Abstract
Neuromyelitis optica spectrum disorder (NMOSD) is a disabling autoimmune astrocytopathic channelopathy, characterized by the presence of pathogenic antibodies to aquaporin-4 (AQP-4) water channels. Several viral infections including HIV, influenza virus, varicella zoster virus, and Epstein Barr virus, among others, have been alleged to trigger NMOSD in both immunocompetent and immunocompromised individuals. Neurological manifestations of coronavirus infectious disease of 2019 (COVID-19) have been ever evolving and the spectrum of neuraxial involvement is broadening. Albeit it may affect any area of the neural axis, the involvement of the spinal cord is rare compared to that of the brain and of the peripheral nervous system. Cases with acute longitudinally extensive transverse myelitis (LETM) have been recently reported in SARS-CoV-2 infection but did not fulfill the international consensus diagnostic criteria for NMOSD. AQP-4-antibody-seropositive NMOSD following SARS-CoV-2 infection had not yet been reported. We herein report a novel case of a previously healthy man who presented with a clinical picture of bouts of vomiting and hiccoughs (area postrema syndrome), which rapidly evolved to acute LETM, all following SARS-CoV-2 infection. He was finally diagnosed to be a case of seropositive NMOSD which presented as area postrema syndrome. The response to immunomodulatory drugs was excellent.
Keywords: Neuromyelitis optica, NMOSD, COVID-19, SARS-CoV-2, Immune-mediated, Longitudinally extensive transverse myelitis
Graphical abstract
1. Introduction
Neurological manifestations of coronavirus infectious disease of 2019 (COVID-19) have been ever evolving and the spectrum of neuraxial involvement is broadening.(Roy et al., 2020) Albeit it may affect any area of the neural axis, the involvement of the spinal cord is rare compared to that of the brain and of the peripheral nervous system.(Ghosh et al., 2020a; Ghosh et al., 2020c; Ghosh et al., 2020d; Gutiérrez-Ortiz et al., 2020; Rábano-Suárez et al., 2020; Roy et al., 2020) SARS-CoV-2 has immense propensity to dysregulate the host immune system resulting in generation of various autoantibodies, which are turning out to be extremely pathogenic (Finsterer et al., 2020; Gutiérrez-Ortiz et al., 2020; Zhang et al., 2020; Zhou et al., 2020). Neuromyelitis optica spectrum disorder (NMOSD) is a disabling autoimmune astrocytopathic channelopathy, characterized by the presence of pathogenic antibodies to aquaporin-4 (AQP-4) water channels, expressed on astrocytic foot processes at blood brain barrier, subpial and subependymal regions.(Fujihara, 2019; Ghosh et al., 2020b; Lucchinetti et al., 2014) Long segments of spinal cord inflammation (myelitis), severe optic neuritis, and/or bouts of intractable vomiting and hiccoughs (area postrema syndrome) are the classic forms of presentation.(Fujihara, 2019; Lucchinetti et al., 2014) From a pathological point of view, NMOSD mainly encompasses AQP-4-antibody-positive astrocytopathy and myelin oligodendrocyte glycoprotein (MOG)-antibody-positive inflammatory demyelinating disease.(Fujihara, 2019, Lucchinetti et al., 2014) Several viral infections including HIV, influenza virus, varicella zoster virus, and Epstein Barr virus, among others, have been alleged to trigger NMOSD in both immunocompetent and immunocompromised individuals.(Lana-Peixoto et al., 2018; Machado et al., 2015; Mathew et al., 2019; Sellner et al., 2010; Tran et al., 2007) It has recently reported a case of a young man presenting with bilateral severe optic neuritis and myelitis, determined to be simultaneously SARS-CoV-2 and MOG IgG antibody positive, i.e. a variant of NMOSD.(Zhou et al., 2020) However, AQP-4-antibody-seropositive NMOSD following SARS-CoV-2 infection had not yet been reported.
We herein report a novel case of a previously healthy man who presented with a clinical picture of bouts of vomiting and hiccoughs (area postrema syndrome), which rapidly evolved to acute LETM, all following SARS-CoV-2 infection. He was finally diagnosed to be a case of seropositive NMOSD which presented as area postrema syndrome. The response to immunomodulatory drugs was excellent.
2. Case report
A previously healthy Asian-Indian 20-year-old man was admitted to the emergency department with rapidly progressive weakness and decreased sensation of all four limbs, urinary retention, and constipation. His past medical history was unremarkable. Ten days prior to his admission, he had developed a mild fever and non-productive cough for 3 days, which resolved spontaneously. Five days after this mild clinical picture, he developed rapidly progressive weakness of his lower limbs, followed by weakness of both upper limbs within 12 h. Further, he complained of persistent hiccough, nausea and vomiting that started a few days before the progressive weakness of his limbs appeared. On the day of admission, on getting up from sleep, he found himself unable to get up from lying down position on his own. He also noticed reduced sensation in all 4 limbs and over his torso to all modalities for the same duration. He also complained of dull aching, deep seated, non-radiating pain over his nape of neck, that appeared the day before he developed weakness. Weakness was also associated with difficulty in voiding, pain and fullness in his lower abdomen, and constipation. There was no history suggestive of any similar previous episode, cranial nerve involvement, headache, eye pain, visual symptoms, diplopia, swallowing difficulty, seizures, loss of consciousness, vaccination, trauma, skin rash, joint pain, photosensitivity, orogenital ulcers, dry eyes, dry mouth, band/girdle-like sensation, root pain, thinning or twitching of muscles, flexor spasms, and respiratory distress. Family history was non-contributory. On physical examination, he was fully awake, conscious, afebrile, and normotensive (recorded supine blood pressure was 120/70 mm of Hg). His respiratory rate was 16/min and oxygen saturation of 96% in room air. Neurological examination revealed intact cognitive and cranial nerve functions. Motor system examination revealed asymmetric flaccid hypo-to-areflexic quadriparesis (muscle power estimation by MRC scale showed 1/5 in all 4 limbs). Sensory system examination revealed patchy areas of decreased sensation to all modalities over torso and extremities with reduced vibration sense and proprioception. Cerebellar functions could not be tested.
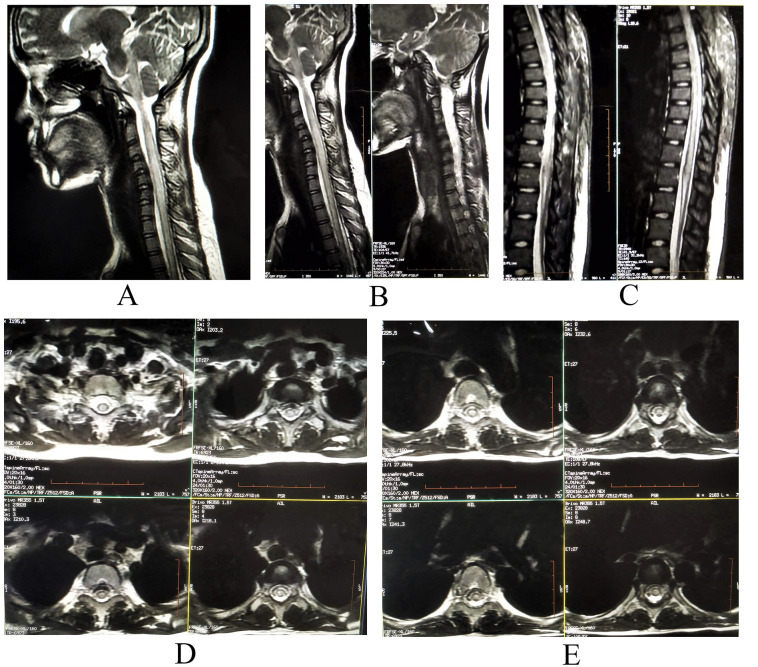
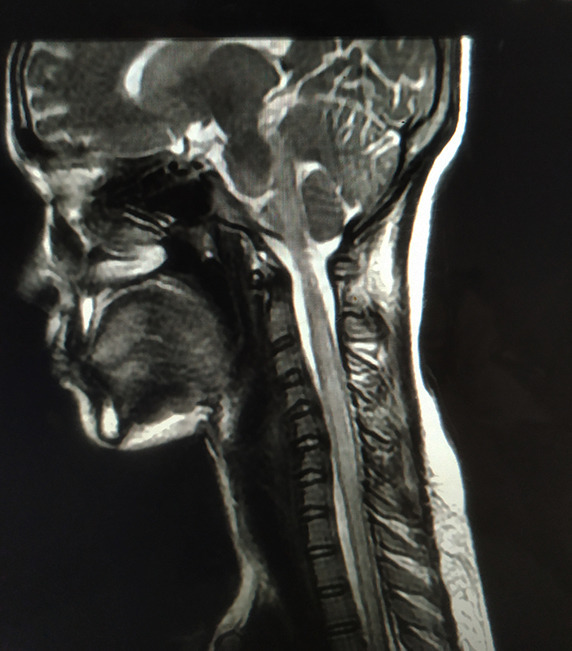
The patient's nasopharyngeal and oropharyngeal swabs tests for SARS-CoV-2 by qualitative real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay (Xpert® Xpress SARS-CoV-2 test) were positive. Blood analysis was remarkable for lymphocytopenia (800 cells/μl). Pro-inflammatory markers (ferritin, C-reactive protein, and erythrocyte sedimentation rate) were mildly raised; however, D-dimer and IL-6 levels were otherwise normal. Magnetic resonance imaging (MRI) of the spine revealed a hyperintense, on T2-weighed images, longitudinally extensive spinal cord lesion, with mild enlargement of the caliber of the cord, without contrast enhancement, extending from lower medulla to D12 level, and predominantly involving the central portion of the cord (Fig. 1 ). MRI of the brain was normal. The lesion was considered to be a result of SARS-CoV-2 associated long segment myelitis, but history of persistent hiccough and involvement of lower medulla and AQP-4 rich area postrema on MRI, warranted testing for anti-AQP-4 antibodies. Cerebrospinal fluid (CSF) study revealed mild pleocytosis, with raised protein levels (white blood cell count = 10/ μl, all lymphocytes, protein = 80 mg/d, glucose = 70 mg/dl, without oligoclonal bands). CSF and paired sera were tested for relevant viral (including HIV), bacterial and parasitic infections, tuberculosis, as well as autoimmune encephalitis and paraneoplastic encephalitis; all results were negative. Tests for autoimmune connective tissue disorders (i.e. systemic lupus erythematosus, Sjögren syndrome, Behçet's disease, sarcoidosis, and antiphospholipid antibody syndrome) were negative. Anti-MOG antibodies were also negative. However, transfected HEK293 cell-based assay from paired sera for anti-AQP-4-antibodies was found to be positive. He was then diagnosed to be a case of seropositive NMOSD presenting with area postrema syndrome. MRI of the orbits and visual evoked potentials were otherwise normal. Albeit the diagnosis of long segment myelitis was obvious, the patient was also tested for nerve conduction studies, which unequivocally ruled out the possibility of co-existing SARS-CoV-2 related immune-mediated Guillain-Barré syndrome.
Fig 1.
MRI of the spine revealing a hyperintense, on T2-weighted images (A and B-cervical spine sagittal view; C-dorsal spine sagittal view; and D and E-cervico-dorsal spine axial view), longitudinally extensive spinal cord lesion, with mild enlargement of the caliber of the cord, extending from lower medulla to D12 level, and predominantly involving the central portion of the cord.
As COVID-19-related illness was almost asymptomatic, he did not receive any specific therapy. However, even before the result of anti-AQP-4 antibodies was received, he was put on intravenous high dose methylprednisolone (1 g/day for 5 consecutive days) for treating the inflammatory myelitis. There was some improvement of the motor power in all limbs and resolution of the sensory symptoms on day 12 of admission. The patient's nasopharyngeal and oropharyngeal swabs tests for SARS-CoV-2 by RT-PCR on day 14 were negative. Then, we put him on rituximab (1 g intravenous infusion, repeated after two weeks as a single course, and in every 6 months intervals till 2 years). After two doses of rituximab, he regained full motor power and catheter could be removed successfully on day 30 of admission. After 4 months follow-up, the patient was maintaining well on rituximab without any further attack. MRI revealed marked resolution of previous lesions and absence of new lesions.
3. Discussion
Historical background, clinical and radiological features and response to immunomodulatory drugs supported a diagnosis of autoimmune inflammatory disease of the central nervous system involving lower brainstem and spinal cord. The key for the diagnosis was the presence of intractable hiccough and nausea, preceding the quadriparesis, which was further substantiated by MRI showing involvement of area postrema. This, in turn, intrigued us to search for NMOSD associated with area postrema syndrome, instead of considering the case as a post-viral LETM. The diagnosis was finally established by serologic testing.
Over the last 20 years, pathogenesis, clinical-radiological spectrum, diagnostic criteria, and therapeutics of NMOSD have been changing and evolving (Fujihara, 2019). Recent evidences suggest that NMOSD is an autoimmune astrocytopathic channelopathy, mediated by anti-AQP-4-autoantibodies,(Fujihara, 2019; Ghosh et al., 2020b; Lucchinetti et al., 2014), having predilection to selected areas of nervous system (i.e. neocortex, hippocampus, cerebellum, optic nerve, glial lamellae of the supraoptic nucleus, subfornical organ, ependymal and meningeal cells, and Muller cells of the retina).(Roemer et al., 2007; Sellner et al., 2010).
Pathogenetic mechanisms for the development of NMOSD following SARS-CoV-2 infection may be either mediated by direct neurotropism or by aberrant immune mediated injury, the latter being the most likely. An aberrant immune response arguably manifest either in the form of molecular/immunological mimicry, bystander activation of immune responses following COVID-19 or aggravation and/or triggering/precipitation of pre-existing disease after systemic infection. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against nervous system.(Angileri et al., 2020; Cappello et al., 2020; Marino Gammazza et al., 2020) Neuroinvasion by SARS-CoV-2 may cause leakage of central nervous system antigens (i.e. AQP-4 peptides) to systemic circulation leading to bystander activation of immune responses.(Azizi and Azizi, 2020; Baig and Sanders, 2020; Yachou et al., 2020) Again, systemic infection by SARS-CoV-2 could elicit an inflammatory response damaging the several AQP-4 expressing epithelial cells and thus triggering a bystander immune cascade.(Azizi and Azizi, 2020; Yachou et al., 2020).
In our patient there was no evidence for prior negativity to anti-AQP-4-antibodies; indeed, the patient could have been positive for the antibodies before SARS-CoV-2 infection. Certain immunologic events (possibly infections, such as COVID-19, and other immune activations) might be needed to allow anti-AQP-4 antibodies to cross the blood-brain barrier to bind to AQP4 on astrocytes.(Nishiyama et al., 2009) In this sense, emerging evidences suggest that, in genetically susceptible individuals, SARS-CoV-2 infection could trigger autoimmune responses.(Caso et al., 2020; Galeotti and Bayry, 2020; Kondo et al., 2020) Besides, SARS-CoV-2 infection induces a cytokine storm,(Ye et al., 2020) which results in an abundance of pro-inflammatory cytokines and, in turn, may damage the astrocytes directly or via immune mechanisms (Fig. 1).
Several case reports have already described acute transverse myelitis associated with SARS-CoV-2 infection, most of them being LETM.(AlKetbi et al., 2020; Chakraborty et al., 2020; Chow et al., 2020; Kaur et al., 2020; Maideniuc and Memon, 2020; Munz et al., 2020; Sotoca and Rodríguez-Álvarez, 2020; Valiuddin et al., 2020; Zachariadis et al., 2020; Zhou et al., 2020; Zoghi et al., 2020) However, only one case was found to be seropositive NMOSD, precisely MOG IgG antibody positivity, in a young Hispanic man with bilateral optic neuritis and LETM. Our case is novel in this aspect. From the erstwhile discussion, a plausible nexus between NMOSD and SARS-CoV-2 could be considered. This taken together with obvious temporality in the course of the disease, anti-AQP-4 seropositivity and response to immunomodulators drugs, provide ample evidence emphasizing a causal link between COVID-19 and anti-AQP-4-antibody mediated astrocytopathy. To our knowledge, this is the first case of NMOSD preceded by area postrema syndrome where there was a clear temporal sequence between SARS-CoV-2 infection and disease onset. The current case stands out as a genuinely rare manifestation of COVID-19. It adds to the tally of cases reporting acute transverse myelitis in COVID-19 and, more importantly, brings to attention the potential involvement of area postrema in the cases of NMOSD associated with this novel infection.
References
- AlKetbi R., AlNuaimi D., AlMulla M., AlTalai N., Samir M., Kumar N. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol. Case Rep. 2020;15:1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angileri F., Legare S., Marino Gammazza A., Conway de Macario E., Jl Macario A., Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020;102591:19. doi: 10.1016/j.autrev.2020.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi S.A., Azizi S.A. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J. Neuro-Oncol. 2020;26:631–641. doi: 10.1007/s13365-020-00903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Sanders E.C. Potential neuroinvasive pathways of SARS-CoV-2: Deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19) J. Med. Virol. 2020 Jun 3 doi: 10.1002/jmv.26105. Epub ahead of print. PMID: 32492193; PMCID: PMC7300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F., Gammazza A.M., Dieli F., de M, Macario A.J. Does SARS-CoV-2 trigger stress-inducedautoimmunity by molecular mimicry? A hypothesis. J. Clin. Med. 2020;9:2038. doi: 10.3390/jcm9072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;102524:19. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U., Chandra A., Ray A.K., Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13:e238668. doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.C.N., Magnussen J., Ip J., Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020:13. doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Scorza F.A., Ghosh R. COVID-19 polyradiculitis in 24 patients without SARS-CoV-2 in the cerebro-spinal fluid. J. Med. Virol. 2020 Jun 4 doi: 10.1002/jmv.26121. Epub ahead of print. PMID: 32497288; PMCID: PMC7300798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara K. Neuromyelitis optica spectrum disorders: still evolving and broadening. Curr. Opin. Neurol. 2019;32:385–394. doi: 10.1097/WCO.0000000000000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Dubey S., Kanti Ray B., Chatterjee S., Benito-León J. COVID-19 Presenting With Thalamic Hemorrhage Unmasking Moyamoya Angiopathy. Can. J. Neurol Sci. 2020:1–3. doi: 10.1017/cjn.2020.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Dubey S., Ray B.K., Purkait S., Pandit A., Benito-León J. Isolated opsoclonus heralding neuromyelitis optica spectrum disorder. J. Neuroimmunol. 2020;577394:348. doi: 10.1016/j.jneuroim.2020.577394. [DOI] [PubMed] [Google Scholar]
- Ghosh R., Lahiri D., Dubey S., Ray B.K., Benito-León J. Hallucinatory palinopsia in COVID-19 induced posterior reversible encephalopathy syndrome. J. Neuroophthalmol. 2020;40:523–526. doi: 10.1097/WNO.0000000000001135. [DOI] [PubMed] [Google Scholar]
- Ghosh R., Roy D., Sengupta S., Benito-León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J. Neurovirol. 2020;26:964–966. doi: 10.1007/s13365-020-00908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Mendez A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Manas R. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 Aug 4;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Kaur H., Mason J.A., Bajracharya M., McGee J., Gunderson M.D., Hart B.L. Transverse myelitis in a child with COVID-19. Pediatr. Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Kaneko Y., Oshige T., Fukui H., Saito S., Okayama M. Exacerbation of immune thrombocytopaenia triggered by COVID-19 in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2020 Aug 5 doi: 10.1136/annrheumdis-2020-218157. [DOI] [PubMed] [Google Scholar]
- Lana-Peixoto M.A., Pedrosa D., Talim N., Amaral J., Horta A., Kleinpaul R. Neuromyelitis optica spectrum disorder associated with dengue virus infection. J. Neuroimmunol. 2018;318:53–55. doi: 10.1016/j.jneuroim.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C.F., Guo Y., Popescu B.F., Fujihara K., Itoyama Y., Misu T. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. (Zurich, Switzerland) 2014;24:83–97. doi: 10.1111/bpa.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C., Amorim J., Rocha J., Pereira J., Lourenço E., Pinho J. Neuromyelitis optica spectrum disorder and varicella-zoster infection. J. Neurol. Sci. 2015;358:520–521. doi: 10.1016/j.jns.2015.09.374. [DOI] [PubMed] [Google Scholar]
- Maideniuc C., Memon A.B. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J. Neurol. 2020 doi: 10.1007/s00415-020-10145-6. Epub ahead of print. PMID: 32772172; PMCID: PMC7415129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino Gammazza A., Légaré S., Lo Bosco G., Fucarino A., Angileri F., Conway de Macario E. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell Stress Chaperones. 2020;25:737–741. doi: 10.1007/s12192-020-01148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew T., Avati A., D'Souza D., Therambil M., Baptist A.A., Shaji A. HIV infection associated neuromyelitis optica spectrum disorder: Clinical features, imaging findings, management and outcomes. Mult. Scler Relat. Disord. 2019;27:289–293. doi: 10.1016/j.msard.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Munz M., Wessendorf S., Koretsis G., Tewald F., Baegi R., Krämer S. Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 2020;267:2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama S., Ito T., Misu T., Takahashi T., Kikuchi A., Suzuki N. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology. 2009;72:1960–1961. doi: 10.1212/WNL.0b013e3181a82621. [DOI] [PubMed] [Google Scholar]
- Rábano-Suárez P., Bermejo-Guerrero L., Méndez-Guerrero A., Parra-Serrano J., Toledo-Alfocea D., Sánchez-Tejerina D. Generalized myoclonus in COVID-19. Neurology. 2020;95:e767–e772. doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer S.F., Parisi J.E., Lennon V.A., Benarroch E.E., Lassmann H., Bruck W. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- Roy D., Ghosh R., Dubey S., Dubey M.J., Benito-León J., Kanti Ray B. Neurological and Neuropsychiatric Impacts of COVID-19 Pandemic. Can. J. Neurol Sci. 2020 doi: 10.1017/cjn.2020.173. Epub ahead of print. PMID: 32753076; PMCID: PMC7533477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J., Hemmer B., Mühlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (Devic) syndromes. J. Autoimmun. 2010;34:371–379. doi: 10.1016/j.jaut.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Sotoca J., Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e803. doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C., Du Pasquier R.A., Cavassini M., Guex-Crosier Y., Meuli R., Ciuffreda D. Neuromyelitis optica following CMV primo-infection. J. Intern. Med. 2007;261:500–503. doi: 10.1111/j.1365-2796.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- Valiuddin H., Skwirsk B., Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: A Case-Report. Brain Behav. Immun. Health. 2020;100091:5. doi: 10.1016/j.bbih.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachou Y., El Idrissi A., Belapasov V., Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020;41:2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19`. J Infect. 2020 Jun;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariadis A., Tulbu A., Strambo D., Dumoulin A., Di Virgilio G. Transverse myelitis related to COVID-19 infection. J. Neurol. 2020;267:3459–3461. doi: 10.1007/s00415-020-09997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020;e38:382. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J. Neuro-Ophthalmol. 2020;40:398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi A., Ramezani M., Roozbeh M., Darazam I.A., Sahraian M.A. `. Mult. Scler Relat. Disord. 2020;102324:44. doi: 10.1016/j.msard.2020.102324. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]