Abstract
Exogenous signals induce cells to enter the specialized cell division process of meiosis, which produces haploid gametes from diploid progenitor cells. Once cells initiate the meiotic divisions, it is imperative that they complete meiosis. Inappropriate exit from meiosis and entrance into mitosis can create polyploid cells and can lead to germline tumors. S. cerevisiae cells enter meiosis when starved of nutrients but can return to mitosis if provided nutrient-rich medium before a defined commitment point. Once past the meiotic commitment point in prometaphase I, cells stay committed to meiosis even in the presence of a mitosis-inducing signal. Recent research investigated the maintenance of meiotic commitment in budding yeast and found that two checkpoints that do not normally function in meiosis I, the DNA damage checkpoint and the spindle position checkpoint, have crucial functions in maintaining meiotic commitment. Here, we review these findings and discuss how the mitosis-inducing signal of nutrient-rich medium could activate these two checkpoints in meiosis to prevent inappropriate meiotic exit.
Keywords: meiosis, mitosis, return-to-growth, RTG, spindle position checkpoint, DNA damage checkpoint, meiotic commitment
Introduction
During sexual reproduction, exogenous signals induce cells to exit mitosis and initiate meiosis, a specialized cell division process in which two rounds of chromosome segregation follow one round of DNA replication to produce haploid gametes. Although the meiosis-inducing signal differs among organisms, the cell-cycle regulation of meiosis is highly conserved (Kimble 2011, Page and Orr-Weaver 1997). How cells integrate external signals with intrinsic cell-cycle control networks is an active area of research and is important for understanding how cells ensure the fidelity of meiosis.
Many organisms including yeast, flies, worms, frogs, mice, and humans have key transition points at the end of prophase I, just before entering into the meiotic divisions, which are regulated by exogenous signals (Kimble 2011, Nebreda and Ferby 2000, Page and Orr-Weaver 1997). During prophase I, homologous chromosomes pair, undergo programmed double-strand breaks, assemble synaptonemal complex, and initiate repair of double-strand breaks off their homolog to form crossovers (Subramanian and Hochwagen 2014). These events prepare chromosomes for proper segregation in meiosis I. To exit prophase I, a cell must satisfy a network of checkpoints that ensure that DNA damage is repaired before entering into the meiotic divisions. In addition, meiotic cells often require exogenous signals to initiate meiotic divisions. For example, mouse and human oocytes need hormone stimulation to exit prophase I and undergo meiosis I (Nebreda and Ferby 2000, Page and Orr-Weaver 1997). In C. elegans oogenesis, prophase I progression is coupled with nutritional status, such that oogenesis stalls in the absence of food, which inactivates insulin-like signaling pathways (Lopez, et al. 2013). In the presence of food, prophase I and oogenesis progress. Budding yeast also respond to nutritional status as a signal for meiotic initiation and progression. However, in contrast to C. elegans, budding yeast undergo meiosis when starved, as a survival mechanism (Neiman 2011). The starvation response induces sporulation, a process coupling meiosis with the cellular differentiation process of spore formation. The spores can survive harsh environmental conditions and then germinate when nutrients return.
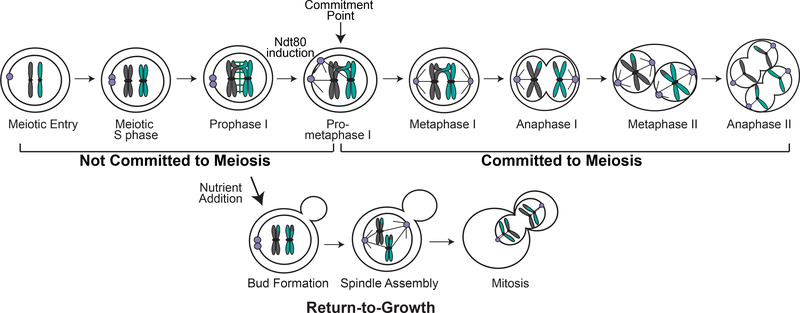
Budding yeast cells require the continued presence of the starvation signal until passing a defined meiotic commitment point in prometaphase I. Interestingly, if provided nutrient-rich medium before the commitment point, budding yeast cells have the unique ability to exit meiosis and return to vegetative divisions in a process called return-to-growth (RTG; Figure 1) (Ganesan, et al. 1958, Sherman and Roman 1963, Simchen, et al. 1972, Winter 2012). Cells undergo RTG even from prophase I, a stage in which many meiosis-specific events have occurred. With the introduction of nutrient-rich medium, cells in prophase I exit meiosis, disassemble their synaptonemal complex, repair joint molecules as crossovers or noncrossovers, and undergo a mitotic division in which sister chromatids separate (Dayani, et al. 2011, Laureau, et al. 2016, Winter 2012). The G2/M checkpoint protein Swe1 safeguards RTG by ensuring that cells form a bud before undergoing chromosome segregation (Gihana, et al. 2018, Tsuchiya and Lacefield 2013).
Figure 1: Cartoon of meiosis and Return-to-growth.
Cells become committed to meiosis in prometaphase I. With nutrient-rich medium addition before commitment, cells will exit meiosis and return to mitosis. Once cells pass the commitment point, they will stay in meiosis, even with the addition of nutrient-rich medium.
After passing through the commitment point in mid-prometaphase I, cells become committed and no longer exit meiosis when challenged with nutrient-rich medium (Tsuchiya, et al. 2014). These cells will complete meiosis and form spores. Assessment of transcriptional profiles showed that cells both before and after meiotic commitment changed their gene expression profiles in response to the addition of nutrients (Friedlander, et al. 2006). Therefore, committed cells are responsive to the nutrient shift, but the metabolic responses in uncommitted and committed cells are different. Surprisingly, both uncommitted and committed cells down-regulated the expression of sporulation-induced genes. These results suggest that committed cells likely have enough protein product to finish meiosis and form spores.
The middle meiosis transcription factor Ndt80 induces genes needed for the meiotic divisions and spore formation, and is required for meiotic commitment (Winter 2012). A positive feedback loop, in which Ndt80 can bind to elements within its own promoter, leads to a burst of Ndt80 activity and a strong up-regulation of Ndt80-transcribed genes. This burst of expression is likely important for producing enough gene product for meiotic commitment. Ablation of this positive feedback loop by mutating the elements within the promoter that Ndt80 binds resulted in cells that fail to commit to meiosis (Tsuchiya, et al. 2014). With addition of nutrient-rich medium, these cells return to mitosis from stages beyond prometaphase I, creating multi-nucleate polyploid cells. Therefore, a failure to maintain meiotic commitment can be dangerous to the cell.
The molecular mechanisms underlying commitment are poorly understood. Recently, we investigated the role of cell-cycle checkpoints in regulating meiotic commitment (Ballew and Lacefield 2019). Because checkpoints establish dependencies between cell cycle events, we hypothesized that checkpoints could be important for coupling meiotic commitment with meiotic progression (Ballew and Lacefield 2019, Hartwell and Weinert 1989). Here, we discuss our findings that the canonical DNA damage checkpoint together with the spindle position checkpoint maintain meiotic commitment. Interestingly, both of these checkpoints do not normally function in meiosis I. Therefore, we further discuss how the addition of nutrient-rich medium alters their activity to ensure that cells remain committed to meiosis.
The Canonical DNA Damage Checkpoint in Meiosis
In prophase I of meiosis, DNA damage is monitored by several checkpoints, collectively called the meiotic checkpoint network (Subramanian and Hochwagen 2014, Tsubouchi, et al. 2018). This checkpoint network was thought to prevent cells with any remaining programmed double-strand breaks from entering into the meiotic divisions by inhibiting Ndt80. Without active Ndt80, the cells remain in prophase I until damage is repaired. However, our analysis shows that cells can exit prophase I with residual damage. Using time-lapse microscopy of meiosis, we monitored Rad52-GFP, a protein that marks double-strand breaks in the process of repair. Approximately 40% of cells exit prophase I with Rad52-GFP foci and most of those foci persist into the meiotic divisions (Ballew and Lacefield 2019). The foci were dependent on Spo11, the enzyme that makes programmed double-strand breaks. Therefore, cells can exit prophase I and enter the meiotic divisions with unrepaired double-strand breaks.
Once meiotic cells exit prophase I, there is not another DNA damage checkpoint before the first meiotic division. In mitosis, a highly-conserved DNA damage checkpoint monitors lesions and arrests the cell cycle in metaphase to ensure that breaks are repaired before the onset of anaphase (MacQueen and Hochwagen 2011). The sensor kinases Mec1 and Tel1, ATR and ATM homologs, bind to the lesion and activate effector kinases Rad53 and Chk1 (Nyberg, et al. 2002). Both effector kinases prevent the destruction of securin, an inhibitor of separase, blocking chromosome segregation (Agarwal, et al. 2003, Cohen-Fix and Koshland 1997, Nyberg, et al. 2002, Sanchez, et al. 1999, Wang, et al. 2001). Rad53 also inhibits cyclin-dependent kinase (CDK) and the mitotic exit network (Agarwal, et al. 2003, Hu, et al. 2001, Palou, et al. 2015, Palou, et al. 2017). However, the canonical DNA damage checkpoint does not delay cells in meiosis I (Cartagena-Lirola, et al. 2008, Lydall, et al. 1996). Previous work demonstrated that with the introduction of a DNA damaging agent, the DNA damage checkpoint did not cause a meiosis I delay, but did cause a meiosis II delay (Cartagena-Lirola, et al. 2008). Similarly, we found that the cells with persistent Rad52-GFP foci did not have a delay in meiosis I but did have a delay in meiosis II (Ballew and Lacefield 2019). Therefore, cells with persistent DNA damage undergo a round of chromosome segregation before delaying the cell cycle for repair.
The current model in the field is that the DNA damage checkpoint is kept inactive in meiosis I through the activity of protein phosphatase 4 (PP4), a phosphatase whose activity is required to promote crossover repair and centromere pairing in prophase I (Falk, et al. 2010). PP4 removes phosphorylation on several key proteins involved in the DNA damage response (Keogh, et al. 2006, Lee, et al. 2010, O’Neill, et al. 2007). One substrate of PP4 is the checkpoint kinase Rad53, whose phosphorylation is essential for checkpoint signaling (Nyberg, et al. 2002, O’Neill, et al. 2007, Sanchez, et al. 1999). By keeping Rad53 unphosphorylated, it is inactive and does not propagate a checkpoint signal.
To our surprise, we found that the addition of nutrient-rich medium allowed committed cells with DNA damage to activate the DNA damage checkpoint and delay cells at metaphase I instead of waiting until metaphase II (Ballew and Lacefield 2019). A comparison of cells with and without Rad52-GFP foci showed that cells with persistent Rad52-GFP were slower to undergo anaphase I than cells without Rad52-GFP. This delay was dependent on Mec1.
How does the addition of nutrient-rich medium lead to the activation of the canonical DNA damage checkpoint in meiosis I? We propose that the addition of nutrients in meiosis I leads to cellular changes that allow the activation of the DNA damage checkpoint when DNA breaks are present. Previous work from mitosis has shown that the addition of glucose and a DNA damaging agent leads to the Mec1/Tel1-dependent increased phosphorylation of Mms21, a SUMO ligase involved in switching from respiration to fermentation in the presence of DNA damage (Simpson-Lavy, et al. 2015). These results suggest that Mec1/Tel1 activity can be enhanced with the addition of both glucose and DNA damage, at least towards some substrates. In addition, the protein kinase A (PKA) pathway crosstalks with the DNA damage checkpoint pathway to restrain anaphase onset in the presence of DNA damage (Searle, et al. 2004, Searle, et al. 2011). Mec1 activity leads to PKA phosphorylation. PKA activation also requires cAMP, a small molecule whose transient increase is regulated by the Ras pathway, a glucose-induced signaling pathway (Broach 2012). Inactive PKA forms a tetramer with two catalytic subunits and two inhibitory regulatory subunits. cAMP binds the inhibitory regulatory subunits, releasing the active catalytic subunits. The combined activity of cAMP and Mec1 is thought to activate PKA specifically during the DNA damage response to phosphorylate Cdc20, a co-activator of the anaphase promoting complex (APC/C) (Searle, et al. 2004, Searle, et al. 2011). Normally the APC/C, a ubiquitin ligase, targets proteins for ubiquitination and subsequent proteasomal degradation to promote the transition from metaphase to anaphase. The phosphorylation of Cdc20 restrains APC/C activity to prevent anaphase onset.
We propose that the addition of nutrient-rich medium to committed cells activates the PKA pathway to restrain anaphase onset in the presence of DNA damage. The nutrient-rich medium that contains glucose likely increases cAMP in the cell through the Ras pathway. DNA damage activates Mec1, which phosphorylates PKA, and this phosphorylation together with cAMP binding to the regulatory subunits leads to activated PKA. PKA then phosphorylates Cdc20 and prevents APC/C activity to allow additional time for DNA damage repair.
The DNA Damage Checkpoint and The Spindle Position Checkpoint are Needed for Meiotic Commitment
We initially investigated the role of the DNA damage checkpoint in meiotic commitment due to its role in establishing dependencies between cell cycle processes (Ballew and Lacefield 2019). We first deleted the checkpoint protein MEC1, and because MEC1 is essential, we also deleted SML1, which suppresses the lethality (Weinert, et al. 1994, Zhao, et al. 1998). Our studies revealed an unexpected phenotype: a proportion of mec1Δ sml1Δ cells in prometaphase I failed to stay committed to meiosis (Ballew and Lacefield 2019). Instead, these cells rapidly underwent anaphase I, and then budded. Some cells exited meiosis after anaphase I and some exited after metaphase II. Similarly, loss of RAD53 and SML1 also led to a similar percentage of cells that underwent anaphase I and then budded, losing meiotic commitment with addition of nutrient-rich medium in prometaphase I. This phenotype was not the consequence of loss of SML1 because sml1Δ cells did not lose meiotic commitment. Furthermore, we used a technique called anchor-away to deplete Mec1 from the nucleus just before nutrient-rich medium addition (Ballew and Lacefield 2019, Haruki, et al. 2008). With Mec1 nuclear depletion, cells also displayed an uncommitted phenotype, indicating that cells needed Mec1 at the time of commitment (Ballew and Lacefield 2019). Significantly, these phenotypes only occur when nutrient-rich medium is added to cells that are in mid-prometaphase I, coinciding with the commitment point. The results suggest that a DNA damage checkpoint delay at the commitment point prevents cells from inappropriately exiting meiosis.
We further analyzed why some cells that lack the DNA damage checkpoint exited meiosis I and some cells continued through meiosis I but exited in meiosis II. We hypothesized that another checkpoint might keep some cells from escaping meiosis I. The cells that exited meiosis after anaphase I did not become multi-nucleate. Instead, they underwent anaphase I, budded, and repositioned one nucleus into the daughter cell. This phenotype is reminiscent of mitotic cells with mis-positioned spindles. In mitosis, the spindle position checkpoint ensures cells with mis-positioned spindles do not exit mitosis until the proper positioning of one spindle pole body into the bud. The checkpoint serves as a mechanism to inhibit the mitotic exit network, a signaling cascade that promotes spindle disassembly and cytokinesis (Botchkarev and Haber 2018, Scarfone and Piatti 2015). According to the current model, the zone model, the inhibitory proteins are present in the mother, but the bud provides an activating zone (Chan and Amon 2010, Falk, et al. 2016). Once signaling molecules on the spindle pole body enter the bud, they are no longer inhibited by spindle position checkpoint components. Instead, activating signals in the bud lead to activation of the mitotic exit network and cells exit mitosis. Although the spindle position checkpoint does not function in meiosis I, in which there is no bud and no activation of the mitotic exit network (Attner and Amon 2012, Kamieniecki, et al. 2005), we hypothesized that both the spindle position checkpoint components and the mitotic exit network became active with the mitosis-inducing signal of nutrient-rich medium addition.
To our surprise, loss of both the spindle position checkpoint as well as the DNA damage checkpoint caused a commitment defect: approximately 30% of rad53Δ sml1Δ bub2Δ cells failed to stay committed to meiosis with nutrient addition in metaphase I or anaphase I (Ballew and Lacefield 2019). These cells finished meiosis I, and then exited meiosis and budded at metaphase II. In the next mitotic division, the mother cell contained two nuclei and divided both nuclei, creating a multi-nucleate mother cell. Similarly, deletion of other components of the spindle position checkpoint, such as KIN4 and BFA1, also led to a loss of commitment in rad53Δ sml1Δ cells. Loss of either checkpoint individually did not have a significant effect on commitment in metaphase I and anaphase I, suggesting that both checkpoints function somewhat redundantly to maintain commitment.
It is currently unclear how the addition of nutrient-rich medium leads to spindle position checkpoint activity. In normal meiosis, cells do not activate the mitotic exit network at the end of anaphase I (Attner and Amon 2012, Kamieniecki, et al. 2005). Therefore, the role of the spindle position checkpoint to inhibit the mitotic exit network is not needed. Our results suggest that the mitotic exit network can become active in meiosis with nutrient-rich medium addition and the spindle position checkpoint components are required to prevent cells from undergoing an inappropriate exit from meiosis when the DNA damage checkpoint is not functional.
Overall, our results demonstrate that two checkpoints that do not normally function in meiosis I are crucial for meiotic commitment. These results further define the requirements for meiotic commitment. We previously showed that high levels of Ndt80, through activation of a positive feedback loop, are essential for meiotic commitment (Tsuchiya, et al. 2014). We propose that Ndt80 is important for commitment by turning on genes required for the meiotic divisions and promoting meiotic progression. In contrast, the DNA damage checkpoint and the spindle position checkpoint together are important for meiotic commitment by preventing meiotic exit and entrance into mitosis. Normally, the two checkpoints are not active during meiosis I; however, in the presence of nutrients, they become essential. The presence of a mitosis-inducing signal likely activates the DNA damage checkpoint and the mitotic exit network and therefore, loss of both checkpoints leads to a failure of cells to stay committed to meiosis. Instead, they inappropriately exit meiosis after undergoing anaphase I, leading to polyploid cells. Furthermore, our work provides a mechanism for the maintenance of meiotic commitment: the checkpoints block cells from an inappropriate exit from meiosis I or meiosis II which keeps them in the meiotic program.
Acknowledgements:
This work was supported by National Institutes of Health grant GM105755.
References
- Agarwal R, Tang Z, Yu H, Cohen-Fix O (2003) Two distinct pathways for inhibiting pds1 ubiquitination in response to DNA damage. J Biol Chem 278: 45027–45033 doi: 10.1074/jbc.M306783200 [DOI] [PubMed] [Google Scholar]
- Attner MA, Amon A (2012) Control of the mitotic exit network during meiosis. Mol Biol Cell 23: 3122–3132 doi: 10.1091/mbc.E12-03-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew O, Lacefield S (2019) The DNA Damage Checkpoint and the Spindle Position Checkpoint Maintain Meiotic Commitment in Saccharomyces cerevisiae. Current biology : CB 29: 449–460 e442 doi: 10.1016/j.cub.2018.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VV Jr., Haber JE (2018) Functions and regulation of the Polo-like kinase Cdc5 in the absence and presence of DNA damage. Curr Genet 64: 87–96 doi: 10.1007/s00294-017-0727-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach JR (2012) Nutritional control of growth and development in yeast. Genetics 192: 73–105 doi: 10.1534/genetics.111.135731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartagena-Lirola H, Guerini I, Manfrini N, Lucchini G, Longhese MP (2008) Role of the Saccharomyces cerevisiae Rad53 checkpoint kinase in signaling double-strand breaks during the meiotic cell cycle. Molecular and cellular biology 28: 4480–4493 doi: 10.1128/MCB.00375-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LY, Amon A (2010) Spindle position is coordinated with cell-cycle progression through establishment of mitotic exit-activating and -inhibitory zones. Mol Cell 39: 444–454 doi: 10.1016/j.molcel.2010.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Fix O, Koshland D (1997) The anaphase inhibitor of Saccharomyces cerevisiae Pds1p is a target of the DNA damage checkpoint pathway. Proc Natl Acad Sci U S A 94: 14361–14366 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayani Y, Simchen G, Lichten M (2011) Meiotic recombination intermediates are resolved with minimal crossover formation during return-to-growth, an analogue of the mitotic cell cycle. PLoS Genet 7: e1002083 doi: 10.1371/journal.pgen.1002083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JE, Chan AC, Hoffmann E, Hochwagen A (2010) A Mec1- and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Developmental cell 19: 599–611 doi: 10.1016/j.devcel.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Falk JE, Tsuchiya D, Verdaasdonk J, Lacefield S, Bloom K, Amon A (2016) Spatial signals link exit from mitosis to spindle position. Elife 510.7554/eLife.14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander G, Joseph-Strauss D, Carmi M, Zenvirth D, Simchen G, Barkai N (2006) Modulation of the transcription regulatory program in yeast cells committed to sporulation. Genome Biol 7: R20 doi: 10.1186/gb-2006-7-3-r20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan AT, Holter H, Roberts C (1958) Some observations on sporulation in Saccharomyces. C R Trav Lab Carlsberg Chim 31: 1–6 doi: [PubMed] [Google Scholar]
- Gihana GM, Musser TR, Thompson O, Lacefield S (2018) Prolonged cyclin-dependent kinase inhibition results in septin perturbations during return to growth and mitosis. J Cell Biol 217: 2429–2443 doi: 10.1083/jcb.201708153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634 doi: [DOI] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK (2008) The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell 31: 925–932 doi: 10.1016/j.molcel.2008.07.020 [DOI] [PubMed] [Google Scholar]
- Hu F, Wang Y, Liu D, Li Y, Qin J, Elledge SJ (2001) Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107: 655–665 doi: [DOI] [PubMed] [Google Scholar]
- Kamieniecki RJ, Liu L, Dawson DS (2005) FEAR but not MEN genes are required for exit from meiosis I. Cell Cycle 4: 1093–1098 doi: [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, Onishi M, Datta N, Galicia S, Emili A, Lieberman J, Shen X, Buratowski S, Haber JE, Durocher D, Greenblatt JF, Krogan NJ (2006) A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature 439: 497–501 doi: 10.1038/nature04384 [DOI] [PubMed] [Google Scholar]
- Kimble J (2011) Molecular regulation of the mitosis/meiosis decision in multicellular organisms. Cold Spring Harb Perspect Biol 3: a002683 doi: 10.1101/cshperspect.a002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureau R, Loeillet S, Salinas F, Bergstrom A, Legoix-Ne P, Liti G, Nicolas A (2016) Extensive Recombination of a Yeast Diploid Hybrid through Meiotic Reversion. PLoS Genet 12: e1005781 doi: 10.1371/journal.pgen.1005781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Pan Y, Kanner S, Sung P, Borowiec JA, Chowdhury D (2010) A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat Struct Mol Biol 17: 365–372 doi: 10.1038/nsmb.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AL 3rd, Chen J, Joo HJ, Drake M, Shidate M, Kseib C, Arur S (2013) DAF-2 and ERK couple nutrient availability to meiotic progression during Caenorhabditis elegans oogenesis. Developmental cell 27: 227–240 doi: 10.1016/j.devcel.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall D, Nikolsky Y, Bishop DK, Weinert T (1996) A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature 383: 840–843 doi: 10.1038/383840a0 [DOI] [PubMed] [Google Scholar]
- MacQueen AJ, Hochwagen A (2011) Checkpoint mechanisms: the puppet masters of meiotic prophase. Trends Cell Biol 21: 393–400 doi: 10.1016/j.tcb.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Ferby I (2000) Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol 12: 666–675 doi: [DOI] [PubMed] [Google Scholar]
- Neiman AM (2011) Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189: 737–765 doi: 10.1534/genetics.111.127126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA (2002) Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet 36: 617–656 doi: 10.1146/annurev.genet.36.060402.113540 [DOI] [PubMed] [Google Scholar]
- O’Neill BM, Szyjka SJ, Lis ET, Bailey AO, Yates JR 3rd, Aparicio OM, Romesberg FE (2007) Pph3-Psy2 is a phosphatase complex required for Rad53 dephosphorylation and replication fork restart during recovery from DNA damage. Proc Natl Acad Sci U S A 104: 9290–9295 doi: 10.1073/pnas.0703252104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AW, Orr-Weaver TL (1997) Stopping and starting the meiotic cell cycle. Curr Opin Genet Dev 7: 23–31 doi: [DOI] [PubMed] [Google Scholar]
- Palou G, Palou R, Zeng F, Vashisht AA, Wohlschlegel JA, Quintana DG (2015) Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast. PLoS Genet 11: e1005468 doi: 10.1371/journal.pgen.1005468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou R, Palou G, Quintana DG (2017) A role for the spindle assembly checkpoint in the DNA damage response. Curr Genet 63: 275–280 doi: 10.1007/s00294-016-0634-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge SJ (1999) Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science 286: 1166–1171 doi: [DOI] [PubMed] [Google Scholar]
- Scarfone I, Piatti S (2015) Coupling spindle position with mitotic exit in budding yeast: The multifaceted role of the small GTPase Tem1. Small GTPases 6: 196–201 doi: 10.1080/21541248.2015.1109023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle JS, Schollaert KL, Wilkins BJ, Sanchez Y (2004) The DNA damage checkpoint and PKA pathways converge on APC substrates and Cdc20 to regulate mitotic progression. Nature cell biology 6: 138–145 doi: 10.1038/ncb1092 [DOI] [PubMed] [Google Scholar]
- Searle JS, Wood MD, Kaur M, Tobin DV, Sanchez Y (2011) Proteins in the nutrient-sensing and DNA damage checkpoint pathways cooperate to restrain mitotic progression following DNA damage. PLoS Genet 7: e1002176 doi: 10.1371/journal.pgen.1002176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Roman H (1963) Evidence for two types of allelic recombination in yeast. Genetics 48: 255–261 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G, Pinon R, Salts Y (1972) Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res 75: 207–218 doi: [DOI] [PubMed] [Google Scholar]
- Simpson-Lavy KJ, Bronstein A, Kupiec M, Johnston M (2015) Cross-Talk between Carbon Metabolism and the DNA Damage Response in S. cerevisiae. Cell Rep 12: 1865–1875 doi: 10.1016/j.celrep.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian VV, Hochwagen A (2014) The meiotic checkpoint network: step-by-step through meiotic prophase. Cold Spring Harb Perspect Biol 6: a016675 doi: 10.1101/cshperspect.a016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Argunhan B, Tsubouchi T (2018) Exiting prophase I: no clear boundary. Curr Genet 64: 423–427 doi: 10.1007/s00294-017-0771-y [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Lacefield S (2013) Cdk1 modulation ensures the coordination of cell-cycle events during the switch from meiotic prophase to mitosis. Current biology : CB 23: 1505–1513 doi: 10.1016/j.cub.2013.06.031 [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Yang Y, Lacefield S (2014) Positive feedback of NDT80 expression ensures irreversible meiotic commitment in budding yeast. PLoS Genet 10: e1004398 doi: 10.1371/journal.pgen.1004398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu D, Wang Y, Qin J, Elledge SJ (2001) Pds1 phosphorylation in response to DNA damage is essential for its DNA damage checkpoint function. Genes Dev 15: 1361–1372 doi: 10.1101/gad.893201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH (1994) Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev 8: 652–665 doi: [DOI] [PubMed] [Google Scholar]
- Winter E (2012) The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 76: 1–15 doi: 10.1128/MMBR.05010-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Muller EG, Rothstein R (1998) A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell 2: 329–340 doi: [DOI] [PubMed] [Google Scholar]