Abstract
Serious of unpredictable drawbacks of Coronavirus 2019 (COVID-19) infectious disease caused by SARS-COV-2 on the nervous system, have been widely noticed among the huge number of infected people. It was found that this type of newly revolving pandemic infection mainly infects the human respiratory tract causing mild to moderate symptoms, however, the hidden door side of COVID-19 is via penetrating the brain, revealing a huge threat especially to elderly people who are more susceptible to its severe side effects and even death to more extent. Almost 80% of COVID-19 patients suffer from severe neurological manifestations including dizziness, headache, unconscious, irritability, dysfunction in smell, and taste accompanied by muscle fatigue.
Herein, we are trying to address the direct neuroinvasive pathway of COVID-19 into human brain cells which is mainly through the olfactory route leading to long-term neurological complications. In addition to highlighting the ability of COVID-19 infection to intensify a pre-existing AD to a more prominent severe stage. The other thing to emphasize is whether AD patients with a highly prominent activation of local immune responses are more or less exposed to getting infected with COVID-19. Along with underlying the hypothesis that the susceptibility to COVID-19 infection may lead to a future risk for neurodegenerative diseases including Alzheimer's disease (AD).
Keywords: Anosmia, Alzheimer's disease, COVID-19, Olfactory bulb
Highlights
-
•
The hidden door side of COVID-19 to the brain is via penetrating the olfactory bulb.
-
•
Cytokine storm may occur following covid-19 infection, leading to neurodegenerative diseases.
1. Introduction
Coronavirus (COVID-19) is a novel kind of pandemic betacoronavirus that results in wide different symptoms including neurological complications by penetrating the central nervous system (CNS) via uncommon pathways including olfactory system [1,2]. Covid-19 patients may be symptomatic but the initial common symptoms which may appear after 5 days incubation period include cough, fever, fatigue, and shortness of breath. The main way for COVID-19 transmission among people is via sneezing-coughing droplets [[3], [4], [5]]. Partial or complete loss in smell and taste following COVID-19 infection is a high frequent diagnostic symptom among infected patients which may last for several weeks [6]. Numerous evidence demonstrated a direct link of neurological disorders to the novel COVID-19 pandemic infection, which can aggravate the manifestations of neurological diseases. Coronaviruses were found to be detected in the central nervous system (CNS) of Alzheimer's (AD) and multiple sclerosis (MS) of both human and animal models accompanied by neurological lesions indicating positive brain infection [7].
In this viewpoint review, we are addressing the vulnerability of COVID-19 infection to increase the incidence of AD and its potential towards the modification of pre-existing AD.
2. Central nervous system cellular immune surveillance
The central nervous system (CNS), controls a wide serious of cellular and molecular interactions for the aim of orchestrating homeostasis. Although the CNS lacks the lymphatic attacking system with a minor histocompatibility complex (MHC) molecules, it is usually being protected from invasion by the blood-brain barrier (BBB) and the blood-cerebrospinal fluid (CSF) under continual controlled immune surveillance system through brain resident macrophage type and immune regulatory system [8,9]. These brain type macrophages required to be stimulated in case of triggered inflammatory cascading pathways or as a drawback of infection crossed the brain via BBB or even through the olfactory system [[8], [9], [10]]. Alzheimer's disease (AD) is the most common type of neurodegenerative disorder for an old population mainly characterized by the presence of Aβ and tau protein particularly in the hippocampus and cerebral cortex. AD directly targets the CNS accompanied by severe neuroinflammation with the probability of being provoked by general systemic body injuries or infection [11,12]. Once senile plaques and neuroinflammatory tangles are deposited on different brain tissues and triggered by the exaggerated neural-immune system, a frequent disturbance in CNS homeostasis and gut type immune homeostasis is usually observed. Disturbances in M1-M2 homeostasis involves many pro-inflammatory and anti-inflammatory cascading pathways depending on brain mediating environment including nuclear factor kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), mitogen-activated protein kinases (MAPK), interferon type II (IFN-γ), and chemokine ligand-2(CCL2) [[13], [14], [15]].
The innate immune system of the body recruits a serious of different defensive cells to make a response to inflammation or pathogen. Such cells include many circulating lymphocytes (B-cells, T-cells, NK cells) and monocytes cells that can develop into both dendritic cells and macrophages. They also include tissue associated bone marrow-derived mast cells. Systemically, these actions are initiated from bone marrow-derived leucocytes reservoir originating as macrophage circulating in the blood to the site of inflammation resulting in the destruction, engulfing, and clearance of invading pathogenic infection [16,17]. In the CNS, microglia cells function as brain type macrophages involved in different systemized regulatory processes essential for brain tissue development, maintenance of the neural healthy environment, and inflammatory remodeling system depending on the surrounding brain tissue local environment. Accordingly, these Microglia cells are usually found in microglia homeostasis balanced system M1-M2 expressed as M1 (pro-inflammatory) and M2 (anti-inflammatory) where both M1 and M2 level is mainly based on the surrounding brain tissue condition. It has been previously observed that once disturbances in microglia brain type homeostasis have been reached, the brain becomes more susceptible to infection and for being irreversibly self-damaged [17].
3. COVID-19 related manifestations
Meanwhile, respiratory tract viral infections represent a major problem for humans resulting in huge health and economic burden effects. These respiratory viral infections induce the most common causes of high morbidity and mortality rate worldwide especially in infants, elderly and immune-deficient individuals [18]. COVID-19 is a large family of enveloped RNA Beta-type which results in severe respiratory tract infection (RTI), suggested being transmitted from bats to humans without finding out if there is a mammalian intermediate host. COVID-19 known severe symptoms include pneumonia, acute repertory, severe distress syndrome, hypercoagulation, and maybe death [[19], [20], [21], [22]]. On the other hand, infected patients may also be asymptomatic without cough or even fever. This kind of atypical viral infection can be described as a large genome (25-30 kb), single-stranded RNA containing all the information necessary for viral components. The RNA is coded with structural proteins forming a capsid called nucleocapsid which is enclosed in a lipid membrane envelope encoded with embedded proteins. From the envelope, projected probes in a spiked glycoproteins crown shape responsible for maintaining viral structural integrity [[19], [20], [21], [22]].
For COVID-19 to infect the host, the spikes of the virus must bind to a receptor on the host-specific cell surface. Mutation in spike protein along with binding receptors ends with a newly infected host cell. Novel COVID-19 appears to use the same receptors as a severe acute respiratory syndrome (SARS) to enter human cells through angiotensin-converting enzyme-2 (ACE2) starting with infecting cells located in respiratory mucosa, then epithelial cells of alveoli in the lungs followed by fusion of the virus with the host membrane ending with host cell penetration [[23], [24], [25]]. The virus then uses the host machinery to get replicated producing viral RNA and proteins followed by being assembled to new viral particles called virion resulting in cell death. The uncontrolled viral spread usually destroys infected organs and tissues triggering aggressive inflammatory cascades identified as body defense mechanism response [[23], [24], [25]].
4. COVID-19 and olfactory bulb
Anosmia which is a complete loss of smell sensation can be majorly linked with abnormalities in flavor perception where both can be related to olfactory nerve damage. Scientists are targeting the respiratory tract as the initial primary site of infection, but recently other organs, including the brain, are infected as well resulting in severe neurological manifestations including anosmia, brain injuries, nerve deficits, meningitis, and paralysis. For viruses to get access to CNS, exploit specific neural pathways including the olfactory bulb which put the CNS at major risk for neurological damage [[26], [27], [28]].
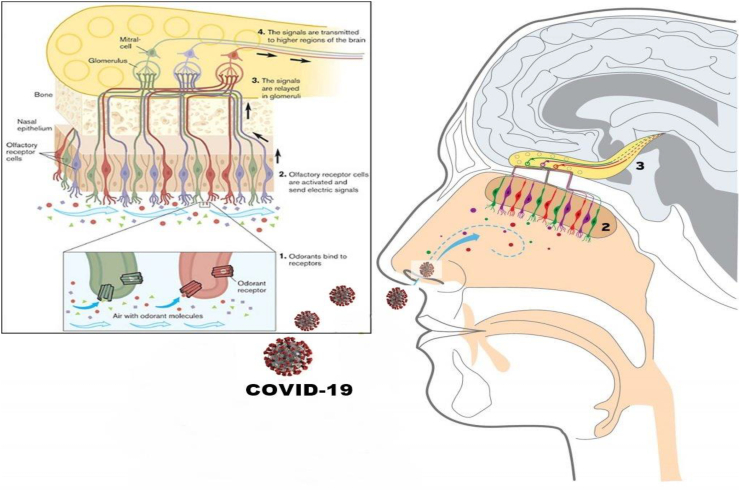
The olfactory nerve is the first cranial nerve responsible for the transmission of information about olfaction or smell to the brain. The nerve begins in the specific olfactory epithelium which is a specialized collection of cells that lies in the nasal cavity of humans. The olfactory epithelium contains millions of olfactory receptors cells. The axons of the olfactory receptors cells form a group of bundles called Fila which travels through a structure called the cribriform plate. The cribriform plate is part of a bone called Ethmoid bone separating the nasal cavity from the brain forming the olfactory nerve [28,29]. COVID-19 pathogen can cross the cribriform plate followed by olfactory bulb neurons resulting in major olfactory dysfunction. The olfactory sensory neurons are mainly exposed to a high extent to the external environment via the nasal cavity, therefore it is suggested that COVID-19 penetrates the brain via the olfactory route (Fig. 1) resulting in a rapid penetration to the brain with extensive tissue damage as well as serious of attained inflammatory cascade accompanied with various neurological complications [[26], [27], [28], [29]].
Fig. 1.
Systemic figure representing the olfactory system and the ability of COVID-19 to penetrate the brain via the olfactory bulb.
5. COVID-19 and neurological manifestations
A recent association between COVID-19 and neurological manifestations has been well documented. This non-casual association brought up many concerns to be explored whether COVID-19 infection results in or even exaggerates neurological drawbacks or it just a kind of positive correlation where one condition intensifies the other [30,31]. Due to its neuroinvasive potentials in certain animals and recently in humans, a suggested positive correlation between the role of ubiquitous human coronaviruses infection in the triggering of neurodegenerative human pathologies has been recently found [[30], [31], [32]]. Even though the high neuroprotective uniqueness capacity of the olfactory bulb region, COVID-19 has a high invasive ability to penetrate the cerebrospinal fluid (CSF) explaining the reason why the nasopharyngeal swab results may be incorrectly false negative with a probability towards undetected true positive to be found in CSF as demonstrated in (Table 1). It is now accepted that COVID-19 is not always confined to the upper respiratory tract and that they can invade the CNS resulting in neuronal complications including aging, cognitive decline, nerve deficits, paralysis, seizures, and meningitis with the risk of Parkinson's disease and Alzheimer's disease as long term complications [[32], [33], [34], [35]].
Table 1.
Neursological manifestations found in COVID-19 research studies
| Study type | Origin of study | Other manifestations | Neurological drawbacks | References |
|---|---|---|---|---|
| Letter to the editor | Switzerland | -Taste and/or smell loss may be observed following SARS-CoV-2 infection. - Observations suggested that the transient anosmia can be recovered following several weeks along with a probability that the impairment remains irreversible. |
- Olfactory epithelium bulb can serve as a direct pathway for COVID-19 to penetrate the brain via the nose. | [36] |
| Review | Liverpool, UK | - Fever and respiratory distress syndrome were observed during the first 14 days of infection. | - Observed neurological manifestations following COVID-19 viral infection which was detected in the CSF followed by neck stiffness, headache, seizures, neuro-muscular disease, motor neuropathy, myopathy, intracerebral hemorrhages, and dementia neurocognitive Syndrome. | [37] |
| Case report | Japan | - Fever, fatigue, headache, fever, and sore throat. | - Covid-19 was found to be negative in nasopharyngeal swab and positive in CSF. - Seizures and loss of consciousness were clinically observed. |
[38] |
| Case report | China | -Fever and dry cough were observed in the first few days following neurological symptoms along with fatigue and areflexia. | - Positive COVID-19 in the oropharyngeal swab. -Several Neurological complications were clinically observed including inflammatory demyelinating polyneuropathy. |
[39] |
| Case report | USA | - Respiratory distress syndrome with altered mental and elevated CRP. | - Seizures accompanied by exaggerated cytokines release. | [40] |
| Perspective | China | - No observed clinical symptoms including fever and cough fever with no shortness of breath except on physical activities. | - Several neurological manifestations were observed including headache, dizziness, mild cognitive impairment, blurred vision, and musculoskeletal damage. | [41] |
| Letter to the editor | USA | - Fever, cough, and shortness of breath. | - Dizziness, headache, altered mental status, nerve pain, and acute cerebrovascular disease were clinically observed along with dementia in some cases. | [42] |
6. Neurodegenerative diseases and COVID-19
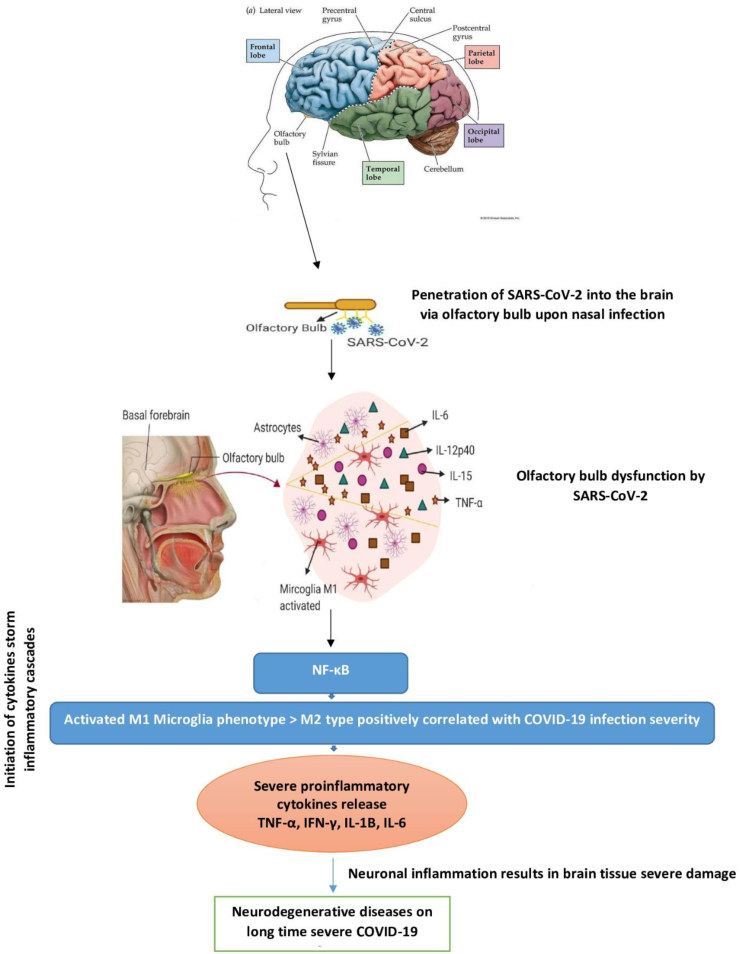
Two of the most repeated COVID-19 olfactory bulb infection signs are anosmia and disorientation. Both signs can be considered as primary outlines for neurodegenerative diseases in case of neuron cells being invaded by COVID-19 and remains inside for a long time resulting in severe toxic neurodegenerative effects. Some of these effects can be even noticed in patients several months following infection recovery [[43], [44], [45], [46]]. Exaggerated cytokines release accompanied by COVID-19 viral infection via the olfactory bulb can be majorly implicated in neuronal and synapses death as a result of modifications in the innate immune responses with the probability of sudden brain occlusion as a drawback of blood hypercoagulation activated pathways. Extended sustained exposure to severe inflammation and high levels of cytokines in COVID-19 patients may contribute to different neuropsychiatric and neurocognitive symptoms in the long term. The storm of elevated cytokines level includes tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin-1 beta (IL-1B), and interleukin-6 (IL-6) which can damage the blood-brain barrier (BBB) by activating microglia M1 phenotype. This may lead to the rapid accumulation of insoluble toxic aggregates in different brain regions where these neuropathological changes can be related to olfaction brain modifications following COVID-19 olfactory bulb penetration [[46], [47], [48], [49]].
It was previously found in cases suffering from damage in olfactory bulb neurons via viral infection that neurofibrillary tangles (NFTs) have been majorly identified within the olfactory bulb, olfactory tract, olfactory nucleus, amygdala, and entorhinal cortex regions. The number of NFTs within such regions has been correlated with dementia severity. Recently neuropathological studies suggest that AD-related brain tissue damage may begin with viral infection within the olfactory cortex followed by spread to different brain areas as a result of elevated inflammatory cytokines release and microglia M1 brain type ending with Alzheimer's disease inflammatory type on the long run [44,50,51]. Consequently, this highlights that long-term exposure to extensive neuroinvasive capabilities of COVID-19 may result in an exaggerated risk of AD as its severity is positively correlated with infection degree (Fig. 2).
Fig. 2.
Hypothetical illustration of the possible role of COVID-19 to result in neurodegenerative diseases via penetrating the CNS upon nasal infection.
7. AD patients infected with COVID-19 drawbacks
Besides its unpredictable complications, it is suggested that COVID-19 patients suffering from AD neuropathological hallmarks patterns are more susceptible to neuroinflammatory drawbacks including neuronal and synapse damage [[52], [53], [54]]. Hereby, COVID-19 virus with pathogen-associated molecular patterns (PAMPs) results in the exaggerated release of innate cytokines via olfactory bulb dysfunction including interleukin 6, IFNγ, NF-κB, and TNF-α which may lead to severe neurological manifestations such as brain tissue irreversible damage. In AD patients, the imbalance between pro- and anti-inflammatory cytokines with more expression of M1 proinflammatory cytokines and chemokines could contribute to the progression of the AD stage. Previous studies have demonstrated that an exaggerated expression of IL-6, IFNγ, and TNF-α levels were observed in AD patient's resulting in dysregulation amyloid precursor protein (APP) along with synaptic and AD neuronal dysfunction [12,51,55].
Consequently, the present review suggests that COVID ‐−19 infection through nasal or olfactory bulb routes can act as a predisposing factor to AD due to triggered inflammatory cytokines release and brain tissue oxidative damage accompanied with taste and smell sensation lost on the first few days as an early sign of viral infection if it is not already lost in elderly AD patients on the long term [27,28,[55], [56], [57], [58], [59]]. The present review also reveals that viral COVID-19 infection may increase senile exaggerated plaques formation maintained mainly by high IL-6 accelerating AD brain hallmarks pathology. For that reason, it is suggested that amyloid plaques deposition in the CNS is more significantly higher in COVID-19 positive patients than in non-viral infected AD patients as a drawback of the serious proinflammatory cytokines production leading to disturbances in both the initiative and adaptive immune responses especially mediated by the exaggerated release of NF-κB, TNF-α, and IL-6 [[53], [54], [55], [56], [57], [58], [59]].
8. Conclusion
Eventually, neurological manifestations may be frequently found among COVID-19 patients along with hospitalization in high-risk patients due to the exaggerated cytokines release and highly triggered neuroinflammation resulting in severe brain tissue damage. Understanding the ability of the pandemic COVID-19 to invade the brain and its mechanism of involvement in neuron damage leading to prolong exaggerated neurological disorders are being crucial to avoid rare complications that could lead to a long-term cascade of neuropathological drawbacks. A required on set management of COVID-19 patients is a vital request with the mandatory of including repeated clinical examination, brain imaging, and essential laboratory-related neurological findings. Along with crucial general routine checkup for an extended duration of time even after recovery, to avoid the probability of sudden future neurological complications. In conclusion, careful clinical and epidemiological studies are highly essential to reveal more mysterious neurological manifestations of COVID-19 and its implications in neurodegenerative diseases.
Disclosure statement
The authors report no conflicts of interest in this work.
Author contributions
All authors contributed to and approved this version of the manuscript.
References
- 1.Dai L., Zheng T., Xu K., Han Y., Xu L., Huang E. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722–733. doi: 10.1016/j.cell.2020.06.035. (e11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fodoulian L., Tuberosa J., Rossier D., Boillat M., Kan C., Pauli V. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv. 2020;03(31):0132–0168. doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohmwald K., Gálvez N.M.S., Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new-onset anosmia during the COVID-19 pandemic – an observational cohort study. J. Otolaryngol. Head Neck Surg. 2020;49(1):26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matías-Guiu J., Gomez-Pinedo U., Montero-Escribano P., Gomez-Iglesias P., Porta-Etessam J., Matias-Guiu J.A. Es esperable que haya cuadros neurológicos por la pandemia por SARS-CoV-2. Neurología. 2020;35(3):170–175. doi: 10.1016/j.nrl.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ní Chasaide C., Lynch M.A. The role of the immune system in driving neuroinflammation. Brain Neurosci. Adv. 2020;4 doi: 10.1177/2398212819901082. (2398212819901082-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulos Z., Herz J., Kipnis J. Meningeal lymphatics: from anatomy to central nervous system immune surveillance. J. Immunol. 2020;204(2):286–293. doi: 10.4049/jimmunol.1900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015;235(2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 11.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217(2):459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan L., Mao C., Hu X., Zhang S., Yang Z., Hu Z. New insights into the pathogenesis of alzheimer’s disease. Front. Neurol. 2020;10:1312. doi: 10.3389/fneur.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anwar M.M. 7(1) 2018. Regulation of miRNA-124, Nuclear Factor-Kappa B and β-Catenin Expression in Response to Novel Therapeutic Protocol in LPS Induced Alzheimer’s Disease in Rats Research in Neuroscience; pp. 14–30. [Google Scholar]
- 14.Akiyama H. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browne T.C., McQuillan K., McManus R.M., O’Reilly J.A., Mills K.H., Lynch M.A. IFN-γ Production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol. (Baltimore, Md:1950) 2013;190(5):2241–2251. doi: 10.4049/jimmunol.1200947. [DOI] [PubMed] [Google Scholar]
- 16.Perry V.H., Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013;35(5):601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak D., Roth T.L., McGovern D.B. Microglia development and function. Annu. Rev. Immunol. 2014;32(1):367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Big C., Reineck L.A., Arono D.M. Viral infections of the central nervous system: a case-based review. Clin. Med. Res. 2009;7(4):142–146. doi: 10.3121/cmr.2009.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jartti T., Jartti L., Ruuskanen O., Soderlund-Venermo New respiratory viral infections. Curr. Opin. Pulm. Med. 2012;18(3):271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 20.Lyons J., McArthur J. Emerging infections of the central nervous system. Curr. Infect. Dis. Rep. 2013;15(6):576–582. doi: 10.1007/s11908-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 21.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin D.E. Emergence and re-emergence of viral diseases of the central nervous system. Prog. Neurobiol. 2010;91(2):95–101. doi: 10.1016/j.pneurobio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jartti T., Jartti L., Ruuskanen O., Soderlund-Venermo New respiratory viral infections. Curr. Opin. Pulm. Med. 2012;18(3):271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 25.Lyons J., McArthur J. Emerging infections of the central nervous system. Curr. Infect. Dis. Rep. 2013;15(6):576–582. doi: 10.1007/s11908-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 26.Aragão M.F.V.V., Leal M.C., Cartaxo Filho O.Q., Fonseca T.M., Valença M.M. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am. J. Neuroradiol. 2020;41(9):1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 28.Li C.-W., Syue L.-S., Tsai Y.-S., Li M.-C., Lo C.-L., Tsai C.-S. Anosmia and olfactory tract neuropathy in a case of COVID-19. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butowt R., Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020;11(9):1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 30.Mao X.-Y., Jin W.-L. The COVID-19 pandemic: consideration for brain infection. Neuroscience. 2020;437 doi: 10.1016/j.neuroscience.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav. Immun. 2020;88:11–16. doi: 10.1016/j.bbi.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita M., Yamate M., Li G.M., Ikuta K. Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus. Biochem. Biophys. Res. Commun. 2005;334:79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong S.S.Y., Yuen K.Y. The severe acute respiratory syndrome (SARS) J. Neuro-Virol. 2005;11(5):455–468. doi: 10.1080/13550280500187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler T. Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells. J. Virol. 2005;79(21):13800. doi: 10.1128/JVI.79.21.13800-13805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365(2):324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marinosci A., Landis B.N., Calmy A. Possible link between anosmia and COVID-19: sniffing out the truth. Eur. Arch. Otorhinolaryngol. 2020;277(7):2149–2150. doi: 10.1007/s00405-020-05966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sohal S., Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Li W., Wang D., Mao L., Jin H., Li Y. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc. Neurol. 2020;5(2):177. doi: 10.1136/svn-2020-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal P., Ray S., Madan A., Tyson B. Neurological manifestations in 404 COVID-19 patients in Washington State. J. Neurol. 2020 doi: 10.1007/s00415-020-10087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav. Immun. 2020;88:11–16. doi: 10.1016/j.bbi.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuffaro L., Di Lorenzo F., Bonavita S., Tedeschi G., Leocani L., Lavorgna L. Dementia care and COVID-19 pandemic: a necessary digital revolution. Neurol. Sci. 2020;41(8):1977–1979. doi: 10.1007/s10072-020-04512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippi A., Domingues R., Setz C., Outeiro T.F. 35(5) 2020. SARS-CoV-2: At the Crossroad Between Aging and Neurodegeneration; pp. 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naughton S.X., Raval U., Pasinetti G.M. Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J. Alzheimers Dis. 2020;76:21–25. doi: 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toljan K. Letter to the editor regarding the viewpoint “evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism”. ACS Chem. Neurosci. 2020;11(8):1192–1194. doi: 10.1021/acschemneuro.0c00174. [DOI] [PubMed] [Google Scholar]
- 50.Chen K. CD40/CD40L dyad in the inflammatory and immune responses in the central nervous system. Cell. Mol. Immunol. 2006;3(3):163–169. [PubMed] [Google Scholar]
- 51.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bohmwald K., NMS Gálvez, Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12(386) doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abboud H., Abboud F.Z., Kharbouch H., Arkha Y., El Abbadi N., El Ouahabi A. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naughton S.X., Raval U., Pasinetti G.M. Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J. Alzheimers Dis. 2020;76:21–25. doi: 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hickman S.E., Allison E.K., El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Wagoner N.J., Oh J.-W., Repovic P., Benveniste E.N. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J. Neurosci. 1999;19(13):5236–5244. doi: 10.1523/JNEUROSCI.19-13-05236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X., Wu K., Wang D., Yue X., Song D., Zhu Y. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365(2):324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S.-Y., Chen T.-F., Lai L.-C., Chen J.-H., Sun Y., Wen L.-L. Sequence variants of interleukin 6 (IL-6) are significantly associated with a decreased risk of late-onset Alzheimer’s disease. J. Neuroinflammation. 2012;9:21. doi: 10.1186/1742-2094-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020;15(1):40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]