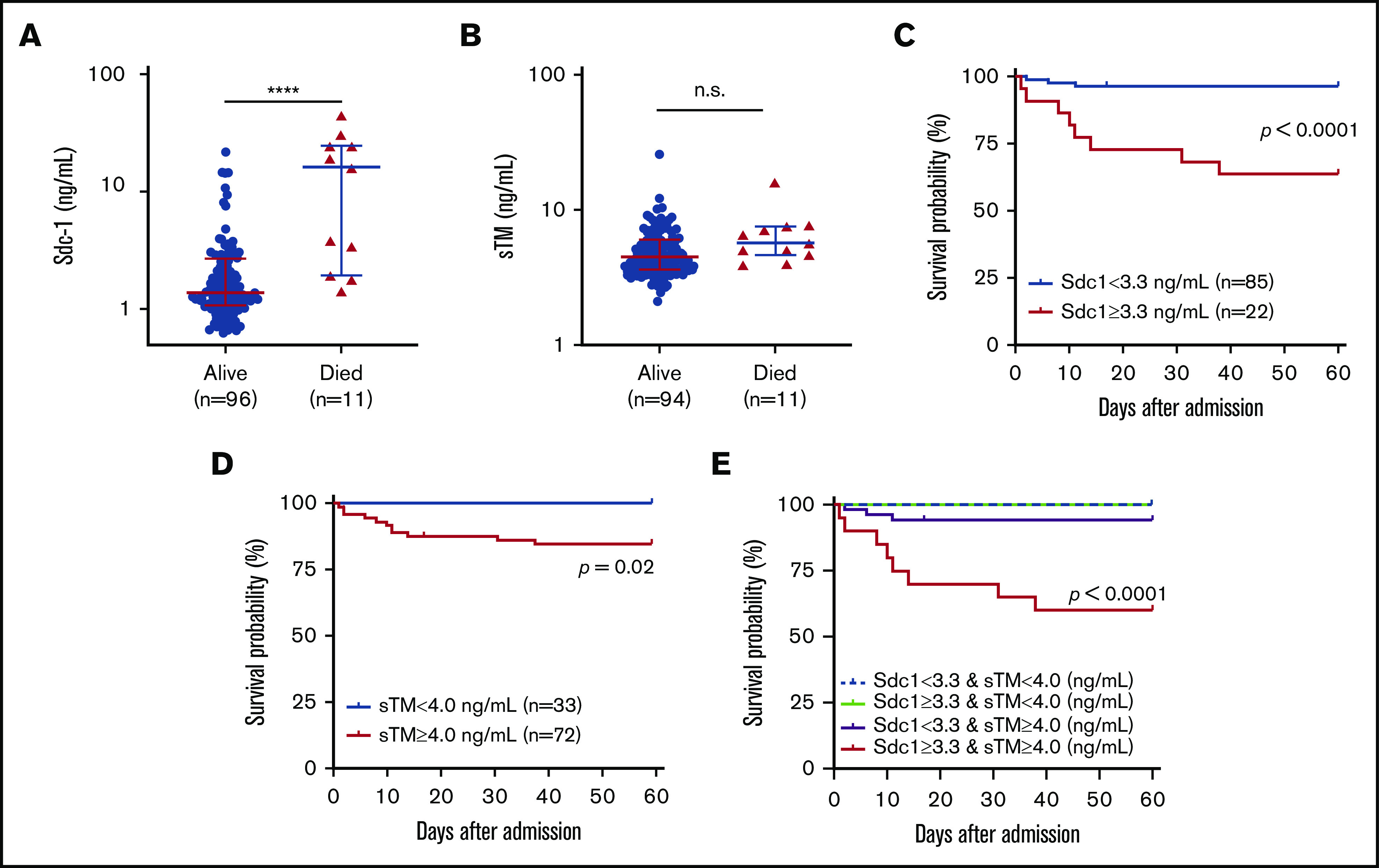
Elevated plasma levels of Sdc-1, sTM, or both are associated with mortality. Plasma levels of Sdc-1 (A), but not sTM (B), are higher in patients who died than in those who survived. Kaplan-Meier survival analyses in patients with Sdc-1 <3.3 ng/mL vs Sdc-1 ≥3.3 ng/mL (C) or sTM <4.0 ng/mL vs ≥4.0 ng/mL (D) or with a double increase in Sdc-1 (≥3.3 ng/mL) and sTM (≥4.0 ng/mL) vs a single change in either Sdc-1 or sTM in the same direction or the opposite direction (E). A Mantel-Cox test was performed to determine statistical significance. Values of P < .05 and P < .01 are considered to be statistically significant and highly significant, respectively. ****P < .0001.
Using the receiver operating characteristic curve, we were able to identify optimal cutoffs of 3.3 ng/mL for Sdc-1 and 4.0 ng/mL for sTM for predicting the probability of death. Kaplan-Meier survival analysis demonstrated that patients with elevated plasma levels of Sdc-1 (≥3.3 ng/mL) (P < .0001) (Figure 5C) or sTM (≥4.0 ng/mL) (P = .020) (Figure 5D) had a significantly lower 60-day survival probability than those with values below the cutoff for Sdc-1 or sTM. When combined, plasma levels of Sdc-1 and sTM elevated above the cutoffs were much a better predictor of 60-day mortality (P < .0001) (Figure 5E). These results demonstrate for the first time that elevated plasma levels of Sdc-1 or sTM, alone or in combination, may be predictive of an adverse acute iTTP outcome.
Increased plasma levels of both Sdc-1 and sTM in clinical remission predict disease recurrence
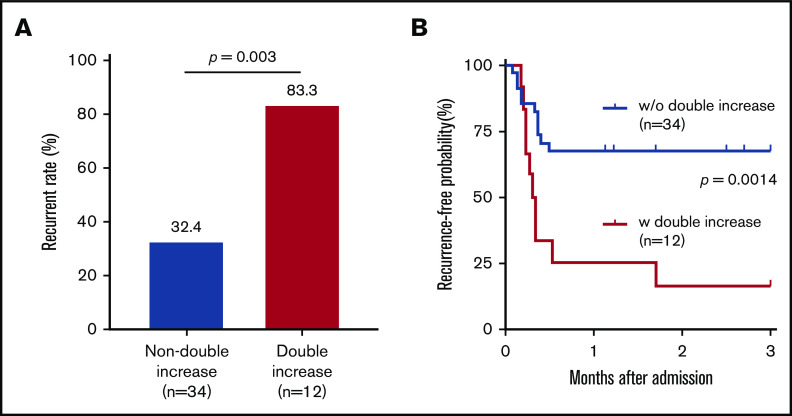
Plasma levels of Sdc-1 and/or sTM decreased in some, but not all, patients during clinical remission. In fact, some patients exhibited increased levels of plasma Sdc-1 or sTM or both when compared with their initial levels on admission. The clinical significance of such a dynamic change in iTTP patients is not known. As shown, 34 of 46 patients (73.9%) had lower plasma levels of either Sdc-1 or sTM, while 12 of 46 patients (26.1%) had higher plasma levels of both Sdc-1 and sTM during clinical remission. Interesting, those with increases in both Sdc-1 and sTM levels at clinical remission had a much higher rate of disease recurrence (either exacerbation or relapse) at 3 months (83.3%) than those without the double increase in these 2 markers (32.4%) (Figure 6A) (P = .003). These results were consistent with those obtained with the Kaplan-Meier survival analysis with the Mantel-Cox test (Figure 6B) (P = .0014). Together, our results demonstrate for the first time that persistent endotheliopathy is a risk factor for iTTP exacerbation and/or relapse.
Figure 6.
Plasma levels of Sdc-1 or sTM in clinical response/remission are associated with disease recurrence in iTTP. (A) Bar graph shows the disease recurrence rate at 3 months after admission in patients with a double increase vs no double increase in plasma Sdc-1 and sTM during clinical response or remission as compared with their admission values. (B) Kaplan-Meir survival analysis demonstrates the recurrence-free probabilities (%) in patients with a double increase in both Sdc-1 and sTM vs those with no double increase in both Sdc-1 and sTM (P = .0014). A Mantel-Cox test was performed to compare the 2 different groups (2 tailed and nonparametric). P > .05 (not significant); P < .05 and P < .01 are considered statistically significant and highly significant, respectively.
Discussion
The present study demonstrates that plasma levels of Sdc-1 and/or sTM are dramatically increased in patients with acute iTTP on admission and remain elevated during clinical response/remission. Furthermore, the admission plasma levels of Sdc-1 and sTM appear to be associated with many routine biomarkers indictive of organ damage and severity of the disease. These include GSC, eGFR, LDH, coagulopathy, ICU admission, and intubation. Consistent with what is in the literature, the episodic mortality rate in our cohort is ∼10%. Interestingly, plasma levels of Sdc-1 and/or sTM on admission are associated with mortality in patients with acute disease. Most importantly, a simultaneous increase in plasma levels of Sdc-1 and sTM on admission is more predictive than a single elevation of either Sdc-1 or sTM for in-hospital acute iTTP mortality.
Once clinical response or remission (ie, normalization of platelet counts and LDH) is achieved, 40% to 50% of patients may experience exacerbation or relapse if not on caplacizumab.2 Laboratory markers predicting iTTP recurrence after clinical remission include low ADAMTS13 activity and antigen and the presence of anti-ADAMTS13 antibodies.56 In this study, we demonstrate that plasma levels of Sdc-1 and/or sTM remain substantially elevated or higher in some patients during clinical remission. A simultaneous increase in plasma Sdc-1 and sTM during clinical response/remission is significantly associated with an increased risk of disease recurrence (ie, exacerbation or relapse) at the 3-month follow-up. These results suggest the persistence of microvascular endothelial damage (ie, endotheliopathy) after achieving clinical response/remission.
Severe deficiency of plasma ADAMTS13 activity is necessary, but may not be sufficient, to cause acute iTTP. Infection or systemic inflammation often precedes an acute episode of iTTP. Studies have demonstrated that Sdc-1 and sTM are involved in the inflammatory process. For example, leukocyte-endothelium adhesion in postcapillary venules is an essential step in the inflammatory process. Alterations in the glycocalyx attendant to endothelial cell activation may affect leukocyte rolling and adhesion to endothelial surface. Plasma Sdc-1 levels may reflect the endothelial damage that results from neutrophil activation, neutrophil gelatinase-associated lipocalin, resistin, and myeloperoxidase.44 MMP-7 also cleaves the juxtamembrane domain of Sdc-1, liberating an intact ectodomain.57 CXCL1, an acute-phase neutrophil chemokine, has neutrophil chemoattractant activity.58 It is bound to the glycosaminoglycan chains of shed Sdc-1, where it supports neutrophil adhesion, but not activation. When Sdc-1 is cleaved, the released Sdc-1/CXCL1 complexes promote neutrophil activation.59 It was reported that the lectin‐like domain of TM is involved in the suppression of inflammation via multiple mechanisms, which include the suppression of leukocyte adhesion to endothelial cells,60 interference with complement activation,32,61 inactivation of proinflammatory nuclear proteins (eg, high mobility group box 1 and histones),62 and inactivation of bacterial endotoxin.63 Our findings may not only provide a tool for risk stratification but also provide a molecular basis for therapeutic intervention. A previous study has shown that an infusion of tissue matrix metalloprotease inhibitors, such as doxycycline or the zinc chelator GM6001, suppresses Sdc-1 shedding, thus reducing the rolling velocity of leukocytes.64
In summary, we have for the first time demonstrated that elevated plasma levels of Sdc-1 and sTM in patients with acute iTTP on admission predict disease severity and in-hospital mortality; a simultaneous increase in both plasma Sdc-1 and sTM during clinical response/remission is associated with an increased rate of recurrence of acute iTTP. Our findings demonstrate the persistent endotheliopathy in acute iTTP, which may guide us when learning how to best treat and monitor acute iTTP to reduce mortality and morbidity rates.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the University of Alabama at Birmingham pathology physicians, residents, fellows, apheresis nurses, and medical technologists in the coagulation laboratory for their assistance in obtaining informed consent, blood samples, sample preparation, and storage.
This study was supported, in part, by the National Institutes of Health/National Heart, Lung, and Blood Institute (HL126724) and the Answering TTP Foundation.
Footnotes
Send data sharing requests via e-mail to the corresponding author, X. Long Zheng (longzheng01@gmail.com).
Authorship
Contribution: R.L., J.S., and X.L.Z. designed the research, performed experiments, analyzed the results, and wrote the manuscript; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: X.L.Z. is a speaker and/or consultant for Alexion, Sanofi, and Takeda and co-founder of Clotsolution. The remaining authors declare no competing financial interests.
Correspondence: X. Long Zheng, Department of Pathology and Laboratory Medicine, The University of Kansas School of Medicine, 5016 Delp, MS 3045, Kansas City, KS 66160; e-mail. xzheng2@kumc.edu or longzheng01@gmail.com.
References
- 1.Terrell DR, Williams LA, Vesely SK, Lämmle B, Hovinga JA, George JN. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost. 2005;3(7):1432-1436. [DOI] [PubMed] [Google Scholar]
- 2.Staley EM, Cao W, Pham HP, et al. Clinical factors and biomarkers predict outcome in patients with immune-mediated thrombotic thrombocytopenic purpura. Haematologica. 2019;104(1):166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng XL, Wu HM, Shang D, et al. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010;95(9):1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M, Cataland S, Coppo P, et al. ; International Working Group for Thrombotic Thrombocytopenic Purpura . Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. [DOI] [PubMed] [Google Scholar]
- 6.Saha M, McDaniel JK, Zheng XL. Thrombotic thrombocytopenic purpura: pathogenesis, diagnosis and potential novel therapeutics. J Thromb Haemost. 2017;15(10):1889-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488-494. [DOI] [PubMed] [Google Scholar]
- 8.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325(6):398-403. [DOI] [PubMed] [Google Scholar]
- 9.Rock GA, Shumak KH, Buskard NA, et al. ; Canadian Apheresis Study Group . Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(6):393-397. [DOI] [PubMed] [Google Scholar]
- 10.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103(11):4043-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblom A, Thorsen S, Hillarp A, Björk P. Minor stroke as singular manifestation of hereditary thrombotic thrombocytopenic purpura in a young man. Int Angiol. 2009;28(4):336-339. [PubMed] [Google Scholar]
- 13.Aksay E, Kiyan S, Ersel M, Hudaverdi O. Thrombotic thrombocytopenic purpura mimicking acute ischemic stroke. Emerg Med J. 2006;23(9):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazelton J, Oster RA, McCleskey B, Fuller J, Adamski J, Marques MB. Increased troponin I is associated with fatal outcome in acquired thrombotic thrombocytopenic purpura. J Clin Apher. 2017;32(5):311-318. [DOI] [PubMed] [Google Scholar]
- 15.George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116(20):4060-4069. [DOI] [PubMed] [Google Scholar]
- 16.Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: toward targeted therapy and precision medicine. Res Pract Thromb Haemost. 2018;3(1):26-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng XL, Vesely SK, Cataland SR, et al. Good practice statements (GPS) for the clinical care of patients with thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2503-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500-1511, quiz 1662. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramaniyam N, Kolte D, Palaniswamy C, et al. Predictors of in-hospital mortality and acute myocardial infarction in thrombotic thrombocytopenic purpura. Am J Med. 2013;126(11):1016.e1011-1017. [DOI] [PubMed] [Google Scholar]
- 20.Goel R, King KE, Takemoto CM, Ness PM, Tobian AA. Prognostic risk-stratified score for predicting mortality in hospitalized patients with thrombotic thrombocytopenic purpura: nationally representative data from 2007 to 2012. Transfusion. 2016;56(6):1451-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhamou Y, Assié C, Boelle PY, et al. ; Thrombotic Microangiopathies Reference Center . Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Haematologica. 2012;97(8):1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benhamou Y, Boelle PY, Baudin B, et al. ; Experience of the French Thrombotic Microangiopathies Reference Center . Cardiac troponin-I on diagnosis predicts early death and refractoriness in acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2015;13(2):293-302. [DOI] [PubMed] [Google Scholar]
- 23.Alwan F, Vendramin C, Vanhoorelbeke K, et al. Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2017;130(4):466-471. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Jin M, Lin S, Cataland S, Wu H. ADAMTS13 activity and antigen during therapy and follow-up of patients with idiopathic thrombotic thrombocytopenic purpura: correlation with clinical outcome. Haematologica. 2011;96(10):1521-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondelaud F, Ricard-Blum S. Structures and interactions of syndecans. FEBS J. 2019;286(15):2994-3007. [DOI] [PubMed] [Google Scholar]
- 26.Kainulainen V, Nelimarkka L, Järveläinen H, Laato M, Jalkanen M, Elenius K. Suppression of syndecan-1 expression in endothelial cells by tumor necrosis factor-alpha. J Biol Chem. 1996;271(31):18759-18766. [DOI] [PubMed] [Google Scholar]
- 27.Sadler JE. Thrombomodulin structure and function. Thromb Haemost. 1997;78(1):392-395. [PubMed] [Google Scholar]
- 28.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440(5):653-666. [DOI] [PubMed] [Google Scholar]
- 29.Fuentes-Prior P, Iwanaga Y, Huber R, et al. Structural basis for the anticoagulant activity of the thrombin-thrombomodulin complex. Nature. 2000;404(6777):518-525. [DOI] [PubMed] [Google Scholar]
- 30.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34(1):107-125. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Kusumoto H, Deyashiki Y, et al. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987;6(7):1891-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(4):345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286(5):H1672-H1680. [DOI] [PubMed] [Google Scholar]
- 34.Pruessmeyer J, Martin C, Hess FM, et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285(1):555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99(4):1471-1476. [DOI] [PubMed] [Google Scholar]
- 36.Diebel LN, Diebel ME, Martin JV, Liberati DM. Acute hyperglycemia exacerbates trauma-induced endothelial and glycocalyx injury: an in vitro model. J Trauma Acute Care Surg. 2018;85(5):960-967. [DOI] [PubMed] [Google Scholar]
- 37.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114(14):3033-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez Rodriguez E, Cardenas JC, Cox CS, et al. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med. 2018;26(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svennevig K, Hoel T, Thiara A, et al. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23(3):165-171. [DOI] [PubMed] [Google Scholar]
- 40.Li D, Wu Y, Guo S, et al. Circulating syndecan-1 as a novel biomarker relates to lung function, systemic inflammation, and exacerbation in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1933-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry-Stanley MJ, Zhang B, Erlandsen SL, Wells CL. Synergistic effect of tumor necrosis factor-alpha and interferon-gamma on enterocyte shedding of syndecan-1 and associated decreases in internalization of Listeria monocytogenes and Staphylococcus aureus. Cytokine. 2006;34(5-6):252-259. [DOI] [PubMed] [Google Scholar]
- 42.Boehme MW, Deng Y, Raeth U, et al. Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: in vivo and in vitro studies. Immunology. 1996;87(1):134-140. [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108(3):347. [DOI] [PubMed] [Google Scholar]
- 44.Smart L, Bosio E, Macdonald SPJ, et al. Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J Crit Care. 2018;47:93-98. [DOI] [PubMed] [Google Scholar]
- 45.Kozar RA, Pati S. Syndecan-1 restitution by plasma after hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(6Suppl 1):S83-S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda M, Matsumoto H, Ogura H, et al. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J Crit Care. 2018;43:48-53. [DOI] [PubMed] [Google Scholar]
- 47.Puskarich MA, Cornelius DC, Tharp J, Nandi U, Jones AE. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J Crit Care. 2016;36:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takano S, Kimura S, Ohdama S, Aoki N. Plasma thrombomodulin in health and diseases. Blood. 1990;76(10):2024-2029. [PubMed] [Google Scholar]
- 49.Gando S, Nakanishi Y, Kameue T, Nanzaki S. Soluble thrombomodulin increases in patients with disseminated intravascular coagulation and in those with multiple organ dysfunction syndrome after trauma: role of neutrophil elastase. J Trauma. 1995;39(4):660-664. [DOI] [PubMed] [Google Scholar]
- 50.Ikegami K, Suzuki Y, Yukioka T, Matsuda H, Shimazaki S. Endothelial cell injury, as quantified by the soluble thrombomodulin level, predicts sepsis/multiple organ dysfunction syndrome after blunt trauma. J Trauma. 1998;44(5):789-794, NaN-795. [DOI] [PubMed] [Google Scholar]
- 51.Lin SM, Wang YM, Lin HC, et al. Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med. 2008;36(3):683-689. [DOI] [PubMed] [Google Scholar]
- 52.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81-84. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Lawson HL, Harish VC, Huff JD, Knovich MA, Owen J. Creation of a recombinant peptide substrate for fluorescence resonance energy transfer-based protease assays. Anal Biochem. 2006;358(2):298-300. [DOI] [PubMed] [Google Scholar]
- 54.Raife TJ, Cao W, Atkinson BS, et al. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009;114(8):1666-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savery MD, Jiang JX, Park PW, Damiano ER. The endothelial glycocalyx in syndecan-1 deficient mice. Microvasc Res. 2013;87:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sui J, Cao W, Halkidis K, et al. Longitudinal assessments of plasma ADAMTS13 biomarkers predict recurrence of immune thrombotic thrombocytopenic purpura. Blood Adv. 2019;3(24):4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635-646. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher C, Clark-Lewis I, Baggiolini M, Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci USA. 1992;89(21):10542-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill SE, Nadler ST, Li Q, et al. Shedding of syndecan-1/CXCL1 complexes by matrix metalloproteinase 7 functions as an epithelial checkpoint of neutrophil activation. Am J Respir Cell Mol Biol. 2016;55(2):243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conway EM, Van de Wouwer M, Pollefeyt S, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196(5):565-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van de Wouwer M, Plaisance S, De Vriese A, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4(8):1813-1824. [DOI] [PubMed] [Google Scholar]
- 62.Ito T, Kakihana Y, Maruyama I. Thrombomodulin as an intravascular safeguard against inflammatory and thrombotic diseases. Expert Opin Ther Targets. 2016;20(2):151-158. [DOI] [PubMed] [Google Scholar]
- 63.Shi CS, Shi GY, Hsiao HM, et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008;112(9):3661-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu Z, Song N, Shen B, et al. Syndecan-1 shedding inhibition to protect against ischemic acute kidney injury through HGF target signaling pathway. Transplantation. 2018;102(7):e331-e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.