Abstract
The Ca2+-permeable, non-selective cation channel, TRPA1 (transient receptor potential ankyrin 1), is the sole member of the ankyrin TRP subfamily. TRPA1 channels are expressed on the plasma membrane of neurons as well as non-neuronal cell types, such as vascular endothelial cells. TRPA1 is activated by electrophilic compounds, including dietary molecules such as allyl isothiocyanate, a derivative of mustard. Endogenously, the channel is thought to be activated by reactive oxygen species and their metabolites, such as 4-hydroxynonenal (4-HNE). In the context of the vasculature, activation of TRPA1 channels results in a vasodilatory response mediated by two distinct mechanisms. In the first instance, TRPA1 is expressed in sensory nerves of the vasculature and, upon activation, mediates release of the potent dilator, calcitonin gene-related peptide (CGRP). In the second, work from our laboratory has demonstrated that TRPA1 is expressed in the endothelium of blood vessels exclusively in the cerebral vasculature, where its activation produces a localized Ca2+ signal that results in dilation of cerebral arteries. In this chapter, we provide an in-depth overview of the biophysical and pharmacological properties of TRPA1 channels and their importance in regulating vascular tone.
1. Introduction
Transient receptor potential (TRP) channels allow movement of cations across membranes to mediate various cellular functions. TRP channels were discovered during investigations of a spontaneous Trp-mutant line of Drosophila melanogaster that exhibited a transient response to light (hence the name); as a result, mutant flies could see normally in dim light, but behaved as if blind under bright light (Cosens & Manning, 1969). The genomes of most mammals encode 28 individual TRP subunits, which together constitute the TRP superfamily; in humans, only 27 TRP subunits are expressed (TRPC2 is a non-functional pseudogene in humans). TRP subunits have been assigned into six subfamilies based on sequence homology. The ankyrin (TRPA) subfamily is the smallest, consisting of only a single member (Story et al., 2003). In this chapter, we provide a review of the current literature on TRPA1 channels that briefly introduces the structural and biophysical properties of the channel and delves deeply into the importance of TRPA1 in regulating vascular function in physiological and pathological settings. Further details regarding the structure, properties, and function of TRPA1 channels in many organ systems can be found in an outstanding recent review by Talavera et al. (2019).
The basic structure and function of TRPA1 channels are evolutionarily conserved (Kang et al., 2010; Story et al., 2003). Despite being among the last TRP channels to be discovered, TRPA1 has received a great deal of interest (Story et al., 2003). The Trpa1 gene has been cloned from several mammalian species, including human, mouse and rat, as well as non-mammalian species, such as insects, fish, birds, and amphibians. Mammals have one Trpa1 gene homolog, whereas several non-mammalian species have more than one homolog. For example, D. melanogaster has four TRPA homologs. TRPA1 was originally thought to be predominantly expressed in sensory neurons of the dorsal root ganglia, trigeminal ganglia and nodose ganglia, as well as in hair cells (Bautista et al., 2005; Nagata, Duggan, Kumar, & Garcia-Anoveros, 2005; Story et al., 2003). However, TRPA1 has since been identified in non-neuronal tissue, such as alveolar epithelial cells (Nassini et al., 2012), cardiomyocytes (Andrei, Sinharoy, Bratz, & Damron, 2016), cardiac fibroblasts (Oguri et al., 2014), and cerebral artery endothelial cells (Earley, Gonzales, & Crnich, 2009).
2. TRPA1 architecture
The functional TRPA1 ion channel is a homotetrameric protein complex (Cvetkov, Huynh, Cohen, & Moiseenkova-Bell, 2011). The high-resolution (~4Å) structure of fully assembled TRPA1 channels, revealed by cryo-electron microscopy (cryo-EM) (Fig. 1), shows that each TRPA1 channel subunit consists of six transmembrane domains (TM1-TM6), with a pore-forming loop between TM5 and TM6, and intracellular N- and C-termini (Paulsen, Armache, Gao, Cheng, & Julius, 2015). Assembly of four TRPA1 subunits forms a Ca2+ permeable non-selective cation channel. The human TRPA1 monomer (hTRPA1) is a 1119-amino acid protein with a molecular mass of 127.4 kDa (Nilius, Appendino, & Owsianik, 2012; Story et al., 2003). The N-terminus forms the largest part of the protein, accounting for 64% of its total mass, whereas the C-terminus only accounts for 14% (Cvetkov et al., 2011). The distinguishing and eponymous feature of TRPA1 is the large number of ankyrin repeat domains (ARDs) within the N-terminus. The human TRPA1 protein contains 16 ARDs—more than any other known protein (Gaudet, 2008; Mosavi, Minor, & Peng, 2002; Story et al., 2003). ARDs are 33-amino acid sequences that may be involved in elasticity, protein-protein interactions, stability, and structural integrity (Lee et al., 2006). Because ankyrin is a linear spring with a tension proportional to its extension, ARDs have a strong tendency to refold (Lee et al., 2006). Each domain forms two short anti-parallel helix-turn-helix structures, followed by a β-hairpin loop (Lee et al., 2006; Sotomayor, Corey, & Schulten, 2005). The N-terminal domain also contains a large number of cysteine residues that can interact intramolecularly or between monomers through formation of disulfide bridges (Cvetkov et al., 2011; Eberhardt et al., 2012; Gaudet, 2008; Wang, Cvetkov, Chance, & Moiseenkova-Bell, 2012). Moreover, conserved cysteine or lysine residues also provide target sites for potent electrophilic TRPA1 activators, which act through covalent modification of the receptor (Macpherson et al., 2007; Takahashi et al., 2011). Cryo-EM images have revealed that some cysteine and lysine residues are also located in the transmembrane core facing the lipid environment, where they might react with lipophilic electrophiles (Paulsen et al., 2015). Zn2+, a potent TRPA1 activator, can bind to cysteine and histidine residues in the C-terminus (Andersson, Gentry, Moss, & Bevan, 2009; Hu, Bandell, Petrus, Zhu, & Patapoutian, 2009). Both N- and C-termini have been suggested to contain binding sites for Ca2+, which can either activate or deactivate TRPA1 (Doerner, Gisselmann, Hatt, & Wetzel, 2007; Jordt et al., 2004; Wang, Chang, Waters, McKemy, & Liman, 2008; Zurborg, Yurgionas, Jira, Caspani, & Heppenstall, 2007).
Fig. 1.
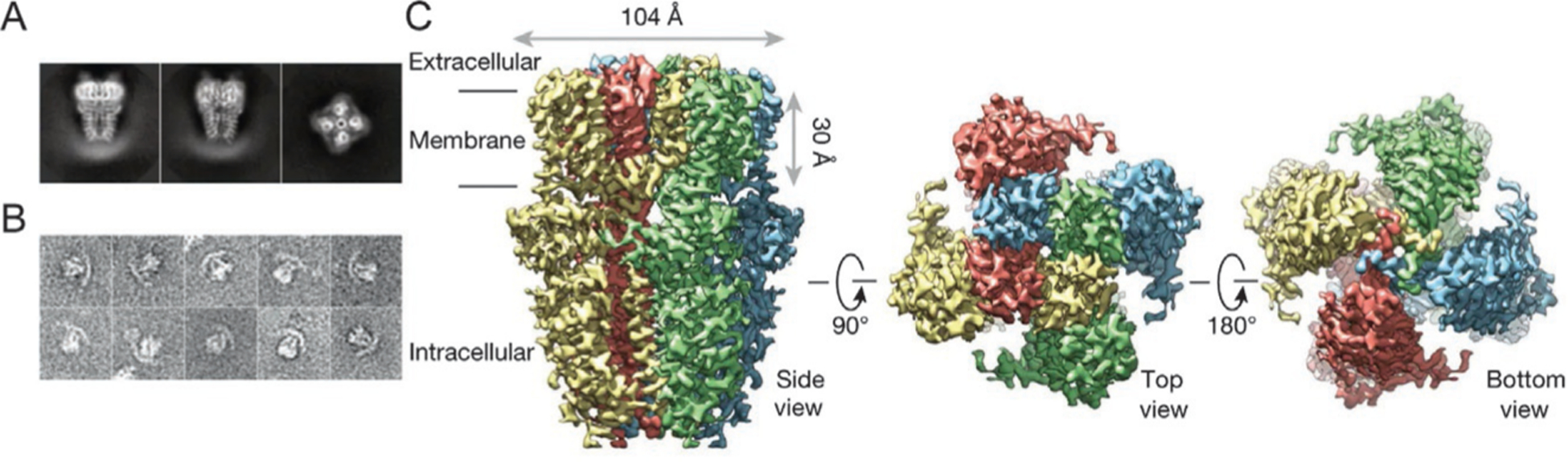
High resolution cryo-EM structure of TRPA1. (A) Representative cryo-EM 2-dimensional image of TRPA1 (left and middle, side view; right, end-on view). (B) Representative image of negative stain particles. (C) 3-Dimensional density map of TRPA1 with each subunit color coded (left, side view; middle, top view; right, bottom view). Figure derived from Paulsen, C. E., Armache, J. P., Gao, Y., Cheng, Y., & Julius, D. (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature, 520(7548), 511–517. https://doi.org/10.1038/nature14367.
3. Biophysical properties of the TRPA1 channel
3.1. Permeation and gating characteristics
TRPA1 is a voltage-gated non-selective cation channel that is permeable to both monovalent and divalent ions, including K+, Na+ and Ca2+. The relative permeability for Ca2+ is higher than that for Na+ or K+ (PCa:PNa: PK = ~7.9:1.0:0.98) (Bobkov, Corey, & Ache, 2011; Nilius et al., 2012; Nilius, Prenen, & Owsianik, 2011), and the Ca2+ fraction of the mixed cation current is approximately 20% (Karashima et al., 2010). The mouth of the pore contains a string of negatively charged residues (E920, E924 and E930) that attract cations and repel anions (Christensen, Akyuz, & Corey, 2016; Paulsen et al., 2015). The permeation pathway has two major constriction sites. First, the upper gate is formed by the D915 residues of individual subunits, which are located at the entrance of the pore. In a tetrameric configuration, this forms a ring of negative charges that allows attraction of cations. The diameter of the pore at this region is 7 Å, which is wide enough to accommodate Ca2+. The lower gate is formed by I957 and V961 residues and has a diameter of only 6 Å, which might represent the closed structure of the channel (upper gate, open; lower gate, closed) (Christensen et al., 2016; Paulsen et al., 2015).
Interestingly, agonist stimulation causes a change in the selectivity filter that results in progressive, but reversible, pore dilation, thereby allowing influx of large molecules such as N-methyl-d-glucamine (NMDG) and the cationic dye Yo-Pro. For example, activation of TRPA1 by allyl isothiocyanate (AITC) dilates the pore diameter up to an additional 2–3 Å over the normal open configuration (Banke, Chaplan, & Wickenden, 2010; Bobkov, Corey, & Ache, 2011; Chen et al., 2009). A separate model of the progressive increase in the permeability of large molecules over time indicates that this phenomenon may result from extensive changes in intracellular ion concentrations, rather than changes in channel permeability (Li, Toombes, Silberberg, & Swartz, 2015).
3.2. Regulation of TRPA1 by Ca2+
TRPA1 channels are highly permeable to Ca2+ ions relative to most other TRP channels (Nilius et al., 2011). Under physiological conditions, their unitary conductance is ~70 and ~110 pS in the inward and outward directions, respectively (Nilius et al., 2012, 2011). Both intracellular and extracellular Ca2+ regulate TRPA1 activity. Increases in intracellular Ca2+ initiate and potentiate TRPA1 current responses elicited by different agonists, including AITC and cannabinoids (Jordt et al., 2004). Similarly, increase in extracellular Ca2+ also potentiate TRPA1 currents stimulated by electrophilic and non-electrophilic agonists (Wang et al., 2008). It has been proposed that three residues, D466, L474 and D477, within the Ca2+-binding EF-hand domain located in the N-terminus are responsible for Ca2+-dependent effects (Doerner et al., 2007; Hinman, Chuang, Bautista, & Julius, 2006; Nagata et al., 2005; Zurborg et al., 2007). Simulation studies have confirmed that, when bound to Ca2+, the EF-hand induces a local conformational change that stiffens the ankyrin repeats structure and therefore affects the force exerted onto the gate and thus opening of the channel (Zayats et al., 2013). A separate study demonstrated that mutation of a single amino acid residue in the pore region of hTRPA1 (D915) disrupts Ca2+-induced activation and inactivation, suggesting that influx of Ca2+ is responsible for mediating these two effects (Wang et al., 2008).
3.3. Voltage gating of TRPA1 channels
TRPA1 channels are not voltage activated in a classical sense, but their activity can be modulated by voltage under certain conditions. The half-maximum voltage required for its activation in the absence of agonist has been reported to be +90 to +170 mV; thus, it is unlikely to be activated at physiological potentials (Karashima et al., 2007; Kremeyer et al., 2010; Meents, Fischer, & McNaughton, 2016). However, it has been demonstrated that the TRPA1 channel displays a shift in its voltage-dependent gating toward physiological membrane potentials during activation by electrophilic and non-electrophilic agonists, cold temperature, and Ca2+ (Fajardo, Meseguer, Belmonte, & Viana, 2008; Karashima et al., 2007). Mutations at several residues in the C-terminus (K1048, K1052, K1092 and T1099) were found to affect AITC and voltage-dependent gating (Samad et al., 2011). In general, the movement of positively charged residues in TM4 of voltage-gated ion channels is responsible for activation; however, TRPA1 does not contain any charged residues in the TM4 region, suggesting that the voltage sensor resides elsewhere. Specifically, it has been proposed that two positively charged residues, R975 and K989, within the C-terminus are responsible for voltage sensitivity. In addition, single alanine mutations of residues K969, K1009, K1071, E1077, T1078, and R1099 have been shown to abolish the voltage sensitivity of the channel (Samad et al., 2011). However, these residues are less likely to be directly affected by changes in the electric field of the cell membrane during depolarization because of their distal location, suggesting that downstream conformational changes that occur during activation of the channel could be involved in providing the connection between the distal region and the proximal putative voltage sensor. For instance, the coiled-coil domain of the C-terminus directly links the distal C-terminal to the putative voltage sensor domain, and also forms the connection with the N-terminal ankyrin repeat domain (Paulsen et al., 2015).
4. Pharmacology of TRPA1 channels
4.1. Activators
TRPA1 agonists can be categorized into two groups: electrophilic compounds (e.g., AITC, allicin and cinnamaldehyde) that covalently modulate the channel, and non-electrophilic compounds (e.g., thymol, menthol and carvacrol) that modulate the channel non-covalently. Electrophilic agonists interact with the thiol group of cysteine residues C621, C641 and C665, which are located in the N-terminal domain of TRPA1 (Bahia et al., 2016; Hinman et al., 2006). The crystal structure of TRPA1 suggests that these three cysteine residues come into close proximity, potentially forming a ligand-binding pocket (Cvetkov et al., 2011; Paulsen et al., 2015; Zayats et al., 2013). For example, JT010, a novel TRPA1 agonist (EC50 ≈ 65 nM) opens the channel by selectively and covalently binding to residue C621 (Takaya et al., 2015). A report by Cordero-Morales et al. suggests that certain ARDs in the N-terminus are involved in TRPA1 activation. AR3–8 and AR10–15 in TRPA1 from Crotalus atrox (western diamondback rattlesnake) are involved in heat sensation, whereas AR11–16 in hTRPA1 are sensitive to AITC (Cordero-Morales, Gracheva, & Julius, 2011).
TRPA1 responds to a wide variety of chemically diverse compounds, including environmental irritants, such as acrolein (Bautista et al., 2006) and diesel exhaust (Hazari et al., 2011), anesthetics (Leffler, Lattrell, Kronewald, Niedermirtl, & Nau, 2011), and the non-steroidal anti-inflammatory compound acetaminophen (Andersson et al., 2011), to name a few (summarized in Table 1). In addition to chemical activators, physical factors such as heat (Cordero-Morales et al., 2011; Rosenzweig et al., 2005) and cold (Kwan et al., 2006; Story et al., 2003) also activate TRPA1. However, responsiveness to cold remains controversial, with several studies failing to detect changes in TRPA1 activity in response to a cold stimulus (Bautista et al., 2006; Caspani & Heppenstall, 2009; Jordt et al., 2004).
Table 1.
List of TRPA1 channel activators.
| Agonist | Source | EC50; isoform, expression system, technique | References |
|---|---|---|---|
| Allyl isothiocyanate (AITC) | Mustard | 33μM; mTRPA1, CHO cells, Ca2+ imaging (FLIPR) 11μM; rTRPAl, oocytes, electrophys (−60 mV) 64μM; hTRPAl, oocytes, electrophys (+80 mV) | Bandell et al. (2004), Hinman et al. (2006), and jordt et al. (2004) |
| Cinnamaldehyde | Cinnamon | 100μM; mTRPA1, CHO cells, Ca2+ imaging (FLIPR) | Bandell et al. (2004) |
| Allicin | Garlic | 1.3μM; mTRPA1, CHO cells, Ca2+ imaging (FLIPR) 1.9μM; hTRPA1, CHO cells, Ca2+ imaging (FLIPR) | Bautista et al. (2005) and Macpherson et al. (2005) |
| Propofol | Anesthetic | 17μM; mTRPA1, Sf21 cells, Ca2+ imaging (FlexStation 3) | Woll et al. (2017) |
| Lidocaine | Anesthetic | 5.7mM; rTRPA1, HEK293, electrophys (−60 mV) 24mM; hTRPAl, HEK293, electrophys (−60 mV) | Leffler et al. (2011) |
| 4-HNE | Reactive oxygen species | 13–20μM; mTRPA1, CHO cells, Ca2+ imaging (FLIPR or FlexStation 3) 27μM; rTRPA1, HEK293, Ca2+ imaging 5μM; hTRPA1, HEK293, Ca2+ imaging | Andersson, Gentry, Moss, and Bevan (2008), Macpherson et al. (2007), Taylor-Clark et al. (2008), and Trevisani et al. (2007) |
| 4-ONE | Reactive oxygen species | 1.9μM; mTRPA1, CHO cells, Ca2+ imaging (FlexStation 3) 5.8μM; hTRPAl, HEK293, Ca2+ imaging | Andersson et al. (2008) and Taylor-Clark et al. (2008) |
| 4-HHE | Reactive oxygen species | 38.9μM; mTRPA1, CHO cells, Ca2+ imaging (FlexStation 3) ≥4.3μM; hTRPA1, HEK293, Ca2+ imaging | Andersson et al. (2008) and Taylor-Clark et al. (2008) |
| H2O2 | Reactive oxygen species | 230μM; mTRPA1, CHO cells, Ca2+ imaging (FlexStation 3) | Andersson et al. (2008) |
| 15-Deoxy-delta(12,14)-prostaglandin J(2) [15d-PGJ(2)] | Reactive oxygen species | 5.6μM; mTRPA1, CHO cells, Ca2+ imaging (FlexStation 3) | Andersson et al. (2008) |
| ASP7663 | Synthetic | 0.50μM; mTRPA1, HEK293, Ca2+ imaging (FLIPR) 0.54μM; rTRPA1, HEK293, Ca2+ imaging (FLIPR) 0.51μM; hTRPA1, HEK293, Ca2+ imaging (FLIPR) | Kojima et al. (2014) |
| JT010 | Synthetic | 65nM; species not specified, HEK293, Ca2+ imaging | Takaya et al. (2015) |
Several studies have reported that reactive oxygen species (ROS) and their metabolites activate TRPA1 channels and are likely to be endogenous activators under physiological conditions. Macpherson et al. and Trevisani et al. were the first to report that the lipid peroxidation product, 4-hydroxy-2-nonenal (4-HNE), stimulates TRPA1 channels (Macpherson et al., 2007; Trevisani et al., 2007). Subsequently, two similar lipid peroxidation products, 4-oxononenal (4-ONE) and 4-hydroxyhexenal (4-HHE) were also found to activate TRPA1 (Taylor-Clark et al., 2008). Additionally, it has been reported that TRPA1 is activated by hydrogen peroxide (H2O2) (Andersson et al., 2008; Bessac et al., 2008), the hydroxyl radical (•OH) (Bessac et al., 2008), the cyclopentenone prostaglandin 15-deoxy-delta(12,14)-prostaglandin J(2) [15d-PGJ(2)] (Andersson et al., 2008), nitric oxide and peroxynitrite (Miyamoto, Dubin, Petrus, & Patapoutian, 2009).
4.2. Inhibitors
Several selective small-molecule TRPA1 inhibitors are available (Table 2). The first-described and most commonly used TRPA1 inhibitor is HC-030031 (McNamara et al., 2007). Chembridge-5861528, a derivative of HC-030031, has similar potency and specificity, but better solubility (Wei et al., 2009). Interestingly, AP-18, a partial agonist of TRPA1, exerts a net inhibitory effect on TRPA1 activity by desensitizing the channel (Defalco et al., 2010; Petrus et al., 2007). A-967079, Compound 10, and Compound 31 are potent available TRPA1 inhibitors with half-maximal inhibitory concentration (IC50) values within the nanomolar range (Chen et al., 2011; Copeland et al., 2014; McGaraughty et al., 2010; Rooney et al., 2014). Previously, compounds such as camphor (Macpherson et al., 2006) and ruthenium red (Nagata et al., 2005) were used to block TRPA1 channels. However, these inhibitors lack specificity and thus are not suitable for experimental or clinical purposes (Story et al., 2003).
Table 2.
List of TRPA1 channel antagonists.
| Antagonist | IC50; isoform | References |
|---|---|---|
| HC-030031 | 7.6μM; rTRPA1 5.3–6.2 uM; hTRPAl | McNamara et al. (2007) |
| Chembridge-5861528 | 14.3–18.7μM; hTRPAl | Wei et al. (2010) and Wei, Hamalainen, Saarnilehto, Koivisto, and Pertovaara (2009) |
| AP-18 | 4.5μM; mTRPA1 3.1μM; hTRPA1 | Defalco et al. (2010) and Petrus et al. (2007) |
| A-967079 | 289nM; rTRPA1 67 nM; hTRPAl | McGaraughty et al. (2010) |
| Compound 10 | 45nM; rTRPA1 170 nM; hTRPAl | Copeland et al. (2014) |
| Compound 31 | 85nM; rTRPA1 15nM; hTRPA1 | Rooney et al. (2014) |
5. TRPA1 channels and vascular control
5.1. TRPA1 channels in sensory neurons
Bautista et al. (2005) provided the first evidence demonstrating a role for TRPA1 channels in the regulation of vascular tone through the release of sensory nerve-derived neuropeptides. They discovered that exogenous application of AITC, purified allicin, and diallyl disulfide (DADS) relaxed pre-contracted rat mesenteric arteries (Bautista et al., 2005). This response was diminished by the non-selective TRP channel antagonist ruthenium red, capsaicin-induced sensory nerve denervation, and the calcitonin gene-related peptide (CGRP) receptor antagonist CGRP8–37, but was unaffected by the TRPV1 (TRP vanilloid 1) inhibitor capsazepine (Bautista et al., 2005). TRPA1 was found to be expressed in a subpopulation of adventitial sensory neurons surrounding rat mesenteric arteries. Furthermore, TRPA1 was detected in sensory neurons that also contain the potent vasodilator CGRP (Bautista et al., 2005; Brain, Williams, Tippins, Morris, & MacIntyre, 1985). These data suggest that TRPA1 is localized to sensory nerve terminals of the vasculature and their activation results in CGRP-dependent vasodilation, suppositions validated by Pozsgai et al. (2010). Cinnamaldehyde was found to induce a relaxation response in isolated mesenteric arteries that was unaffected by endothelium removal, but was significantly diminished in preparations from global TRPA1-knockout (KO) mice (Pozsgai et al., 2010). In vivo, topical application of cinnamaldehyde or AITC onto the ears of anesthetized mice increases blood flow in wild-type mice, but not TRPA1-KO mice (Pozsgai et al., 2010). A follow-up study by this group demonstrated that cinnamaldehyde-induced increases in blood flow were diminished by HC-030031 and CGRP8–37, and were blunted in CGRP-KO mice (Aubdool et al., 2016). Intraplantar injections of 4-ONE into the hind paw of anesthetized mice was shown to increase blood flow, a response that was absent in TRPA1- and CGRP-KO mice, but was unaffected in TRPV1-KO mice (Graepel et al., 2011). Kunkler et al. investigating a role for TRPA1 channels in meningeal vasodilation, reported that AITC, cinnamaldehyde, and acrolein induced an increase in release of CGRP from cultured rat trigeminal neurons that was sensitive to TRPA1 inhibition with HC-030031 (Kunkler, Ballard, Oxford, & Hurley, 2011). Moreover, nasal administration of these TRPA1 agonists induced a transient increase in meningeal blood flow that was blocked by CGRP8–37 and HC-030031 (Kunkler et al., 2011). Collectively, these data support the hypothesis proposed by Bautista et al. (2005) that TRPA1 channels are expressed on sensory neurons of the adventitia of blood vessels. Upon activation, Ca2+ influx causes CGRP to be released and bind its G protein-coupled receptor on the underlying smooth muscle cells to cause membrane hyperpolarization and relaxation (Fig. 2).
Fig. 2.
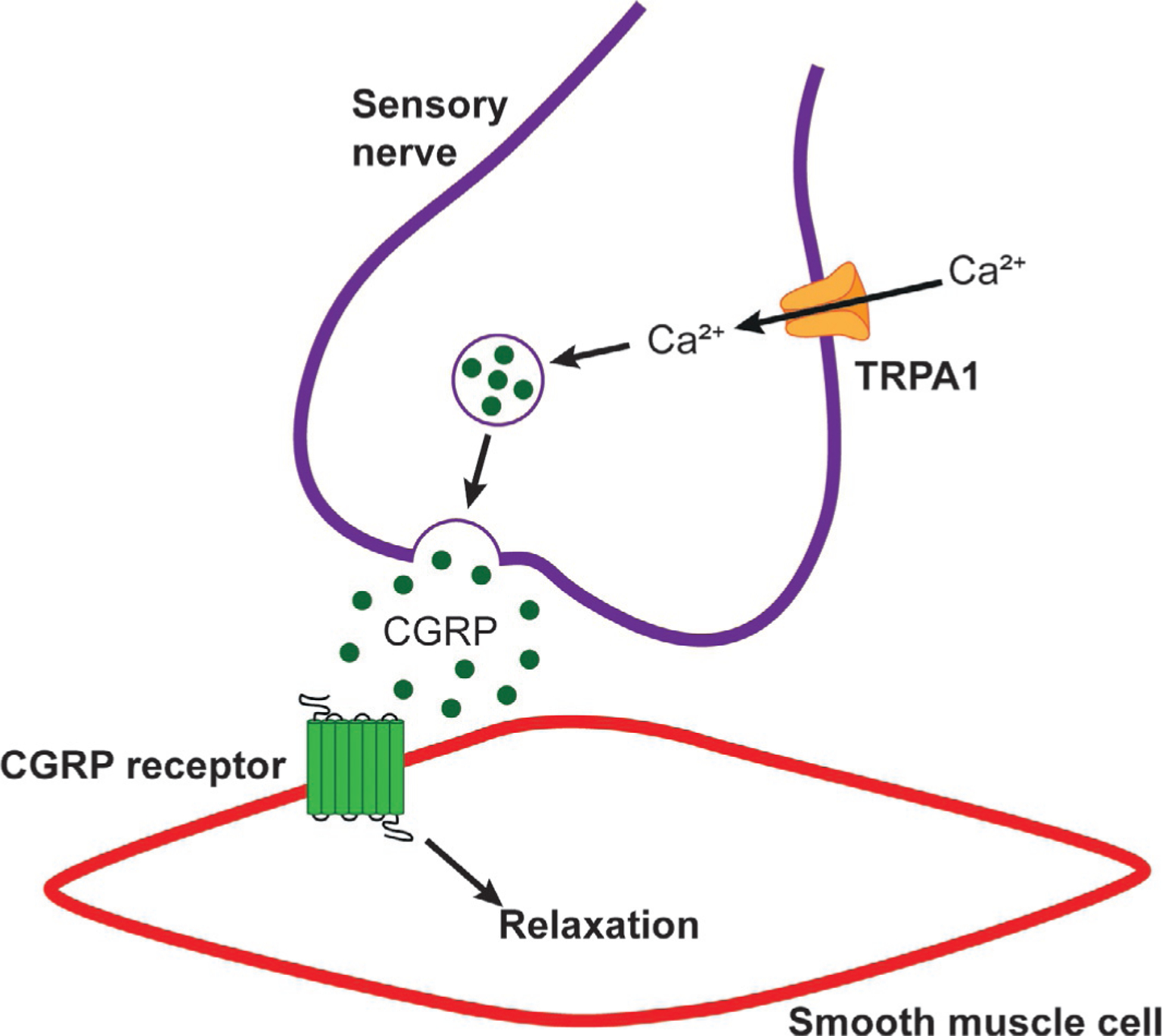
Sensory nerve TRPA1-dependent vasodilation. Activation of TRPA1 channels on pre-synaptic sensory nerves leads to Ca2+ influx. This causes the release of CGRP, which binds CGRP receptors on vascular smooth muscle cells and mediates hyperpolarization and relaxation.
In addition to CGRP, neuronal nitric oxide (NO) and ROS are involved in TRPA1-dependent neurogenic dilation. Cinnamaldehyde-induced increases in mouse ear blood flow were attenuated by the selective neuronal nitric oxide synthase (nNOS) inhibitor, S-methyl-l-thiocitrulline (SMTC), and by ROS scavenging (Aubdool et al., 2016). The authors of this study proposed that superoxide and NO react to form peroxynitrite, which mediates dilation. Peroxynitrite is known to relax blood vessels in a cGMP-dependent manner (Casey et al., 2012; Dowell & Martin, 1997). Increased protein nitrosylation was observed following cinnamaldehyde treatment, and cinnamaldehyde-induced dilation was reduced in mice treated with the peroxynitrite scavenger FeTPPs (Aubdool et al., 2016). However, the precise mechanism by which superoxide is produced following TRPA1 activation, which was not assessed in this study, remains unclear.
The peripheral vascular response to noxious cold has been studied for several decades (Daanen, 2003; Keatinge, 1957). Local cold exposure produces an initial rapid vasoconstriction lasting approximately 5–10 min, followed by a vasodilatory recovery phase that protects against cold-induced injury, such as chilblains (Daanen, 2003; Keatinge, 1957). Aubdool et al. (2014) proposed that TRPA1 channels are the cold sensors that initiate this vascular response. They showed that exposure of the mouse hind paw to 10 °C produced a biphasic vascular response that was absent in TRPA1-KO mice or wild-type animals treated with HC-030031 (Aubdool et al., 2014). The authors proposed that the initial vasoconstriction is dependent on TRPA1 activation-dependent production of ROS, which in turn causes release of norepinephrine from sympathetic neurons. Norepinephrine binds to α2C-adrenoceptors, activating Rho-kinase to mediate vasoconstriction. However, sympathetic nerve denervation with guanethidine or blockade of α-adrenoceptors with phentolamine partially attenuated the contractile response, suggesting involvement of other contractile pathways (Aubdool et al., 2014). Moreover, neither the precise cellular location of TRPA1 responsible for mediating the contractile phase nor the mechanisms by which ROS was produced downstream of TRPA1 activation was determined. Aubdool et al. (2014) found that TRPA1 is also involved in the vasodilatory phase, showing that intravenous administration of HC-030031 immediately following the peak contraction prevented cold-induced vasodilation. Sensory nerves were found to be responsible for mediating the vasodilation phase, as evidenced by the fact that sensory nerve denervation, as well as CGRP and nNOS inhibition, attenuated the response. The role of TRPA1 channels as cold sensors remains controversial, with several reports suggesting that the channel in not activated by exposure to cold temperatures (Bautista et al., 2006; Caspani & Heppenstall, 2009; Jordt et al., 2004). Further investigation will be required to provide a definitive answer to this question.
5.2. TRPA1 channels in the endothelium
Work from our laboratory has shown that TRPA1 channels are localized to the endothelium of the cerebral vasculature of humans and rodents, but are not present in the endothelium of mesenteric, coronary, dermal, or renal arteries (Fig. 3A) (Earley et al., 2009; Sullivan et al., 2015). Interestingly, TRPA1 is predominantly found in endothelial cell plasma membrane projections that penetrate through the internal elastic lamina, where they are positioned proximal to vascular smooth muscle cells (Earley et al., 2009). These structures, known as myoendothelial projections (MEPs), house important signaling complexes that regulate the membrane potential and contractility of underlying smooth muscle cells (Griffith, Chaytor, Taylor, Giddings, & Edwards, 2002; Sandow & Hill, 2000). Ledoux et al. demonstrated that the endoplasmic reticulum (ER) of endothelial cells projects into MEPs and that inositol trisphosphate receptors (IP3R) are enriched within this region (Ledoux, Bonev, & Nelson, 2008). This study also reported that spontaneous, transient subcellular Ca2+ events caused by Ca2+ release through IP3Rs on the ER occurred at MEPs. Several studies have reported that intermediate-conductance Ca2+-activated K+ channels (KCa3.1) and connexin proteins 40 and 37 (Cx40 and Cx37) are also expressed at high levels within MEPs (Ledoux et al., 2008; Sandow, Neylon, Chen, & Garland, 2006). Ledoux et al. (2008) proposed that Ca2+ signals within MEPs hyperpolarize the plasma membrane of endothelial cells by activating nearby KCa3.1 channels. The resulting hyperpolarization is subsequently conducted through myoendothelial gap junctions (MEGJs) within MEPs composed of Cx40 and/or Cx37 hemichannels to underlying smooth muscle cells to evoke relaxation.
Fig. 3.
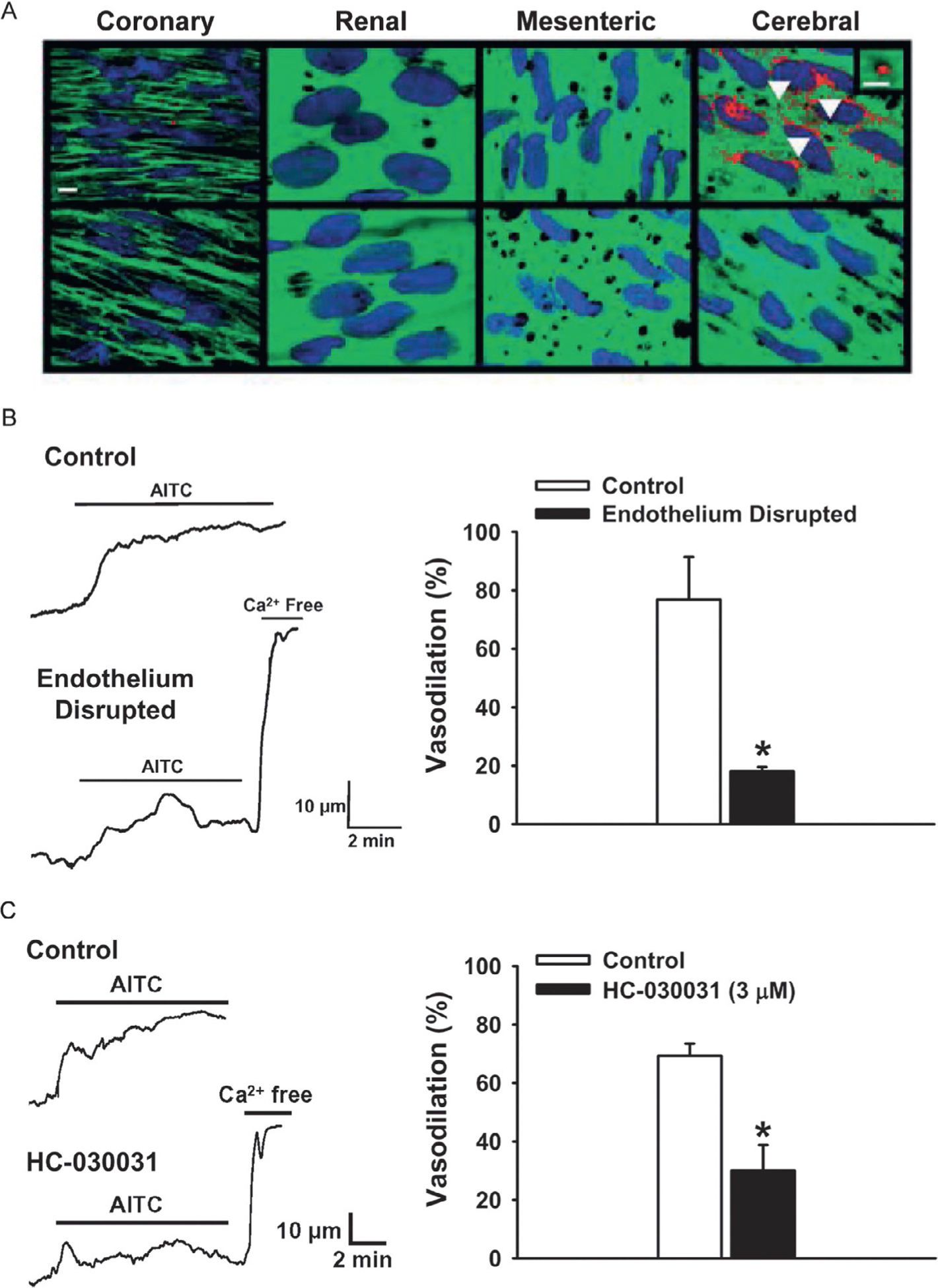
TRPA1 activation causes endothelium-dependent vasodilation in cerebral arteries. (A) (Top) Immunolabeling for TRPA1 (red) in rat coronary, renal, mesenteric and cerebral arteries (scale bar, 10 μm). Green is autofluorescence produced by the internal elastic lamina. TRPA1 was only detected in the endothelium of the cerebral vasculature and not in other vascular beds. Bottom: omission of primary antibody. (B) Representative trace and summary data demonstrating that the TRPA1 agonist AITC (100 μM) induces vasodilation of rat cerebral arteries, which was significantly reduced following disruption of the endothelium (n = 3, *P ≤ 0.05 vs control). (C) Representative trace and summary data showing the vasodilation to AITC is blocked by the selective TRPA1 antagonist HC-030031 (3 μM) (n = 3, *P ≤ 0.05 vs control). Panel (A) image derived from Sullivan, M. N., Gonzales, A. L., Pires, P. W., Bruhl, A., Leo, M. D., Li, W., et al. (2015). Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Science Signaling, 8(358), ra2. doi: https://doi.org/10.1126/scisignal.2005659 (web archive link), Panels (B and C) images derived from Earley, S., Gonzales, A. L., & Crnich, R. (2009). Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circulation Research, 104(8), 987–994. https://doi.org/10.1161/CIRCRESAHA.108.189530 (web archive link).
TRPA1 channels colocalize with KCa3.1 channels within the MEPs of cerebral arteries (Earley et al., 2009; Sullivan et al., 2015). Activation of TRPA1 in isolated rat cerebral arteries with AITC induced a relaxation response that was sensitive to inhibition of TRPA1 channels with HC-030031 and removal of the endothelium (Fig. 3B and C). Inhibition of KCa3.1 and small-conductance Ca2+-activated K+ channels (KCa2.3) with TRAM-34 and apamin, respectively, and inhibition of inwardly rectifying K+ (KIR) channels with Ba2+ also attenuated relaxation to AITC (Earley et al., 2009). These data suggest that Ca2+ influx through TRPA1 channels evokes endothelial cell hyperpolarization through activation of KCa3.1 and KCa2.3 (Fig. 4). This hyperpolarizing signal is propagated through MEGJs to underlying smooth muscle cells. KIR channels amplify the transmitted hyperpolarizing signal, resulting in smooth muscle relaxation (Smith et al., 2008). Qian et al. further characterized the Ca2+-signaling events stimulated by TRPA1 channel activity in isolated rat cerebral arteries, showing that AITC evoked a concentration-dependent increase in Ca2+-signaling events that were localized to MEPs and dependent on IP3R-mediated release of Ca2+ from the ER (Qian, Francis, Solodushko, Earley, & Taylor, 2013). The authors suggested that Ca2+ influx through TRPA1 channels present within MEPs stimulated the formation of larger Ca2+-signaling events by stimulating IP3R activity through a Ca2+-induced Ca2+-release mechanism. The physiological significance of this mechanism was revealed by the observation that the frequency of IP3R-dependent Ca2+-signaling events generated by stimulating TRPA1 channels was directly correlated with the vasodilatory response.
Fig. 4.

Endothelial TRPA1-dependent dilation of cerebral arteries. Activation of TRPA1 channels present in myoendothelial projections (MEPs) causes Ca2+ influx. Ca2+ is released from the endothelial cell ER through IP3Rs via a Ca2+-induced Ca2+-release mechanism. Intermediate conductance Ca2+-activated K+ channels (KCa3.1) are activated and the resulting K+ efflux causes hyperpolarization of the endothelial cell plasma membrane (EC PM). The change in membrane potential (ΔEM) is conducted to the underling smooth muscle through myoendothelial gap junctions (MEGJs), resulting in hyperpolarization of the smooth muscle plasma membrane (SM PM) and relaxation.
Distinctive Ca2+-signaling events reflecting Ca2+ influx through single TRP channels can be optically recorded in isolated cells and the intact endothelium using TIRF (total internal reflection fluorescent) microscopy (Sullivan & Earley, 2013; Sullivan, Francis, Pitts, Taylor, & Earley, 2012; Sullivan et al., 2015) or high-speed high-resolution spinning-disk confocal microscopy (Sonkusare et al., 2012). These Ca2+-signaling events are generically called “sparklets” and are typically associated with the name of the conducting channel (e.g., “TRPA1 sparklets”). Sullivan et al. recorded TRPA1 sparklets in native cerebral endothelial cells using TIRF microscopy (Sullivan et al., 2015), reporting that TRPA1 sparklets are large Ca2+ influx events, with a unitary amplitude approximately twice that of TRPV4 sparklets, probably due to the larger single-channel conductance and greater Ca2+ fraction conducted by TRPA1 (Story et al., 2003; Sullivan et al., 2012, 2015). The signal mass of a single TRPA1 sparklet was estimated to be 200-fold greater than that of a single l-type Ca2+ channel sparklet (Sullivan et al., 2015). Moreover, the most frequently occurring TRPA1 sparklets recorded had amplitudes that were consistent with the simultaneous opening of two TRPA1 channels and a doubling of the amount of Ca2+ influx during an event. This suggests that TRPA1 channels exist in a functionally linked binary configuration on the plasma membrane and that opening of one channel triggers cooperative gating of the adjacent channel, possibly through binding of incoming Ca2+ to the EF-hand domain located on the N-terminus of the adjacent TRPA1 channel (Jordt et al., 2004).
Sullivan et al. demonstrated that TRPA1 sparklet activity is low under basal conditions, and that the large localized increases in subcellular Ca2+ concentration induced by TRPA1 agonists occurs through recruitment of previously inactive TRPA1 channels, rather than through increases in the frequency of basally active TRPA1 channels (Sullivan et al., 2015). Notably, Sullivan et al. found that only 4–8 TRPA1 sparklets sites per cell were active following stimulation with a concentration of AITC that produced maximal dilation of isolated cerebral arteries (Sullivan et al., 2015), implying that the vast majority of TRPA1 channels are inactive even under optimal stimulation conditions. This phenomenon of “silent” channels has been observed for several types of ion channels. For example, in studies of TRPV4 sparklets in primary human endothelial cells, Sullivan et al. showed that only a few sparklet sites were detectable even during maximal stimulation with the TRPV4 agonist GSK1016790A, despite immunolabeling experiments revealing a cell-wide distribution of TRPV4 channels (Sullivan et al., 2012). An examination of l-type Ca2+ channels in rat cerebral artery smooth muscle cells by Navedo et al. revealed similar characteristics, further suggesting that ion channels present in the plasma membrane may exist in a “silent” state (Navedo, Amberg, Votaw, & Santana, 2005). Although the reason for this is not clear, it may reflect nanoscale association of channels with other signaling elements (Mercado et al., 2014; Navedo et al., 2005; Tajada et al., 2017).
TRPA1 is activated by the endogenously generated ROS metabolites, 4-HNE, 4-ONE, and 4-HHE (Andersson et al., 2008; Macpherson et al., 2007; Trevisani et al., 2007; Uchida, 2003). Sullivan et al. demonstrated that exogenous application of 4-HNE increased TRPA1 sparklet frequency and relaxed isolated cerebral arteries, and further showed that these responses were abolished by HC-030031 and were absent in tissues isolated from endothelial cell-specific TRPA1-KO (Trpa1-ecKO) mice (Sullivan et al., 2015). TRPA1 was found to colocalize with the ROS-generating enzyme, NADPH oxidase 2 (NOX2), in MEPs of isolated cerebral arteries (Sullivan et al., 2015). Exogenous administration of the NOX2 substrate NADPH increased TRPA1 sparklet frequency and dilated cerebral arteries isolated from wild-type mice, but not those from Trpa1-ecKO mice (Sullivan et al., 2015). Moreover, the response to NADPH was inhibited by catalase and iron chelation, indicating that extracellular H2O2 and hydroxyl radicals (•OH) were generated—intermediates that are involved in the production of 4-HNE. These data suggest that NOX2-derived lipid peroxidation products are endogenous activators of TRPA1 channels in the cerebral endothelium and cause endothelium-dependent dilation in this vascular segment.
5.3. TRPA1 channels in vascular smooth muscle cells
There is little evidence to support the expression of functional TRPA1 channels on vascular smooth muscle cells. In an examination of relaxation of the rat aorta to cinnamaldehyde, Yanaga et al. showed that relaxation responses were impaired by endothelium removal or NOS inhibition, but were not completely abolished (Yanaga et al., 2006). This implies that cinnamaldehyde is able to dilate arteries through direct effects on the smooth muscle. However, this study did not test the effect of TRPA1 inhibitors on this response, leaving doubt about the specific involvement of TRPA1 channels. Bodkin et al. further demonstrated that cinnamaldehyde-induced relaxation of mouse mesenteric arteries was independent of the endothelium (Bodkin et al., 2014). However, relaxation persisted in the presence of HC-030031 and in preparations from TRPA1-KO mice, indicating that cinnamaldehyde-induced relaxation occurs independently of TRPA1. TRPA1 is not found in the smooth muscle of the cerebral vasculature, and these cells do not exhibit a Ca2+ response to AITC (Earley et al., 2009). Thus, it remains unlikely that TRPA1 channels are expressed in vascular smooth muscle cells, and observations of direct vasorelaxant effects by TRPA1 agonists are likely attributable to off-target actions.
5.4. TRPA1 and integrative cardiovascular physiology
It is clear that activation of TRPA1 channels mediates dilation of the peripheral vasculature, but what are the integrative effects of TRPA1 channel activity on global cardiovascular hemodynamics? Pozagai et al. reported comparable mean arterial pressure (MAP) and heart rate in anesthetized wild-type and global TRPA1-KO mice (Pozsgai et al., 2010). A follow-up study from this group validated these findings in freely-moving, conscious mice using radio-telemetry devices (Bodkin et al., 2014). Interestingly, this latter study noted increased locomotor activity in global TRPA1-KO mice, although the underlying mechanism was not investigated. These studies suggest that unstressed mice are able to compensate for loss of TRPA1 activity and maintain basal cardiovascular function.
Pozagai et al. (2010) examined the effects of TRPA1 activation on the systemic circulation. The authors noted a bimodal effect of intravenous cinnamaldehyde administration in anesthetized female mice, with an initial, transient reduction in MAP and heart rate (depressor response) that was dose-independent, followed by a subsequent dose-dependent sustained rise in both parameters (pressor response) (Pozsgai et al., 2010). Further experiments in TRPA1-KO mice revealed that the depressor response was diminished in knockout mice, but the pressor response was absent following a moderate cinnamaldehyde dose (80 μM/kg body weight). However, at higher doses (320 μM/kg body weight) the pressor response, but not the depressor response, was blunted in TRPA1-KO mice. On the basis of experiments performed using TRPV1- and CGRP-KO mice and inhibitors of substance P, the authors proposed that the effects of cinnamaldehyde administration were independent of sensory nerve-derived vasodilators. Cholinergic receptor blockade diminished the pressor response to cinnamaldehyde, but had little effect on the pressor response. α-Adrenergic inhibition attenuated both pressor and depressor responses, but combined ganglionic blockade and cholinergic receptor blockade did not influence the pressor response. The authors concluded that the depressor response was due to activation of the vasovagal reflex, while the depressor response was mediated by peripheral sympathetic activation (Pozsgai et al., 2010). However, because of the transient nature of the depressor response, it is difficult to draw meaningful conclusions. Moreover, the lack of a relationship between cinnamaldehyde dose and magnitude of the reduction in MAP and heart rate, as well as the absence of a discernable difference between wild-type and TRPA1-KO mice at higher cinnamaldehyde doses further confound data interpretation. Additional experiments, in particular, investigations of both short- and long-term effects of TRPA1 agonists and antagonists on cardiovascular parameters in conscious animals, are required to better understand the significance of systemic TRPA1 activation in the regulation of cardiovascular hemodynamics.
In an examination of the role of TRPA1 channels in renal sympathetic nerve activity (RSNA), Koda et al. showed that intra-arterial injection of AITC into decerebrate rats significantly increased RSNA (Koba, Hayes, & Sinoway, 2011). This response was prevented by sectioning the sciatic nerve, indicating a muscle-based reflex response. Sustained static contraction of the hindlimb muscle, induced by electrical stimulation, resulted in increased RSNA and blood pressure that were diminished by TRPA1 blockade with HC-030031. Moreover, HC-030031 reduced RSNA in response to administration of arachidonic acid, bradykinin or diprotonated phosphate, all metabolic byproducts of muscle contraction. These findings were further supported by Xing et al. who observed similar TRPA1-dependent increases in RSNA, MAP, and heart rate (Xing, Lu, & Li, 2015). These latter authors proposed that TRPA1 channels are present on afferent nerves of muscle and may contribute to increased sympathetic nerve activity as well as associated increases in cardiovascular parameters during exercise.
6. TRPA1 in vascular pathology
6.1. Inflammation
Neuronal TRPA1 channels may play an important role in the vascular component of neurogenic inflammation by stimulating the release of substance P, CGRP, and other proinflammatory vasoactive neuropeptides. This was first shown by Trevisani et al. who reported that the TRPA1 agonist 4-HNE caused edema formation and pain-related behavior following intraplantar administration to the hind paw of rats (Trevisani et al., 2007). Further evidence was provided by Andre et al. in a model of cigarette smoke inhalation-induced inflammation, tracheal plasma extravasation was attenuated by TRPA1 inhibition with HC-030031 (Andre et al., 2008). The authors also demonstrated that capillary permeability was increased in wild-type mice following treatment with an aqueous extract of cigarette smoke, but not in global TRPA1-KO mice (Andre et al., 2008), suggesting that TRPA1 is involved in the breakdown of the endothelial cell-to-cell barrier. TRPA1 has been reported to mediate neurogenic inflammation in response to other stimuli, including N-acetyl-p-benzoquinone imine (NAPQI), a toxic by-product of acetaminophen, and the bacterial endotoxin lipopolysaccharide (Meseguer et al., 2014; Nassini et al., 2010). In contrast, Graepel et al. suggested that TRPA1 is important for the vasodilatory effect of neurogenic inflammation, but not edema formation in the mouse hind paw (Graepel et al., 2011). The reasons for these discrepancies are unclear, but these observations may suggest that TRPA1 channel activity mediates neurogenic inflammation in a tissue-specific manner. TRPA1 channels may provide a novel therapeutic target for treating pathological increases in capillary permeability and edema formation associated with inflammation.
A recent study by Zhao et al. suggested that TRPA1 channels have a protective role against the progression of atherosclerosis (Zhao, Shyue, Kou, Lu, & Lee, 2016). Atherosclerosis is a progressive, chronic inflammatory condition that is associated with an accumulation of fatty and/or fibrous material in the innermost layer of the artery that eventually impedes blood flow. It can lead to a number of other complications, such as aneurysms and stroke, and is the leading cause of vascular disease worldwide (Libby et al., 2019). Activation of TRPA1 channels with AITC has been shown to suppress atherosclerotic progression in apolipoprotein E (apoE)-KO mice, a commonly used mouse model of atherosclerosis (Plump et al., 1992; Zhang, Reddick, Piedrahita, & Maeda, 1992; Zhao et al., 2016). Moreover, apoE-KO mice treated with HC-030031 or lacking TRPA1 channels exhibit exacerbated atherosclerotic lesions, hyperlipidemia, and elevated circulating levels of inflammatory cytokines (Zhao et al., 2016). Interestingly, TRPA1 was found to be expressed in macrophages. Macrophages play a pivotal role in the pathogenesis of atherosclerosis by accumulating lipoproteins and forming foam cells, which are retained in the plaque and lead to further plaque growth (Bobryshev, Ivanova, Chistiakov, Nikiforov, & Orekhov, 2016). Moreover, infiltrated macrophages have a reduced ability to migrate, which further contributes to the proinflammatory environment, as well as plaque growth and instability. Zhao et al. proposed that TRPA1 activation prevents TNF-α (tumor necrosis factor-α)-dependent activation of macrophages, thereby reducing ingestion of lipoproteins and plaque formation (Zhao et al., 2016). These results are interesting, but further investigation is required to understand the role of TRPA1 signaling in macrophages and the effects of TRPA1 activation on macrophage recruitment and polarization.
6.2. Hypertension
Hypertension, characterized by elevated systolic and/or diastolic blood pressure, is a prevalent condition that is a major risk factor for multiple cardiovascular morbidities, such as coronary heart disease, heart failure and stroke (Taler, 2018). Studies have shown an exacerbated hypertensive response in mice lacking CGRP, a neuropeptide that is released following TRPA1 activation (Gangula et al., 2000; Smillie et al., 2014). However, Bodkin et al. demonstrated that TRPA1 may be involved in the inflammatory component, but not the blood pressure increase associated with the pathology. Blood pressure increases following subcutaneous infusion of angiotensin II over 14 days were comparable in wild-type and global TRPA1-KO mice, but the proinflammatory cytokine interleukin-6 was found to be reduced in hypertensive TRPA1-KO mice (Bodkin et al., 2014). Ma et al. (2019) further explored this concept in the context of kidney function following angiotensin II infusion over 28 days. Similar to the report of Bodkin et al. (2014), these authors found that systolic blood pressure was comparable between TRPA1-KO mice and wild-type controls (Ma, Zhang, He, Wang, & Wang, 2019). However, kidney dysfunction was exacerbated in knockout mice. Blood urea nitrogen, serum creatinine, renal fibrosis and renal inflammatory cytokine levels were enhanced in hypertensive TRPA1-KO mice (Ma et al., 2019). The authors proposed that TRPA1 inhibits macrophage-dependent production of proinflammatory mediators that provide protection against kidney dysfunction. These data imply that, although targeting TRPA1 may not reduce blood pressure in hypertensive individuals, it may provide benefit in reducing the inflammatory component of the disease.
6.3. Stroke
Stroke is a condition in which sudden interruption of regional blood flow within the brain results in tissue damage, which can lead to a number of long-lasting disabilities. The majority of strokes occur due to blockage of arteries and lead to tissue ischemia and hypoxia. Cerebral blood vessels dilate in response to hypoxia, a response that serves to enhance oxygen delivery to ischemic brain regions (Kontos et al., 1978). Takahashi et al. suggested that TRPA1 channels are maximally activated by a reduction in the partial pressure of O2 (pO2) from ~150 to ~80 mmHg, defined by the authors of this study as hypoxia (Takahashi et al., 2011). However, under normoxic conditions, pO2 levels range from ~100 mmHg within the lungs to ~20–40 mmHg in the venous system (Tsai, Johnson, & Intaglietta, 2003). According to the model proposed by Takahashi et al. (2011), this would suggest that TRPA1 channels are constitutively active, an inference inconsistent with the literature (Pires & Earley, 2017). It is more likely that, in the study by Takahashi et al. (2011), TRPA1 channels are activated by ROS generated by the transition from hyperoxic (pO2, 150 mmHg) to normoxic (pO2, 80 mmHg) conditions. In a previous study from our lab examining the effects of acute hypoxia on TRPA1 activity (Pires & Earley, 2018), a pO2 of ~10–15 mmHg, which is comparable to the level in the peri-infarct area following an ischemic stroke, was used as a pathophysiologically relevant pO2 level (Liu et al., 2004; Pires & Earley, 2018). Acute hypoxia increased TRPA1 sparklet frequency and dilated pressurized mouse cerebral arteries and penetrating parenchymal arterioles. Additionally, hypoxia increased 4-HNE levels in the cerebral endothelium (Pires & Earley, 2018). As noted above, NOX2 is responsible for producing ROS and driving TRPA1 activity under physiological conditions within the cerebral vasculature (Sullivan et al., 2015). However, hypoxia-induced dilation was unaffected by NOX inhibition with apocynin or extracellular superoxide dismutase (SOD), but was attenuated by membrane-permeant PEG-SOD and mitochondrial-targeted antioxidant mitoTEMPO (Pires & Earley, 2018). These observations suggest that hypoxia increases mitochondrial ROS generation. It has been reported that mitochondria are localized near MEPs, providing an alternative pathway of TRPA1-dependent vasodilation in the cerebral vasculature during hypoxic conditions (Maarouf, Sancho, Furstenhaupt, Tran, & Welsh, 2017). The in vivo importance of hypoxia-induced activation of TRPA1 channels was assessed in Trpa1-ecKO mice using the middle cerebral artery occlusion model of ischemic stroke (Pires & Earley, 2018). Interestingly, the infarct area produced following occlusion was significantly greater in Trpa1-ecKO mice, suggesting that TRPA1 protects against tissue damage associated with ischemia. Furthermore, treatment of control mice with the TRPA1 agonist cinnamaldehyde 15 min post-occlusion reduced infarct area. These data suggest that endothelial TRPA1 channels evoke vasodilation and increase blood flow in response to hypoxic conditions. TRPA1 is important for an early adaptive response to an acute reduction in pO2 levels that serves to improve collateral blood flow to affected regions and improve tissue outcome.
7. Conclusions
This chapter has described the importance of TRPA1 channels in vascular physiology and pathophysiology. It is evident that these channels regulate vascular tone in a tissue-dependent manner. Activation of TRPA1 channels using pungent dietary compounds evokes a robust vasodilatory response either through release of vasoactive neuropeptides from sensory nerves or via endothelial cell-dependent hyperpolarization pathways. Under physiological conditions, TRPA1 is likely to be regulated endogenously by ROS and their metabolites, rather than by consumed dietary molecules. Novel therapeutic strategies may target TRPA1 channels in the treatment of certain diseases, such as neurogenic inflammation, atherosclerosis, and ischemic stroke. However, whether such treatment strategies will prevent the development of disease or provide treatment for existing conditions remains unclear. Moreover, understanding the cardiovascular effects of short- and long-term TRPA1 activation and inhibition on basal function and pathology remains unclear, and should be a focus of studies going forward.
Acknowledgments
Support for this chapter was provided by grants from the National Heart, Lung, and Blood Institute (R01HL091905, R01HL137852, and R01HL139585), the National Institute of Neurological Disorders and Stroke (RF1NS110044 and R61NS115132), and the National Institute of General Medicine (P20GM130459) to S.E.
Footnotes
Disclosures
The authors have no conflicts of interest, financial or otherwise, to report.
References
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, et al. (2011). TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nature Communications, 2, 551 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, & Bevan S (2008). Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. The Journal of Neuroscience, 28(10), 2485–2494. 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, & Bevan S (2009). Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proceedings of the National Academy of Sciences of the United States of America, 106(20), 8374–8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. (2008). Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. The Journal of Clinical Investigation, 118(7), 2574–2582. 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei SR, Sinharoy P, Bratz IN, & Damron DS (2016). TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: Co-localization at z-discs, costameres and intercalated discs. Channels (Austin, Tex.), 10(5), 395–409. 10.1080/19336950.2016.1185579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, et al. (2014). TRPA1 is essential for the vascular response to environmental cold exposure. Nature Communications, 5, 5732 10.1038/ncomms6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubdool AA, Kodji X, Abdul-Kader N, Heads R, Fernandes ES, Bevan S, et al. (2016). TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. British Journal of Pharmacology, 173(15), 2419–2433. 10.1111/bph.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia PK, Parks TA, Stanford KR, Mitchell DA, Varma S, Stevens SM Jr., et al. (2016). The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1. The Journal of General Physiology, 147(6), 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron, 41(6), 849–857. 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Banke TG, Chaplan SR, & Wickenden AD (2010). Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. American Journal of Physiology-Cell Physiology, 298(6), C1457–C1468. 10.1152/ajpcell.00489.2009. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell, 124(6), 1269–1282. 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. (2005). Pungent products from garlic activate the sensory ion channel TRPA1. Proceedings of the National Academy of Sciences of the United States of America, 102(34), 12248–12252. 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, & Jordt SE (2008). TRPA1 is a major oxidant sensor in murine airway sensory neurons. The Journal of Clinical Investigation, 118(5), 1899–1910. 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkov YV, Corey EA, & Ache BW (2011). The pore properties of human nociceptor channel TRPA1 evaluated in single channel recordings. Biochimica et Biophysica Acta, 1808(4), 1120–1128. 10.1016/j.bbamem.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, & Orekhov AN (2016). Macrophages and their role in atherosclerosis: Pathophysiology and trans-criptome analysis. BioMed Research International, 2016, 9582430 10.1155/2016/9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JV, Thakore P, Aubdool AA, Liang L, Fernandes ES, Nandi M, et al. (2014). Investigating the potential role of TRPA1 in locomotion and cardiovascular control during hypertension. Pharmacology Research & Perspectives, 2(4). e00052. 10.1002/prp2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, & MacIntyre I (1985). Calcitonin gene-related peptide is a potent vasodilator. Nature, 313(5997), 54–56. 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Casey DB, Pankey EA, Badejo AM, Bueno FR, Bhartiya M, Murthy SN, et al. (2012). Peroxynitrite has potent pulmonary vasodilator activity in the rat. Canadian Journal of Physiology and Pharmacology, 90(4), 485–500. 10.1139/Y2012-012. [DOI] [PubMed] [Google Scholar]
- Caspani O, & Heppenstall PA (2009). TRPA1 and cold transduction: An unresolved issue? The Journal of General Physiology, 133(3), 245–249. 10.1085/jgp.200810136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, et al. (2011). Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain, 152(5), 1165–1172. 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, et al. (2009). Pore dilation occurs in TRPA1 but not in TRPM8 channels. Molecular Pain, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AP, Akyuz N, & Corey DP (2016). The outer pore and selectivity filter of TRPA1. PLoS One, 11(11), e0166167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KW, Boezio AA, Cheung E, Lee J, Olivieri P, Schenkel LB, et al. (2014). Development of novel azabenzofuran TRPA1 antagonists as in vivo tools. Bioorganic & Medicinal Chemistry Letters, 24(15), 3464–3468. 10.1016/j.bmcl.2014.05.069. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales JF, Gracheva EO, & Julius D (2011). Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proceedings of the National Academy of Sciences of the United States of America, 108(46), E1184–E1191. 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosens DJ, & Manning A (1969). Abnormal electroretinogram from a Drosophila mutant. Nature, 224(5216), 285–287. 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Cvetkov TL, Huynh KW, Cohen MR, & Moiseenkova-Bell VY (2011). Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. The Journal of Biological Chemistry, 286(44), 38168–38176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daanen HA (2003). Finger cold-induced vasodilation: A review. European Journal of Applied Physiology, 89(5), 411–426. 10.1007/s00421-003-0818-2. [DOI] [PubMed] [Google Scholar]
- Defalco J, Steiger D, Gustafson A, Emerling DE, Kelly MG, & Duncton MA (2010). Oxime derivatives related to AP18: Agonists and antagonists of the TRPA1 receptor. Bioorganic & Medicinal Chemistry Letters, 20(1), 276–279. 10.1016/j.bmcl.2009.10.113. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, & Wetzel CH (2007). Transient receptor potential channel A1 is directly gated by calcium ions. The Journal of Biological Chemistry, 282(18), 13180–13189. [DOI] [PubMed] [Google Scholar]
- Dowell FJ, & Martin W (1997). The effects of peroxynitrite on rat aorta: Interaction with glucose and related substances. European Journal of Pharmacology, 338(1), 43–53. 10.1016/s0014-2999(97)01320-4. [DOI] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, & Crnich R (2009). Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circulation Research, 104(8), 987–994. 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt MJ, Filipovic MR, Leffler A, de la Roche J, Kistner K, Fischer MJ, et al. (2012). Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): A possible mechanism of metabolic neuropathies. The Journal of Biological Chemistry, 287(34), 28291–28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo O, Meseguer V, Belmonte C, & Viana F (2008). TRPA1 channels: Novel targets of 1,4-dihydropyridines. Channels (Austin, Tex.), 2(6), 429–438. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, et al. (2000). Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension, 35(1 Pt. 2), 470–475. 10.1161/01.hyp.35.1.470. [DOI] [PubMed] [Google Scholar]
- Gaudet R (2008). A primer on ankyrin repeat function in TRP channels and beyond. Molecular BioSystems, 4(5), 372–379. 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graepel R, Fernandes ES, Aubdool AA, Andersson DA, Bevan S, & Brain SD (2011). 4-Oxo-2-nonenal (4-ONE): Evidence of transient receptor potential ankyrin 1-dependent and -independent nociceptive and vasoactive responses in vivo. The Journal of Pharmacology and Experimental Therapeutics, 337(1), 117–124. 10.1124/jpet.110.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, & Edwards DH (2002). cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proceedings of the National Academy of Sciences of the United States of America, 99(9), 6392–6397. 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. (2011). TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environmental Health Perspectives, 119(7), 951–957. 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, & Julius D (2006). TRP channel activation by reversible covalent modification. Proceedings of the National Academy of Sciences of the United States of America, 103(51), 19564–19568. 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Bandell M, Petrus MJ, Zhu MX, & Patapoutian A (2009). Zinc activates damage-sensing TRPA1 ion channels. Nature Chemical Biology, 5(3), 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature, 427(6971), 260–265. 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, et al. (2010). Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature, 464(7288), 597–600. 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, et al. (2007). Bimodal action of menthol on the transient receptor potential channel TRPA1. The Journal of Neuroscience, 27(37), 9874–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, & Nilius B (2010). Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophysical Journal, 98(5), 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge WR (1957). The effect of general chilling on the vasodilator response to cold. The Journal of Physiology, 139(3), 497–507. 10.1113/jphysiol.1957.sp005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba S, Hayes SG, & Sinoway LI (2011). Transient receptor potential A1 channel contributes to activation of the muscle reflex. American Journal of Physiology. Heart and Circulatory Physiology, 300(1), H201–H213. 10.1152/ajpheart.00547.2009. [DOI] [PubMed] [Google Scholar]
- Kojima R, Nozawa K, Doihara H, Keto Y, Kaku H, Yokoyama T, et al. (2014). Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. European Journal of Pharmacology, 723, 288–293. 10.1016/j.ejphar.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Raper AJ, Rosenblum WI, Navari RM, & Patterson JL Jr. (1978). Role of tissue hypoxia in local regulation of cerebral microcirculation. The American Journal of Physiology, 234(5), H582–H591. 10.1152/ajpheart.1978.234.5.H582. [DOI] [PubMed] [Google Scholar]
- Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, et al. (2010). A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron, 66(5), 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Ballard CJ, Oxford GS, & Hurley JH (2011). TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain, 152(1), 38–44. 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. (2006). TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron, 50(2), 277–289. 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Bonev AD, & Nelson MT (2008). Ca2+-activated K+ channels in murine endothelial cells: Block by intracellular calcium and magnesium. The Journal of General Physiology, 131(2), 125–135. 10.1085/jgp.200709875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, & Marszalek PE (2006). Nanospring behaviour of ankyrin repeats. Nature, 440(7081), 246–249. 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- Leffler A, Lattrell A, Kronewald S, Niedermirtl F, & Nau C (2011). Activation of TRPA1 by membrane permeable local anesthetics. Molecular Pain, 7, 62 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Toombes GE, Silberberg SD, & Swartz KJ (2015). Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nature Neuroscience, 18(11), 1577–1583. 10.1038/nn.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. (2019). Atherosclerosis. Nature Reviews Disease Primers, 5(1), 56 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, & Liu KJ (2004). Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism, 24(3), 343–349. 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Ma S, Zhang Y, He K, Wang P, & Wang DH (2019). Knockout of TRPA1 exacerbates angiotensin II-induced kidney injury. American Journal of Physiology. Renal Physiology, 317(3), F623–F631. 10.1152/ajprenal.00069.2019. [DOI] [PubMed] [Google Scholar]
- Maarouf N, Sancho M, Furstenhaupt T, Tran CH, & Welsh DG (2017). Structural analysis of endothelial projections from mesenteric arteries. Microcirculation, 24(3), e12330 10.1111/micc.12330. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. (2005). The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Current Biology, 15(10), 929–934. 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, & Story GM (2006). More than cool: Promiscuous relationships of menthol and other sensory compounds. Molecular and Cellular Neurosciences, 32(4), 335–343. 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, et al. (2007). An ion channel essential for sensing chemical damage. The Journal of Neuroscience, 27(42), 11412–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, & Kym PR (2010). TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Molecular Pain, 6, 14 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. (2007). TRPA1 mediates formalin-induced pain. Proceedings of the National Academy of Sciences of the United States of America, 104(33), 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents JE, Fischer MJ, & McNaughton PA (2016). Agonist-induced sensitisation of the irritant receptor ion channel TRPA1. The Journal of Physiology, 594(22), 6643–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, et al. (2014). Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. The Journal of General Physiology, 143(5), 559–575. 10.1085/jgp.201311050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, et al. (2014). TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nature Communications, 5, 3125 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Dubin AE, Petrus MJ, & Patapoutian A (2009). TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One, 4(10), e7596 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosavi LK, Minor DL Jr., & Peng Z-Y (2002). Consensus-derived structural determinants of the ankyrin repeat motif. Proceedings of the National Academy of Sciences of the United States of America, 99(25), 16029–16034. 10.1073/pnas.252537899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, & Garcia-Anoveros J (2005). Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. The Journal of Neuroscience, 25(16), 4052–4061. 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Andre E, Sartiani L, Aldini G, Trevisani M, et al. (2010). Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. The FASEB Journal, 24(12), 4904–4916. 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, et al. (2012). Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One, 7(8), e42454 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Votaw VS, & Santana LF (2005). Constitutively active L-type Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 11112–11117. 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Appendino G, & Owsianik G (2012). The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflügers Archiv, 464(5), 425–458. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, & Owsianik G (2011). Irritating channels: The case of TRPA1. The Journal of Physiology, 589(Pt. 7), 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguri G, Nakajima T, Yamamoto Y, Takano N, Tanaka T, Kikuchi H, et al. (2014). Effects of methylglyoxal on human cardiac fibroblast: Roles of transient receptor potential ankyrin 1 (TRPA1) channels. American Journal of Physiology. Heart and Circulatory Physiology, 307(9), H1339–H1352. 10.1152/ajpheart.01021.2013. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Armache JP, Gao Y, Cheng Y, & Julius D (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature, 520(7548), 511–517. 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. (2007). A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Molecular Pain, 3, 40 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires PW, & Earley S (2017). Redox regulation of transient receptor potential channels in the endothelium. Microcirculation, 24(3), e12329 10.1111/micc.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires PW, & Earley S (2018). Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. eLife, 7, e35316 10.7554/eLife.35316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, et al. (1992). Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell, 71(2), 343–353. 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, & Brain SD (2010). Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovascular Research, 87(4), 760–768. 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- Qian X, Francis M, Solodushko V, Earley S, & Taylor MS (2013). Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation, 20(2), 138–148. 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney L, Vidal A, D’Souza AM, Devereux N, Masick B, Boissel V, et al. (2014). Discovery, optimization, and biological evaluation of 5-(2-(trifluoromethyl) phenyl)indazoles as a novel class of transient receptor potential A1 (TRPA1) antagonists. Journal of Medicinal Chemistry, 57(12), 5129–5140. 10.1021/jm401986p. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, & Garrity PA (2005). The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes & Development, 19(4), 419–424. 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad A, Sura L, Benedikt J, Ettrich R, Minofar B, Teisinger J, et al. (2011). The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. The Biochemical Journal, 433(1), 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, & Hill CE (2000). Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circulation Research, 86(3), 341–346. 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, & Garland CJ (2006). Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: Possible relationship to vasodilator function? Journal of Anatomy, 209(5), 689–698. 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie SJ, King R, Kodji X, Outzen E, Pozsgai G, Fernandes E, et al. (2014). An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension, 63(5), 1056–1062. 10.1161/HYPERTENSIONAHA.113.02517. [DOI] [PubMed] [Google Scholar]
- Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, et al. (2008). KIR channels function as electrical amplifiers in rat vascular smooth muscle. The Journal of Physiology, 586(4), 1147–1160. 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, et al. (2012). Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science, 336(6081), 597–601. 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Corey DP, & Schulten K (2005). In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure, 13(4), 669–682. 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. (2003). ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell, 112(6), 819–829. 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Sullivan MN, & Earley S (2013). TRP channel Ca(2+) sparklets: Fundamental signals underlying endothelium-dependent hyperpolarization. American Journal of Physiology. Cell Physiology, 305(10), C999–C1008. 10.1152/ajpcell.00273.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MN, Francis M, Pitts NL, Taylor MS, & Earley S (2012). Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Molecular Pharmacology, 82(3), 464–472. 10.1124/mol.112.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, et al. (2015). Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Science Signaling, 8(358), ra2 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajada S, Moreno CM, O’Dwyer S, Woods S, Sato D, Navedo MF, et al. (2017). Distance constraints on activation of TRPV4 channels by AKAP150-bound PKCalpha in arterial myocytes. The Journal of General Physiology, 149(6), 639–659. 10.1085/jgp.201611709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, et al. (2011). TRPA1 underlies a sensing mechanism for O2. Nature Chemical Biology, 7(10), 701–711. 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Takaya J, Mio K, Shiraishi T, Kurokawa T, Otsuka S, Mori Y, et al. (2015). A potent and site-selective agonist of TRPA1. Journal of the American Chemical Society, 137(50), 15859–15864. 10.1021/jacs.5b10162. [DOI] [PubMed] [Google Scholar]
- Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, et al. (2019). Mammalian transient receptor potential TRPA1 channels: From structure to disease. Physiological Reviews. 10.1152/physrev.00005.2019. [DOI] [PubMed] [Google Scholar]
- Taler SJ (2018). Initial treatment of hypertension. The New England Journal of Medicine,378(7), 636–644. 10.1056/NEJMcp1613481. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, et al. (2008). Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. The Journal of Physiology, 586(14), 3447–3459. 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. (2007). 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proceedings of the National Academy of Sciences of the United States of America, 104(33), 13519–13524. 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AG, Johnson PC, & Intaglietta M (2003). Oxygen gradients in the microcirculation. Physiological Reviews, 83(3), 933–963. 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- Uchida K (2003). 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Progress in Lipid Research, 42(4), 318–343. doi: S0163782703000146 [pii]. [DOI] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Waters HN, McKemy DD, & Liman ER (2008). The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. The Journal of Biological Chemistry, 283(47), 32691–32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cvetkov TL, Chance MR, & Moiseenkova-Bell VY (2012). Identification of in vivo disulfide conformation of TRPA1 ion channel. The Journal of Biological Chemistry, 287(9), 6169–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Chapman H, Saarnilehto M, Kuokkanen K, Koivisto A, & Pertovaara A (2010). Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil-treated non-diabetic rat. Neuropharmacology, 58(3), 578–584. 10.1016/j.neuropharm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, & Pertovaara A (2009). Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology, 111(1), 147–154. 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- Woll KA, Skinner KA, Gianti E, Bhanu NV, Garcia BA, Carnevale V, et al. (2017). Sites contributing to TRPA1 activation by the anesthetic propofol identified by photoaffinity labeling. Biophysical Journal, 113(10), 2168–2172. 10.1016/j.bpj.2017.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, & Li J (2015). TRPA1 mediates amplified sympathetic responsiveness to activation of metabolically sensitive muscle afferents in rats with femoral artery occlusion. Frontiers in Physiology, 6, 249 10.3389/fphys.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, & Shimada Y (2006). Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biological & Pharmaceutical Bulletin, 29(12), 2415–2418. 10.1248/bpb.29.2415. [DOI] [PubMed] [Google Scholar]
- Zayats V, Samad A, Minofar B, Roelofs KE, Stockner T, & Ettrich R (2013). Regulation of the transient receptor potential channel TRPA1 by its N-terminal ankyrin repeat domain. Journal of Molecular Modeling, 19(11), 4689–4700. 10.1007/s00894-012-1505-1. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, & Maeda N (1992). Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science, 258(5081), 468–471. 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zhao JF, Shyue SK, Kou YR, Lu TM, & Lee TS (2016). Transient receptor potential ankyrin 1 channel involved in atherosclerosis and macrophage-foam cell formation. International Journal of Biological Sciences, 12(7), 812–823. 10.7150/ijbs.15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, & Heppenstall PA (2007). Direct activation of the ion channel TRPA1 by Ca2+. Nature Neuroscience, 10(3), 277–279. [DOI] [PubMed] [Google Scholar]


