Abstract
Fungal infections cause substantial morbidity and mortality. However, the burden of deep fungal infections is not well described in Uganda. We aimed to estimate the burden and etiology of histologically diagnosed deep fungal infections in Uganda. We retrospectively reviewed histology reports at the Pathology Reference Laboratory, Department of Pathology, Makerere University, Kampala, Uganda from January 1950 to September 2019 to identify any reports that had a fungal infection as the diagnosis. Over the study period, 697 cases of deep fungal infections were identified with an average incidence of 0.73/100,000 persons per decade. There was a general decline in the number of cases detected. Median age of the cases was 28 years (IQR: 11–40) and majority (59%) were male. The age group of 0–10 years were the most affected. The foot was the most affected part of the body (26%). Deep mycoses identified include eumycetoma (32%), subcutaneous phycomycosis (26%), histoplasmosis (9.2%), chromoblastomycosis (4.6%), aspergillosis (3.3%), cryptococcosis (3.3%), blastomycosis (1.6%), subcutaneous mycosis (1.4%), dermatomycosis (1.3%), coccidioidomycosis (0.6%), mucormycosis (0.6%), and sporotrichosis (0.1%). Histoplasma was the commonest causative agent (9.2%) followed by Aspergillus (3.4%) and Cryptococcus (3.3%), while 81% of the fungal pathogens were not identified to genus/species level. Only 31% of the cases were diagnosed clinically as deep fungal infections. There is a substantial burden of deep fungal infections caused by multiple fungal pathogens in Uganda. There is need to build local capacity for mycology so as to improve on the index of clinical suspicion and diagnostic capabilities.
Keywords: deep mycoses, histology, opportunistic mycoses, endemic mycoses, Uganda
Introduction
Deep fungal infections range from deep cutaneous and subcutaneous mycoses to multi-organ diseases caused by primary or opportunistic fungal pathogens.1 Globally, there has been a rise in the incidence and a change in the pattern of fungal diseases in the past few decades posing unique clinical and public health problems due to the diagnostic challenges and the high morbidity and mortality associated with fungal diseases.2–5 Epidemiological studies are important for fungal diseases surveillance and institution of tailored interventions. However, routine surveillance of fungal diseases to inform policy and public health actions are nonexistent in most of the nations of the world.6
The fungal genera Cryptococcus, Aspergillus, Candida, Histoplasma, and Pneumocystis are the most common causes of opportunistic fungal infections worldwide.2,3,7 Meanwhile, the agents of endemic mycoses, Blastomyces, Paracoccidioides, Coccidioides, Talaromyces, and Emergomyces are more geographically restricted in certain areas of the world.3,8–10 On the other hand, heterogeneous group of implantation subcutaneous mycoses caused by transcutaneous inoculation of fungal spores, particularly sporotrichosis, chromoblastomycosis, eumycetoma, and subcutaneous zygomycoses are common in sub-tropical and tropical regions of the world.11,12
Histopathological examination of biopsy or surgically resected abnormal tissues with fungal stains such as periodic acid- Schiff, methenamine silver or fluorescent stains, in parallel with regular stains provides a rapid and frequently definitive diagnostic features such as muriform cells and tissue spherules seen in chromoblastomycosis and coccidiodomycosis respectively.13 In addition, tissue examinations may help to distinguish yeast from a septate or nonseptate mould infection. This distinction is critical in the initial selection of an appropriate antifungal therapy prior to receipt of sensitivity results.
Since most systemic mycoses present with clinically indistinguishable mucocutaneous manifestations which can be easily biopsied or surgically excised,14,15 histopathology plays a vital role in the initial or definitive diagnosis of both subcutaneous and systemic fungal infections. However, there is scarcity in published literature on the histological spectrum of deep mycoses in Africa. The present single-centre study sought to retrospectively describe the incidence and aetiology of deep fungal infections in Uganda based on histological examination of clinical specimens.
Methods
Study design and setting
This was a 70-year descriptive retrospective chart review of histology results at the Pathology Reference Laboratory, Department of Pathology, Makerere University, Kampala, to estimate the burden and the spectrum of deep fungal infections in Uganda. The Department of Pathology was established in 1937 with the aim of ensuring the provision of medical education, research and diagnostic services at the highest standards. It provides diagnostic pathology services to Mulago National Referral Hospital and majority of other hospitals in Uganda in the areas of general surgical pathology, cytopathology and autopsy pathology.
Review of histology reports
We manually reviewed archived records containing biopsy reports at the Pathology Reference Laboratory, Department of Pathology, Makerere University, Kampala, Uganda, from January 1950 to September 2019 to identify reports that had a fungal infection as the final diagnosis. We excluded all superficial fungal infections identified; all of which were superficial oral or genital Candidiasis (n = 21). Anonymised data were captured from these reports and entered into Microsoft Excel® spreadsheet. We captured data on age, sex, tribe, referring unit, clinical diagnosis, histology diagnosis, causative agent, body part with lesion, and district of residence. We retained the original fungal names used in these reports, rather than adopting likely modern taxonomic synonyms. We then retrieved some archived slides for some of these reports to take pictures using a digital microscope.
Ethical consideration
Ethics approval for this study was received from the School of Biomedical Sciences, Higher Degrees, Research and Ethics Committee of Makerere University, Kampala, Uganda (SBS-712).
Statistical analysis
Data were analyzed using STATA version 14 (STATA, College Station, TX, USA). Statistical analysis aimed at establishing the incidence of different deep mycoses over the 70-year period. To estimate the incidence, the number of cases diagnosed by histology per decade were divided through the mid-decade population for each decade. Population figures were derived from World Bank (https://www.google.com/publicdata/explore?ds=d5bncppjof8f9_&met_y=sp_pop_totl&idim=country:UGA:RWA:COD&hl=en&dl=en#!ctype=l&strail=false&bcs=d&nselm=h&met_y=sp_pop_totl&scale_y=lin&ind_y=false&rdim=region&idim=country:UGA&ifdim=region&hl=en_US&dl=en&ind=false). This site only gives data from 1960 up to 2017. For years 1950 to 1959 and 2018 to 2019, we used population figures from Worldometers (https://www.worldometers.info/world-population/uganda-population/).We analyzed the distribution of cases by sex, age, tribe, and lesion site. We then described the spatial distribution by district of residence.
Results
Incidence of deep fungal infections in Uganda
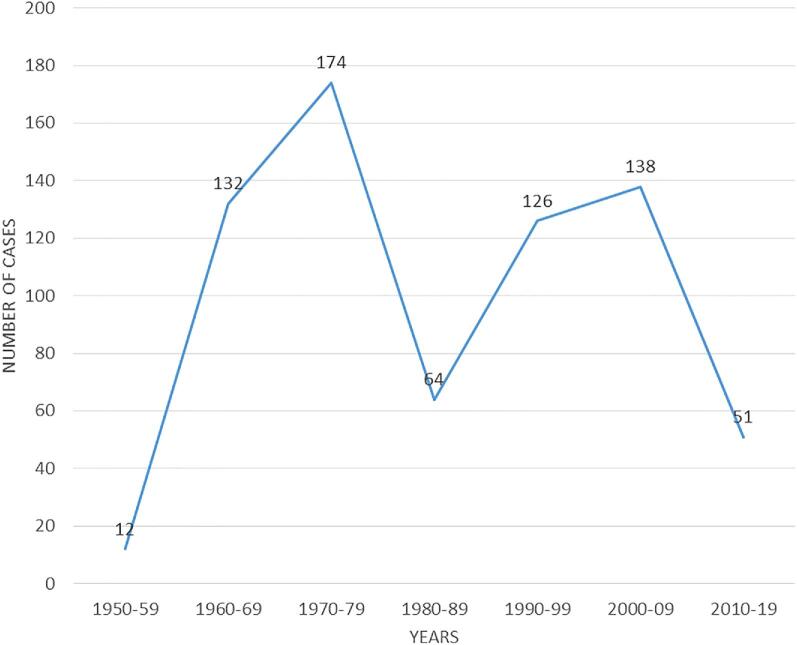
Over the 70-year study period (1950–2019), we identified 697 cases of deep fungal infections diagnosed by histology (Fig. 1). The number of fungal infections ranged from zero (0) to 27 cases per year with an average of 10 cases per year. The incidence per decade ranged from 0.13/100,000 to 1.65/100,000 persons with an average of 0.73/100,000 persons (Table 1). With the current population of Uganda in 2019 (44,269,594), this translates into approximately 323 new cases of deep fungal infections annually.
Figure 1.
Trend in number of deep fungal infections: There has been a gradual decrease in the number of cases identified over the years. This Figure is reproduced in color in the online version of Medical Mycology.
Table 1.
Incidence and the aetiology of deep fungal infections in Uganda.
| Causative agents | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | Mid-decade population | Number of biopsy samples processed | Number of deep fungal cases identified | Decade incidence per 100,000 persons | Histoplasma species | Aspergillus species | Cryptococcus species | Blastomyces species | Dermatophyte | Coccidioides species | Sporothrix species | Unidentified fungi |
| 1950–59 | 5,888,793 | 3280 | 12 | 0.20 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| 1960–69 | 8,014,400 | 68,407 | 132 | 1.65 | 3 | 5 | 0 | 2 | 0 | 3 | 0 | 119 |
| 1970–79 | 10,827,100 | 77,814 | 174 | 1.61 | 11 | 1 | 1 | 1 | 0 | 0 | 0 | 160 |
| 1980–89 | 14,646,600 | 47,473 | 64 | 0.44 | 3 | 2 | 2 | 6 | 0 | 0 | 0 | 51 |
| 1990–99 | 20,550,300 | 65,761 | 126 | 0.61 | 26 | 4 | 4 | 0 | 7 | 0 | 0 | 85 |
| 2000–09 | 28,543,900 | 61,079 | 138 | 0.48 | 12 | 7 | 7 | 1 | 1 | 0 | 1 | 109 |
| 2010–19# | 40,144,900 | 52,185 | 51 | 0.13 | 8 | 5 | 9 | 1 | 1 | 1 | 0 | 26 |
#Data are up to September 2019.
Causative agents of deep fungal infections in Uganda
As seen in Table 1, majority (81% [561/697]) of the fungal pathogens were not reported to genus or species level from the histology tissue sections. Periodic acid-Schiff (PAS) staining was used as a special stain to confirm presence of fungal pathogens. However, for fungi that were identified by histology, Histoplasma species were the most common causative agents (9.2% [64/697]) followed by Aspergillus (3.4% [24/697]), Cryptococcus (3.3% [23/697]), Blastomyces (1.6% [11/697]), dermatophytes (1.3% [9/697]), Coccidioides (0.6% [4/697]), and Sporothrix species (0.1% [1/697]) (Table 1).
Index of clinical suspicion
Only 218 cases (31%) were provisionally diagnosed as fungal infections clinically. Majority of the cases were clinically misdiagnosed as Kaposi's sarcoma (59 cases), tuberculosis (24 cases), unspecified tumor (23 cases), actinomycosis (15 cases), carcinoma (10 cases), malignant ulcer (12 cases), buruli ulcer (10 case), granuloma (eight case), prurigo (five cases), fibroma (four cases), lymphoma (three cases), melanoma (three cases), rhabdomyosarcoma (three cases), sarcoma (three cases), Burkitt's lymphoma (two cases), cyst (two cases), ganglion (two cases), keloid (two cases), lipoma (two cases), lupus vulgaris (two cases), neurofibromatosis (two cases), abscess (one case), atheroma (one case), bronchiectasis (one case), chicken pox (one case), dermoid carcinoma (one case), epithelioma (one case), fibrolipoma (one case), fibrosarcoma (one case), hypernephroma (one case), lepralepromatosa (one case), leprosy (one case), liposarcoma (one case), lymphadenitis (one case), molluscum contagiosum (one case), squamous carcinoma (one case), subcutaneous necrosis (one case), trauma (one case), and ulcer (one case).
Two hundred sixty five (38%) cases did not have a clinical diagnosis documented. More than half (53%, 369 cases) of the cases or their specimens were referred from surgical units of both private and public hospitals around the country. Other referring units included medical outpatient (40 cases), medical inpatient (35 cases), pediatric (36 cases), ear, nose and throat (nine cases), oral surgery (seven cases), skin clinics (eight cases) cardiothoracic (five cases), eye clinic (seven cases), orthopedic (five cases), gynecology (three cases), pulmonology (three cases), antenatal (one case), burns (one case), pathology (one case), and urology (one case).
Spatial distribution of deep fungal infections in Uganda
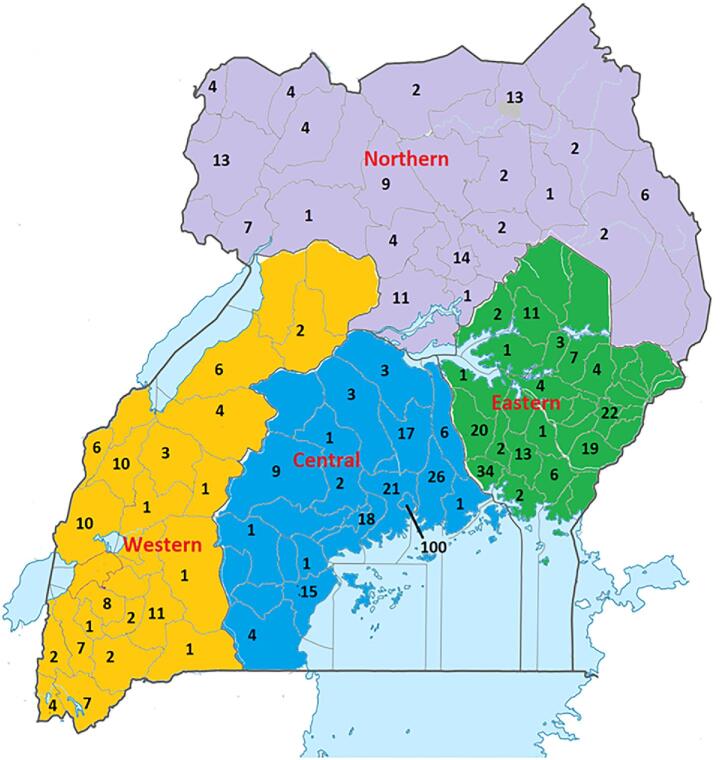
With regard to the tribe of the patients, majority of the cases were from the tribes of Baganda (28.3%, n = 197), Basoga (10.6% n = 74), Lango (6.9%, n = 48), Itesot (6%, n = 42), Banyankole (5.5% n = 38), Acholi (5.2% n = 36), Kiga (2.9%, n = 21) and Nyarwanda (2.9%, n = 20). The rest of the tribes had less than 20 cases each. Uganda is divided into 134 districts, which are grouped into four administrative regions, that is, Northern, Western, Eastern, and Central region. However, cases were registered from only 77/134 districts. Using data from the district of residence of the patients, we plotted the number of cases per district and region to determine the spatial distribution. The highest number of cases were recorded from Kampala district (100 cases). However, the eastern region had more cases per district (Fig. 2).
Figure 2.
Spatial distribution of deep fungal infections: Figures shows a map of Uganda indicating the number of cases identified per district. There were 127 cases without a record of district of residence and these were not included in this map. Districts with no cases were left blank. Map was created using the Microsoft Paint app in Windows 10. This Figure is reproduced in color in the online version of Medical Mycology.
Distribution of deep fungal infections by age, sex, and lesion site
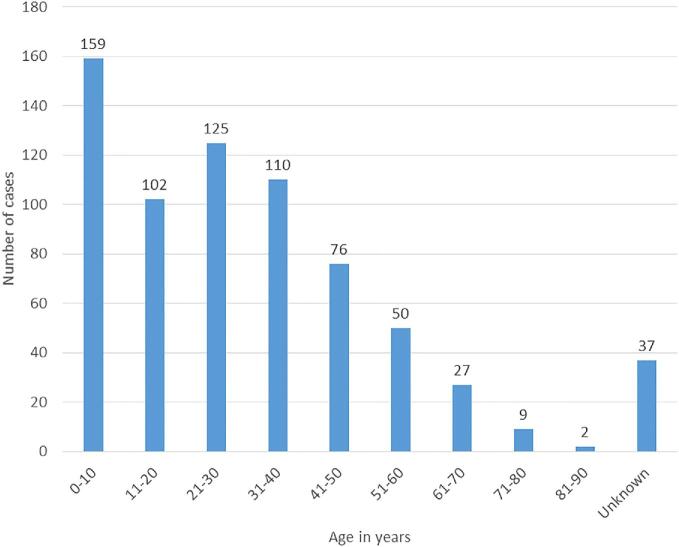
With regard to the lesion site for the fungal infections, the foot (183 cases) was the most affected part of the body followed by the thigh (72 cases), leg (68 cases), forearm (44 cases), buttock (36 cases), skin (33 cases), and the lungs (22 cases). Other body parts had less than 20 cases each over the study period. Majority of the cases were males (59.1% [412/697]) with an overall median age for the all cases being 28 years (interquartile range [IQR] = 11–40, n = 660). Adults (≥18 years) comprised of 67.7% (472/697) of all cases; 37/472 (7.8%) adults had missing age and were recorded as “adult” in the reports. The age group most affected by deep fungal infections was 0–10 years (Fig. 3), mostly suffering from subcutaneous phycomycosis (78.6%) and majority residing in rural areas.
Figure 3.
Age distribution of patients with fungal infections: children were mostly affected. This Figure is reproduced in color in the online version of Medical Mycology.
Distribution of individual fungal infections in Uganda
Aspergillosis
We identified 23 cases of aspergillosis (Fig. 4) of which nine (39%) were aspergillomas. All 23 cases were caused by members of the genus Aspergillus without identifying the species. Of the 23 cases, 14 were diagnosed using lung biopsy suggestive of pulmonary aspergillosis. Two cases were diagnosed from brain tissue (both aspergillomas) showing septate regular and branching fungus in the brain. Two cases were from the eye, one from ethmoid sinus (aspergilloma), one from nasal cavity and one from the maxillary sinus. Two of the cases had no lesion site recorded. Only four cases of aspergillosis were correctly diagnosed clinically. Three of the pulmonary cases were clinically misdiagnosed as pulmonary tuberculosis (PTB) while six cases had no clinical diagnosis. Six of the 14 pulmonary cases were aspergillomas suggestive of chronic pulmonary aspergillosis (CPA). Four of the CPA cases had documented evidence of pulmonary cavities containing masses of hyphae/ spores. One of the CPA cases had a mass in the upper right lobe and direct microscopy on bronchoalveolar lavage (BAL) showed branched septate hyphea. Only one case had a documentation of cavities with negative tuberculosis sputum smears. The remaining eight pulmonary cases were suggestive of invasive pulmonary aspergillosis (IPA). Two of the IPA cases had documented evidence of bronchiectasis. One of the IPA cases was human immunodeficiency virus (HIV) positive with negative sputum acid- fast bacilli (AFBs), while one was HIV positive on PTB treatment. Only one aspergillosis case was a European (Dutch) who was diagnosed with a brain aspergilloma.
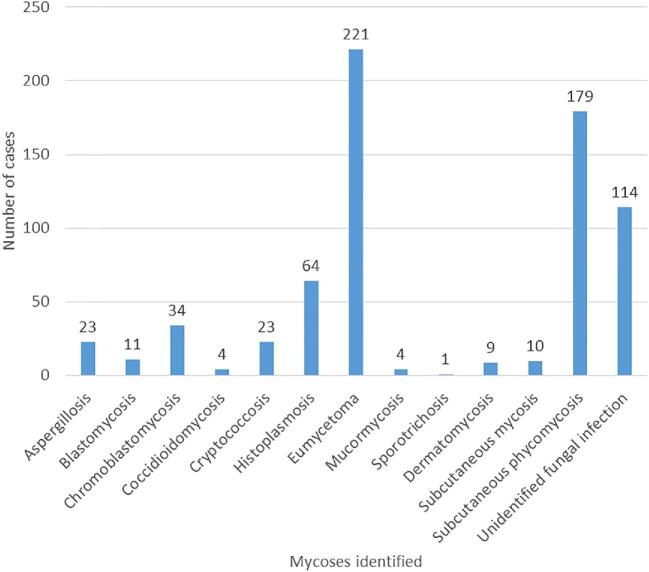
Figure 4.
Number of cases for each fungal infection: Eumycetoma and subcutaneous phycomycosis had the highest number of cases over the 70-year period. This Figure is reproduced in color in the online version of Medical Mycology.
Blastomycosis
We identified 11 cases of Blastomycosis diagnosed by histology and all cases were caused by Blastomyces dermatitidis. Seven of the cases had biopsies taken from the lower limbs (mostly the foot), one from the upper limbs, one from the abdominal wall, and one from the lungs (smear on BAL). One of the cases had no lesion site recorded. Cases mostly presented with a chronic skin ulcer and histology revealed micro abscesses and foreign body giant cells containing greenish spores. None of the cases were correctly diagnosed clinically. Majority of the cases came from the Eastern region of Uganda.
Histoplasmosis
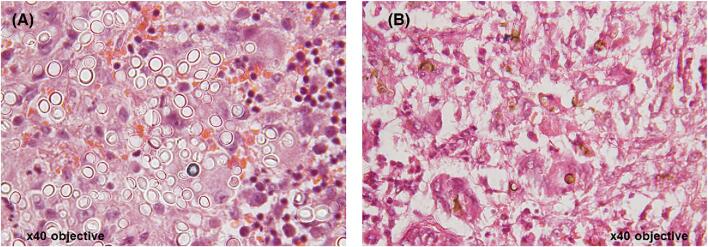
There were 64 cases of histoplasmosis identified of which 23 cases were caused by Histoplasma capsulatum and 41 cases caused by Histoplasma duboisii (African type). Only eight cases were correctly diagnosed clinically. Most of the presentation was cutaneous. The forearm, neck, and shoulder were the most affected parts of the body. Most patients presented with nodular cystic swellings and histology revealed a chronic granulomatous inflammatory reaction with foreign body giant cells containing large fungal spores (Fig. 5A). One case had bone involvement.
Figure 5.
Histology images for fungal infections: (A) African histoplasmosis; Hematoxylin and eosin (H&E) stained sections showing multinucleate giant cells containing numerous large yeast cells in their cytoplasm. The yeast cells are approximate 10–15um in diameter and are thick walled. (B) Chromoblastomycosis; H&E stained sections showing pigmented, brown, round organisms with thick walls ‘Copper bodies’. This Figure is reproduced in color in the online version of Medical Mycology.
Chromoblastomycosis
We identified 34 cases of chromoblastomycosis diagnosed by histology. All cases were caused by unidentified fungi seen with PAS staining. Lower limbs were the most affected. Only one case was correctly diagnosed clinically, while the rest were clinically misdiagnosed; mostly as tumors, malignant ulcers, or Kaposi's sarcoma. Some slides revealed chest nut shaped fungus cells while others showed brown fungal spores and hyphae (Fig. 5B).
Coccidioidomycosis
Only four cases of coccidioidomycosis were identified using histology. All four cases were caused by unidentified Coccidioides species. None of the cases had a correct clinical diagnosis. One case was clinically suspected to be histoplasmosis. Lesions were seen on the abdominal wall, buttock, and shoulder.
Cryptococcosis
There were 23 cases of cryptococcosis diagnosed by histology. This, however, did not include cases of cryptococcal meningitis, which is usually diagnosed using microbiology techniques in a different laboratory. Six cases were confirmed to be cryptococcomas with one having bone involvement on the forearm. Cutaneous cryptococcosis was the commonest manifestation of disseminated disease. The body parts mostly affected were lymph node, face, lung, liver, foot, fore arm, neck, jaw, nasal cavity, armpit, abdominal wall, and skin. One patient had lesions on the whole body. Seven cases were caused by Cryptococcus neoformans while others were caused by unidentified Cryptococcus species. Only one patient had a documentation of being HIV positive with a foot lesion. Histology of the skin lesions often showed thick walled fungal spores. Only one case was correctly diagnosed clinically.
Maduromycosis (eumycetoma)
There were 221 cases of eumycetoma all of which were caused by unidentified fungal pathogens diagnosed histologically using PAS staining and tissue reaction without identifying the genus or species of the fungus. Only 96 cases had a correct clinical diagnosis, and the majority were mostly misdiagnosed as Kaposi's sarcoma. The foot was the most affected part of the body (74%). Twenty-two cases had bone involvement, of which 13 got amputation. The majority of cases presented with a chronic swelling with multiple discharging sinuses. Histology showed granulation tissue with micro abscesses and foreign body giant cells. Histology also revealed presence of granules containing colonies of a fungus.
Mucormycosis
Only four cases of mucormycosis were identified all caused by unidentified fungi, diagnosed by PAS stain and microscopic appearance of the fungi. None of the cases had a correct clinical diagnosis. Body parts affected included stomach, forearm, face, and maxillary sinus.
Sporotrichosis
Only one case of sporotrichosis was identified caused by Sporothrix species in a 60-year-old female around the knee. The tissue section showed round spores suggestive of sporotrichosis. Clinical diagnosis suggested a fungal infection.
Dermatomycosis
Nine cases of dermatomycoses were identified all of which were caused by unidentified dermatophytes. Three patients had lesions of fungal dermatitis on the whole body. Majority of the cases were referred from eastern Uganda. Only one case was correctly diagnosed clinically while the rest were mainly clinically misdiagnosed as prurigo. Cases were surprisingly middle-aged adults with a mean age of 28 years.
Subcutaneous mycosis
We found 10 cases of subcutaneous mycosis diagnosed by histology. One case was caused by Aspergillus species, while the rest were caused by unidentified fungi. Only one case was correctly diagnosed clinically as a fungal infection. Lesions were mostly confined to the lower limbs.
Subcutaneous phycomycosis
There were 179 cases of subcutaneous phycomycosis all caused by unidentified fungi. Only 88 of the cases had correct clinical diagnosis with a very large differential. The thigh was the most affected part of the body. Histology mostly showed eosinophilic Splendore-Hoeppli material coating these hyphae and giant cells containing fungal spores and/or septate hyphae. One patient got amputation of the forearm.
Unidentified fungal infection
We found 114 cases of fungal infections that were diagnosed histologically using PAS staining and tissue reaction without identifying the genus or species of the fungus. Only 14 of these were correctly diagnosed clinically as a fungal infection. Samples were collected from different parts of the body where the lesions were located. However, lower limbs were the most affected. Most of these were deep fungal infections showing septate or nonseptate filamentous fungus or PAS positive material.
Discussion
We attempted to estimate the burden of deep fungal infections diagnosed by histology in Uganda. We identified a total of 697 cases of deep fungal infections over a period of 70 years (1950–2019) with an average incidence of 0.73 cases per 100,000 persons. Our finding suggests a substantial burden of deep fungal infections, mainly subcutaneous disease in Uganda causing more than 300 new infections annually. However, this seemed an underestimate since histology is not routinely used to diagnose fungal infections in Uganda and fungal skin, nail, and hair infections, which are more common, are diagnosed and treated clinically. Besides, even though histopathology is essential to demonstrate fungal elements in the infected tissues and to exclude other noninfectious disease conditions sometimes mimicking as neoplastic disorders, it should be used together with other tests such as culture and serology (where applicable). Also, in the past two or three decades, HIV-associated mycoses notably cryptococcal disease and Pneumocystis jirovecii pneumonia have been the most important causes of deep mycoses in Uganda.16 A recent review estimated that fungal infections affect up to 2.5 million people (6.5%) in Uganda resulting to 18,000 deaths per year.16
For most of the deep or invasive fungal infections, a definite diagnosis can be got by isolating the fungus in culture from the tissue; despite the limitations for culture. There was a general decline in the number of cases detected recently. This was probably due to a reduction in clinical suspicion or just poor health seeking habits. Besides, the index of clinical suspicion was very low (31%). Similarly, the level of laboratory expertise to identify fungal pathogens could have been low. This is so because majority of the fungal pathogens could not be identified to genus and/or species level from the histology tissue sections and only recorded as fungal infection based on PAS staining. Formal training in mycology techniques for the laboratory technicians may help to address this gap.
With the exception of cryptococcal disease, which can be diagnosed reliably by antigen testing,17–20 diagnosis of other deep fungal infections is not straightforward. Conventional culture on Sabouraud dextrose agar is the gold standard diagnosis for each of the deep fungal infections and also allows antifungal susceptibility testing; however, they display low sensitivities and take days to weeks to yield positive results.13 Molecular-based techniques are becoming increasingly important in the species-level identification of invasive fungal disease. However, they currently lack methodological standardization and validation, and unclear interpretation of the results is thus not widely used routinely in the clinics.21 Serology and biomarkers also play an important role in the diagnosis of invasive fungal diseases owing to their speed and specificity.22
The spatial distribution of cases was not even across the four regions. The 100 cases identified in Kampala district could be explained by the ease of access of the reference pathology laboratory in the same district and the proximity to the National Referral Hospital. However, the eastern region had more cases per district. Similarly, the tribes from the central and eastern region had the highest number of cases. Males and the age group of 0–10 years were most affected by fungal infections mostly suffering from subcutaneous phycomycosis, similar to previous studies.23 The foot was the most affected part of the body followed by the thigh. This could probably be due to the large number of eumycetoma cases, which mostly affects the lower limbs.
Eumycetoma (maduromycosis) was the commonest mycosis identified by histology. Mycetoma is a neglected tropical disease globally,24 and data are limited about its burden and associated complications in Uganda. There are currently only two published papers about the disease in Uganda.25,26 In the current study, the foot was the most affected part of the body. This is probably because the causative agents, which are mostly present in the soil, are introduced into the subcutaneous tissue by traumatic injury.27,28 In Uganda, the disease is mainly confined to barefooted cultivators and more common among men than women.25 In Africa, majority of the mycetoma cases are reported from Sudan which boarders with Uganda in the north.29 However, the spatial distribution did not show any clustered cases on and around this boarder as would be expected.
Subcutaneous phycomycoses were the second commonest mycoses identified. They are fungal infection of subcutaneous tissues.30 Most of these cases were children with a mean age of 11 years. Males were more affected than females. The causative agents are often Basidiobolus haptosporus/Basidiobolus ranarum or Conidiobolus species.23 However, in the records we reviewed, the causative agent was not identified and only recorded as unidentified fungi based on PAS staining.
Histoplasmosis was relatively more common with 64 cases, mostly caused by Histoplasma capsulatum var. duboisii (African type), and clinical presentation was mostly cutaneous. Data about the pathogenesis, epidemiology, prevalence, and mortality due to the African histoplasmosis remain scarce.31 It is present exclusively in the African continent. Pulmonary disease is rare in this form of histoplasmosis. It involves mainly skin, bone, and subcutaneous tissues. However, it may involve the liver, spleen, and other organs causing a fatal febrile illness. It is rare in HIV patients. Although uncommon, it was interesting to note that endemic mycoses such as blastomycosis and coccidioidomycosis, which are thought to be geographically restricted to North America, were observed in the Ugandan setting, and some cases were clinically misdiagnosed as histoplasmosis.
Limitations
Cases were found by searching archives from a single reference laboratory. There were missing data due to blank entries on some reports. Majority of the fungal pathogens were not identified to genus or species level. There were no data about treatment of the cases.
Despite the limitations to the study, we retrospectively gave a good estimate for the burden and etiology of deep fungal infections in Uganda, highlighting the low index of clinical suspicion and the important role played by histology in the diagnosis of these infections. However, histology should be used together with other tests such as culture and serology (where applicable). There is also a need to build local capacity for mycology so as to improve on the index of clinical suspicion and diagnostic capabilities beyond the conventional methods. Further epidemiological studies are needed to verify theses estimates. Literature review on blastomycocis and coccidioidomyosis in African continent is required before concluding that these two infections are endemic in Africa.
Acknowledgments
We thank institutional support from the department of pathology, Makerere University, and the Infectious Diseases Institute. Richard Kwizera is currently supported through the DELTAS Africa Initiative grant DEL-15-011 to THRiVE-2, from Wellcome Trust grant 107742/Z/15/Z, and the UK government.
Contributor Information
Richard Kwizera, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Felix Bongomin, Department of Medical Microbiology & Immunology, Faculty of Medicine, Gulu University, Gulu, Uganda.
Robert Lukande, Department of Pathology, School of Biomedical Sciences, College of Health Sciences, Makerere University, Kampala, Uganda.
Authors’ contributions
R.K.and R.L. conceived and designed concept/protocol. R.K. performed literature search. R.K. reviewed biopsy reports. R.K. analyzed data. R.K. participated in initial manuscript drafting. R.K., F.B., and R.L. participated in critical revisions for intellectual content. R.L. participated in administrative, technical, or material support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Richardson MD, Warnock DW. Fungal Infection: Diagnosis and Management. New York: John Wiley & Sons; 2012. [Google Scholar]
- 2. Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transll Med. 2012; 4: 165rv13. [DOI] [PubMed] [Google Scholar]
- 3. Vallabhaneni S, Mody RK, Walker T, Chiller T. The global burden of fungal diseases. Infect Di Clin. 2016; 30: 1–11. [DOI] [PubMed] [Google Scholar]
- 4. Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005; 56: i5–i11. [DOI] [PubMed] [Google Scholar]
- 5. Kwizera R, Musaazi J, Meya DB et al.. Burden of fungal asthma in Africa: a systematic review and meta-analysis. PloS One. 2019; 14: e0216568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denning D. Calling upon all public health mycologists. Eur J Clin Microbiol Infect Dis. 2017; 36: 923–924. [DOI] [PubMed] [Google Scholar]
- 7. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases: estimate precision. J Fungi. 2017; 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maphanga TG, Britz E, Zulu TG et al.. In vitro antifungal susceptibility of yeast and mold phases of isolates of dimorphic fungal pathogen Emergomyces africanus (formerly Emmonsia sp.) from HIV-infected South African patients. J Clin Microbiol. 2017; 55: 1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol. 2011; 49: 785–798. [DOI] [PubMed] [Google Scholar]
- 10. Chakrabarti A, Slavin M. Endemic fungal infections in the Asia-Pacific region. Med Mycol. 2011; 49: 337–344. [DOI] [PubMed] [Google Scholar]
- 11. Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017; 17: e367–e377. [DOI] [PubMed] [Google Scholar]
- 12. Hay R, Denning DW, Bonifaz A et al.. The diagnosis of fungal neglected tropical diseases (fungal NTDs) and the role of investigation and laboratory tests: an expert consensus report. Trop Med Infect Dis. 2019; 4: E122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schelenz S, Barnes RA, Barton RC et al.. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect Dis. 2015; 15: 461–474. [DOI] [PubMed] [Google Scholar]
- 14. Smith JA, Riddell J, Kauffman CA. Cutaneous manifestations of endemic mycoses. Current Infect Dis Rep. 2013; 15: 440–449. [DOI] [PubMed] [Google Scholar]
- 15. Guidry JA, Downing C, Tyring SK. Deep fungal infections, blastomycosis-like pyoderma, and granulomatous sexually transmitted infections. Dermatol Clin. 2015; 33: 595–607. [DOI] [PubMed] [Google Scholar]
- 16. Parkes-Ratanshi R, Achan B, Kwizera R, Kambugu A, Meya D, Denning DW. Cryptococcal disease and the burden of other fungal diseases in Uganda: where are the knowledge gaps and how can we fill them? Mycoses. 2015; 58: 85–93. [DOI] [PubMed] [Google Scholar]
- 17. Kozel TR, Bauman SK. CrAg lateral flow assay for cryptococcosis. Expert Opin Med Diagn. 2012; 6: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boulware DR, Rolfes MA, Rajasingham R et al.. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis. 2014; 20: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwizera R, Nguna J, Kiragga A et al.. Performance of cryptococcal antigen lateral flow assay using saliva in Ugandans with CD4< 100. PloS One. 2014; 9: e103156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams DA, Kiiza T, Kwizera R et al.. Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis. 2015; 61: 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bašková L, Buchta V. Laboratory diagnostics of invasive fungal infections: an overview with emphasis on molecular approach. Folia Microbiologica. 2012; 57: 421–430. [DOI] [PubMed] [Google Scholar]
- 22. Richardson M, Page I. Role of serological tests in the diagnosis of mold infections. Current Fungal Infect Rep. 2018; 12: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thotan SP, Kumar V, Gupta A, Mallya A, Rao S. Subcutaneous Phycomycosis—fungal infection mimicking a soft tissue tumor: a case report and review of literature. J Trop Pediatr. 2009; 56: 65–66. [DOI] [PubMed] [Google Scholar]
- 24. WHO Addressing the Burden of Mycetoma 2016; https://www.who.int/neglected_diseases/mediacentre/WHA_69.21_Eng.pdf?ua=1. Accessed 26 March 2019. [Google Scholar]
- 25. Davies AG. The bone changes of Madura foot: observations on Uganda Africans. Radiology. 1958; 70: 841–847. [DOI] [PubMed] [Google Scholar]
- 26. Wilson AM. The aetiology of mycetoma in Uganda compared with other African countries. East Afr Med J. 1965; 42: 182–190. [PubMed] [Google Scholar]
- 27. WHO Mycetoma. 2019; https://www.who.int/buruli/mycetoma/en/. Accessed 26 March 2019. [Google Scholar]
- 28. Fahal AH. Mycetoma: a thorn in the flesh. Trans R Soc Trop Med Hyg. 2004; 98: 3–11. [DOI] [PubMed] [Google Scholar]
- 29. van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013; 7: e2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burkitt D, Wilson A, Jelliffe D. Subcutaneous phycomycosis: a review of 31 cases seen in Uganda. Br Med Journal. 1964; 1: 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bongomin F, Kwizera R, Denning DW. Getting histoplasmosis on the map of international recommendations for patients with advanced HIV disease. J Fungi (Basel). 2019; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]