Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a pandemic infection in 2020 has presented many therapeutic challenges. Not least among these is the importance of abnormal host response to infection that is one of the main drivers of more severe disease. Despite significant research endeavours, very few effective therapies have been identified, in part related to the different pathogenic mechanisms underlying different stages of clinical COVID-19. This mini review summarises data related to current and potential future therapies for COVID-19 and highlights the many challenges inherent in developing effective therapeutic options for new pandemic infection.
Keywords: SARS-CoV-2, COVID-19, Clinical trials, Therapeutics, Immunothearpies
1. Introduction
The outbreak of the novel coronavirus, first identified in Wuhan, China was declared a Public Health Emergency of International Concern by the World Health Organisation (WHO) on the 31st of January 2020. Subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), this virus has, as of the 5th of November 2020, infected more than 47 million people, caused massive economic disruption worldwide and prompted a wide range of restrictions to be imposed by governing bodies globally. Here we provide an overview of the clinical presentation and pathogenesis of COVID-19 and the main therapeutic avenues currently under investigation.
1.1. Pathophysiology
Coronaviruses are enveloped RNA viruses first described in 1968, named for their appearance by electron microscopy which is similar to the solar corona [1]. There are seven coronaviruses (CoV) that cause human disease. Human CoV-OC43, HCoV-229E, HCoV-HKU1 and HCoV-NL63 are all endemic HCoVs that cause mainly mild upper respiratory tract infections. SARS-CoV-2 is the third zoonotic CoV to emerge in the last 20 years. Severe Acute Respiratory Syndrome (SARS)-CoV was the cause of the SARS outbreak in Guandong province, China in 2002 and 2003 [2], and Middle East Respiratory Syndrome (MERS)-CoV was first reported in Saudi Arabia in 2012 [3] and cases continue to be reported throughout the Middle East.
SARS-CoV-2 shares 79% genome sequence identity with SARS-CoV and targets cells via the same entry receptor, angiotensin-converting enzyme 2 (ACE2) which is displayed on airway epithelial cells, alveolar epithelial cells, vascular endothelial cells and macrophages in the lung. Host cellular entry is mediated by the viral Spike (S) protein, with the receptor binding domain on subunit 1 (S1), binding to ACE2, and proteolytic cleavage of S protein by the cellular serine protease TMPRSS2 facilitating viral activation [4].
1.2. Clinical presentation
The first case series of 41 patients with COVID19 infection described an illness similar to SARS with most patients presenting with fever, dry cough, dyspnea and bilateral pneumonia, with characteristic ground glass opacities noted on computed tomography (CT) scan [5]. Since this initial report it has emerged that a large proportion of COVID19 cases are asymptomatic or mildly symptomatic. Estimating the truly asymptomatic fraction is difficult, not least because of inconsistent symptom reporting between countries, with atypical symptoms sometimes excluded, but also the relative scarcity of follow up data on contacts of confirmed cases to identify transmission and differentiate between asymptomatic and pre-symptomatic infection. A systematic review of papers that account for a potential pre-symptomatic period estimated that 20% individuals affected by SARS-CoV-2 remain asymptomatic for the entire infection [6].
Symptomatic COVID19 typically follows a triphasic course. Day one to seven comprise influenza like symptoms with headache, fatigue, dry cough and sore throat all being frequent. Gastrointestinal symptoms are relatively common and may occur in the absence of respiratory symptoms [7]. Diarrhoea, nausea/vomiting or abdominal pain were present in 19%, 11% and 7% respectively in a cohort of 370,000 confirmed cases reported to the Centre for Disease Control and prevention (CDC) in the United States [8]. Anosmia has emerged as a distinctive feature of COVID19 and is estimated to occur in 55% of infections [9]. Presence of anosmia in a cohort of healthcare workers tested for COVID19 gave an adjusted odds ratio of 7.21 of a positive test [10]. The pathophysiology of anosmia has not been fully elucidated, but post mortem samples have found an olfactory neuropathy [11].
A proportion of patients will develop severe disease, with approximately 17% of those hospitalised requiring intensive care unit (ICU) admission [12]. In hospitalised patients, dyspnea develops 5–8 days post onset of symptoms. Progression to critical disease with development of acute respiratory distress syndrome (ARDS) or ventilation occurs approximately 10 days post symptom onset [5]. Age is strongly associated with risk for progression to severe disease, as are comorbidities such as obesity, hypertension and diabetes [12]. The severe/critical phase of disease is characterised by severe interstitial pneumonia with high oxygen requirements and elevations in inflammatory markers such as C-reactive protein, d-dimer, fibrinogen, lactate dehydrogenase and interleukin (IL)-6. Active viral replication in the lower respiratory tract, and viral particles are found in pneumocytes on post mortem examination [[13], [14]]. However these particles are sparse and the pathogenesis of this stage seems to be predominantly immune-mediated rather than due to direct viral cytoxicity, with a dysfunctional immune response leading to uncontrolled inflammation and end-organ damage. The mechanisms of immune dysregulation have not yet been fully elucidated, and studies to date have suggested heterogenous patterns of dysfunction in patients with severe disease [[15], [16]]. Patients with severe disease have a sustained elevation of a broader range of cytokines compared to those with moderate disease. Although viral RNA load by nasopharyngeal swab does not correlate with overall outcome, it does correlate with elevation of IFNα, IFNγ, tumour necrosis factor (TNF) and tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), and patients with severe disease exhibit a slower decline in viral load, suggesting that viral persistence could drive ongoing inflammation. Lymphopenia is a common feature, with some studies showing a preferential effect on CD8+ T cells and others on CD4+ T cells, CD8+ T cells, NK cells and B cells, potentially reflecting recruitment to inflamed respiratory tissue [17]. CD4+ and CD8+ cells in patients with severe COVID19 display markers of activation or exhaustion but a significant minority show a minimal response [16]. Male sex is a risk factor for poor outcome in COVID19 and early work has identified sex differences in immune response, with female patients showing higher levels of activated and terminally differentiated T cells, particularly CD8+ cells, and males showing higher levels of innate immune cytokines IL-8, IL-18 and CCL5. Lower levels of T cell activation were correlated with deterioration in males but not females, where higher levels of CCL5, TRAIL and IL-15 were associated with deterioration [18].
Thrombotic complications are prominent in COVID-19 with the risk being highest in those with critical disease within the ICU. Thrombotic complications occur in up to 43% of ICU patients with COVID19 [19], despite prophylactic low molecular weight heparin. Pulmonary embolism is particularly common, occurring twice as frequently compared to patients admitted to the ICU with influenza [19, 20]. Arterial thrombosis has also been observed. One centre reported 20 COVID-19 patients presenting with acute limb ischaemia in a 3 month period, an increase in incidence from 1.8% to 16.3% compared to the same period the prior year [21]. Coagulopathy plays a major role in the pathogenesis of COVID-19 with platelet-fibrin thrombi shown on post mortem examination at a much greater frequency than those with influenza pneumonia [22]. Direct infection of the endothelium by SARS-CoV-2 has been demonstrated [23], and endothelial disruption is postulated to activate the coagulation cascade leading to a prothrombotic state.
SARS-CoV-2 causes direct and indirect cardiac complications. Myocardial injury, manifested by elevation of cardiac biomarkers were reported in an initial case series from Wuhan, China [5]. Echocardiographic abnormalities were observed in 55% of patients in a global observational study of COVID-19 patients who underwent routine echocardiography, 46% of whom had no pre-existing cardiovascular disease [24]. Right ventricular dysfunction may be a result of pulmonary embolism or pneumonia, and myocardial infarction related to the prothrombotic state. Direct infection of the coronary vasculature may account for some of the observed cardiac complications. A study of cardiac magnetic resonance imaging in convalescent COVID-19 patients, the majority of whom had not been hospitalised, demonstrated changes in more than three quarters, suggestive of cardiac inflammatory involvement [25], and viral RNA has been found in the myocardium of patients who died of COVID-19. Dramatic increases in the rate of out of hospital cardiac arrests was observed in New York, Paris and Lombardy, Italy during the early peak of COVID-19 diagnoses [[26], [27], [28]]. While some of the observed increase was likely related to disruptions to normal medical care, COVID-19 was considered directly responsible for a proportion of cases.
Diverse neurological presentations have been observed. Most common are non-specific symptoms such as headache, fatigue and dizziness, while confusion or impaired consciousness is seen in 15% of those with severe disease [29]. Acute stroke has been observed, again more commonly in severe infection [29] and COVID-19 has been demonstrated to be an independent risk factor for acute ischaemic stroke [30]. Cases of acute inflammatory demyelinating polyneuropathy, meningoencephalitis, haemorrhagic posterior reversible encephalopathy syndrome and acute necrotizing encephalopathy have all been reported in association with COVID-19 [31]. Although multiple routes of entry to the central nervous system (CNS) have been proposed, including trans-synaptic spread by the olfactory nerve and an increase in blood brain barrier permeability through endothelial disruption, cerebrospinal fluid and autopsy studies do not provide consistent evidence of direct CNS invasion [32].
1.3. Therapeutic considerations
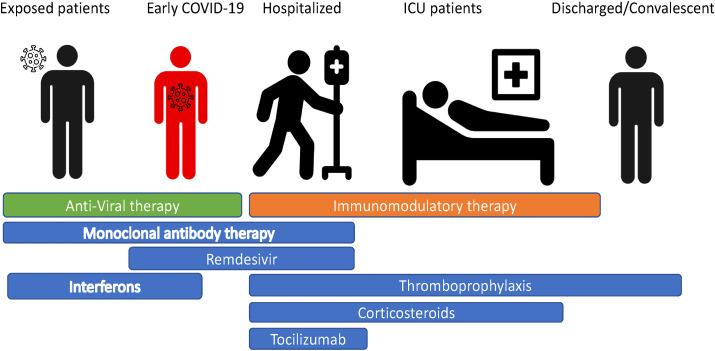
Increased understanding of the pathophysiology of COVID-19 has revealed a range of therapeutic targets. In addition to preventative therapeutics and vaccines, which are not the focus of this review, additional therapeutic targets can be broadly divided into three categories. Firstly anti-viral therapies that inhibit viral replication, shorten the infectious period and inhibit progression to severe disease, targeted mainly at the pre-symptomatic or early symptomatic period. Secondly immunomodulatory agents that target the maladaptive host immune response observed in moderate and severe COVID19 that aim to interrupt pro-inflammatory feedback loops to hasten recovery and prevent transition to critical severity and ICU admission. Lastly supportive care to manage the consequences of COVID-19, including end organ damage, such as ARDS and VTE (Fig. 1 ).
Fig. 1.
Timing of different therapeutic targets for COVID-19.
1.4. Supportive care
Despite the use of many investigational agents during the first eight months of the pandemic, treatment options are limited, and supportive care remains the mainstay of management.
Respiratory support is commonly needed in hospitalised patients. The WHO recommends an oxygenation target of 94% or above during initial resuscitation and 90% or above thereafter. An early intubation strategy was used by many centres early in the pandemic, due to the observation that patients often deteriorate precipitously and may be more safely intubated while clinically stable, and to minimise infection risk by avoiding aerosol generation due to the use of high flow oxygen or non-invasive ventilation (NIV). However mechanical ventilation carries its own risks and is a limited resource. This and some limited evidence that NIV may avoid ventilation [33] has led to a shift towards trialling NIV prior to a decision to proceed to invasive ventilation.
Prone positioning is used in both ventilated and non-ventilated patients due to its proven benefit in ARDS due to causes other than COVID-19, and the observation that it improves oxygenation in COVID-19 patients [34], although it is unclear if this influences overall clinical outcome.
All patients without contraindications should receive thromboprophylaxis and a there should be a low threshold to assess for venous thromboembolism. There are ongoing studies to assess the correct type and optimal dose of anticoagulation to maximise clinical outcomes [35].
2. Pharmaceutical interventions
2.1. Antiviral therapies
2.1.1. Remdesivir
Remdesivir is a monophosphoramidate prodrug of an adenosine analogue that was developed as a treatment for Ebola Virus Disease (EVD), and has a broad anti-viral spectrum inhibiting filoviruses, paramyxoviruses, pneumoviruses and coronaviruses. Despite its efficacy in an animal model of SARS-CoV-2 [36], its role in management of COVID-19 is still evolving. Three randomised controlled trials (RCT) of remdesivir administered as an intravenous infusion to hospitalised patients have been published, none of which have shown a mortality benefit. Wang et al. compared 10 days of remdesivir to placebo in 236 hospitalised patients with a median age of 66 years in the remdesivir group, the majority (82%) of whom required supplemental oxygen but not NIV or higher levels of respiratory support. No difference it time to clinical improvement was demonstrated, but their study may have been underpowered [37]. The international, multicentre Adaptive COVID-19 Treatment Trial (ACTT-1) enrolled 1062 patients with a mean age of 58.9 years, with a range of disease severities; 13% required no oxygen, 41% required supplemental oxygen, 18.2% NIV or high flow oxygen and 26.8% required mechanical ventilation or extracorporeal membrane oxygenation (ECMO). This larger study showed recovery was shorter by four days in patients treated with ten days of remdesivir compared to placebo [38]. The third RCT compared five and ten day courses of remdesivir with standard care in 584 patients with moderate COVID-19 (defined by the presence of pulmonary infiltrates on radiographic imaging and oxygen saturations >94%). The patient group randomized to 5 days of remdesivir achieved a statistically significant improvement in clinical status distribution at day 11 from start of treatment compared to standard of care, with clinical status measured on a 7 point ordinal scale ranging from death (point 1) to discharged from hospital (point 7). However there was no difference in clinical status distribution at day 11 in the group randomized to a ten day course compared to standard of care, and no difference in any of the secondary end-points including all-cause mortality, duration of hospitalization or time to clinical improvement [39]. Thus despite some evidence of benefit, results are conflicting, and optimal duration of therapy and ideal time along the disease course to introduce therapy has not been fully elucidated.
2.2. Hydroxychloroquine and chloroquine
Hydroxychloroquine and chloroquine inhibit SARS-CoV-2 replication in-vitro, and both drugs were used widely in the early months of the pandemic. Chloroquine is an anti-malarial drug and its use was recommended by Chinese expert consensus in March 2020. Hydroxychloroquine, a chloroquine analoque used in systemic lupus erythematous and other rheumatologic conditions has a preferable safety profile and was given emergency use authorisation by the federal drug agency (FDA) in March 2020. A small French study of hydroxychloroquine used in association with azithromycin showed faster reduction in viral RNA measured in nasopharyngeal swabs, leading to increased concomitant use of both drugs for a period [40]. However a number of randomised controlled trials (RCTs) of hydroxychloroquine have subsequently shown no clinical benefit in COVID19. For example, the RECOVERY trial showed no benefit in mortality in 1542 hospitalised patients randomised to receive hydroxychloroquine versus 3132 who received usual care [41]. A study of 504 hospitalised patients with mild to moderate COVID-19 showed no improvement in clinical status with hydroxychloroquine with or without azithromycin [42]. A trial of hydroxychloroquine as post exposure prophylaxis in patients with household or workplace exposure to COVID-19 also showed no benefit [43]. These negative data, along with safety concerns relating to prolonged QTc interval, especially with concomitant use of azithromycin, have led most countries to no longer recommend use of either drug in the management of COVID19.
2.3. Interferons
Interferons (IFNs) are endogenous cytokines that constitute a major first line antiviral defence. Recognition of viral pathogen-associated molecular patterns triggers type I IFN responses. Interferon binding to the ubiquitously expressed type I IFN receptor activates interferon stimulated genes (ISGs), which collectively establish cellular resistance to viral infection. SARS-CoV-2 elicits a very low IFN-I and IFN-III response and a limited ISG response [44], and evasion of the IFN response has also been observed with SARS and MERS [45]. However SARS-CoV-2 exhibits sensitivity to type I IFNs in vitro [46] suggesting exogenous interferon administration could be an effective therapeutic strategy. A randomised trial of interferon beta-1b with lopinavir-ritonavir versus lopinavir-ritonavir alone in 127 patients demonstrated shorter time to SARS-CoV-2 negativity by nasopharyngeal swab and faster time to clinical improvement, defined as a national early warning score of 0, in the interferon group [47]. This study screened 144 patients which accounted for 80% of all confirmed COVID19 cases in Hong Kong during the study period, and disease severity was mild with a median sequential organ failure assessment (SOFA) score of 0 at enrolment. A retrospective study 77 hospitalised patients in Wuhan, China who all received arbidol, nebulised interferon alpha 2b or a combination of both drugs showed similar results with faster viral clearance and reduction of systemic inflammation in patients treated with nebulised interferon [48]. A pilot trial of inhaled interferon beta in 101 hospitalised patients with COVID-19 reported significant reduction in progression to severe disease in a press release [49], a larger trial is ongoing and published results are awaited.
2.4. Other anti-viral agents
Lopinavir/ritonavir is a protease inhibitor used to treat HIV infection, which has in vitro activity against SARS-CoV-2, however multiple RCTs failed to show benefit [[41], [50]]. Camostat mesylate is a serine protease protease inhibitor that has been shown to inhibit SARS-CoV-2 interaction with TMPRSS2 in vitro [4] and is undergoing trial in outpatients and hospitalised patients with COVID19. Favipiravir is a pyrazine carboxamide derivative developed as an antiviral against influenza, inhibiting viral RNA-dependent RNA polymerase, licensed for use in influenza Japan. Umifenovir is a small, indole-derivative molecule licensed for treatment of influenza in China. An RCT comparing favipravir and umifenovir in 240 patients with moderate, severe or critical COVID-19 showed no difference in clinical recovery at day 7 (defined as >72 h of normalisation of body temperature, respiratory rate and oxygen saturation) [51].
2.5. Immune based therapies and immunomodulators
2.5.1. Dexamethasone
Dexamethasone is the only medication to date that has been shown to reduce mortality in COVID-19 infection in a large RCT. The Randomised Evaluation of COVID-19 therapy (RECOVERY) multicentre trial which included 6425 patients showed a significant reduction in mortality at 28 days in patients receiving oxygen or invasive mechanical ventilation when administered 6 mg of dexamethasone daily (or equivalent) for up to ten days [52]. This contradicted initial WHO guidance to avoid corticosteroids, a recommendation based on lack of benefit observed in SARS, MERS and influenza pneumonia. The benefit observed in the RECOVERY trial was greater in those recruited more than seven days after onset of illness, and the authors hypothesise that the benefit of corticosteroids is due to the prominent immune mediated pathology at this phase of the disease. The benefit of corticosteroids has since been further supported by other RCTs and meta-analyses [53,54].
2.6. Tocilizumab
The rationale for use of tocilizumab is derived from its approved use in cytokine release syndrome associated with chimeric antigen receptor (CAR) T cell therapy, an IL-6 mediated process that resembles the systemic inflammatory response in severe COVID-19. Tocilizumab is a humanized monoclonal antibody (mAb) against the IL-6 receptor, also approved for the treatment of rheumatoid arthritis and giant cell arteritis. Benefit has been suggested in case series and retrospective cohort studies of tocilizumab [55,56], however the Roche funded RCT COVACTA reported no benefit in the primary endpoint of difference in clinical endpoint at four weeks in a population of patients hospitalised with COVID-19, or in the secondary endpoint of overall mortality [57]. However, a secondary endpoint analysis showed significantly shorter stays in ICU and quicker hospital discharge in those who received tocilizumab, with adverse events being equal between the two groups. Importantly, a post hoc analysis also showed significantly less clinical failure in patients not mechanically ventilated at baseline in the tocilizumab arm compared to placebo, with a 40% reduction in progression to ICU within this subgroup of patients. Further trials targeting this pre-ICU group are underway [58]. Additionally, the COVACTA trial recruited patients based on oxygenation requirements and did not specifically target patients with evidence a systemic inflammatory syndrome, and it is possible that a greater benefit may be observed in this specific group. Other IL-6 inhibitors, sarilumab, another anti-IL-6 receptor mAb and siltuximab, an antI-IL-6 mAb are also under investigation.
2.7. Convalescent plasma and passive antibody therapy
The use of passive antibody therapy predates modern antimicrobial therapy with the use of antibody preparations derived from immune donors known as “serum therapy” dating back to the 1930s [59]. More recently antibody therapies have been used for viral infections such as influenza, Ebola and respiratory syncytial virus, either in the form of convalescent plasma or monoclonal antibody therapy [[60], [61], [62]]. Convalescent plasma has been widely used for COVID19 with the suggestion of benefit in large observational trials [63], although there was no benefit on outcome observed in one RCT [64]. Risks associated with plasma infusion include transfusion related acute lung injury, transfusion associated circulatory overload, allergic transfusion reactions and thrombotic events. Although these reactions are infrequent with the use of convalescent plasma in COVID19, they can be fatal [65] and underly the need for data from RCT to confirm benefit from this approach. The development of monoclonal antibody (mAb) therapy with neutralizing activity against SARS-CoV-2 could avoid many of the adverse effects inherent in the use of transfusion therapy. The intensive focus on line of therapy has led to the development eleven mAb therapies that are currently undergoing clinical trials for SARS-CoV-2, eight in phase one clinical trials and three in phase three clinical trials, with multiple other mAbs in preclinical development [66] (Table 1 ).
Table 1.
Monoclonal antibodies in Phase 3 trials.
| Name | Sponsor | Target | Trial number | Study population |
|---|---|---|---|---|
| REGN-COV2 | Regeneron pharmaceuticals | SARS-CoV-2 RBD | NCT04519437 NCT04452318 NCT04425629 NCT04426695 |
Healthy volunteers Household contacts of patients with confirmed COVID-19 Outpatients with COVID-19 Hospitalised patients with COVID-19 |
| LY-CoV555 | AbCellera Biologics, Eli Lilly and Company | SARS-CoV-2 Spike protein | NCT04411628 NCT04427501 BLAZE-1 NCT04497987 BLAZE-2 NCT04518410 ACTIV-2 NCT04501978 ACTIV-3 |
Hospitalised COVID-19 patients Outpatients with COVID19 Nursing home residents and staff Outpatients with COVID-19 Inpatients with COVID-19 |
| VIR-7831 | Vir Biotechnology, Inc and GlaxoSmithKline plc | SARS-CoV-2 RBD | NCT04545060 | Outpatients with COVID-19 |
The three mAbs in phase three clinical trials all target the spike protein. REGN-COV2 developed by Regeneron Pharmaceuticals consists of two human antibodies REGN10933 and REGN10987. These were identified from a library of anti-Spike antibodies generated both from immunized genetically humanized VelocImmune mice, and RBD specific B cells isolated from COVID-19 recovered donors. The two antibodies were chosen for their high potency in neutralisation assays, capability to mediate antibody-dependent cellular cytoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) and their binding to distinct epitopes on RBD [67]. Four clinical trials of this antibody cocktail are ongoing, a phase one/phase two trial of tolerability and efficacy in healthy volunteers, a trial of post exposure prophylaxis in asymptomatic household contacts of confirmed SARS-CoV-2 infection, a trial in symptomatic and asymptomatic outpatients with confirmed early SARS-CoV-2 and a trial in patients hospitalised with COVID-19. The trial in hospitalised patients recently received a recommendation from the independent data monitoring committee to hold further enrolment in patients receiving high flow oxygen or mechanical ventilation based on a potential safety signal and unfavourable risk/benefit ratio [68].
Another mAb, LY3819253 (LY-CoV555), developed from a recovered donor by AbCellera and Eli Lilly in collaboration with the Vaccine Research Centre at the National Institute of Allergy and Infectious Diseases has finished recruiting its phase one trial and has four other active trials; one in mild to moderate early outpatient COVID-19 in comparison to another Lilly developed mAb LY3832479 or placebo, one as pre-exposure prophylaxis in residents and staff in nursing homes, one in comparison to remdesivir in hospitalised patients, and a second outpatient study compared to placebo alone.
In addition, VIR-7831, developed by Vir Biotechnology, Inc and GlaxoSmithKline plc was developed using peripheral blood mononuclear cells from a donor recovered from SARS, and targets an epitope of RBD that is conserved within the Sarbecovirus genus [69]. VIR-7831 is undergoing a phase two/phase three trial in outpatients with early COVID-19.
2.8. Other immunomodulatory agents
Granulocyte-macrophage colony-stimulating factor (GM-CSF), a myelpoietic growth factor is a pro-inflammatory cytokine involved in alveolar homeostasis. Human recombinant GM-CSF (sargramostim) is being trialled in hypoxic respiratory failure with the aim of stabilising alveolar macrophage and epithelial cell function and preventing against secondary infection [70]. Conversely inhibition of GM-CSF is effective in a number of inflammatory conditions that resemble the systemic inflammatory response in COVID-19, including ARDS, haemophagocytic lymphohistiocytosis (HLH) and rheumatoid arthritis, and anti GM-CSF mAbs could be useful in COVID-19 [70]. Research into use of agents targeting GM-CSF is ongoing.
Janus Kinase (JAK) inhibitors such as Barcitinib and Ruxolitinib inhibit transmembrane proteins that mediate and amplify extracellular signals from growth factors and cytokines. They are licensed for rheumatoid arthritis and myeloproliferative disorders respectively but have also been used for HLH. A recent meta-analysis of JAK inhibitors in COVID-19 showed a significantly reduced odds ratio of mortality or ICU admission, although only two small RCTs were included in the analysis with 41 and 17 patients recruited in each [71].
3. Conclusion
Despite the wealth of data generated on potential therapies for COVID-19 many questions remain. The early course of disease has been well described but the frequency and duration of long term effects is unknown. The treatments described above only represent a fraction of all those under investigation, but treatment options remain limited. With global transmission remaining high, the search for effective therapies targeted at each stage of disease must remain a priority.
Funding
This work was supported by Science Foundation Ireland [grant number 20/COV/0305]. G.K. is supported through a COVID Fellowship awarded by the United States Embassy in Ireland.
Declaration of competing interest
P.M. has received honoraria and/or travel grants from Gilead Sciences, MSD, Bristol Myers Squibb and ViiV Healthcare.G.K. – no conflicts declared.
References
- 1.Virology Coronaviruses. Nature. 1968;220(5168):650. doi: 10.1038/220650b0. [Internet] Available from. [DOI] [Google Scholar]
- 2.N S.Z., B J.Z., Y M.L., L L.M.P., Z H.X., K H.C., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003 Elsevier Connect , the company ’ s public news and information. Lancet. 2020;362(January):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerowitz Eric, Richterman Aaron, Bogoch Isaac, Low Nicola, Cevik M. Towards an accurate and systematic characterization of persistently asymptomatic infection with SARS-CoV-2. SSRN Electron J. 2020;280(7506):717. doi: 10.2139/ssrn.3670755. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., et al. Coronavirus disease 2019 case surveillance — United States, january 22–may 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(24):759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker A., Pottinger G., Scott A., Hopkins C. Anosmia and loss of smell in the era of covid-19. BMJ. 2020;370(table 2):1–4. doi: 10.1136/bmj.m2808. [DOI] [PubMed] [Google Scholar]
- 10.Lan F.Y., Lan F.Y., Filler R., Filler R., Mathew S., Buley J., et al. COVID-19 symptoms predictive of healthcare workers’ SARS-CoV-2 PCR results. PloS One. 2020;15(6):1–12. doi: 10.1371/journal.pone.0235460. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschenbaum D., Imbach L.L., Ulrich S., Rushing E.J., Keller E., Reimann R.R., et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396:166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:1–19. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wölfel Roman, Corman Victor M., Guggemos Wolfgang, Michael Seilmaier, Zange Sabine, Marcel A., Müller D.N., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 14.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M. 2020. Pulmonary Post-mortem Findings in a Series of COVID-19 Cases from Northern Italy: a Two-Centre Descriptive Study; pp. 19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(June) doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;1209(September) doi: 10.1126/science.abc8511. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z., Wherry J. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;published online Aug 26 doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med [Internet] 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 21.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J. Vasc. Surg. 2020 doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen A., Md M.S., Smulian John C., Mph M.D., John A., Lednicky, PhD, Tony S., Wen M.D., Denise J., Jamieso M. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Hear J - Cardiovasc Imaging. 2020:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020:1–9. doi: 10.1001/jamacardio.2020.3557. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., Scherschel K., Kirchhof P., Escher F., Schultheiss H.P., Blankenberg S. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldi E., Sechi G.M., Mare C., Canevari F., Brancaglione A., Primi R., Klersy C., Palo A., Contri E., Ronchi V., Beretta G. Out-of-Hospital cardiac arrest during the covid-19 outbreak in Italy. N. Engl. J. Med. 2020;383(5):494–496. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai P.H., Lancet E.A., Weiden M.D., Webber M.P., Zeig-Owens R., Hall C.B., Prezant D. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York city. JAMA Cardiol. 2020;5(10):1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belani P., Schefflein J., Kihira S., Rigney B., Delman B.N., Mahmoudi K., et al. COVID-19 is an independent risk factor for acute ischemic stroke. Am. J. Neuroradiol. 2020:1361–1364. doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020:1–12. doi: 10.1016/j.cell.2020.08.028. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schünemann H.J., Khabsa J., Solo K., Khamis A.M., Brignardello-Petersen R., El-Harakeh A., Darzi A., Hajizadeh A., Bognanni A., Bak A., Izcovich A. Ventilation techniques and risk for transmission of coronavirus disease, including COVID-19. Ann. Intern. Med. 2020;173(3) doi: 10.7326/M20-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coppo A., Bellani G., Winterton D., Di Pierro M., Soria A., Faverio P., et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765–774. doi: 10.1016/S2213-2600(20)30268-X. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.InterMediate ProphylACtic versus therapeutic dose anticoagulation in critically ill patients with COVID-19: a prospective randomized study (the impact trial) https://clinicaltrials.gov/ct2/show/NCT04406389 [Internet]. Available from.
- 36.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., Van Doremalen N., Leighton I., Yinda C.K., Pérez-Pérez L., Okumura A. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.04.15.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (London, England) 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. http://www.ncbi.nlm.nih.gov/pubmed/32423584 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19 — preliminary report. N. Engl. J. Med. 2020;1–12 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 39.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., et al. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. Jama. 2020:94404. doi: 10.1001/jama.2020.16349. http://www.ncbi.nlm.nih.gov/pubmed/32821939 Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents [Internet] 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on Hydroxychloroquine, 5 June 2020 No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19 [Internet]. [cited 2020 Aug 30]. Available from: https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf.
- 42.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N. Engl. J. Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. [Internet] e9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park A., Iwasaki A. Type I and type III interferons – induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27(6):870–878. doi: 10.1016/j.chom.2020.05.008. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020;179(April):104811. doi: 10.1016/j.antiviral.2020.104811. [Internet] Available from: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung I.F.N., Lung K.C., Tso E.Y.K., Liu R., Chung T.W.H., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet [Internet] 2020;395(10238) doi: 10.1016/S0140-6736(20)31042-4. 1695–704. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X., et al. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020;11(May):1–6. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Synairgen Announces Positive Results from Trial of SNG001 in Hospitalised COVID-19 Patients. [Internet]. [cited 2020 Nov 5]. Available from: https://www.synairgen.com/wp-content/uploads/2020/07/200720-Synairgen-announces-positive-results-from-trial-of-SNG001-in-hospitalised-COVID-19-patients.pdf.
- 50.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., et al. 2020. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. [Google Scholar]
- 52.Dexamethasone in hospitalized patients with covid-19 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Writing Committee for the REMAP-CAP Investigators. Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. Jama. 2020:15261. doi: 10.1001/jama.2020.17022. http://www.ncbi.nlm.nih.gov/pubmed/32876697 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Jama. 2020:1–12. doi: 10.1001/jama.2020.17023. http://www.ncbi.nlm.nih.gov/pubmed/32876694 [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy C., Savinelli S., Feeney E.R., Butler M.W., O’Broin C., Ryan S., et al. Tocilizumab therapy in individuals with COVID-19 infection and hyperinflammatory state. Respirology. 2020;2019 doi: 10.1111/resp.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., Santoro A. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;(August) doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roche Provides an Update on the Phase III COVACTA Trial of Actemra/RoActemra in Hospitalised Patients with Severe COVID-19 Associated Pneumonia [Internet]. [cited 2020 Aug 30]. Available from:: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm.
- 58.Cotter A., Wallace D., McCarthy C., Feeney E., O’Neill L., Stack J., et al. The COVIRL002 Trial-Tocilizumab for management of severe, non-critical COVID-19 infection: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):20–22. doi: 10.1186/s13063-020-04680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casadevall A., Dadachova E., Pirofski L.A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004;2(9):695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 60.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J. Infect. Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulangu S., Dodd L.E., Davey R.T., Mbaya O.T., Proschan M., Mukadi D., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 63.Joyner M.J., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. 2020 doi: 10.1101/2020.08.12.20169359. 2020.08.12.20169359. [DOI] [Google Scholar]
- 64.Li L., Li L., Zhang W., Zhang W., Hu Y., Tong X., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joyner Michael J., Bruno Katelyn A., Klassen Stephen A., Kunze Katie L., Patrick W. Johnson, Elizabeth R., Lesser C.C.W., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Covid-19 Biologics Tracker https://www.antibodysociety.org/covid-19-biologics-tracker/ [Internet]. [cited 2020 Sep 6]. Available from.
- 67.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 80- 2020;1014(August) doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.REGN-COV2 Independent Data Monitoring Committee Recommends Holding Enrollment in Hospitalized Patients with High Oxygen Requirements and Continuing Enrollment in Patients with Low or No Oxygen Requirements [Internet]. [cited 2020 Nov 5]. Available from: https://investor.regeneron.com/news-releases/news-release-details/regn-cov2-independent-data-monitoring-committee-recommends.
- 69.Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815) doi: 10.1038/s41586-020-2349-y. Internet]290–5. Available from. [DOI] [PubMed] [Google Scholar]
- 70.Lang F.M., Lee K., Teijaro J.R., Burkhard B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walz L., Cohen A.J., Rebaza A.P., Vanchieri J., Slade M.D., Dela Cruz C.S., et al. Janus kinase-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. medRxiv. 2020;1101 doi: 10.1101/2020.08.10.20172189. Internet]08.10.20172189. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]