Highlights
-
•
Patients with haematological malignancies and COVID-19 are characterized by T lymphopenia and reduction in NK cell numbers.
-
•
T lymphopenia in haematological patients with COVID-19 include reduction in CD4+ T cells numbers and lowered CD4/CD8 ratio.
-
•
Increase in activated T cells with low CD4/CD8 ratio are crucial for the early stages of COVID-19 in haematological patients.
-
•
Low levels of both TCRγ/δ and CD3+CD25+ T cells may indicate impaired cellular response to COVID-19 in haematological patients.
Keywords: Lymphocyte subsets, T cells, CD4/CD8 ratio, NK cells, TCR ɣ/ƍ cells, Haematological malignancies, COVID-19
Abstract
The role of immune dysregulation in the course and prognosis of COVID-19 is not clearly established. In particular, immune status in specific populations such as haematological patients, who have an impaired immunological system, has not been described so far. Here, we performed a comprehensive analysis of peripheral blood lymphocyte subsets in 27 SARS-CoV-2-infected patients, including 16 patients with haematological malignancies. We identified T cell subpopulations, B cells, NK cells and TCR α/ß and ɣ/ƍ-expressing T cells during COVID-19 infection, with significant changes observed in immune profiles during the course of disease, especially in haematological patients. We observed an increase in activated T lymphocytes (CD3+HLA-DR+ and CD3+CD8+HLA-DR+) in the early stages of SARS-CoV-2 infection with a concomitant decrease in the CD4/CD8 ratio in haematological patients compared to non-haematological patients affected by COVID-19. We also found a decrease in ɣ/ƍ T cells in both studied groups of patients, with lower numbers of CD25+ T cells and CD16+CD56+ NK cells in haematological patients compared to non-haematological patients with COVID-19. Our findings demonstrate, for the first time, impaired adaptive immunity in patients with haematological malignancies infected with COVID-19, resulting in impaired cellular immune responses to SARS-CoV-2. This warrants further investigation of this disease group in COVID-19 patient cohorts
Introduction
First discovered in December 2019 in China, a novel type of coronavirus, SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) quickly spread worldwide and caused a pandemic in just a few months. The complications of COVID-19 (coronavirus disease 2019), such as ARDS (acute respiratory distress syndrome), respiratory failure and sepsis were associated with the need for intensive care treatment in approximately 5–11% of cases. Deaths, in general, reached a level of about 3% of all diagnosed patients [1,2], and as high as 97% in patients requiring invasive mechanical ventilation [3].
Previous studies emphasized the role of disturbed leucocyte homoeostasis during the course of COVID-19 infection [8], [9], [10], [11], [12], [13], [14]. In most cases, total lymphocyte count were clearly reduced. In contrast, the concentration of ILs such as IL-6, IL-7 or IL-10 was relatively high. Both of these phenomena were correlated with severity of infection and the need for intensive treatment [5,6]. Moreover, there was a trend toward higher lymphocyte baseline levels in survivors compared to non-survivors, which demonstrates the predictive value of the parameter [3].
However, little is known about dysregulation of the immune system, especially in the context of lymphocyte profiles, in patients with haematological diseases. Due to therapies that target B lymphocytes in haematological patients, immune changes occurring during the course of COVID-19 were mainly assessed with respect to populations of T lymphocytes and NK cells. In our study, however, only 13% of patients with haematological diseases were treated with a monoclonal antibody against CD20 found on B lymphocytes. CD19+ B cells were slightly decreased in haematological patients with COVID-19 and remained within normal ranges in non-haematological patients, however there were no significant differences in the levels of CD19+ B cells and CD19+CD20+ B cells between haematological versus non-haematological patients with COVID-19. This indicates that B lymphopenia does not constitute the essence of immune disturbances observed in COVID-19, which is consistent with previous studies [9,15].
In this multicentre study, we examined the immune profiles of Polish haematological patients affected by COVID-19. To the best of our knowledge, this study of haematological patients infected with SARS-CoV-2 is the first report from Central and Eastern Europe that compared lymphocyte profiles in haematological versus non-haematological patients with COVID-19. The exact effect of SARS-CoV-2 infection on the human immune system still remains unknown. Patients with haematological malignancies represent a distinct group of patients with compromised immunological processes, and hence require more specialized clinical approaches for COVID-19 treatment.
Materials and methods
Study population
We prospectively examined 41 patients with COVID-19, including 30 patients with haematological diseases from three medical centres in Poland (Department of Haematology, Blood Neoplasms and Bone Marrow Transplantation Wroclaw Medical University, Department of Infectious Diseases, Liver Diseases and Acquired Immune Deficiencies Wroclaw Medical University, and Regional Specialist Hospital in Grudziadz) hospitalised from April 1 to June 22, 2020.
Study design
A confirmed COVID-19 case was defined as positive in a real-time reverse-transcriptase polymerase-chain reaction (RT-PCR) assay using nasal and pharyngeal swab specimens.
Demographic data, symptoms, signs and laboratory measures were collected on admission to hospital. Collected data did not include any personally identifiable information. The lymphocyte subsets in peripheral blood were assessed by FACS Canto II in 27 patients including 16 patients with concomitant haematological diseases and 11 patients without haematological burden at several time points: after confirmation of the SARS-CoV-2 infection (day 1), thorough its duration (day 7, 14, 21) and on recovery (day 28, 35). The following mouse anti-human monoclonal antibodies, all purchased from Becton Dickinson and Company (BD), San Jose, CA, were used for analysis: CD8 FITC, CD16 PE, CD4 PerCP-Cy5.5, CD56 PC-7, CD7 APC, CD14 APC-H7, HLA-DR V450, CD3 V500, TCRαβ FITC, CD5 PerCP-Cy5.5, TCRγδ PC-7, CD19 APC, CD38 APC-H7, CD4 V450 and CD20 V450. For FACS analysis, 20 mL of blood was collected in EDTA tubes (BD). Human peripheral blood mononuclear cells (PBMC) were separated using Ficoll-Hypaque (Sigma, St Louis, MO). Cells were surface stained with the following fluorescence-conjugated mouse anti-human monoclonal antibodies in one tube: CD8, CD16, CD4, CD56, CD7, CD14, HLA-DR and CD3, and in a second tube cells, were stained with the following antibodies: TCRαβ, CD5, TCRγδ, CD19, CD38, CD20, CD4 and CD3. The evaluation of nucleated cells was carried out on a 8-color FACS Canto II flow cytometer (BD). The data were analysed using BD FACSDiva software v 8.0 – the gating strategy is shown in Fig. 1. The severity of COVID-19 was assessed according to the Guidelines for the Diagnosis and Treatment of COVID-19 (Trial Version 7) [7].
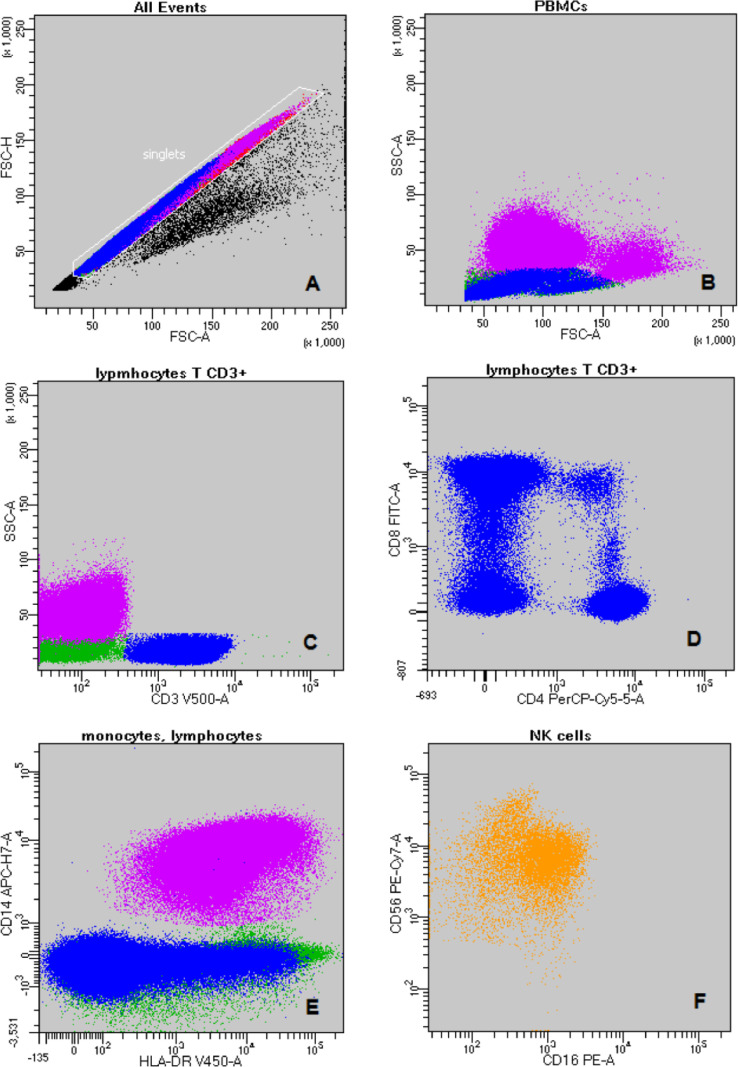
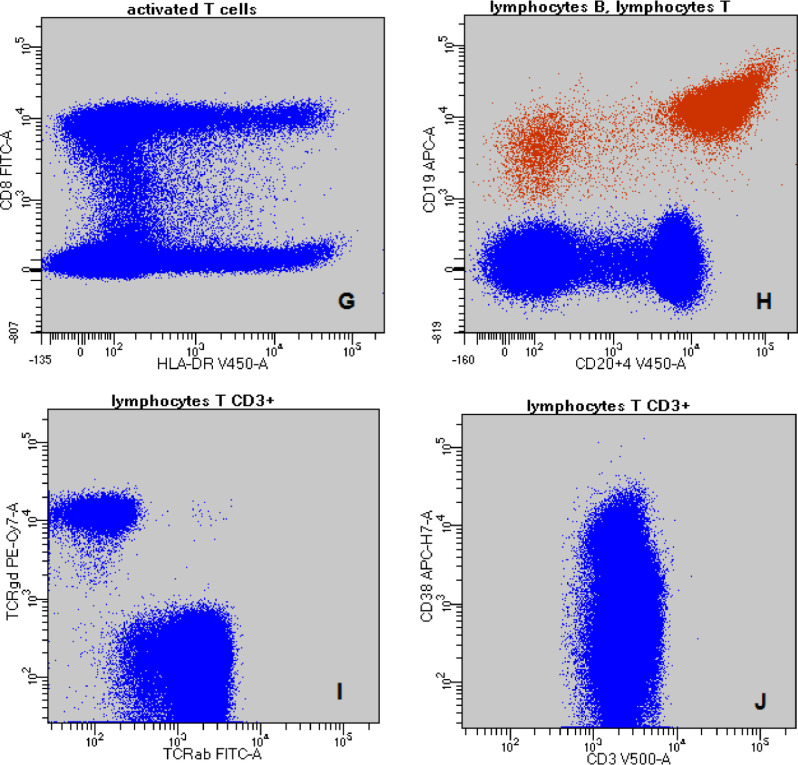
Fig. 1.
The gating strategy: A-discrimination of doublets (FSC-A vs. FSC-H); B - PBMCs: purple - monocytes; blue - lymphocytes T (FSC-A vs. SSC-A); C - blue - lymphocytes T CD3+; purple - monocytes; green – lymphocytes B and NK cells (CD3 vs. SSC-A); D – lymphocyte T subpopulations (CD4 vs. CD8); E – monocytes and lymphocytes (HLA-DR vs. CD14); F – NK cell subpopulations (CD16 vs. CD56); G - activated T CD3+CD8+ cells (HLA-DR vs. CD8); H – blue - lymphocytes T and red - lymphocytes B (CD20+4 vs. CD19); I – lymphocytes T CD3+ (TCRα/β vs. TCRγ/δ); J – lymphocytes T CD3+ (CD3 vs. CD38). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Written informed consents were obtained and accepted by the Wroclaw Medical University Ethics Committee. The study was performed in accordance with the Wroclaw Medical University Ethics Committee (Consent no 315/2020).
Statistical analysis
Statistical analysis was performed using Statistica 13.1 software for Windows. Categorical variables were presented as frequencies with percentages, whereas median and interquartile range (IQR) or range (min-max) were used to describe continuous variables. Categorical variables were compared using χ2 test or Fisher's exact test. Evaluation of data normality was performed using Shapiro-Wilk test. Non-normally distributed continuous variables were analysed using U Mann Whitney test and Spearman's rank correlation. Receiver Operating Characteristics (ROC) curves were used to assess the sensitivity, specificity and area under the ROC curve (AUC) of the investigated parameters. P < 0.05 was considered statistically significant.
Results
Clinical characteristics of patients infected with SARS-CoV-2
We prospectively analysed 41 patients infected with COVID-19, including 30 patients with haematological malignancies hospitalised in three polish medical centres between 1 April and 22 June 2020. The baseline clinical characteristics of 41 patients included in the analysis were given in Table 1.
Table 1.
Baseline clinical characteristics of 41 patients with COVID-19.
| Variable | All patients, n = 41 | Haematological patients with covid-19, n = 30 | Non-haematological patients with COVID-19, n = 11 | p |
|---|---|---|---|---|
| Age (years) | 60 ± 15 | 58 ± 15 | 64 ± 15 | 0.65 |
| male, n (%) | 21 (51%) | 17 (57%) | 4 (36%) | 0.25 |
| Haematological malignancy | ||||
| Acute leukaemia/MDS EB2, n (%) | 12 (40%) | |||
| Chronic leukaemia/indolent lymphoma, n (%) | 7 (23.3%) | |||
| Agressive lymphoma, n(%) | 6 (20%) | |||
| Multiple myeloma, n(%) | 4 (13.3%) | |||
| Other, n (%) | 1 (3.3%) | |||
| Comorbidities | ||||
| 0, n(%) | 12 (29.3%) | 9 (30%) | 3 (27.3%) | 0.86 |
| 1–2, n (%) | 22 (53.7%) | 15 (50%) | 7 (63.6%) | 0.44 |
| ≥3, n(%) | 7 (17%) | 6 (20%) | 1 (9.1%) | 0.41 |
| Hypertension, n(%) | 15 (36.6%) | 13 (43.3%) | 2 (18.2%) | 0.14 |
| Diabetes mellitus, n(%) | 9 (21.9%) | 7 (23.3%) | 2 (18.2%) | 0.72 |
| Heart diseases, n(%) | 7 (17.1%) | 4 (13.3%) | 3 (27.3%) | 0.29 |
| Symptoms/signs | ||||
| cough, n(%) | 23 (56.1%) | 17 (56.7%) | 6 (54.5%) | 0.90 |
| dyspnoea, n(%) | 22 (53.6%) | 16 (53.3%) | 6 (54.5%) | 0.94 |
| Fever, n(%) | 19 (46.3%) | 12 (40%) | 7 (63.6%) | 0.18 |
| Pneumonia, n(%) | 27 (65.8%) | 18 (60%) | 9 (81.8%) | 0.19 |
| White blood cells [x103/uL] | 3.3 (2.2–5.6) | 2.92 (1.3–6.2) | 3.9 (2.7–5.6) | 0.48 |
| Lymphocytes [x103/uL] | 0.76 (0.43–1.44) | 0.75 (0.26–1.61) | 0.76 (0.6–1.44) | 0.63 |
| Neutrophiles [x103/uL] | 1.86 (0.83–3.2) | 1.5 (0.17–2.29) | 2.4 (1.8–4.1) | 0.07 |
| White blood cells <1.5 × 103/uL | 8 (19.5%) | 8 (26.7%) | 0 (0%) | 0.056 |
| Neutrophiles <1.0 × 103 /uL | 13 (31.7%) | 12 (40%) | 1 (9.1%) | 0.059 |
| Lymphocytes <1.0 × 103/uL | 26 (63.4%) | 18 (60%) | 8 (72.7%) | 0.45 |
| Platelets [x103/uL] | 115 (30–225) | 76 (25–183) | 218 (110–296) | 0.038 |
| Platelets <20 × 103/uL (n,%) | 6 (14.6%) | 6 (20%) | 0 (0%) | 0.11 |
| CRP [mg/l] | 44.5 (26–141) | 47 (10–259) | 40 (26–64) | 0.42 |
| COVID-19 severity | ||||
| Mild, n (%) | 10 (24.4%) | 9 (30%) | 1 (9.1%) | 0.17 |
| Moderate, n (%) | 9 (21.9%) | 5 (16.7%) | 4 (36.4%) | 0.18 |
| Severe, n (%) | 17 (41.5%) | 12 (40%) | 5 (45.4%) | 0.75 |
| Critical, n (%) | 5 (12.2%) | 4 (13.3%) | 1 (9.1%) | 0.71 |
| Time of SARS-CoV-2 infection | 27 ± 14 | 29 ± 15 | 25 ± 10 | 0.53 |
| Time of SARS-CoV-2 infection >28 days | 18 (44%) | 14 (46.7%) | 4 (36.4%) | 0.55 |
| Clinical outcome, death, n (%) | 10 (24.4%) | 10 (33.3%) | 0 (0%) | 0.02 |
| Treatment | ||||
| Oxygen therapy, n(%) | 23 (56.1%) | 16 (53.3%) | 7 (63.6%) | 0.55 |
| High-Flow Nasal Oxygen, n(%) | 6 (14.6%) | 5 (16.7%) | 1 (9.1%) | 0.54 |
| Mechanical ventilation, n(%) | 5 (12.2%) | 4 (13.3%) | 1 (9.1%) | 0.71 |
| Hydroxychloroquine, n(%) | 25 (61%) | 23 (76.7%) | 2 (18.2%) | 0.0007 |
| Lopinavir/Ritonavir, n(%) | 6 (14.6%) | 2 (6.7%) | 4 (36.4%) | 0.02 |
| Remdesivir, n(%) | 1 (2.4%) | 1 (3.3%) | 0 (0%) | 0.54 |
| Fresh frozen plasma, n(%) | 7 (17.1%) | 3 (10%) | 4 (36.4%) | 0.046 |
| Tocilizumab, n(%) | 4 (9.7%) | 3 (10%) | 1 (9.1%) | 0.93 |
Males comprised 57% of haematological patients in the study cohort and 36% of patients without haematological diseases. The most common diagnosis among patients with haematological malignancies was acute leukaemia, accounting for 40%, followed by chronic leukaemia and/or indolent lymphoma, which was observed in 26.6% of patients in the group. Only one third of patients in both groups was not burdened with comorbidities. Just over two thirds (63.6%) of non-haematological patients and half of haematological patients had one or two comorbidities. The most common comorbidity in haematological patients was hypertension (43.3%). Heart diseases were most common in non-haematological patients (27.3%). Both studied groups presented with similar COVID-19 symptoms with cough and dyspnoea observed in just over half of the studied patients. The most common sign of SARS-CoV-2 infection was pneumonia, which was observed in 60% and 82% among haematological and non-haematological patients, respectively. Most of the haematological and non-haematological patients were classified as having severe/critical COVID-19 infection (53.3% and 54.5%, respectively). The mean time of infection was 29 days in haematological patients and 25 days in non-haematological patients. Mortality rates were significantly higher in the haematological group (p = 0.02).
Clinical features and procedures in patients infected with SARS-CoV-2
Laboratory data and applied therapies for the 41 patients included in the study are given in Table 1. Lymphopenia was present in 60% of haematological patients and in 72.7% of non-haematological patients. Neutropenia was observed in 40% of haematological patients and in 9.1% of non-haematological patients. Thrombocytopenia was present in only one fifth of haematological patients. There was no difference between studied groups of patients with respect to the inflammatory marker C-reactive protein (CRP).
The majority of both haematological and non-haematological patients required oxygen therapy during SARS-CoV-2 infection (70% and 72.7%, respectively). Mechanical ventilation was only used in 13.3% of haematological patients and in 9.1% of patients without haematological malignancy. Most of the haematological patients were treated with hydroxychloroquine (76.7%), whereas non-haematological patients were treated mostly with lopinavir/ritonavir (36%) and fresh frozen plasma (36%).
Lymphocyte subpopulation analysis in patients infected with SARS-CoV-2
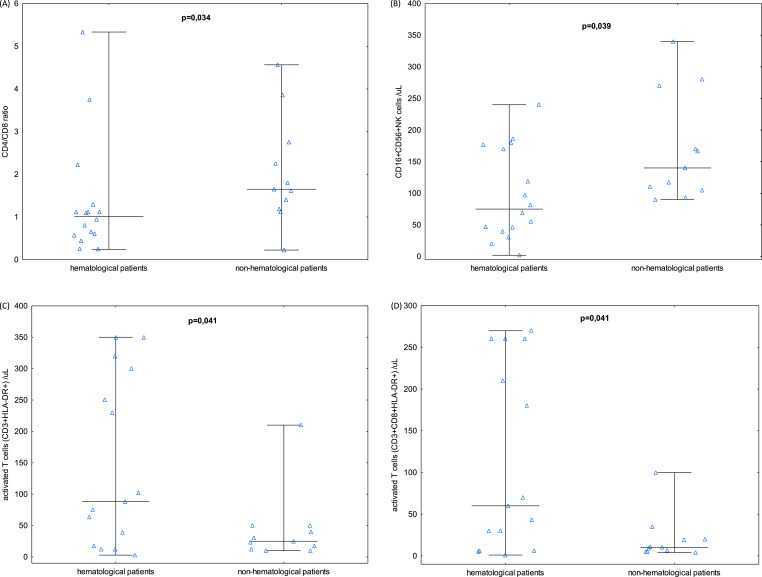
Lymphocyte subpopulations were analysed in 27 patients with COVID-19, including 16 patients with haematological malignancy. The analysis of lymphocyte subsets in 27 patients with COVID-19 is shown in Table 2 and in Fig. 2. The cytometric comparison of relevant lymphocyte subsets is shown in Fig. 3. The total number of T cells was decreased in all patients infected with SARS-CoV-2 (530 cells/µL), whereas the total number of B cells and NK cells were only decreased in haematological patients infected with SARS-CoV-2 (29 and 75 cells/µL, respectively). Analysing different lymphocyte subpopulations, we observed lower than normal levels of helper T cells (CD3+CD4+) in both studied groups of patients. The helper T and suppressor T cell ratio (CD4/CD8) was near the lower limit range, and it was significantly lower in patients with haematological burden (p = 0.03). Levels of activated T cells (CD3+HLA-DR+), as well as levels of activated suppressor T cells (CD3+CD8+HLA-DR+) were significantly higher in haematological patients (both p = 0.041). Levels of TCRα/ß and TCRɣ/ƍ were decreased in both studied groups of patients. Activated CD25+ T cells were near the lower limit normal range in haematological patients and significantly lower than in non-haematological patients (p = 0.008). Regarding other lymphocyte subsets, NK cells were below normal range in haematological patients and significantly decreased compared to non-haematological patients (p = 0.039). Moreover, levels of TCRɣ/ƍ in haematological patients were strongly correlated with CD16+CD56+ NK cells (R = 0.70, p = 0.002).
Table 2.
Comparison of lymphocyte subsets between haematological and non-haematological patients with COVID-19.
| Lymphocyte subsets | Normal ranges /uL | All patients (n = 27) | Haematological patients (n = 16) | Non-haematological patients (n = 11) | p |
|---|---|---|---|---|---|
| T cells (CD3+)/uL | 617–2383 | 530 (200–1000) | 540 (175–1080) | 530 (360–980) | 0.65 |
| Helper T cells (CD3+CD4+)/uL | 424–1513 | 240 (140–530) | 185 (95–525) | 320 (200–530) | 0.23 |
| Suppressor T cells (CD3+CD8+)/uL | 101–955 | 240 (90–510) | 320 (60–630) | 200 (98–380) | 0.88 |
| CD4/CD8 ratio | 0.9–2.0 | 1.11 (0.65–2.22) | 1.01 (0.59–1.2) | 1.65 (1.18–2.75) | 0.03 |
| Activated T cells (CD3+HLA-DR+)/uL | 30–200 | 50 (18–230) | 95 (28.5–310) | 25 (12–50) | 0.041 |
| Activated suppressor T cells (CD3+CD8+HLA-DR+)/uL | 30–180 | 30 (6–180) | 65 (18–260) | 10 (5–20) | 0.041 |
| TCRα/ß/uL | 573–2216 | 510 (200–980) | 510 (170–1045) | 510 (290–880) | 0.64 |
| TCRɣ/ƍ/uL | 30–119 | 20 (8–49) | 18 (7–45) | 20 (9.7–83) | 0.29 |
| CD25+CD3+/uL | 7–94 | 20 (11–41) | 13 (8–27.5) | 41 (20–67) | 0.008 |
| B cells (CD19+)/uL | 31–527 | 68 (10–160) | 29 (0.8–210) | 70 (58–140) | 0.4 |
| B cells (CD19+CD20+)/uL | 66–528 | 50 (2–139) | 9 (0–130) | 65 (50–139) | 0.07 |
| NK cells (CD16+CD56+)/uL | 110–678 | 110 (55–177) | 75 (43–173) | 140 (105–270) | 0.039 |
Fig. 2.
Comparison of lymphocyte subsets between haematological and non-haematological patients with COVID-19: haematological group (n = 16); non-haematological group (n = 11). CD4/CD8 T cells ratio (A), CD16+CD56+ NK cells (B), HLA-DR+ T cells (C), CD8+HLA-DR+ T cells (D). p<0,05.
Fig. 3.
Comparison of lymphocyte subsets between haematological (A,C,E) and non-haematological patients (B,D,F): pink - NK cells CD16+CD56+, orange - NK cells (A,B); blue - lymphocytes T CD3+; yellow - lymphocytes T CD3+HLA-DR+ (C,D); blue - lymphocytes T CD3+; white - activated suppressor lymphocytes T CD3+CD8+HLA-DR+ (E,F). A – haematological patient's NK cells subpopulation CD16+CD56+ consisted 92,9% of all patient's NK cells vs. B – non - haematological patient's NK cells subpopulation CD16+CD56+ consisted 87,3% of all patient's NK cells. C - haematological patient's lymphocytes T CD3+HLA-DR+ consisted 45,9% of patient's all lymphocytes T CD3+ vs. D – non - haematological patient's lymphocytes T CD3+HLA-DR+ consisted 6,6% of patient's all lymphocytes T CD3+. E - haematological patient's activated suppressor lymphocytes T CD3+CD8+HLA-DR+ consisted 21,7% of patient's all lymphocytes T CD3+ vs. F – non - haematological patient's activated suppressor lymphocytes T CD3+CD8+HLA-DR+ consisted 1,4% of patient's all lymphocytes T CD3+. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
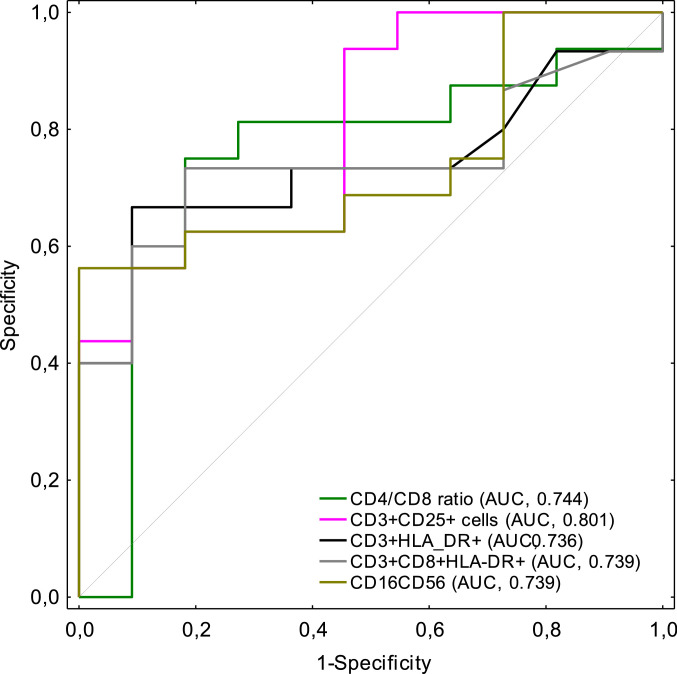
Based on the results, we singled out CD25+ T cells, activated T cells (CD3+HLA-DR+), activated suppressor T cells (CD3+CD8+HLA-DR+), CD4/CD8 ratio and CD16+CD56+ NK cells as markers differentiating between haematological patients and non-haematological patients infected with SARS-CoV-2. ROC curve analysis was conducted to evaluate the relationship between clinical attributes and lymphocyte subsets between haematological and non-haematological patients with COVID-19 (Fig. 4). It was found that CD25+ T cells counts were below the cut-off value of 37 in 15 haematological patients, which constituted 93.8% of all haematological patients. The area under the ROC curve (AUC) was 0.801 (95% CI: 0.632 – 0.970) with 54.5% specificity and 93.8% sensitivity. The optimal cut-off values of CD3+HLA-DR+ T cells and CD3+CD8+HLA-DR+ T cells from the ROC curve were 63 and 30, respectively. These results were elevated in 10 (67%) and 11 (73%) haematological patients with AUC of 0.736 (95% CI: 0.539 – 0.934) and 0.739 (95% CI: 0.542 – 0.937). Our results show that the CD4/CD8 ratio and CD16+CD56+ NK cell values appeared to be lower in the haematological group with a cut off value of 1.12 and 81, respectively, and AUC of 0.744 (95%CI: 0.537 – 0.951) and 0.739 (95% CI: 0.548 – 0.929), respectively. The sensitivity and specificity of the parameters were between 0.379 and 1.0.
Fig. 4.
ROC curve analysis of different lymphocyte subsets (CD4/CD8 T cells ratio - dark green line, CD25+ T cells – pink line, HLA-DR+ T cells – black line, CD8+HLA-DR+ T cells – grey line, CD16+CD56+ NK cells – light green line). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Changes in the counts of lymphocyte subpopulations in patients infected with SARS-CoV-2
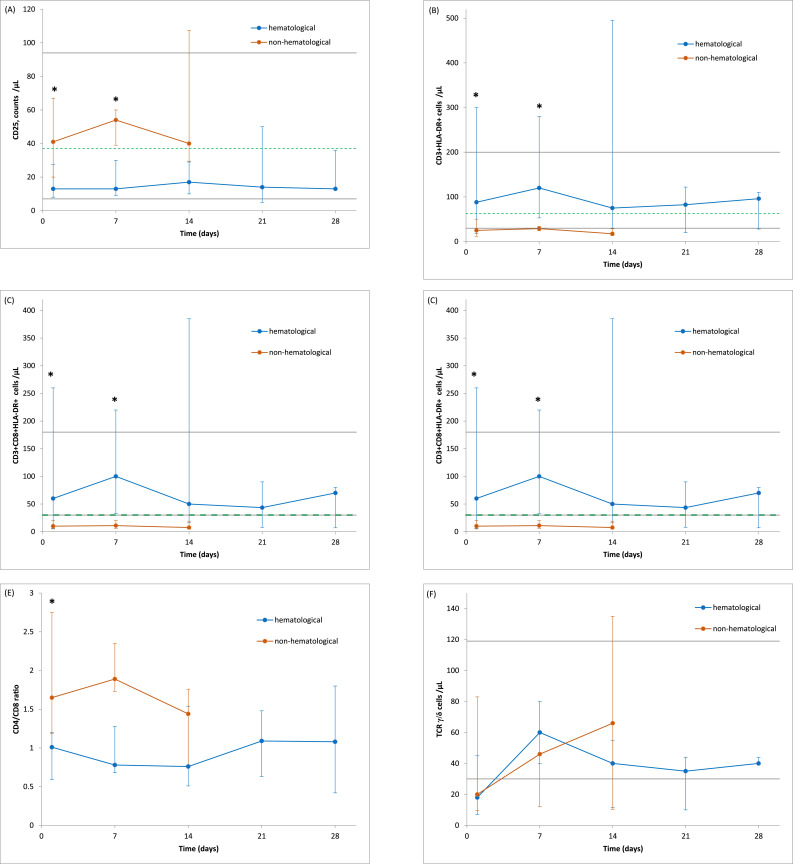
The observed changes in the counts of lymphocyte subsets in patients during SARS-CoV-2 infection are given in Fig. 5. Twenty seven COVID-19 patients (16 with haematological diseases and 11 without haematological burden) were assessed during infection at weekly intervals.
Fig. 5.
Longitudinal analysis of cell counts of different lymphocyte subsets in haematological versus non-haematological patients with COVID-19. The absolute numbers of CD25+ T cells (A), HLA-DR+ T cells (B), CD8+HLA-DR+ T cells (C), CD16+CD56+ NK cells (D), CD4/CD8 T cells ratio (E), TCRγ/δ cells (F) in haematological patients with COVID-19 (blue line), and non-haematological patients with COVID-19 (red line) were assessed at different time points after PCR confirmation of SARS-CoV-2 infection. Median ± IQR. The solid grey lines show the normal reference of each parameter. The dotted green line indicates the cut-off value calculated by ROC analysis. * p < 0,05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Activated T cells (CD3+HLA-DR+) and activated suppressor T cells (CD3+CD8+HLA-DR+) increased significantly on day 1 to day 7 of SARS-CoV-2 infection in haematological patients compared to non-haematological patients (p = 0.043 and p = 0.047, respectively). In parallel, during the initial days of COVID-19, haematological patients had a significantly lowered CD4/CD8 ratio compared to non-haematological patients (p = 0.036).
On the other hand, numbers of CD25+ T cells increased significantly at the beginning of SARS-CoV-2 infection only in non-haematological patients (day 1 and day 7) compared to haematological patients (p = 0.01 and 0.038, respectively).
Numbers of CD16+CD56+ NK cells showed no significant difference between studied groups during infection, except on day 1, when their levels were significantly lower in haematological patients.
ɣ/ƍ T cells showed no significant increase during infection, both in haematological and non-haematological patients.
Discussion
In our multicentre study, we showed a marked dysregulation of the immune system through reduced numbers of T and NK cells in patients with haematological diseases suffering from COVID-19. Our research provides two major findings: first, patients with haematological malignancies affected by COVID-19 are characterized by an increase in activated T lymphocytes (CD3+HLA-DR+ and CD3+CD8+HLA-DR+) in the early stages of SARS-CoV-2 infection, with a concomitant decrease in the CD4/CD8 ratio compared to non-haematological patients. Second, we observed a decrease in ɣ/ƍ T cells in both studied groups of patients, with lower CD25+ T cells in haematological patients and a significant decrease in CD16+CD56+ NK cells in haematological compared to non-haematological patients with COVID-19.
Lymphopenia is an adverse factor in SARS-CoV-2 infection associated with severe presentation of COVID-19, as well as independent risk factor in patients with haematological malignancies, especially in acute myeloid leukaemia [4]. T lymphopenia during COVID-19 is associated with impaired inflammatory response manifested by an increase in proinflammatory cytokines (Il-10, Il-2, Il-4, TNFα, INFγ) which, in turn, could induce severe lung injury during SARS infection [15]. Potential reasons for lymphocyte depletion in the course of SARS-CoV-2 infection might include: suppression of bone marrow, T-cell migration into inflamed tissues, direct (by ACE-2 receptor) and indirect (by inducing proinflammatory cytokines- TNFα, Il-6 or producing metabolic molecules such as lactic acid) destruction of lymphocytes [21], [22], [23].
Available data concerning immune abnormalities during COVID-19 reported decreased levels of T lymphocytes – both CD4+ and CD8+ T cells [[8], [9], [10],[13], [14], [15], [16]], as well as NK cells [[8], [9], [10],13,14], with more pronounced reductions in the counts of CD4+ T cells in severely ill patients [8,13]. Our results showed decreased levels of CD3+ T lymphocytes, especially in the case of CD4+ T cells, both in haematological and non-haematological patients affected by COVID-19. The CD4/CD8 ratio remained in the normal range, but was significantly decreased in haematological patients compared to patients without haematological malignancy. This indicated that haematological patients with COVID-19 were characterized by a more common decline in CD4+ T cells than CD8+ T cells, which is in contrast to previous research describing lymphocyte profiles in COVID-19 patients where mainly a decrease in CD8+ T cells was observed [9]. Interestingly, during the first days of SARS-CoV-2 infection in haematological patients, levels of activated T cells (both CD3+HLA-DR+ and CD3+CD8+HLA-DR+) were significantly higher compared to non-haematological patients. This finding demonstrates similarities with the immune status of HIV-infected patients in whom early increases of activated T cells precede the clinically significant CD4+ T cell depletion [17,18].
T cell responses to many infections are mediated by the expression of TCR α/ß and TCR ɣ/ƍ on their surface, the levels of which increase after infection. ɤ/ƍ T cells play a key role in immunity by exerting effector functions to eliminate pathogens [19]. In the case of SARS-CoV-2 infection, in contrast to other viral infections, ɤ/ƍ T cells were found to be decreased compared to healthy controls [19]. In our study, we observed decreased levels of α/ß and ɤ/ƍ T cells in both studied groups of patients after confirmation of SARS-CoV-2 infection. In the days after infection, absolute levels of ɤ/ƍ T cells increased in both groups of patients, however, without achieving statistical significance. This may indicate that during the course of COVID-19, ɤ/ƍ T cells may become dysfunctional and/or exhausted, leading to lower responsiveness to antigen stimulation. Additionally, no significant increase in the CD25 antigen on T cells (which serves as a marker of activation of ɤ/ƍ T cells) during SARS-CoV-2 infection was observed in haematological patients. Moreover, in haematological patients, CD25+ T cells were significantly decreased compared to patients without haematological malignancy. Therefore, indirectly, we can speculate that in the course of COVID-19, not only levels of ɤ/ƍ T cells, but also their function becomes depleted, especially in patients with haematological malignancies. The role of antigen-stimulated ɤ/ƍ T cells also includes interaction with NK cells. In our study, CD16+CD56+ NK cells were below normal levels and significantly decreased in haematological patients compared to non-haematological patients with COVID-19. This fact reflects immune deficiencies observed in other viral infections, such as HIV, during the course of which the levels and function of ɤ/ƍ T and NK cells are impaired [20].
The sample size in this multicentre study was relatively small. Specific markers of inflammatory activation (i.e. IL-6, IL-10, INFα) were not assessed, therefore, the relationship between particular subsets of lymphocytes and inflammatory markers were not evaluated. No significant correlations between lymphocyte subsets, inflammatory markers (CRP) and in-hospital mortality were observed. This fact may be due to the heterogeneity of the studied group with respect to different treatments and various haematological diagnoses.
Further research with larger sample sizes are needed to assess the potential impact of the mentioned immune disturbances on clinical outcome. Large-scale studies would provide valuable information on immune status in haematological patients through a detailed assessment of the activity of lymphocyte subpopulations by evaluating activation markers and secreted cytokines.
Our study demonstrated impaired adaptive immunity in patients with haematological malignancies affected by COVID-19 with respect to T lymphopenia, particularly decreased CD4+ T cells, lowered CD4/CD8 ratio and a reduction in NK cells. Early features of COVID-19 in haematological patients included increase in activated T cells (CD3+CD8+HLA-DR+ and CD3+HLA-DR+) with a concomitant decrease in the CD4/CD8 ratio, which were specific for this group of patients and distinct from overall population with COVID-19. Low levels of ɣ/ƍ T cells in parallel with low CD3+CD25+ T cells in haematological patients may indicate an impaired cellular immune response to COVID-19.
CRediT authorship contribution statement
Elżbieta Kalicińska: Conceptualization, Investigation, Data curation, Writing - original draft, Writing - review & editing. Donata Szymczak: Methodology, Investigation, Data curation, Visualization. Iga Andrasiak: Formal analysis, Visualization. Aleksandra Bogucka-Fedorczuk: Writing - original draft. Aleksander Zińczuk: Resources. Wojciech Szymański: Resources. Monika Biernat: Resources. Marcin Rymko: Resources, Writing - review & editing. Grażyna Semeńczuk: Resources. Paula Jabłonowska: Resources. Justyna Rybka: Supervision. Krzysztof Simon: Supervision. Tomasz Wróbel: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behl D., Porrata L.F., Markovic S.N., Letendre L., Pruthi R.K., Hook C.C. Absolute lymphocyte count recovery after induction chemotherapy predicts superior survival in acute myelogenous leukemia. Leukemia. 2006;20(1):29–34. doi: 10.1038/sj.leu.2404032. [DOI] [PubMed] [Google Scholar]
- 5.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7) Available on http://en.nhc.gov.cn/2020-03/29/c_78469.htm
- 8.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M., Guo Y., Luo Q., Huang Z., Zhao R., Liu S. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J. Infect. Dis. 2020;222(2):198–202. doi: 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W., Berube J., McNamara M., Saksena S., Hartman M., Arshad T. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020 doi: 10.1002/cyt.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He R., Lu Z., Zhang L., Fan T., Xiong R., Shen X. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R., Wang Y., Li J., Han H., Xia Z., Liu F. Decreased T cell populations contribute to the increased severity of COVID-19. Clin. Chim. Acta. 2020;508:110–114. doi: 10.1016/j.cca.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B., Fan Cun-Yu, Wang An-Lu, Zou Yi-Long, Yu Yi-Han, He C. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Li S., Liu Jia, Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-COV-2 infected patients. EBioMedicina. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogner J.R., Matuschke A., Heinrich B., Schreiber M.A., Nerl C., Goebel F.D. Expansion of activated T lymphocytes (CD3+HLA/DR+) detectable in early stages of HIV-1 infection. Klin. Wochenschr. 1990;68(8):393–396. doi: 10.1007/BF01648577. [DOI] [PubMed] [Google Scholar]
- 18.Mahalingam M., Peakman M., Davies E.T., Pozniak A., McManus T.J., Vergani D. T cell activation and disease severity in HIV infection. Clin. Exp. Immunol. 1993;93(3):337–343. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei L., Qian H., Yang X., Zhang X., Zhang D., Dai T. The phenotypic changes of γ/δ T cells in COVID-19 patients. J. Cell Mol. Med. 2020;24(19):11603–11606. doi: 10.1111/jcmm.15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauza C.D., Poonia B., Li H., Cairo C., Chaudhry S. γδ T cells in HIV disease: past, present, and future. Front. Immunol. 2014;5:687. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Yi-Quan. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Sig. Transduct. Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148y-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamo S., Chevrier S., Cervia C., Zurbuchen R.M., Yang L., Sivapatham S. Lymphopenia-induced T cell proliferation is a hallmark of severe COVID-19. bioRxiv. 2020 doi: 10.1101/2020.08.04.236521. [DOI] [Google Scholar]
- 23.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int. J. Infect. Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]