Key Points
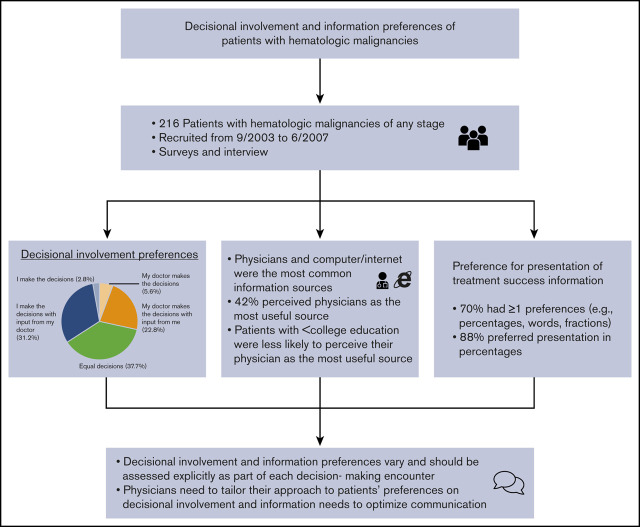
Decisional involvement and information preferences vary among patients and should be assessed as part of decision-making encounters.
Physicians need to tailor their approach to patients’ preferences on decisional involvement and information needs to optimize communication.
Abstract
Understanding decisional involvement and information preferences in patients with hematologic malignancies may help to optimize physician-patient communication about treatment decisions and align the decision-making processes with patients’ preferences. We described and examined factors associated with preferences of patients with hematologic malignancies for decisional involvement, information sources, and presentation of information. In a multicenter observational study, we recruited 216 patients with hematologic malignancies of any stage from September 2003 to June 2007. Patients were asked about their decisional involvement preferences (Control Preferences Scale), information sources (including most useful source of information), and preferences for their oncologists’ presentation of treatment success information. We used multivariate logistic regressions to identify factors associated with decisional involvement preferences and usefulness of information sources (physicians vs nonphysicians). Patient-directed, shared, and physician-directed approaches were preferred in 34%, 38%, and 28% of patients, respectively. Physicians and computer/Internet were the most common information sources; 42% perceived physicians as the most useful source. On multivariate analysis, patients with less than a college education (vs postgraduate education) were less likely to perceive their physician as the most useful source (adjusted odds ratio [AOR], 0.46; 95% confidence interval (CI), 0.21-1.00), whereas patients with acute leukemia (vs other blood cancers) were more likely to perceive their physician as the most useful source (AOR, 2.49; 95% CI, 1.07-5.80). In terms of communicating treatment success rates, 70% preferred ≥1 method(s), and 88% preferred presentation in percentages. Our study suggests that decisional involvement and information preferences vary and should be assessed explicitly as part of each decision-making encounter.
Visual Abstract
Introduction
Treatment options for hematologic malignancies have dramatically increased in the past decade. Targeted therapies, bispecific antibody-based therapy, and chimeric antigen receptor (CAR) T-cell therapy are now options in addition to chemotherapy, radiotherapy, and hematopoietic cell transplantation. Although the availability of more treatment options is desirable, patients may be overwhelmed by the increasingly complicated and pressured treatment decision-making process.1 Previous work in solid tumor populations has shown variability in patients’ preferences for receiving information and making decisions, but less is known about how people with hematologic malignancies educate themselves and choose among available treatments, especially given the acuity of symptoms and diagnostic workup associated with hematologic malignancies compared with solid tumors.
There are 3 main preferences for decisional involvement: physician directed (physician makes decisions), shared (physician and patient make decisions together equally), and patient directed (patient makes decisions), ranging from nonparticipation of the patient to a high level of patient autonomy.2,3 Shared decision making has been the preference of a majority of patients with hematologic malignancies, although they perceived that the degree of information they received was insufficient to facilitate shared decision making.4 To help patients engage in decision making, their information needs and preferences should be assessed and addressed.5 In studies of patients with solid malignancies, most preferred to receive all possible information, both good and bad news, including information about available treatments and prognosis.6 Patients sought information about their disease, further diagnostic workup and management, side effects, effects on sexuality, supportive care, and financial concerns.7 Patients also inquired about diets, complementary therapies, prevention of relapse, adverse effects, and alternative treatment options.8 However, they reported inadequate access to several information sources.7 It is unclear whether patients with hematologic malignancies have similar information needs.
In this study, we investigated decisional involvement and information preferences of patients with hematologic malignancies. In addition, we examined factors associated with decisional involvement preference and usefulness of information sources. Our study findings will help to optimize physician-patient communication about treatment decisions and align the decision-making process with patients’ preferences.
Methods
Study design, setting, and participants
This was a secondary analysis of a multicenter study conducted at 4 academic cancer centers (Dana-Farber Cancer Institute [Boston, MA], Massachusetts General Hospital, Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance [Seattle, WA], and Massey Cancer Center [Richmond, VA]). The parent study focused on physician-patient communication among patients with newly or previously diagnosed hematologic malignancies who were referred for hematology consultation. We have previously described the study methods in detail.9-11 The parent study included patients aged ≥18 years with hematologic malignancies who were able to communicate in English and were referred to the above centers for consultation from September 2003 to June 2007. All participants provided written informed consent. All sites obtained approval from their Institutional Review Board. At enrollment, patients completed surveys prior to their clinical encounter with the hematologist. They were interviewed 0 to 7 days prior to their consultations based on a structured interview guide.
Survey
Comorbidity.
We compiled patients’ self-reported comorbidities into the Charlson Comorbidity Index (CCI).12 This index has been tested for its ability to predict the risk of death from comorbid disease.13
Preferences for decisional involvement.
We asked patients about their preferences for decisional involvement based on the Control Preferences Scale, which is a validated measure to assess “the degree of control an individual wants to assume when decisions are being made about medical treatment.” The Control Preferences Scale consists of 5 statements that describe different roles in medical decision making.14 Specifically, they were asked to select 1 of the following statements that best describes their point of view about decision making: (1) “I prefer to leave decisions about my medical care and treatment up to my doctor,” (2) “I prefer to have my doctor make the decisions with significant input from me,” (3) “I prefer to share equally in decisions about my medical care with my doctor,” (4) “I prefer to make the decisions with significant input from my doctor,” and (5) “I prefer to be the one making decisions about my medical care.”
Interview
Demographics and disease information.
We obtained patients’ demographics and disease information during the structured interview. These data included age, sex, race, marital status, education, work status, annual household income in US dollars, type of hematologic malignancy, social support, whether they had received treatment for their disease in the past, and whether they were receiving treatment or about to undergo treatment at the time of the clinic encounter. Social support was measured using the Medical Outcomes Study Social Support survey, which has 19 items. The total score ranges from 19 to 95, with a higher score indicating greater social support.15
Information sources.
Patients were asked to identify all applicable information sources that they used to learn about their disease and treatment options. Sources included physician(s), nurse(s), other health care professional(s), family/friends, patient support groups, other patients, books/pamphlets/videotape, newspaper/TV/magazines, computer/Internet/world wide Web, or other. In addition, they were asked to choose the most useful source of information.
Preferences for types of information.
Patients were read a list of 11 topics that they might want to discuss during the consultation: treatment options, treatment goals (eg, cure disease or prolong life), impact of disease and treatment on lifestyle, things you can do to help your recovery, physician recommendation for treatment, likelihood of cure, average survival for patients with this disease, clinical trials testing new drugs, complementary and alternative medicines, likelihood of treatment success, and How are you doing emotionally with this disease? Patients were allowed to choose >1 topic.
Preference for presentation of treatment success information by their oncologists.
Patients were asked to select their preference for how their oncologist presents information about their treatment success rates: percentages (eg, 80%), fractions (eg, 8 of 10 people), words (eg, “most”), and stories about previous patients the doctor has treated. Participants were allowed to select >1 preference. The responses were not conveyed to the physician.
Statistical analyses
We used descriptive statistics to summarize our sample, decision-involvement preferences (physician directed vs patient directed or equal participation), information sources, and information preferences. We used multivariate logistic regression to determine factors (age [continuous], sex [male vs female], race [White vs nonwhite], marital status [married vs nonmarried], education [less than college graduate vs college graduate vs postgraduate], annual household income [≥100 000 vs <100 000], CCI [continuous], type of hematologic malignancy [acute leukemia vs lymphoma vs other], and social support [continuous]) associated with decisional-involvement preference and most useful information source.
For exploratory analyses, we conducted bivariate analyses to assess differences in information sources and preferences for presentation of treatment success information by their oncologists by age groups (age <60 years vs ≥60 years; we selected 60 years, because this is a common cutoff for the definition of “older” in studies of hematologic malignancies).16-18 We also conducted bivariate analyses to assess for differences in information sources by education levels.
All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
We included 216 patients; 35.7% (77/216) were aged ≥60 years. More than half (55.1%) were male, and most were White (92.0%; 196/216) and married (76.3%; 164/216) (Table 1). Mean CCI, excluding hematologic malignancy, was 0.4 (standard deviation, 0.9). The most common cancer type was lymphoma (39.4%; 26.9% non-Hodgkin lymphoma, 7.4% chronic lymphocytic leukemia/small lymphocytic lymphoma, and 5.1% Hodgkin lymphoma). Lymphoma was followed by myelodysplastic syndrome (19.0%), acute leukemia (18.5%; 14.4% acute myeloid leukemia and 4.2% acute lymphoblastic leukemia), multiple myeloma (13.0%), chronic myeloid leukemia (7.9%), and other (2.3%). Forty-three percent (93/214) had received some form of treatment for their disease, and 46.2% (99/214) were receiving treatment or about to undergo treatment at the time of the clinic encounter.
Table 1.
Participant demographics (N = 216)
| Variables | All patients |
|---|---|
| Age, mean (SD), y | 53.7 (11.4) |
| Age, y | |
| <60 | 77 (35.7) |
| ≥60 | 139 (64.3) |
| Sex | |
| Male | 119 (55.1) |
| Female | 97 (44.9) |
| Race * | |
| White | 196 (92.0) |
| Nonwhite | 17 (8.0) |
| Marital status † | |
| Married | 164 (76.3) |
| Nonmarried | 51 (23.7) |
| Working † | |
| Yes | 107 (49.8) |
| No | 108 (50.2) |
| Education † | |
| Less than college graduate | 89 (41.4) |
| College graduate | 64 (29.8) |
| Postgraduate | 62 (28.8) |
| Annual household income ‡ | |
| ≥$100 000 | 82 (41.4) |
| >$100 000 | 116 (58.6) |
| CCI, mean (SD)§ | 0.4 (0.9) |
| Cancer type | |
| Acute leukemia | 40 (18.5) |
| Lymphoma | 85 (39.4) |
| Other | 91 (42.1) |
| MOS Social Support, mean (SD)‡ | 81.2 (13.8) |
Unless otherwise noted, data are n (%).
MOS, Medical Outcomes Study.
Three patients had missing data.
One patient had missing data.
Eighteen patients had missing data.
Excluding a history of a hematologic malignancy.
Preferences for decisional involvement
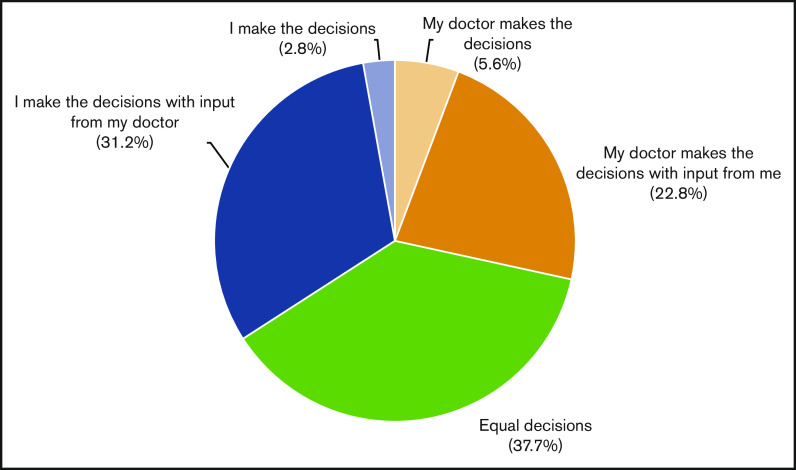
Preferences for decisional involvement varied (Figure 1). One third (34.0%; 73/215) of participants preferred a patient-directed approach, in which they were primarily responsible for treatment decisions. Equal participation was preferred in 37.7% (81/215), in which patients share equal responsibility with their physicians for treatment decisions. The remaining 28.3% (61/215) preferred a physician-directed approach, in which the physician assumes greater responsibility than the patient for making decisions. Of note, very few patients preferred to make decisions without input from their doctor (3%) or wanted their doctor to make decisions without their input (6%). On multivariate analysis, age, sex, race, marital status, education, annual household income, comorbidity, type of hematologic malignancy, and social support were not associated with decisional-involvement preferences.
Figure 1.
Decisional involvement preferences.
Information sources.
Common sources from which patients obtained information on disease or treatment options included their physician (97.7%; 208/213), computer/Internet (87.3%; 186/213), print and broadcast media (70.9%; 151/213), and family/friends/other patients/patient support group (78.4%; 167/213) (Table 2). There was no difference between the older and younger age groups, with the exception of their use of the computer/Internet. Although a high percentage of both age groups used the computer/Internet, utilization was higher among adults aged <60 years compared with those aged ≥60 years (91.2% vs 80.5%; P = .02). Use of the computer/Internet was high across the different education levels, but it was statistically lower in those with lower education levels (80.9% vs 88.9% vs 95.1% for less than college graduate vs college graduate vs postgraduate, respectively; P = .03).
Table 2.
Patient information sources
| Patient information sources | All (N = 213)* | Αge ≥60 y (n = 77) | Age <60 y (n = 136) | P | Less than college graduate (n = 89) | College graduate (n = 63) | Postgraduate (n = 61) | P | Most useful information source (N = 213)† |
|---|---|---|---|---|---|---|---|---|---|
| Physicians | 208 (97.7) | 76 (98.7) | 132 (97.1) | .66 | 86 (96.6) | 61 (96.8) | 61 (100.0) | .52 | 88 (41.3) |
| Computer/internet | 186 (87.3) | 62 (80.5) | 124 (91.2) | .02 | 72 (80.9) | 56 (88.9) | 58 (95.1) | .03 | 64 (30.0) |
| Family, friends, other patients, patient support group | 167 (78.4) | 61 (79.2) | 106 (77.9) | .83 | 65 (73.0) | 52 (82.5) | 50 (82.0) | .31 | 16 (7.5) |
| Print and broadcast media (eg, book, pamphlets, news, TV) | 151 (70.9) | 54 (70.1) | 97 (71.3) | .85 | 64 (71.9) | 43 (68.3) | 44 (72.1) | .88 | 20 (9.4) |
| Nurse and other professionals | 147 (69.0) | 48 (62.3) | 99 (72.3) | .11 | 58 (65.2) | 42 (66.7) | 47 (77.1) | .27 | 9 (4.2) |
With the exception of the P values, all data are n (%).
Three patients had missing data
Sixteen patients chose ≥2 sources as being the most useful information source.
Nearly half (41.9%; 88/210) perceived the physician as the most useful information source, followed by the computer/Internet (30.5%; 64/210), and print and broadcast media (9.5%; 20/210). On multivariate analysis, patients with less than a college education (compared with those with a postgraduate education) were less likely to perceive their physician as the most useful source (adjusted odds ratio [AOR], 0.46; 95% confidence interval [CI], 0.21-1.00; P = .05). Patients with acute leukemia (vs other blood cancer) were more likely to perceive their physician as the most useful source (AOR, 2.49; 95% CI, 1.07-5.80; P = .03) (Table 3). Patients with higher CCI (AOR, 0.61; 95% CI; 0.41-0.92; P = .02) and acute leukemia (vs other blood cancer: AOR, 0.26; 95% CI, 0.10-0.74; P = .01) were less likely to perceive the Internet as the most useful source. None of these factors were associated with the perception of family, friends, other patients, and patient support groups being the most useful source.
Table 3.
Multivariate analysis evaluating factors associated with usefulness of information sources (N = 190)
| Variables | Physician is most useful source | Internet is most useful source | Family/friends/other patients/patient support group is most useful source |
|---|---|---|---|
| Age* | 0.99 (0.96-1.02); .51 | 1.02 (0.99-1.05); .31 | 0.99 (0.94-1.05); .82 |
| Sex | |||
| Male | Ref | Ref | Ref |
| Female | 0.84 (0.45-1.57); .58 | 1.07 (0.55-2.09); .83 | 2.30 (0.60-8.78); .22 |
| Race | |||
| White | Ref | Ref | Ref |
| Nonwhite | 0.50 (0.14-1.86); .30 | 0.80 (0.22-2.92); .73 | 1.00 (0.97-1.02); .81 |
| Marital status | |||
| Married | Ref | Ref | Ref |
| Nonmarried | 0.65 (0.30-1.39); .27 | 1.38 (0.62-3.05); .43 | 1.60 (0.39-6.67); .52 |
| Education | |||
| Postgraduate | Ref; .11† | Ref; .51† | Ref; .34† |
| College graduate | 0.85 (0.38-1.90); .68 | 1.05 (0.43-2.60); .91 | 3.94 (0.40-39.20); .24 |
| Less than college graduate | 0.46 (0.21-0.999); .0496 | 1.53 (0.67-3.48); .31 | 5.32 (0.56-50.26); .14 |
| Annual household income | |||
| ≥$100 000 | Ref | Ref | Ref |
| <$100 000 | 1.23 (0.63-2.39); .55 | 0.71 (0.35-1.43); .33 | 0.60 (0.15-2.41); .48 |
| CCI‡ | 1.20 (0.89-1.62); .24 | 0.61 (0.41-0.92); .02 | 1.09 (0.59-2.01); .76 |
| Cancer type | |||
| Other | Ref; .08† | Ref; .03† | Ref; .56† |
| Acute leukemia | 2.49 (1.07-5.80); .03 | 0.26 (0.10-0.74); .01 | 2.56 (0.46-14.15); .28 |
| Lymphoma | 1.75 (0.88-3.49); .11 | 0.57 (0.28-1.16); .12 | 1.48 (0.33-1.59); .60 |
| MOS social support§ | 1.02 (0.99-1.05); .14 | 1.00 (0.98-1.03); .57 | 0.97 (0.94-1.02); .22 |
Data are AOR (95% CI); P value.
Ref, reference.
One unit increase in number of years.
Overall P value.
One unit increase in CCI.
One unit increase in the MOS Social Support survey.
Preferences for types of information
More than 90% of participants indicated that they would like to discuss each of the following disease and treatment issues: options, goals, impact of disease and treatment on lifestyle, likelihood of treatment success, and average survival. Similarly, >90% of participants were interested in discussing physician recommendations for treatment, things they can do to help with recovery, and emotional response to the disease. Almost 80% (169/216) were interested in discussing clinical trials, 74.1% (160/216) would like to discuss complementary and alternative medicines, and 51.9% (112/216) indicated additional topics they would like to discuss with their oncologist (eg, questions related to bone marrow transplantation, cost and insurance, logistics, living situation, symptoms, novel treatment options, confirmation of diagnosis, and side effects).
Preference for presentation of treatment success information by their oncologists
When asked about their preferences for information presentation about treatment success rates, 70.4% (150/216) had ≥1 preference. Most participants (88%; 190/216) preferred presentation in percentages, 59% (127/216) wanted to hear about a previous patient whom the physician had treated, 37% (80/216) preferred presentation in words, and 30% preferred presentation in fractions (64/216). Compared with adults aged <60 years, those aged ≥60 years were less likely to prefer presentation in percentages (82% vs 91%; P = .04).
Discussion
We found considerable heterogeneity in patients’ preferences for decisional involvement and information needs in this multicenter study conducted in 4 academic medical centers. Almost all patients wanted their input to be considered, as well as their doctors’ input. Preferences for decisional involvement were roughly equally distributed: 37.7% of patients preferred equal participation in decision making, 28.4% preferred a physician-directed approach, and 34.0% preferred a patient-directed approach. Our results are generally similar to findings of a meta-analysis of 6 clinical studies including 3491 patients with primarily solid tumors, in which 34% preferred a shared approach, 36% preferred a physician-directed approach, and 30% preferred a patient-directed approach in decision making.19 However, none of these studies specifically included patients with hematologic malignancies (3 studies included only solid tumors, and the numbers of patients with hematologic malignancies were small and unspecified in the remaining 3 studies). A prior study in patients with hematologic malignancies demonstrated that they may have a weaker desire to participate in medical decisions compared with those with solid tumors.20 Although our study may suggest the opposite, findings emphasize the importance of inquiring about patients’ preferences for decisional involvement because of its heterogeneity so that oncologists can tailor information and decision making.
It is generally thought that patients need a clear understanding of their medical situation and prognosis to participate in shared decision making and to ensure that treatment choices are congruent with their values. In observational studies, treatment choice was associated with patient expectations for outcomes,21-23 suggesting that misperceptions about prognosis and the potential benefits of therapy could lead to treatments that would not be chosen if patients truly understood their medical situations. Complicating this, is that surveyed patients with hematologic malignancies have reported difficulty recalling information (28%), information overload (26%), insufficient opportunity for clarification (23%), and limited information about managing psychosocial symptoms (20%).24 Yet, routinely providing this information effectively during an initial consultation can be challenging. Discussions of complex and emotionally laden issues, such as chance of cure or median life expectancy, are difficult and should take place over time.25,26 Even when a patient is contemplating enrollment in a clinical trial or surgery, there is debate about the wisdom of complete disclosure to every patient.27-29
Discrepancies between patient- and physician-reported prognostic estimates are well documented in the literature, suggesting that conversation about cancer and treatment outcomes may not be adequate to meet the information needs of patients.30,31 Physicians may be expressing prognosis in words that may be interpreted differently from numbers by patients.32-34 Discrepancies in prognostic estimates are amplified in non-Hispanic White patients who have lower income and less social support.35 Although most patients in our sample preferred that treatment success information be presented in percentages, studies have shown that the average American layperson is not functionally numerate, described as the ability to interpret a graph with numeric data, and that lower education is associated with lower numeracy.36-38 Not accounting for numeracy may result in misperceptions about prognosis.38,39 One potential approach is the use of percentages along with other presentation formats (eg, fractions) that may improve transfer of knowledge. The use of the Teach-Back method may be helpful to gauge patient understanding.40
In our study, the majority of patients (>90%) wished to discuss many topics and had already accessed many sources of information. Our results are consistent with a review of 112 articles published over 23 years, in which the most frequent information needs were treatment related (38.1%), cancer specific (12.8%), and prognostic (10.8%).41 The most important information source was health care professionals (27.3%), followed by print materials (26.2%) and interpersonal (18.8%).41 However, the number of patients with hematologic malignancies included was unclear. The literature indicates that seeking out information about cancer is associated with younger age, higher education, and higher income.42 Patients with lower educational levels were less likely to seek out information sources.42-44 In our study, patients with lower educational levels did not view physicians as the most important information source. Our findings further emphasize the need to understand and tailor patient preferences into communication, specifically for people of variable levels of education. For example, health care systems and societies should ensure that consistent and reliable information is provided via various sources. These sources should be complementary to, rather than conflicting with, each other.
A high percentage of older (80.5%) and younger adults (91.2%) in our study used a computer/Internet to obtain information about their cancer and its treatment. Although the utilization was lower in the older age group, the percentage was still relatively high. Similarly, a high percentage (>80%) of patients in both age groups preferred information about treatment success rates to be presented in percentages, although this was lower in older adults. The age-related differences in information seeking and preferences are consistent with prior studies of older women with chronic illnesses and solid malignancies.45,46 Among women with breast cancer, those who were younger were more likely to use the Internet for researching health conditions.45 Older adults were also more likely to seek information from nonmedical sources, such as friends who may have had cancer themselves.46 Older patients were generally less interested than younger patients in knowing specific medical details, such as the medical name of their cancer, all available treatments and their side effects, and their chances for cure.6 However, both age groups were found to want as much information as possible.47 Compared with younger adults, older adults have lower electronic health literacy.48 Age, however, was not associated with decisional involvement preferences and usefulness of information sources on multivariate analyses. It is important to note that computer/Internet utilization in younger and older adults is likely higher now compared with when the study was conducted.
The strengths of our study are that it is a multicenter study, involves a large sample size, and consists of a heterogeneous group of patients with hematologic malignancies. Our study also fills a gap in research on decisional involvement preferences and information needs of patients with hematologic malignancies; however, it has several limitations. First, this study was conducted from 2003 to 2007, and decisional involvement and information preferences may have changed since then with the advent of additional therapeutic options, such as targeted therapies and immunotherapy. Second, the majority of our patients were White, had at least a college education, were married/partnered, and had high incomes. Therefore, our findings might not be generalizable to patients with different demographics. Third, patients were recruited from academic cancer centers and may not be reflective of those seen in community oncology practices. Regardless, given the limited inclusion of patients with hematologic malignancies in decision-making research to date, these findings are important. Fourth, we did not collect the amount of time that the patient had been treated by their oncologist. Finally, it is important to note that decisional involvement preferences represent a dynamic spectrum, and patients may change their preference depending on the decisions and stakes involved, as well as their medical and psychological challenges at the moment in time.
In conclusion, our study suggests that decisional involvement and information preferences vary among patients with hematologic malignancies, are not predictable based on age or other characteristics, and should be assessed periodically as part of decision-making encounters to ensure that patients’ needs are being met. For physicians who use a similar template to discuss treatment options with their patients, they may consider tailoring their approach to their patients’ preferences on decision-making and information needs. Interventions are needed to improve communication about preferences and trust between patients and their oncologists to tailor shared decision-making processes that respect patients’ decisional style preferences and individual information needs.
Acknowledgments
The authors thank Susan Rosenthal for editorial assistance.
This work was supported by National Institutes of Health, National Cancer Institute grant R01CA09848 (S.J.L.). K.P.L. is supported by National Institutes of Health, National Cancer Institute grant K99CA237744 and by a Wilmot Research Fellowship. A.E.-J. is a scholar in clinical research for the Lymphoma and Leukemia Society.
Footnotes
Data sharing requests should be sent to Stephanie J. Lee (sjlee@fredhutch.org).
Authorship
Contribution: K.P.L., T.W.L., A.E.-J., and S.J.L conceived and designed the study; K.P.L. performed the analyses; A.B. and S.J.L. acquired data; K.P.L. and M.T. wrote the manuscript; and all authors interpreted the data and critically revised the manuscript.
Conflict-of-interest disclosure: K.P.L. has served as a consultant for Seattle Genetics and Pfizer. During the past 24 months, T.W.L. has received personal fees/honoraria and/or has been a member of the speaker’s bureau for AbbVie, Agios, AstraZeneca, Amgen, Bristol Myers Squibb, Carevive Systems, Celgene, Daiichi-Sankyo, Flatiron, Helsinn, Heron, Medtronic, Otsuka, Pfizer, Seattle Genetics, UpToDate, and Welvie and has received grants and/or institutional research funding (to Duke University) from the American Cancer Society, AstraZeneca, Bristol Myers Squibb, Duke University, the National Institute of Nursing Research/National Institutes of Health, Jazz Pharmaceuticals, and Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Kah Poh Loh, James P. Wilmot Cancer Institute, University of Rochester Medical Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: kahpoh_loh@urmc.rochester.edu.
References
- 1.LeBlanc TW, Fish LJ, Bloom CT, et al. Patient experiences of acute myeloid leukemia: a qualitative study about diagnosis, illness understanding, and treatment decision-making. Psychooncology. 2017;26(12):2063-2068. [DOI] [PubMed] [Google Scholar]
- 2.Truglio-Londrigan M, Slyer JT, Singleton JK, Worral P. A qualitative systematic review of internal and external influences on shared decision-making in all health care settings. JBI Library Syst Rev. 2012;10(58):4633-4646. [DOI] [PubMed] [Google Scholar]
- 3.Moth EB, Kiely BE, Martin A, et al. Older adults’ preferred and perceived roles in decision-making about palliative chemotherapy, decision priorities and information preferences. J Geriatr Oncol. 2020;11(4):626-632. [DOI] [PubMed] [Google Scholar]
- 4.Rood JAJ, Nauta IH, Witte BI, et al. Shared decision-making and providing information among newly diagnosed patients with hematological malignancies and their informal caregivers: not “one-size-fits-all”. Psychooncology. 2017;26(12):2040-2047. [DOI] [PubMed] [Google Scholar]
- 5.Bol N, Linn AJ, Smets EMA, Verdam MGE, van Weert JCM. Tailored communication for older patients with cancer: using cluster analysis to identify patient profiles based on information needs. J Geriatr Oncol. 2020;11(6):944-950. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins V, Fallowfield L, Saul J. Information needs of patients with cancer: results from a large study in UK cancer centres. Br J Cancer. 2001;84(1):48-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua GP, Tan HK, Gandhi M. What information do cancer patients want and how well are their needs being met? Ecancermedicalscience. 2018;12:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua GP, Tan HK. A qualitative approach in determining the patient-centered information and supportive care needs of cancer patients in Singapore. BMJ Open. 2020;10(2):e034178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loberiza FR Jr., Swore-Fletcher BA, Block SD, et al. Coping styles, health status and advance care planning in patients with hematologic malignancies. Leuk Lymphoma. 2011;52(12):2342-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman RE, Sullivan A, Back AL, Alexander SC, Matsuyama RK, Lee SJ. Patients’ reflections on communication in the second-opinion hematology-oncology consultation. Patient Educ Couns. 2009;76(1):44-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander SC, Sullivan AM, Back AL, et al. Information giving and receiving in hematological malignancy consultations. Psychooncology. 2012;21(3):297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 13.Guzzo TJ, Dluzniewski P, Orosco R, Platz EA, Partin AW, Han M. Prediction of mortality after radical prostatectomy by Charlson comorbidity index. Urology. 2010;76(3):553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21-43. [PubMed] [Google Scholar]
- 15.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. [DOI] [PubMed] [Google Scholar]
- 16.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and d7aunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas X, Olteanu N, Charrin C, Lhéritier V, Magaud JP, Fiere D. Acute lymphoblastic leukemia in the elderly: The Edouard Herriot Hospital experience. Am J Hematol. 2001;67(2):73-83. [DOI] [PubMed] [Google Scholar]
- 18.Straka C, Liebisch P, Salwender H, et al. Autotransplant with and without induction chemotherapy in older multiple myeloma patients: long-term outcome of a randomized trial. Haematologica. 2016;101(11):1398-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh JA, Sloan JA, Atherton PJ, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16(9):688-696. [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst J, Kuhnt S, Schwarzer A, et al. The desire for shared decision making among patients with solid and hematological cancer. Psychooncology. 2011;20(2):186-193. [DOI] [PubMed] [Google Scholar]
- 21.Siminoff LA, Fetting JH. Factors affecting treatment decisions for a life-threatening illness: the case of medical treatment of breast cancer. Soc Sci Med. 1991;32(7):813-818. [DOI] [PubMed] [Google Scholar]
- 22.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709-1714. [DOI] [PubMed] [Google Scholar]
- 23.Meropol NJ, Weinfurt KP, Burnett CB, et al. Perceptions of patients and physicians regarding phase I cancer clinical trials: implications for physician-patient communication. J Clin Oncol. 2003;21(13):2589-2596. [DOI] [PubMed] [Google Scholar]
- 24.Watson R, Bryant J, Sanson-Fisher R, Turon H, Hyde L, Herrmann A. Do haematological cancer patients get the information they need about their cancer and its treatment? Results of a cross-sectional survey. Support Care Cancer. 2019;27(4):1509-1517. [DOI] [PubMed] [Google Scholar]
- 25.Epstein RM, Alper BS, Quill TE. Communicating evidence for participatory decision making. JAMA. 2004;291(19):2359-2366. [DOI] [PubMed] [Google Scholar]
- 26.Helft PR. Necessary collusion: prognostic communication with advanced cancer patients. J Clin Oncol. 2005;23(13):3146-3150. [DOI] [PubMed] [Google Scholar]
- 27.Wallace LM. Informed consent to elective surgery: the “therapeutic” value? Soc Sci Med. 1986;22(1):29-33. [DOI] [PubMed] [Google Scholar]
- 28.Tobias JS, Souhami RL. Fully informed consent can be needlessly cruel. BMJ. 1993;307(6913):1199-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annas GJ. Informed consent, cancer, and truth in prognosis [published correction appears in N Engl J Med. 1994;330(9):651]. N Engl J Med. 1994;330(3):223-225. [DOI] [PubMed] [Google Scholar]
- 30.Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS. Cancer patients’ perceptions of their disease and its treatment. Br J Cancer. 1988;58(3):355-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siminoff LA, Fetting JH. Effects of outcome framing on treatment decisions in the real world: impact of framing on adjuvant breast cancer decisions. Med Decis Making. 1989;9(4):262-271. [DOI] [PubMed] [Google Scholar]
- 32.Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16(2):515-521. [DOI] [PubMed] [Google Scholar]
- 33.Nakao MA, Axelrod S. Numbers are better than words. Verbal specifications of frequency have no place in medicine. Am J Med. 1983;74(6):1061-1065. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland HJ, Lockwood GA, Tritchler DL, Sem F, Brooks L, Till JE. Communicating probabilistic information to cancer patients: is there “noise” on the line? Soc Sci Med. 1991;32(6):725-731. [DOI] [PubMed] [Google Scholar]
- 35.Loh KP, Mohile SG, Lund JL, et al. Beliefs about advanced cancer curability in older patients, their caregivers, and oncologists. Oncologist. 2019;24(6):e292-e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz S. Literacy and numeracy skills of U.S. men and women. Available at https://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2018164. Accessed 5 July 2020.
- 37.Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ. Making numbers matter: present and future research in risk communication. Am J Health Behav. 2007;31(suppl 1):S47-S56. [DOI] [PubMed] [Google Scholar]
- 38.Wong ST, Pérez-Stable EJ, Kim SE, et al. Using visual displays to communicate risk of cancer to women from diverse race/ethnic backgrounds. Patient Educ Couns. 2012;87(3):327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters EA, Sullivan HW, Nelson W, Hesse BW. What is my cancer risk? How internet-based cancer risk assessment tools communicate individualized risk estimates to the public: content analysis. J Med Internet Res. 2009;11(3):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed Pract. 2019;36(6):284-289. [PMC free article] [PubMed] [Google Scholar]
- 41.Rutten LJ, Arora NK, Bakos AD, Aziz N, Rowland J. Information needs and sources of information among cancer patients: a systematic review of research (1980-2003). Patient Educ Couns. 2005;57(3):250-261. [DOI] [PubMed] [Google Scholar]
- 42.Finney Rutten LJ, Agunwamba AA, Wilson P, et al. Cancer-related information seeking among cancer survivors: trends over a decade (2003-2013). J Cancer Educ. 2016;31(2):348-357. [DOI] [PubMed] [Google Scholar]
- 43.Blanch-Hartigan D, Blake KD, Viswanath K. Cancer survivors’ use of numerous information sources for cancer-related information: does more matter? J Cancer Educ. 2014;29(3):488-496. [DOI] [PubMed] [Google Scholar]
- 44.Ramanadhan S, Viswanath K. Health and the information nonseeker: a profile. Health Commun. 2006;20(2):131-139. [DOI] [PubMed] [Google Scholar]
- 45.Sedrak MS, Soto-Perez-De-Celis E, Nelson RA, et al. Online health information-seeking among older women with chronic illness: analysis of the Women’s Health Initiative. J Med Internet Res. 2020;22(4):e15906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinquart M, Duberstein PR. Information needs and decision-making processes in older cancer patients. Crit Rev Oncol Hematol. 2004;51(1):69-80. [DOI] [PubMed] [Google Scholar]
- 47.Butow PN, Maclean M, Dunn SM, Tattersall MH, Boyer MJ. The dynamics of change: cancer patients’ preferences for information, involvement and support. Ann Oncol. 1997;8(9):857-863. [DOI] [PubMed] [Google Scholar]
- 48.Hoogland AI, Mansfield J, Lafranchise EA, Bulls HW, Johnstone PA, Jim HSL. eHealth literacy in older adults with cancer. J Geriatr Oncol. 2020;11(6):1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]