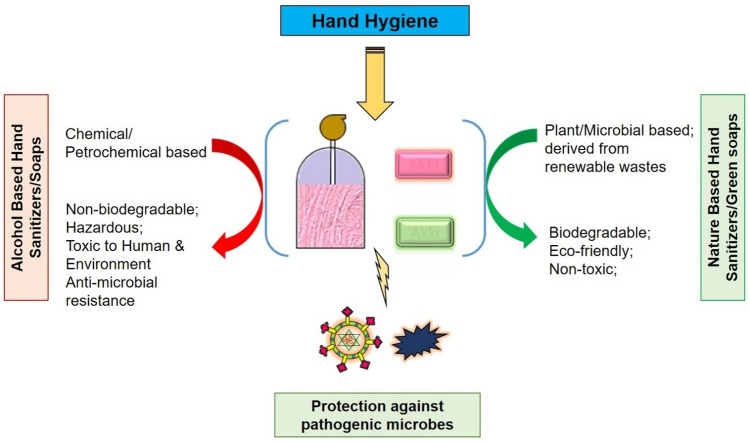
Graphical abstract
Keywords: Hand sanitizers, Alcohol-based hand rub, Chemical surfactants, Biosurfactants, Ecofriendly soaps, Antimicrobial resistance
Abstract
The Coronavirus disease-2019 (COVID-19) outbreak is caused by a highly pathogenic novel coronavirus (SARS-CoV-2). To date, there is no prescribed medicine for COVID-19. Frequent handwashing with soap and the use of alcohol-based hand sanitizers is recommended by WHO for hand hygiene and to prevent the spread of COVID-19. However, there are safety concerns associated with the use of soaps and alcohol-based hand sanitizers. Therefore, the review aims to highlight the health and environmental concerns associated with the frequent use of soaps/detergents and alcohol-based hand sanitizers amid COVID-19. The potential of some of the natural detergents and sanitizing agents as eco-friendly alternatives to petrochemical-based soaps and alcohol-based hand rubs for hand hygiene are discussed. The market of soaps and hand sanitizers is expected to grow in the coming years and therefore, future research should be directed to develop eco-friendly soaps and hand sanitizers for human and environmental safety.
1. Introduction: COVID-19 and hygiene
Coronavirus Diseases 2019 (COVID-19), which was first reported in Wuhan city (China) in December 2019 has already spread around the world within a short time. World Health Organization (WHO) has already declared it a pandemic before the mid-March 2020. Globally number of COVID-19 positive cases has reached up to 20,439,814 including 744,385 deaths worldwide as on August 13, 2020 [1]. The infective agent of COVID-19 is Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a highly pathogenic human coronavirus. Researchers and scientists around the world are searching for the right medication and looking to develop the vaccine to control the disease.
As vaccine or approved drugs are not available for COVID-19, it is advisable to prevent its spread by following simple measures as prescribed by WHO [2]: (a) Hand hygiene: Regularly wash hands with soaps and when washing facility is not available to clean hands with alcohol-based hand rubs; (b) Social distancing: Maintain at least 3 feet distance with others and avoid visiting crowded places, if possible; (c) Respiratory hygiene: Use a tissue or cover your mouth and nose with the bent elbow while sneezing or coughing, or always wear a mask when going outside or visiting any public place; (d) Stay home and stay safe: Stay at home and self-isolate in case of feeling unwell or having any symptoms of cough, fever, headache. In brief, personal hygiene is key to prevent the spread of this contagious disease. Soaps and hand sanitizers play a crucial role in maintaining personal hygiene to fight against COVID-19.
The active components of soaps are detergents derived from the petrochemicals, while the recommended hand sanitizers contain high concentrations of alcohol. The detergents and alcohol cause skin irritation and dryness and are hazardous to the environment [[3], [4], [5],56]. Therefore, there is a need for eco-friendly soaps and hand sanitizers for human and environmental safety.
Various nature-based compounds such as microbial biosurfactants and plant secondary metabolites have been reported to have antimicrobial and virucidal activities [[6], [7], [8]]. These natural compounds are usually non-toxic and easily biodegradable. Therefore, this short-review aims to highlight the health and environmental concerns of using soaps and alcohol-based hand sanitizers amid COVID-19 and to discuss the potential of some of the natural detergents and sanitizing agents as eco-friendly alternatives to petrochemical-based soaps and alcohol-based hand rubs for hand hygiene.
2. Hand hygiene with soaps and alcohol-based sanitizers
Hand hygiene using soaps or hand sanitizers is the first-line defensive measure to fight against COVID-19. Soaps or detergents (chemical surfactants) are surface-active agents. They are synthesized from petrochemicals and act by reducing the surface and interfacial tension between two phases. Linear alkylbenzene sulfonates (LAS) having a chain length of C10-C14 are the most commonly used surfactants in soaps, detergents, shampoos, and personal care products [9]. Handwashing facilities like the availability of water are required for maintaining hand hygiene by soaps/detergents.
Hand sanitizers are popular as they can be carried easily and useful when handwashing facilities are not readily available. Not all sanitizers are effective and therefore, WHO has recommended the use of alcohol-based hand sanitizers which can be easily prepared for local production. The composition of WHO-recommended hand sanitizer formulations contains, either ethanol (96 %; final concentration 80 % v/v) or isopropyl alcohol (99.8 %; final concentration 75 %) along with hydrogen peroxide (0.125 % v/v as a preservative to inactivate bacterial spores) and glycerol (1.45 % v/v as a humectant – moisturizing agent) diluted with sterilized distilled water or boiled water [10]. In commercial products, propylene glycol is being used as a humectant. A viscosity enhancer such as alkyl acrylate cross-polymer, tetrahydroxypropyl-ethylenediamine, etc. is usually added in alcohol-based hand-rub gels. The cost of alcohol-based liquid and gel sanitizers is ranged around US$ 2.5–5.4 and US$ 8, respectively [10].
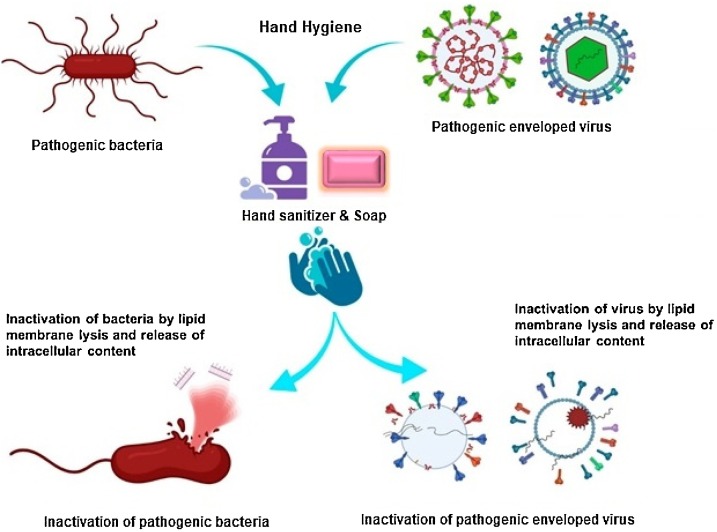
The mechanism of killing the microorganisms by soaps or detergents relies on the fact that they disrupt the lipophilic membrane of the cell wall of bacteria and other microorganisms including enveloped viruses [11]. Similarly, alcohol also dissolves the lipid membrane of microorganisms. Literature suggests that ethanol is highly effective (within 30 s) against almost all clinically relevant enveloped viruses including coronaviruses (SARS-CoV i.e. Severe Acute Respiratory Syndrome Coronavirus and MERS-CoV i.e. the Middle East Respiratory Syndrome Coronavirus, which belong to the same class of viruses as SARS-CoV-2), and influenza viruses [12,13]. Therefore, alcohol-based hand sanitizers with alcohol content >60 % v/v are popular and recommended by WHO and other national organizations such as CDC (Centers for Disease Control and Prevention), USA [14]. The recent study by Kratzel et al. [15] reports that SARS-CoV-2 was efficiently inactivated by ethanol and 2-propanol at a concentration of >30 % v/v and by the two preparations recommended by WHO in 30 s. That's why frequent hand washing with soaps and hand hygiene with alcohol-based hand sanitizers has been recommended. The simplified mechanism of soaps/detergents and alcohol-based hand sanitizers are presented in Fig. 1 .
Fig. 1.
Hand hygiene by washing hands with soap and alcohol based hand sanitizers (modified from [25] under CC BY 4.0 License).
2.1. Soaps and chemical detergents—environmental impact
Chemical detergents or surfactants, one of the emerging contaminants are not easily degradable and persist in the environmental matrices for a long duration and therefore, contribute to environmental pollution. Their toxicity to the living organisms including microorganisms to mammals of the different ecosystems (water, soil, and sediments) are well reported [4,5]. They also increase the concentrations of hydrocarbons and pesticides in the aqueous environment and antibiotic resistance in microorganisms [16,17]. Their concentrations in greywater effluents and natural waters ranged from 0.7 to 70 mg/L and 0.001 to 10 mg/L, respectively [4,18]. Their treatment in wastewater treatment plants is challenging and they also create secondary issues like foaming, interruption in oxygen diffusion, inhibition of the biodegradation of organic compounds, and denitrification process [17]. LAS, anionic surfactants are non-biodegradable anaerobically, though it could be degraded aerobically (80–96 %). Nonylphenol ethoxylates, non-ionic surfactant, is poorly degraded (<20 %) in aerobic wastewater treatment plants [9].
Due to frequent hand washing during the COVID-19 pandemic, the concentrations of surfactants in the wastewater generated from the household and other institutions are expected to increase by several times. Their impact on the performance of the wastewater treatment plants and environment during and the post-COVID-19 pandemic is yet to be quantified.
2.2. Health and environmental concerns of alcohol-based hand sanitizers
The general safety issues associated with alcohol-based hand sanitizers are flammability of alcohol and toxicity due to the accidental ingestion of the sanitizer. In a recent review by Mahmood et al. (2020), the human health risks and environmental concerns of alcohol-based hand sanitizers are highlighted. Alcohol and isopropyl alcohol spills in the water bodies are toxic to aquatic animals, while a large amount of isopropyl alcohol spill at soil may contaminate the groundwater [56].
Though the key components of hand sanitizers, alcohol and H2O2 are in general not toxic externally, there is a concern of skin damage due to excessive use of hand sanitizers, which can lead to an inability of the skin to protect against other microorganisms or viruses [56]. Younger kids (12-year-old children or younger) are at high risk due to accidental ingestion. In children, even a small dose of alcohol can cause alcohol poisoning. Therefore, the American Association of Poison Control Center (AAPCC) regarded hand sanitizers as emerging hazards. In the first six months (January to June 2020), 11,363 cases of exposure due to hand sanitizer have been reported according to AAPCC (https://aapcc.org/track/hand-sanitizer).
Methanol contamination has also been found in hand sanitizers probably due to the high demand for ethyl alcohol and isopropyl alcohol during this pandemic. FDA (U.S. Food & Drug Administration) found methanol contamination in several tested hand sanitizers (77 products as on July 23, 2020) and advises consumers not to use hand sanitizers from certain manufactures [19]. Therefore, there is a dire need to replace alcohol-based hand sanitizers with non-toxic or low-toxic hand sanitizers for human and environmental safety.
2.3. Hand sanitizers and soaps and the risk of antimicrobial resistance in the environment
Antimicrobial resistance due to the rampant use of antibiotics and other antimicrobial agents has become one of the major concerns worldwide. Apart from antimicrobials (antibiotics, antivirals, and antiparasitics), excessive use of surfactants, alcohol, and hydrogen peroxides are also known to cause resistance to microorganisms [[20], [21], [22], [23]]. Recent findings revealed that the pathogen Enterococcus faecium, which is a leading cause of nosocomial infections has become 10-fold more resistant to hand-wash alcohol (70 % isopropanol) [22]. Other reports also suggest the excessive use of alcohol-based hand sanitizers in hospital settings creates a selection pressure to develop more resistant strains of pathogens [24]. Though these reports confined to medical/hospital settings as alcohol-based disinfectants and hand-rubs are commonly used in hospitals, the role of alcohol in causing antimicrobial resistance in natural environments could not be ignored due to their excessive use amid COVID-19.
Antimicrobial agents such as triclosan, triclocarban, chlorhexidine, and quaternary ammonium compounds (benzalkonium chloride) are usually added in medicated hand soaps and non-alcohol based hand rubs [23,25]. Triclosan (2, 4, 4′ – trichloro- 2′-hydroxydiphenyl ether) is the most commonly used antibacterial agent in medicated hand soaps and other personal care products. Triclosan and its related compounds are endocrine disrupters and persistent in the environment, which have the potential to induce multi-antibiotic resistance through genetic mutations in pathogens [23,26]. The environmental toxicity of quaternary ammonium compounds is well-reviewed and documented [27,28]. Several pathogens including Pseudomonas aeruginosa, Bacillus cereus, Escherichia coli, Mycobacterium species, have shown resistance and tolerance to benzalkonium chloride. Not only this, cross-resistance between benzalkonium chloride and antibiotics have also been well documented in the literature [27]. Therefore, excessive use of hand rubs and medicated hand soaps having such antimicrobial agents during and post-COVID-19 pandemic could lead to a much more devastating impact on the environment and humans.
3. Ecofriendly hand hygiene
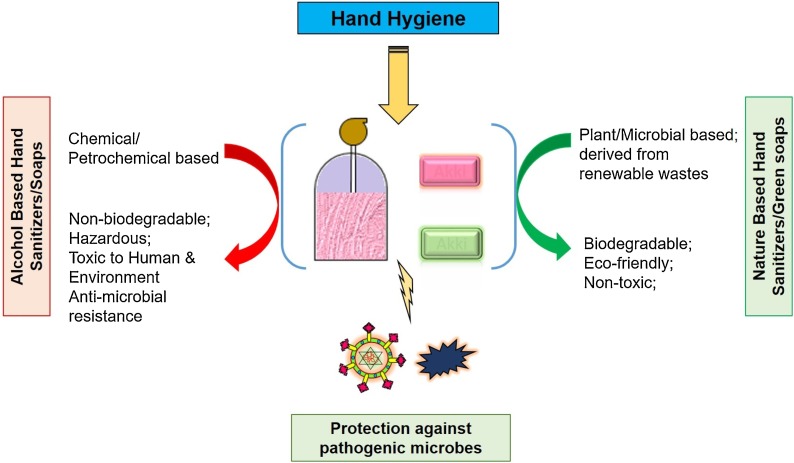
Several natural compounds derived from microbial sources or plants possess detergents, humectants, antibacterial, antifungal, and antiviral properties, and could be potential agents in nature-based soaps and hand sanitizers. The natural compounds are biodegradable, non-toxic to the environment, and usually biocompatible to humans. The main features of ecofriendly hand hygiene by using nature-based soaps and sanitizers are presented in Fig. 2 .
Fig. 2.
Simplified overview of the key points of using alcohol based, and ecofriendly nature based hand sanitizers and soaps for hand hygiene.
3.1. Biosurfactants (microbial and plant-derived surface-active agents)
Many microorganisms (bacteria, yeasts, and fungi) and plants produce surface-active agents called biosurfactants. Chemically they are amphiphilic compounds i.e. have both lipophilic and hydrophilic moieties in their structure. The biosurfactants possess similar properties as their chemical counterpart. For example, they efficiently reduce the surface and interfacial tensions between two phases, act as emulsifiers, and have foaming properties. Therefore, biosurfactants are potential agents to replace synthetic surfactants in soaps and detergents.
3.1.1. Microbial biosurfactants
Based on their chemical structure microbial biosurfactants are classified as glycolipids, lipopeptides, phospholipids, polymeric surfactants, and particulate surfactants [29]. Rhamnolipids, sophorolipids, and surfactin are the most widely studied microbial biosurfactants. Their antimicrobial (antibacterial and antifungal), pharmaceutical and medical applications such as anticancer, anti-inflammatory and immunomodulators, antihypertensive activities are well reported in the literature [30,6,29,31]. More importantly, they have also been active against various viruses. For example, surfactin – a cyclic lipopeptide type of biosurfactant has shown antiviral activities against various enveloped and non-enveloped viruses such as Porcine epidemic diarrhea virus, transmissible gastroenteritis virus, herpes simplex virus, Semliki Forest virus, and vesicular stomatitis virus [32,8]. Similarly, sophorolipid - a glycolipid type of biosurfactant has also shown in-vitro antiviral activity against enveloped viruses (influenza virus, herpes virus, and human immunodeficiency virus) [6].
Many of the microbial biosurfactants are already in the market as household detergents, and active ingredients in cosmetics and personal care products [33,29,31]. For example, sophorolipids are the active ingredients of various cosmetics and personal care products available commercially, which shows they are biocompatible and non-toxic. Rhamnolipids and surfactin also find application in bio-detergent and lip gloss formulations as biocompatible and nonirritant biosurfactants [[34], [35], [36]].
Overall, properties such as antiviral, antimicrobial, low-toxicity, skin biocompatibility, wettability, and detergent properties make microbial biosurfactants as suitable candidates for the key component of hand hygiene formulations (soaps as well as non-alcohol based hand sanitizers). Moreover, they can also be produced by the microbes using renewable waste materials to make them competitive to chemical surfactants [37,38].
3.1.2. Plant-based surfactants
Plants secrete various secondary metabolites and many of them possess surfactant properties. Saponins are the most widely studied plant-derived biosurfactants and >500 plant species secrete them as secondary metabolites. It is found in different parts of the plants (root, stems, bark, leaves, fruits, and seeds) and their concentrations in plant extracts vary from 0.1 to 10 % [7]. Saponins from the tree Quillaja saponaria Molina (Chilean soapbark tress) are commercially available and found applicability in food, cosmetics, and pharmaceutical industries. Apart from antibacterial and pharmaceutical agents, Quillaja saponins also possess antiviral activity against various viruses such as vaccinia virus, herpes simplex virus, HIV, and reovirus [7,39].
Saponins from the root of plants Bupleurum marginatum (saikosaponins) and Parispolyphylla (Polyphylla saponins) are effective against the influenza A virus (H1N1) [40,41]. Saikosaponins also reported having antiviral activity against coronavirus H-CoV-22E9 by preventing the early stage of infection, viral attachment, and penetration [42]. The Camellia sinensis var. assamica seed cake (an unutilized saponin source) based detergent was reported to have LD50 values >14 g/kg in mice with stronger activity than commercial detergent [43]. Plant extracts of horse chestnuts and soap nuts have also been suggested as good candidates for surface hygiene management [44].
In the Kumaun region of Uttarakhand (India) 22 plant species have been used as traditional soaps and detergents. Different plant parts such as seeds, seed coats, barks, leaves, and young shoots, roots, ash have been used for washing and bathing purposes by the local people [45]. Overall, all these properties of plant-derived natural soaps and detergents have the potential to replace the synthetic detergents and alcohol-based sanitizers. However, in-vitro activities of these natural biosurfactants against coronaviruses and SARS-CoV-2 has to be tested before using for hand hygiene.
3.2. Aloe vera gel - antiviral agent, a natural humectant and viscosity enhancer
Propylene glycol is the most commonly used humectant in hand sanitizers due to its low cost. A viscosity enhancer such as carbomer hydroxyethyl cellulose, sodium carboxymethyl cellulose, etc. is also added in the hand rubs. Aloe vera gel, a transparent mucilaginous jelly-like material obtained from the Aloe vera plant is a natural humectant, which can be used as an alternative to conventional humectant and viscosity enhancer [55]. Aloe vera gel contains water (99 %), glucomannans, amino acids, lipids sterols, and vitamins [46]. It has various medicinal properties and therefore, it is used in various pharmaceutical applications. Antiviral and antibacterial effects of Aloe vera has also been reported in the literature [46,47]. Aloin and aloe-emodin are the key molecules for the antiviral activity of Aloe vera. These molecules kill the enveloped viruses including SARS-CoV-1, influenza, and HIV viruses either by destructing the lipid envelope of the virus or inhibiting the viral replication [47]. All these properties make it an attractive choice to be a key component in a non-alcoholic hand rub sanitizer.
3.3. Essential oils and phenolic compounds
Essential oils and phenolic compounds from a variety of plants have been reported to have antiviral activities due to their power to solubilize the lipid membrane of enveloped viruses including human coronaviruses [48,49]. Resveratrol – a triterpenoid found in grape seeds and skin has been reported to prevent the entry and inhibit replication of SARS-CoV (MERS-CoV) [50]. Lemon balm oil – an essential oil, which inhibits the enveloped herpes simplex virus and phenolic compounds from the Isatis indigotica, which inhibits coronavirus, could also inactivate SARS-CoV-2 [49]. However, the low-yield of essential oils and phenolic compounds could be a major factor for their commercial application in hand-sanitizers.
4. Economic aspects of eco-friendly soaps and hand-sanitizers
The main raw material used in soaps and hand sanitizers is petrochemicals based surfactant and ethanol (or isopropanol), respectively. Petrochemical based surfactants and ethanol are priced around US$ 1–3/kg and US$ 1.38/gallon, respectively ([51]; https://tradingeconomics.com/commodity/ethanol). The major limitation of the commercialization of any bio-based product is the cost associated with its production and biosurfactants are not different. However, considering the huge market demand for biosurfactants, several companies have emerged in recent years. These companies are based in Europe, the USA, and Asia [37]. The costs of commercial biosurfactants are reported to be around US$ 2.5–6.3/kg and US$ 5/kg for sophorolipids and rhamnolipids, respectively which is 2–3 times higher than petrochemicals based surfactants [51]. The raw material and downstream processing of bio-product usually account for 10–30 % and 60–70 % of total production cost, respectively [37,51]. Therefore, the production cost of biosurfactants could be reduced further by utilizing low-cost renewable waste materials as substrates, developing innovative and cost-effective separation processes, and bioprocess optimization. The use of solid-state fermentation over submerged fermentation and production of biosurfactant as a coproduct with other industrially important biochemical are the other strategies [52] which could be adopted to make them economically viable for various applications including eco-friendly soaps and sanitizers.
In the case of plant-derived biosurfactants and other antimicrobial agents, the plant processing and extraction of the active compounds using conventional methods such as maceration, soxhlet extraction, hydro-distillation are not cost-effective for industrial-scale application. Such conventional techniques have various limitations including low extraction rate, use of hazardous and costly solvents, and high energy consumption [53]. Therefore, emerging extraction techniques such as microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, and pulsed electric field extraction are gaining interest. These emerging techniques are regarded as green and sustainable extraction techniques [53,54] and could make these plant-derived antimicrobial agents as viable ingredients in natural hand-sanitizers. However, it is important to look indigenous plants for the economic viability of plant-derived biosurfactants and other natural antimicrobials for their utilization as key ingredients in nature-based soaps and hand sanitizers.
5. Conclusion
Good hand hygiene prevents the spread of various diseases including COVID-19. Handwashing with soaps and the use of hand sanitizers to clean hands have increased immensely during the COVID-19 pandemic. The global market of detergents and hand sanitizers is expected to grow in the coming days. However, considering the harmful effects of chemical detergents and hand sanitizers, it is high time to replace them with eco-friendly natural agents. Several microbial biosurfactants and plant secondary metabolites possess detergent, antimicrobial and antiviral activities. Being non-toxic and biodegradable, these eco-friendly agents have tremendous potential to replace conventional soaps and hand sanitizers. Economical production of biosurfactants and extraction of bioactive antimicrobial agents from the plants will play a crucial role in their commercial application and sustainability as eco-friendly soaps and hand sanitizers and therefore further research is needed in this direction.
Declaration of Competing Interest
None
Editor: Z. Wen
References
- 1.WHO . 2020. Coronavirus Disease (COVID-2019) Situation Reports. Situation Report 206.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ 13 August 2020. Retrieved on 14 August 2020 from. [Google Scholar]
- 2.WHO . 2020. Coronavirus Disease (COVID-19) Advice for the Public.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public Last updated 4 June 2020. Retrieved on 29 July 2020 from. [Google Scholar]
- 3.Boyce J.M., Kelliher S., Vallande N. Skin irritation and dryness associated with two hand-hygiene regimens: soap-and-water hand washing versus hand antisepsis with an alcoholic hand gel. Infect. Control Hosp. Epidemiol. 2000;21(7):442–448. doi: 10.1086/501785. [DOI] [PubMed] [Google Scholar]
- 4.Wiel-Shafran A., Ronen Z., Weisbrod N., Adar E., Gross A. Potential changes in soil properties following irrigation with surfactant-rich greywater. Ecol. Eng. 2006;26(4):348–354. doi: 10.1016/j.ecoleng.2005.12.008. [DOI] [Google Scholar]
- 5.Pironti C., Motta O., Ricciardi M., Camin F., Cucciniello R., Proto A. Characterization and authentication of commercial cleaning products formulated with biobased surfactants by stable carbon isotope ratio. Talanta. 2020 doi: 10.1016/j.talanta.2020.121256. [DOI] [PubMed] [Google Scholar]
- 6.Borsanyiova M., Patil A., Mukherji R., Prabhune A., Bopegamage S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol (Praha). 2016;61(1):85–89. doi: 10.1007/s12223-015-0413-z. [DOI] [PubMed] [Google Scholar]
- 7.Reichert C.L., Salminen H., Weiss J. Quillaja saponin characteristics and functional properties. Annu. Rev. Food Sci. Technol. 2019;10:43–73. doi: 10.1146/annurev-food-032818-122010. [DOI] [PubMed] [Google Scholar]
- 8.Yuan L., Zhang S., Wang Y., Li Y., Wang X., Yang Q. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J. Virol. 2018;92(21):e00809–00818. doi: 10.1128/JVI.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camacho-Muñoz D., Martín J., Santos J.L., Aparicio I., Alonso E. Occurrence of surfactants in wastewater: hourly and seasonal variations in urban and industrial wastewaters from Seville (Southern Spain) Sci. Total Environ. 2014;468-469:977–984. doi: 10.1016/j.scitotenv.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 10.WHO . 2010. Guide to Local Production: WHO-Recommended Handrub Formulations.https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf Retrieved from on 26th July 2020. [Google Scholar]
- 11.Ijaz M.K., Nims R.W., Whitehead K., Srinivasan V., Charlesworth B., McKinney J., Rubino J.R., Ripley M., Jones C. Microbicidal actives with virucidal efficacy against SARS-CoV-2. Am. J. Infect. Control. 2020;48(8):972–973. doi: 10.1016/j.ajic.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kampf G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018;98(4):331–338. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun Golin A.P., Choi D., Ghahary A. Hand sanitizers: a review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control. 2020;2020 doi: 10.1016/j.ajic.2020.06.182. [published online ahead of print, 2020 Jun 18]S0196-6553(20)30562-30569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC . 2020. Hand Hygiene Recommendations: Guidance for Healthcare Providers about Hand Hygiene and COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html Updated May 17, 2020. Retrieved from. [Google Scholar]
- 15.Kratzel A., Todt D., V’kovski P., Steiner S., Gultom M., Thao T., et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-Recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26(7):1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer M., Hatley H. The role of surfactants in wastewater treatment: impact, removal and future techniques: a critical review. Water Res. 2018;147:60–72. doi: 10.1016/j.watres.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 17.Kruszelnicka I., Ginter-Kramarczyk D., Wyrwas B., et al. Evaluation of surfactant removal efficiency in selected domestic wastewater treatment plants in Poland. J Environ Health Sci Engineer. 2019;17:1257–1264. doi: 10.1007/s40201-019-00387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X., Wang Z., Sun Y., et al. Surfactants at environmentally relevant concentrations interfere the inducible defense of Scenedesmus obliquus and the implications for ecological risk assessment. Environ Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114131. [DOI] [PubMed] [Google Scholar]
- 19.FDA . 2020. FDA Updates on Hand Sanitizers With Methanol.https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-methanol#products Updated on July 23, 2020. Retrieved from. [Google Scholar]
- 20.Singer A.C., Shaw H., Rhodes V., Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016;7:1728. doi: 10.3389/fmicb.2016.01728. Published 2016 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan W. Heavy use of hand sanitizer boosts antimicrobial resistance. PhysOrg. 2020 https://phys.org/news/2020-04-heavy-sanitizer-boosts-antimicrobial-resistance.html April 17, 2020. Retrieved from on October 5, 2020. [Google Scholar]
- 22.Pidot S.J., Gao W., Buultjens A.H., Monk I.R., Guerillot R., Carter G.P., Lee J.Y., Lam M.M., Grayson M.L., Ballard S.A., Mahony A.A. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018;10(452):p.eaar6115. doi: 10.1126/scitranslmed.aar6115. [DOI] [PubMed] [Google Scholar]
- 23.Atolani O., Baker M.T., Adeyemi O.S., Olanrewaju I.R., Hamid A.A., Ameen O.M., Oguntoye S.O., Usman L.A. COVID-19: critical discussion on the applications and implications of chemicals in sanitizers and disinfectants. EXCLI J. 2020;19(June (15)):785–799. doi: 10.17179/excli2020-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bondurant S.W., Duley C.M., Harbell J.W. Demonstrating the persistent antibacterial efficacy of a hand sanitizer containing benzalkonium chloride on human skin at 1, 2, and 4 hours after application. Am. J. Infect. Contr. 2019;47:928–932. doi: 10.1016/j.ajic.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Jing J.L.J., Pei Yi T., Bose R.J.C., McCarthy J.R., Tharmalingam N., Madheswaran T. Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health. 2020;17(9):3326. doi: 10.3390/ijerph17093326. Published 2020 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J., Jin M., Nguyen S.H., Mao L., Li J., Coin L.J., Yuan Z., Guo J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018;118:257–265. doi: 10.1016/j.envint.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Merchel Piovesan Pereira B., Tagkopoulos I. Benzalkonium chlorides: uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019;85:e00377. doi: 10.1128/AEM.00377-19. -19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 29.Zanotto A.W., Valério A., de Andrade C.J., et al. New sustainable alternatives to reduce the production costs for surfactin 50 years after the discovery. Appl. Microbiol. Biotechnol. 2019;103:8647–8656. doi: 10.1007/s00253-019-10123-7. [DOI] [PubMed] [Google Scholar]
- 30.Ghazala I., Bouassida M., Krichen F., Manuel Benito J., Ellouz-Chaabouni S., Haddar A. Anionic lipopeptides from Bacillus mojavensis I4 as effective antihypertensive agents: production, characterization, and identification. Eng. Life Sci. 2017;17(12):1244–1253. doi: 10.1002/elsc.201700020. Published 2017 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith M.L., Gandolfi S., Coshall P.M., Rahman P.K.S.M. Biosurfactants: a Covid-19 perspective. Front. Microbiol. 2020;11:1341. doi: 10.3389/fmicb.2020.01341. Published 2020 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollenbroich D., Ozel M., Vater J., Kamp R.M., Pauli G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;25(3):289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 33.Patel S., Homaei A., Patil S., Daverey A. Microbial biosurfactants for oil spill remediation: pitfalls and potentials. Appl. Microbiol. Biotechnol. 2019;103(1):27–37. doi: 10.1007/s00253-018-9434-2. [DOI] [PubMed] [Google Scholar]
- 34.Fei D., Zhou G.W., Yu Z.Q., Gang H.Z., Liu J.F., Yang S.Z., Ye R.Q., Mu B.Z. Low‐toxic and nonirritant biosurfactant surfactin and its performances in detergent formulations. J. Surfactants Deterg. 2019;23:109–118. doi: 10.1002/jsde.12356. [DOI] [Google Scholar]
- 35.Helmy Q., Gustiani S., Mustikawati A.T. Application of rhamnolipid biosurfactant for bio-detergent formulation. IOP COnf. Ser.: Mater. Sci. Eng. 2020;823 doi: 10.1088/1757899X/823/1/012014. [DOI] [Google Scholar]
- 36.Jun Eleni Drakontis C., Amin S. Design of sustainable lip gloss formulation with biosurfactants and silica particles. Int. J. Cosmet. Sci. 2020;2020 doi: 10.1111/ics.12642. [published online ahead of print, 2020 Jun 22] doi:10.1111/ics.12642. [DOI] [PubMed] [Google Scholar]
- 37.Geetha S., Banat I.M., Joshi S.J. Biosurfactants: production and potential applications in microbial enhanced oil recovery (MEOR) Biocatal. J. Agric. Biotechnol. Sustain. Dev. 2018;14:23–32. doi: 10.1016/j.bcab.2018.01.010. [DOI] [Google Scholar]
- 38.Jiménez-Peñalver P., Rodríguez A., Daverey A., Font X., Gea T. Use of wastes for sophorolipids production as a transition to circular economy: state of the art and perspectives. Rev. Environ. Sci. Biotechnol. 2019;18:413–435. doi: 10.1007/s11157-019-09502-3. [DOI] [Google Scholar]
- 39.Roner M.R., Sprayberry J., Spinks M., Dhanji S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina) J. Gen. Virol. 2007;88(Pt 1):275–285. doi: 10.1099/vir.0.82321-0. [DOI] [PubMed] [Google Scholar]
- 40.Fang W., Yang Y.J., Guo B.L., Cen S. Anti-influenza triterpenoid saponins (saikosaponins) from the roots of Bupleurum marginatum var. stenophyllum. Bioorg. Med. Chem. Lett. 2017;27(8):1654–1659. doi: 10.1016/j.bmcl.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Pu X., Ren J., Ma X., et al. Polyphylla saponin I has antiviral activity against influenza A virus. Int. J. Clin. Exp. Med. 2015;8(10):18963–18971. [PMC free article] [PubMed] [Google Scholar]
- 42.Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong W., Huang Y., Ji A., et al. Optimisation of saponin extraction conditions with Camellia sinensis var. assamica seed and its application for a natural detergent. J. Sci. Food Agric. 2018;98(6):2312–2319. doi: 10.1002/jsfa.8721. [DOI] [PubMed] [Google Scholar]
- 44.Fink R., Potočnik A., Oder M. Plant-based natural saponins for Escherichia coli surface hygiene management. Lwt - Food Sci. Technol. 2020;122:109018. doi: 10.1016/j.lwt.2020.109018. [DOI] [Google Scholar]
- 45.Mehta P.S., Bhatt K.C. Traditional soap and detergent yielding plants of Uttaranchal. Indian J. Tradit. Know. 2007;6:279–284. [Google Scholar]
- 46.Maan A.A., Nazir A., Khan M.K.I., Ahmad T., Zia R., Murid M., Abrar M. The therapeutic properties and applications of Aloe vera: a review. J. Herb. Med. 2018;12:1–10. doi: 10.1016/j.hermed.2018.01.002. [DOI] [Google Scholar]
- 47.Mpiana P.T., Ngbolua K.-T.-N., Tshibangu D.S.T., Kilembe J.T., Gbolo B.Z., Mwanangombo D.T., Inkoto C.L., Lengbiye E.M., Mbadiko C.M., Matondo A., Bongo G.N., Tshilanda D.D. Aloe vera (L.) burm. F. as a potential Anti-COVID-19 plant: a mini-review of its antiviral activity. European J. Med. Plants. 2020;31(8):86–93. doi: 10.9734/ejmp/2020/v31i830261. [DOI] [Google Scholar]
- 48.Ben-Shabat S., Yarmolinsky L., Porat D., Dahan A. Antiviral effect of phytochemicals from medicinal plants: applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020;10(2):354–367. doi: 10.1007/s13346-019-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jahan I., Ahmet O. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk. J. Biol. 2020;44(3):228. doi: 10.3906/biy-2005-114. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin S.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soares da Silva R.C.F., de Almeida D.G., Brasileiro P.P.F., et al. Production, formulation and cost estimation of a commercial biosurfactant. Biodegradation. 2019;30:191–201. doi: 10.1007/s10532-018-9830-4. [DOI] [PubMed] [Google Scholar]
- 52.Singh P., Patil Y., Rale V. Biosurfactant production: emerging trends and promising strategies. J. Appl. Microbiol. 2019;126(1):2–13. doi: 10.1111/jam.14057. [DOI] [PubMed] [Google Scholar]
- 53.Jiménez-Moreno N., Esparza I., Bimbela F., Gandía L.M., Ancín-Azpilicueta C. Valorization of selected fruit and vegetable wastes as bioactive compounds: opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020;50(20):2061–2108. doi: 10.1080/10643389.2019.1694819. [DOI] [Google Scholar]
- 54.Panzella L., Moccia F., Nasti R., Marzorati S., Verotta L., Napolitano A. Bioactive phenolic compounds from agri-food wastes: an update on green and sustainable extraction methodologies. Front. Nutr. 2020;7 doi: 10.3389/fnut.2020.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berardi A., Perinelli D.R., Merchant H.A., Bisharat L., Basheti I.A., Bonacucina G., Cespi M., Palmieri G.F. Hand sanitisers amid CoViD -19: A critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. International Journal of Pharmaceutics. 2020:p.119431. doi: 10.1016/j.ijpharm.2020.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahmood A., Eqan M., Pervez S., Alghamdi H.A., Tabinda A.B., Yasar A., Brindhadevi K., Pugazhendhi A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Science of the Total Environment. 2020;742:p140561. doi: 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]