Abstract
Pneumocystis jirovecii can cause life-threatening pneumonia in immunocompromised patients. Traditional diagnostic testing has relied on staining and direct visualization of the life-forms in bronchoalveolar lavage fluid. This method has proven insensitive, and invasive procedures may be needed to obtain adequate samples. Molecular methods of detection such as polymerase chain reaction (PCR), loop-mediated isothermal amplification (LAMP), and antibody-antigen assays have been developed in an effort to solve these problems. These techniques are very sensitive and have the potential to detect Pneumocystis life-forms in noninvasive samples such as sputum, oral washes, nasopharyngeal aspirates, and serum. This review evaluates 100 studies that compare use of various diagnostic tests for Pneumocystis jirovecii pneumonia (PCP) in patient samples. Novel diagnostic methods have been widely used in the research setting but have faced barriers to clinical implementation including: interpretation of low fungal burdens, standardization of techniques, integration into resource-poor settings, poor understanding of the impact of host factors, geographic variations in the organism, heterogeneity of studies, and limited clinician recognition of PCP. Addressing these barriers will require identification of phenotypes that progress to PCP and diagnostic cut-offs for colonization, generation of life-form specific markers, comparison of commercial PCR assays, investigation of cost-effective point of care options, evaluation of host factors such as HIV status that may impact diagnosis, and identification of markers of genetic diversity that may be useful in diagnostic panels. Performing high-quality studies and educating physicians will be crucial to improve the rates of diagnosis of PCP and ultimately to improve patient outcomes.
Keywords: Pneumocystis, Pneumocystis pneumonia, immunocompromised host, polymerase chain reaction, antigen-antibody reaction
Introduction
Pneumocystis jirovecii is an opportunistic fungal pathogen that causes life-threatening cases of Pneumocystis pneumonia (PCP) in immunocompromised patients. Pneumocystis was originally named Pneumocystis carinii after Antonio Carinii, the parasitologist who found the life-form in infected rat lungs.1 The organism that causes pneumonia in humans was named Pneumocystis jirovecii in honor of the parasitologist Otto Jirovec who described the first human cases.2
Pneumocystis jirovecii is ubiquitous, and many humans are exposed by 2 years of age.1 Consistent with this, the recent PERCH study assessing molecular diagnostics of pediatric pneumonia in the developing world found that Pneumocystis caused up to 1–2% cases of community-acquired pneumonia in children under 5 with a peak incidence in infants.3 PCP was first identified in immunocompromised children during World War II and became widely recognized in human immunodeficiency virus (HIV)-positive adults during the acquired immunodeficiency syndrome (AIDS) epidemic. With the increasing use of immunosuppressants, the incidence in the HIV-negative population has increased, resulting in significant morbidity and mortality. Early diagnosis is key to allow for early treatment to improve outcomes.
Because Pneumocystis jirovecii is extremely difficult to culture in vitro, the diagnosis has traditionally relied upon clinical symptoms, radiographic findings, and confirmation via visualization of the organisms on staining of lung specimens such as bronchoalveolar lavage fluid or induced sputum. However, these staining methods have been shown to have poor sensitivity for detection of PCP, and new molecular methods including polymerase chain reaction (PCR), loop-mediated isothermal amplification (LAMP), and antibody-antigen testing on less invasive samples have been developed for the diagnosis of PCP. In this review, we summarize the current methods of detection, highlight novel approaches to detection, and discuss the next steps to improve diagnostic methods for PCP.
Pneumocystis jirovecii
Structure and life-forms
Pneumocystis jirovecii was first identified by Carlos Chagas as a protozoan that was thought to be part of the life cycle of Trypanosoma cruzi during the early 20th century.4Pneumocystis was reclassified as a fungus in 1988 when a phylogenetic linkage to the fungal kingdom was established via genomic analysis of the small rRNA subunit.1 The confusion regarding the type of organism stems from the two unique life-forms of Pneumocystis: the cystic life-form (ascus) and the trophozoite life-form (troph).1,5,6 Much is still unknown about these life-forms because it is extremely difficult to culture Pneumocystis jirovecii in vitro.1,7 The ascus life-form is believed to be the environmental and transmissible form based on co-housing studies in immunosuppressed mice and rats, which were unable to transmit the infection after treatment with cyst-depleting medications like echinocandins.8,9 The cysts are inhaled into the lungs, and when they rupture, the trophozoite forms are released.9 The trophozoite form of Pneumocystis adheres to the type I alveolar epithelium as the infection becomes established.1,10 During infection, trophozoite life-forms are much more abundant than cysts and are typically present at a 10:1 or higher ratio.1,7 Trophozoites can then replicate asexually or potentially sexually to form new cysts.7 Functional CD4 + T lymphocytes and alveolar macrophages are key for effective clearance of both life-forms in immunocompetent hosts, but a robust immune response also has the potential to cause lung injury and respiratory impairment.1
Epidemiology and transmission
Pneumocystis jirovecii was first recognized as a clinically relevant pathogen during World War II when it caused pneumonia in patients in orphanages in Europe and life-threatening cases of pneumonia in children with acute lymphoblastic leukemia.11,12 PCP became particularly widespread during the HIV epidemic, being responsible for two-thirds of AIDS-defining illnesses during the 1980s.13 After the advent of highly active antiretroviral therapy (HAART) and Walter Hughes's discovery of trimethoprim-sulfamethoxazole therapy, cases of PCP in HIV-infected patients decreased in developed countries.13,14 However, a significant number of HIV-positive patients are still affected including those not yet diagnosed with HIV or not in medical care, those patients not receiving PCP prophylaxis, and those patients not taking or responding to HAART.13,15,16 With the increasing number of blood and solid organ transplants and the advent of new, more potent immunotherapies, cases in non-HIV infected patients have been noted to be increasing.4,17 These include patients with conditions such as hematologic malignancies, solid tumors, long-term high-dose steroids, stem cell transplantation, solid organ transplantation, connective tissue diseases, and patients taking immunosuppressive drugs such as glucocorticoids and immunotherapy against CD20.17-29
Pneumocystis pneumonia continues to have a high mortality when it does occur. Hospital survival for PCP ranges from 7 to 20% in HIV-positive patients and 29 to 60% in HIV-negative patients.1,17,23,30-33 The mortality may be higher in HIV-negative patients for several reasons. First, HIV-positive patients have much higher burden of Pneumocystis organisms in their lungs with fewer neutrophils, which results in increased diagnostic yield of induced sputum (IS) and bronchoalveolar lavage fluid (BALF) to confirm the diagnosis.1,34,35 In contrast, patients without HIV may have lower fungal burden leading to fewer symptoms and a delayed diagnosis.17,31,36,37,38–41 Second, the lower level of inflammatory cells leads to less lung damage and better oxygenation in HIV-positive patients.1,34 Consideration of HIV status is an important consideration in interpreting risk and diagnostic testing for PCP.
Clinical manifestations
Common symptoms include the subacute onset of dyspnea, nonproductive cough, and low-grade fever. Patients are generally tachypnic, tachycardic, and have normal lung exams.1,42 Notably, HIV-negative patients typically have a more sudden onset of symptoms and more severe clinical presentation than HIV-positive patients.31,38,41 Classic radiographic features include bilateral perihilar interstitial infiltrates with increased involvement of lung fields and homogeneity over time. Chest computed tomography may reveal ground-glass opacification.1,42
Prophylaxis and treatment
All patients with HIV should receive prophylactic therapy when the CD4+ count is less than 200 cells per millimeter.43,44 HIV-negative patients who have an acquired or inherited immunodeficiency should receive prophylaxis, and this population at risk is increasing due to new immunotherapies and a higher number of transplants.4,17,44,45 Prophylaxis rates in these patients are often suboptimal given varying or lack of guideline recommendations for duration of prophylaxis.27,38 Prophylactic therapy may include trimethoprim-sulfamethoxazole, dapsone, atovaquone, and pentamidine with trimethoprim-sulfamethoxazole being the preferred primary prophylactic therapy.46 Notably, patients may develop PCP even while on prophylactic therapy, so it should still be included in the differential diagnosis if clinical suspicion is high.30
There are limited treatment options as Pneumocystis is resistant to most anti-fungal therapies, and trimethoprim-sulfamethoxazole continues to be the first-line therapy.1,44 Trimethoprim-sulfamethoxazole has many potential side effects, including hypersensitivity reactions, hepatitis, myelosuppression, and interstitial nephritis.4,47 Alternate therapies include primaquine plus clindamycin, atovaquone, and intravenous pentamidine.44 However, these medications also can cause a wide range of side effects such as rash, diarrhea, Clostridium difficile infection, hemolytic anemia, methemoglobinemia, kidney injury, leukopenia/bone marrow suppression, hypotension, arrhythmias, hypoglycemia, and insulin-dependent diabetes mellitus.4,47 Patients with severe PCP and HIV-positive patients who have hypoxemia (partial pressure of arterial oxygen less than 70 mm Hg or alveolar-arterial gradient more than 35) also benefit from being treated with corticosteroids to reduce inflammation.1 Given that these therapies are not without risk, confirmation of the diagnosis and cessation of therapy if PCP is not present is vital.
Search methods
Pubmed was searched for all published articles about Pneumocystis jirovecii. Abstracts and related references were reviewed. The authors identified 100 studies that focused on diagnostic methods and compared different types of samples or tests. The full-text articles for these studies were then reviewed. In sum, 36 studies were considered high-impact due to their inclusion of at least 100 patient samples and were highlighted in Table 1.
Table 1.
Comparison of methods of detection for Pneumocystis jirovecii pneumonia.
| Ref | First author | Sample type | Sample | HIV pts | HIV pts | GMS | IF | PCR | BG | LDH | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | Se | Sp | Se | Sp | Se | Sp | Se | Sp | Se | Sp | |||||
| 19 | Azoulay | BAL, IS | 448 | No | Yes | X | X | X | X | 87 | 92 | X | X | X | X |
| 35 | Oren | BAL | 214 | Yes | Yes | X | X | X | X | 75 | 95 | X | X | X | X |
| 50 | Lu | Various | 2505 | Yes | Yes | X | X | X | X | 99 | 90 | X | X | X | X |
| 51 | Fan | BAL | 1793 | Yes | Yes | X | X | X | X | 98 | 91 | X | X | X | X |
| 52 | Summah | Various | 2330 | Yes | Yes | X | X | X | X | 97 | 94 | X | X | X | X |
| 53 | Mc-Taggart | BAL | 105 | U | U | X | X | X | X | 100 | 100 | X | X | X | X |
| 56 | Rohner | BAL | 1843 | Yes | Yes | X | X | X | X | 100 | 92 | X | X | X | X |
| 58 | Jian-cheng | BAL, IS | 1044 | No | Yes | X | X | X | X | 100 | 88 | X | X | X | X |
| 59 | Caliendo | BAL | 112 | Yes | Yes | X | X | X | X | 100 | 98 | X | X | X | X |
| IS | 120 | Yes | Yes | X | X | 82 | X | 95 | 94 | X | X | X | X | ||
| 60 | Cart-wright | BAL | 154 | Yes | Yes | X | X | 100 | 100 | 100 | 99 | X | X | X | X |
| IS | 208 | Yes | Yes | X | X | 78 | 100 | 100 | 98 | X | X | X | X | ||
| 61 | Pinlaor | BAL | 21 | Yes | No | 64 | 100 | 100 | 100 | 100 | 90 | X | X | X | X |
| IS | 139 | Yes | No | 62 | 100 | 85 | 98 | 85 | 98 | X | X | X | X | ||
| 62 | Revathy | Sputum | 150 | Yes | Yes | X | X | X | X | 100 | 93 | X | X | X | X |
| 68 | Fischer | Oral wash | 175 | U | U | X | X | X | X | 75-91 | 94-96 | X | X | X | X |
| 71 | Samuel | Naso-pharynx | 349 | Yes | Yes | X | X | X | X | 86 | 95 | X | X | X | X |
| 89 | Procop | BAL | 313 | U | U | 79 | 99 | 91 | 82 | X | X | X | X | X | X |
| 91 | Tiley | BAL, sputum | 202 | Yes | Yes | 54 | X | 92 | X | X | X | X | X | X | X |
| 92 | Raab | BAL | 243 | U | U | 100 | X | X | X | X | X | X | X | X | X |
| 94 | Stratton | BAL, sputum | 150 | U | U | X | X | 87-96 | 100 | X | X | X | X | X | X |
| 95 | Nato | BAL | U | Yes | Yes | 67 | X | 76-77 | X | X | X | X | X | X | X |
| Sputum | U | Yes | Yes | 87 | X | 94-97 | X | X | X | X | X | X | X | ||
| 96 | Cregan | BAL | 50 | U | U | 86 | 97 | 86-90 | 90-100 | X | X | X | X | X | X |
| Sputum | 50 | U | U | 92 | 92 | 97 | 85-100 | X | X | X | X | X | X | ||
| 97 | Ng | BAL, sputum, lung tissue | 182 | U | U | 75 | 100 | 80 | 90 | X | X | X | X | X | X |
| 98 | Orholm | BAL, sputum | 122 | U | U | 67 | X | 73-95 | X | X | X | X | X | X | X |
| 100 | Aderaye | BAL | 118 | Yes | Yes | X | X | 57 | 99 | X | X | X | X | X | X |
| Sputum | 78 | Yes | Yes | X | X | 48 | 100 | X | X | X | X | X | X | ||
| 103 | Armbrus-ter | BAL | 112 | Yes | No | X | X | 59 | 99 | 66 | 97 | X | X | X | X |
| 104 | Ng | BAL | 37 | U | U | X | X | 100 | 96 | X | X | X | X | X | X |
| Sputum | 125 | U | U | X | X | 72 | 100 | X | X | X | X | X | X | ||
| 107 | Hauser | BAL, sputum | 110 | Yes | Yes | X | X | 93 | 100 | 93 | 91 | X | X | X | X |
| 110 | Gupta | BAL, sputum, naso-pharynx gastric | 143 | Yes | Yes | 31 | 100 | X | X | 100 | 99 | X | X | X | X |
| 111 | Fillaux | BAL | 400 | Yes | Yes | X | X | X | X | 100 | 91 | X | X | X | X |
| 112 | Flori | BAL | 173 | Yes | Yes | X | X | X | X | 100 | 86 | X | X | X | X |
| 131 | Onishi | Serum | 2331 | Yes | Yes | X | X | X | X | X | X | 96 | 84 | X | X |
| 132 | Kara-georgo-poulos | Serum | 2080 | Yes | Yes | X | X | X | X | X | X | 95 | 86 | X | X |
| 133 | Li | Serum | 1362 | Yes | Yes | X | X | X | X | X | X | 91 | 75 | X | X |
| 135 | Tasaka | Serum | 295 | Yes | Yes | X | X | X | X | X | X | X | X | 86 | 45 |
| 138 | Esteves | Serum | 145 | Yes | Yes | X | X | X | X | X | X | X | X | 80 | 52 |
| 139 | Esteves | Serum | 100 | Yes | No | X | X | X | X | X | X | X | X | 91 | 36 |
| 140 | Vogel | Serum | 328 | Yes | Yes | X | X | X | X | X | X | X | X | 66 | 45 |
| Averages: | 72 | 98 | 83 | 97 | 94 | 94 | 94 | 82 | 81 | 45 | |||||
To be included in Table 1, diagnostic tests had to be evaluated in at least two high quality studies, and studies each had to have at least 100 patient samples. GMS, IF, PCR, BG, and LDH are Gomori-methenamine silver stain, immunofluorescent staining, polymerase chain reaction, (1,3)-beta-D-glucan, and lactate dehydrogenase testing. Se is sensitivity, and Sp is specificity. U is defined as unspecified in the study, and X was defined as not included in the study. BAL = bronchoalveolar lavage; HIV = human immunodeficiency virus; IS = induced sputum.
Current methods of diagnosis
Sample considerations
Pneumocystis jirovecii is a near obligate alveolar pathogen with only rare cases of dissemination.48 In the early days of diagnosis, lung biopsy procedures were used to obtain large specimens of tissue to stain for organism identification. As diagnostic methods have become more sophisticated and technical expertise has improved, biopsy has been replaced with more minimally invasive sampling techniques as noted below.48 See Figure 1 for a description of sampling techniques.
Figure 1.
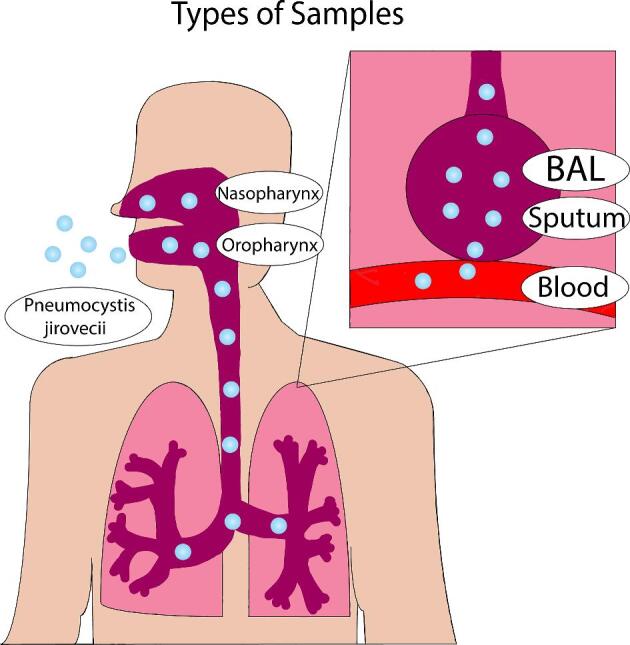
Illustration of the current samples used for potential diagnosis of Pneumocystis pneumonia.
Bronchoalveolar lavage fluid (BALF)
The current gold standard sample for diagnosis of PCP is BALF, which is considered to be the highest quality respiratory sample.49–53 However, the lack of a standardized sampling technique can impact test performance. Bronchoscopy, when performed after negative induced sputum testing, has been noted to yield a diagnosis in 51% of cases and, if negative for PCP, allows for discontinuation of treatment.54–56 Limitations include the fact that this invasive procedure is expensive, carries more of a risk to the patient, may not always be feasible for patients with severe pulmonary disease, and may not be available in resource-poor settings.13 Nondirected bronchoalveolar lavage may deserve further study, as it does not require use of a bronchoscope.
Sputum
Attempts have been made to develop less invasive techniques for obtaining samples for testing. According to a meta-analysis that included 322 individuals, performance of immunofluorescent staining on IS has been found to have a >95% negative predictive value in low prevalence situations (<10% prevalence), making a negative test adequate for ruling out PCP.55 However, in high prevalence areas and cases of high suspicion of PCP, a bronchoscopy with BAL should be performed when negative IS results are obtained.1,55,57,58 Notably, IS has also been found to have 85–100% sensitivity and good concordance with BALF results when methods of detection such as PCR are utilized.19,59–63
Oral washing
Pneumocystis jirovecii may be found in oral washes if the organism has been coughed or recently inhaled into the oropharyngeal tract. Oral washes can be obtained quickly and noninvasively, and positive tests may reflect an even higher fungal burden in the lower respiratory tract. However, there are theoretical disadvantages such as increased degree of PCR inhibition due to dilution from pharyngeal secretions, the inability of organisms to reach the oral cavity in low fungal burden infections.64 Multiple studies have evaluated the ability to detect Pneumocystis jirovecii in oral wash specimens using highly sensitive PCR detection systems.65–69 When compared with sputum and BAL, oral wash PCR has been noted to have a sensitivity of 75–91% and a specificity of 68–100%.66–68,70 Oral wash samples may be most useful in supporting a diagnosis of PCP if positive, but a negative result cannot reliably rule out PCP in symptomatic patients.65
Nasopharyngeal aspirate
Lower respiratory tract specimens such as BALF and sputum are difficult to obtain in children. In one study, MSG PCR on nasopharyngeal samples was found to have an 86% sensitivity and 95% specificity for detecting PCP when compared to BALF and sputum samples.71 PCR was specifically noted to have a higher detection rate than immunofluorescence staining techniques, which may explain the low sensitivities reported in previous studies that used these older staining methods.71,72 Therefore, PCR on nasopharyngeal samples, if positive, may obviate the need to obtain more invasive samples. For example, positivity in nasopharyngeal samples was defined in the recent PERCH study as a threshold of at least 104 copies per milliliter.3
Blood/serum
Blood/serum has the significant advantage of being easily obtained and inexpensive. The presence of Pneumocystis jirovecii in the blood reflects disease progression, as the pathogen is no longer limited to the respiratory tract. Several studies have suggested that detection of Pneumocystis jirovecii DNA in the serum may be a useful diagnostic marker for PCP.66,73–76 Earlier studies did not detect Pneumocystis jirovecii DNA in the serum of patients with known PCP, which may have been due to use of conventional PCR.77,78 One study demonstrated positive Pneumocystis jirovecii nested PCR in blood from patients with PCP, colonized patients, and patients who were neither infected nor colonized.79 A recent report from Sweden revealed that real-time PCR analysis on serum samples had a very high sensitivity (100%) and negative predictive value (99%) for the diagnosis of PCP in HIV-infected patients. The major differences in the findings of these studies have not yet been reconciled, and use of serum PCR is not currently recommended for detection of PCP. Other serum diagnostic tests including serum enzyme-linked immunosorbent assay (ELISA) for antibodies and antigens associated with PCP are more promising and are further described below.
Urine
Our literature search revealed no data on urine testing for diagnosis of PCP. Urine antigen testing has proven valuable in the diagnosis of histoplasmosis, and preliminary studies have shown that other fungal infections such as cryptococcosis, blastomycosis, and aspergillosis may be detectable by urine lateral-flow assay testing.80–87 Urine testing for PCP may represent a new frontier for development of noninvasive molecular diagnostic techniques, but studies are needed to ascertain feasibility.
Traditional diagnostic tests
Pneumocystis jirovecii is extremely difficult to culture and has therefore been classically diagnosed by clinical symptoms and radiographic findings with confirmation via visualization of the stained organism.1 However, it is notable that these methods are not sensitive due to dependence on the quality and type of samples and the skill of observers reviewing slides. The more prevalent cyst-staining techniques may underestimate rates of active infection, which is characterized by predominance of the trophozoite life-forms.1,50 Additionally, when fungal burdens are low, as in HIV-negative patients and patients taking PCP chemoprophylaxis, microscopic diagnosis may be falsely negative.17,88 This is why molecular testing is becoming increasingly important in the diagnosis of PCP. Figure 2 depicts the traditional staining methods for detection of PCP.
Figure 2.
Summary of current methods to detect and diagnose Pneumocystis infection.
Nonimmunofluorescent staining
The cyst life-form can be detected with many stains. Giemsa, Diff-Quik, and Wright stains can detect the cyst but do not stain its wall. The Gomori-methenamine-silver (GMS) stain, Gram-Weigert, cresyl echt violet, toluidine blue O (TBO), and calcofluor white (CW) stains stain the cell wall of the cyst.1,49 The stains for the cyst cell wall have traditionally been preferred due to the ability for rapid analysis and minimal expertise needed for interpretation. These stains will stain both live and dead cysts. The trophozoite life-form can be detected with Giemsa, Diff-Quik, Wright-Giemsa stain, modified Papanicolaou, or Gram-Weigert stains.1 However, due to its small size and nonspecific staining pattern, this is not the life-form typically used in diagnosis.
Studies comparing staining methods report that the highest sensitivity methods are CW, GMS stain, and TBO stain.47,89–93 Per Procop et al., only CW and GMS had positive and negative predictive values >90% when performed on BALF. The sensitivity of the CW stain has ranged from 57 to 78%.56,89,91,94 The sensitivity of GMS ranges from 31 to 97% with lower sensitivities being present in studies including poor quality samples or a large number of noninvasive samples such as IS and nasopharyngeal aspirates.61,89,91,95–99 TBO staining has also been reported to have lower sensitivity ranging from 49 to 94%.56,90,91,97,98,100,101 These staining methods are specific for the presence of organisms but if negative, do not rule out the presence of PCP. While these tests are easy to perform, they are reliant on the quality of the sample and subjective due to dependence on stain interpretation.
Immunofluorescent staining
Immunofluorescent stains via monoclonal antibodies to Pneumocystis jirovecii have a higher sensitivity and specificity than conventional stains. Sensitivity ranges from 48 to 100%, and specificity from 82 to 100%.55,60,61,89–91,94–98,100,102–107 They are easier to perform, more repeatable, and less reliant on technical skill for performance and interpretation.105 They can also stain both trophozoites and cysts.1 Comparisons of GMS and immunofluorescent stains are depicted in Table 1.
Novel methods of detection
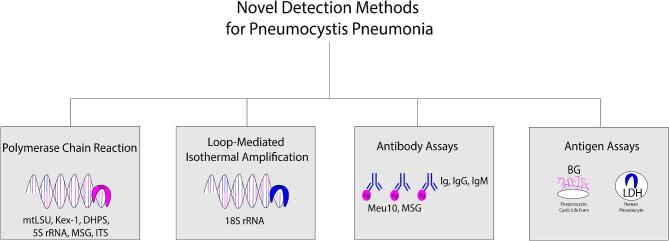
Polymerase chain reaction
PCR for Pneumocystis jirovecii was initially developed in the 1980s with primers testing for the gene for pneumocystis mitochondrial large-subunit ribosomal RNA (mtLSU rRNA).1 Nested PCR was one of the first techniques developed but has since been shown to be more labor intensive, more expensive, less quantitative, and less specific than real-time PCR, which will be the focus of this review.108 mtLSU real-time PCR has remained one of the most popular methods with other gene assays being developed including Kex-1, dihydroperoate synthase (DHPS), 5S rRNA, mitochondrial ribosomal rRNA, major surface glycoprotein (MSG), and internal transcribed spacer.9,51,109
PCR has been shown to be more sensitive for detection of PCP than staining methods in patients with and without HIV.1,3,18,110–112 Some studies suggest that it may be 104 to 106 times as sensitive.18 Three meta-analyses published in recent years reported a pooled sensitivity of 98%, 99%, and 97% with a pooled specificity of 91%, 90%, and 94% with most samples being BALF.50–52 This high sensitivity and specificity persisted in both the HIV-positive and HIV-negative populations.50,52 Notably, the highest sensitivity and specificity were found in studies using quantitative PCR methods.50–52 Because the sensitivity is high, a false negative test result is rare. Therefore, a negative PCR on BALF means that PCP is an unlikely diagnosis, and other diagnoses should be considered as the etiology for the patient's symptoms. In contrast, the high specificity means that a positive PCR on BALF is highly suggestive of the presence of Pneumocystis jirovecii.50–52
Loop-mediated isothermal amplification (LAMP)
Loop-mediated isothermal amplification (LAMP) provides an alternative to PCR as it can amplify a target gene with only a heating device and isothermal conditions.113 Sensitivity ranges from 87.5 to 95.4%, and LAMP has been shown to be relatively specific with no cross-reactivity to other fungal species.114–116 In small studies, LAMP has been shown to have higher rates of detection of PCP than conventional stains and rates similar to those of PCR.113,115,116 In some cases, visual detection with LAMP is possible as a particularly rapid and easy assay with only a black light and heating block.115
Flow cytometry
Flow cytometry can detect single or multiple microbes in an easy, reliable, and fast way. These organisms can be identified by cytometric parameters, fluorochromes such as CW, or monoclonal antibodies to Pneumocystis jirovecii.117,118 Flow cytometers can also detect antibodies against Pneumocystis and comment on antifungal susceptibility.117 Barbosa et al. have developed a method that uses immunofluorescent staining with the Detect IF kit (Axis-Shield Diagnostics Limited, UK) followed by flow cytometry.118,119 This method allows for detection of Pneumocystis jirovecii in clinical BAL and bronchial samples with 100% sensitivity and specificity when compared to immunofluorescent staining.118,119 While the applications are vast, the data are limited, and this is not currently recommended as a diagnostic method.117
Antibody assays
A promising diagnostic approach is to use an antigenic tool in an ELISA technique to detect immunoglobulin (Ig), IgM, and IgG antibodies against Pneumocystis jirovecii.120,121 Multiple potential immunogenic antigens have been described, including natural antigens such as Meu10 and recombinant synthetic antigens designed from the MSG gene.120–122 One study has shown that ELISA IgM anti-P. jirovecii has a sensitivity of 100% and a specificity of 81% when testing serum samples from 88 patients.120 Notably, the immune response may be variable depending on the nature of the immunocompromise and may affect the sensitivity of this assay in certain populations. Previous studies have shown alterations in immune response in patients with HIV, patients with a history of transplant, patients with cancer, patients who fail to adhere to prophylactic therapy, patients who smoke, patients with chronic obstructive pulmonary disease, patients with hazardous alcohol use, patients with injection drug use, and even patients from different geographic areas.123–128 Previous clinical infection or subclinical exposure to Pneumocystis may also impact immune response and lead to false positive tests.126,128–130 Additional elucidation of the complex host and environmental factors that affect antibody formation will be required before this and similar tests are considered for widespread utilization.
Antigen and biomarker assays
(1,3)-Beta D-Glucan (BG) is a cell wall constituent in the ascus life-form of Pneumocystis jirovecii and multiple other fungal pathogens. Various assays that detect BG in the serum have been developed with the most popular in the Western hemisphere being the Fungitell test, a chromogenic kinetic test approved in 2003 by the US Food and Drug Administration.131,132 In several meta-analyses, this assay was found to be 91%, 96%, and 95% sensitive with high sensitivity being demonstrated in both HIV positive and negative patients.131–133 However, BG was only 75%, 84%, and 86% specific for definite PCP because the assay could be positive in other fungal infections in patients with gram-negative endotoxinemia, in patients on certain antibiotics, in patients on albumin or globulin therapy, and in patients undergoing HD due to cellulose membranes and filters.131–133 Notably, the true value for specificity is more likely closer to 75% because the other two meta-analyses excluded the patients diagnosed with other invasive fungal diseases which likely exaggerated specifity.131,132 The significant advantage is the noninvasive nature of the test and the ability of a negative test to make PCP highly unlikely. The European Conference on Infections in Leukemia even stated that a negative serum BG was adequate to rule out PCP.134 The disadvantage is that a positive BG is not specific for the diagnosis of PCP, so further testing would need to be performed for validation of the diagnosis.133
Lactate dehydrogenase (LDH) is an intracellular enzyme found in almost all tissues.135 Serum levels of LDH have been found to be significantly elevated in patients with PCP relative to patients negative for PCP.135–138 The sensitivity and specificity of this marker for PCP have been estimated to be 66%–91% and 36–52%, respectively.135,138–140 In one study, the sensitivity of LDH elevation was found to be 100% in HIV-positive patients but only 63% in HIV-negative patients, indicating that this marker may only be useful in detecting PCP in HIV-positive patients.140 Oxygenation and BAL neutrophil levels have also been found to correlate with LDH levels.135,141 Therefore, LDH levels are likely a reflection of the underlying lung inflammation and injury and are not specific to PCP.135
Other antigens such as KL-6 and S-adenosylmethionine have also been evaluated as prospective markers. KL-6 antigen is a mucin-glycoprotein expressed on type 2 alveolar pneumocytes and bronchiolar epithelial cells.135 Serum levels of KL-6 have been found to be elevated in patients with PCP, but this marker has low specificity due to its elevation in any interstitial lung disease and other infectious diseases.135,138,141 S-adenosylmethionine (SAM) is an intermediate in multiple cellular functions that Pneumocystis cannot synthesize and must extract from the plasma of its host.142 Some studies have demonstrated significantly lower serum SAM levels in patients infected with PCP relative to patients infected with other pathogens and control patients.138,143,144 In other studies, this marker failed to discriminate patients with PCP from those without PCP.138,145 These biomarkers cannot be recommended for use at this time.
Challenges and next steps to improve diagnosis
Detection of low fungal burdens (colonization)
With the advent of more sensitive diagnostic testing, Pneumocystis jirovecii is increasingly being identified in asymptomatic individuals or individuals without the symptoms classically associated with PCP. This phenomenon has been termed colonization, and while it affects anywhere from 9 to 69% of immunocompromised patients, the clinical significance is unknown.13,36,37,146–149 There is concern that colonization may increase the risk for progression to PCP, and the phenotypes that progress are poorly defined.13,146,150 However, it is widely considered likely that colonization in these patients is likely to progress to active infection as they are unable to mount a defense to the increasing fungal burden.146,151 These patients are also at risk for developing inflammation that is detrimental to their lungs or transmitting the infection to others. Identification of the patients at highest risk for progression and initiation of prophylaxis or treatment may prevent significant morbidity and mortality.
Notably, the rates of colonization may be even higher than those reported. It is possible that increased use of prophylactic medications, changing strains of Pneumocystis, and changing patient factors (more non-HIV patients) may be resulting in a reduction in symptoms overall during cases of true pneumonia. The classic symptoms associated with PCP were developed when it was most prevalent in HIV-positive patients during the AIDS epidemic.152 In this case, many patients may be falsely classified as colonized if done according to symptomatology, and these colonized patients still have radiographic abnormalities and a high mortality rate of 16–22%, which may be due to PCP.17,88 One meta-analysis identified that 31.8% of the patients considered colonized have either had PCP or will develop PCP. The authors noted that the discrepancies were due to the higher sensitivity of PCR than staining methods, which allowed for detection of PCP several weeks before or after definitive PCP.50 The current diagnostic gold standard (staining for PCP) is suboptimal, and its use in place of PCR may delay diagnosis and treatment of active infection.
Given that symptoms may be unreliable, diagnostic testing for colonization should be considered. Methods proposed in the literature for distinguishing active infection from colonization include cutoffs in the copy number levels considered positive (<103 copies per capillary of DNA as colonized) by real-time PCR, cutoffs in the cycle threshold for true infection positivity, BG serum levels, copies per milliliter, picogram/microliter, and calculations of trophic forms per milliliter.36,37,111,112,153–157 These cutoffs have been extremely variable due to differences in technique by both PCR platform and gene tested. There may also be differences in HIV-positive and HIV-negative patients due to the known difference in fungal burden. Use of multiple diagnostic assays should be further investigated as a combination of PCR and BG may be more effective in determination of the significance of weakly positive PCR results.155
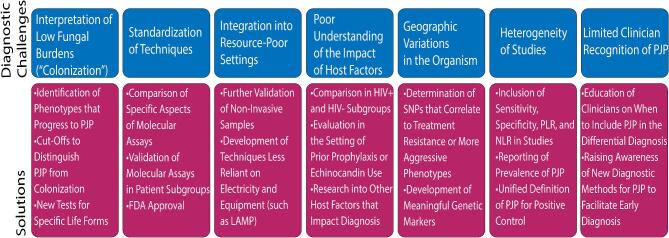
Interpretation of colonization presents a diagnostic challenge, and potential solutions deserve further investigation (Fig. 3). Research should aim to identify ‘colonization’ phenotypes that are likely to progress to PCP. Because symptomatology may be a poor marker for infection, better diagnostic tests should be developed with the ability to distinguish active infection from colonization. Future studies should further validate the cutoffs above and consider options for innovative new markers. The field may also be advanced by the recent genomic and transcriptomic data that have been recently published.158–160 Two of these papers performed RNAseq after antibiotic pressure with echinocandins that target the ascus life-form. These studies revealed unique genes that are expressed in trophs that may serve as novel diagnostics. Thus, PCR markers for the trophozoite life-form may be a useful diagnostic for infection.158
Figure 3.
Summary of current challenges and potential solutions to improve detection and diagnosis of Pneumocystis pneumonia.
Standardization of techniques
Multiple organizations have issued consensus guidelines that PCR should be the standard diagnostic method for PCP, and many PCR assays have been CE marked and adopted in Europe.7,134,161 However, no molecular assays have been approved to date by the FDA, resulting in limited adoption of these methods in hospitals in the United States.7 One difficulty in obtaining FDA approval is the wide range of commercially available PCR testing platforms available. These platforms vary in their recommended protocols of RNA and DNA extraction prior to testing, concentrations of samples, genes tested, thermocycling procedures, and methods for quantifying/validating the results of their assays.162 Future studies should focus on comparisons of various aspects of the platforms and testing in patient subgroups to identify the optimal molecular method for PCP diagnosis, which may improve the likelihood of FDA approval (Fig. 3). The recent multicenter study by the Fungal PCR Initiative is one of the first to start comparing quantitative PCR assays using known negative and positive BALF samples.109
Integration into resource-poor settings
Certain sampling techniques and diagnostic methods may not be feasible in resource-poor settings. With regard to sampling, bronchoscopy is required for BAL, and its cost, invasiveness, and expertise make it impractical for resource-poor settings. In these settings, induced sputum is typically preferred.47 Nasopharyngeal aspirates and oral washes are good options, particularly in pediatric patients, but they may not have adequate sensitivity without molecular testing method availability.
The conventional staining techniques are typically more affordable than the IFA and real time PCR.47 They require minimal equipment, only a microscope and experienced microscopist.138 PCR is not technically or financially viable due to lack of reliable electricity supplies and the ability to ship reagents in dry ice through customs.47 PCR also requires expensive equipment such as thermal cyclers, electrophoresis apparatus, and transilluminators as well as complicated DNA purification procedures.138 However, in settings where PCR is possible, it can prevent the need to obtain invasive specimens like BAL. PCR on noninvasive samples is considered the most cost-effective technique.163 In resource-poor settings, LAMP may be a good alternative to PCR as it has similar detection rates and can be performed in an isothermal environment.115 Detection can be performed visually or with fluorescence. Antigen serologic assays such as BG are much cheaper to perform than traditional staining or PCR. However, their overall cost-effectiveness is limited by lower specificity, and they may not be appropriate for resource-poor settings due to the requirement of expensive equipment (microplate reader).138 As diagnostic method options continue to evolve, additional studies should include information on cost-effectiveness and potential for being incorporated in resource-poor settings.
Poor understanding of the impact of host factors
Future studies should also include host factors that may impact sensitivity and specificity of detection (Fig. 3). For example, studies should compare diagnostic methods in HIV-positive and HIV-negative patients in their cohorts, as it has been well demonstrated that these two populations have different phenotypes of PCP and therefore may require different diagnostic techniques. Other host conditions including obstructive lung diseases like obliterative bronchiolitis may reduce the BALF return and impact diagnostic yield.164 Prophylaxis should be specified as it may lead to a predominance of trophozoite life-forms, which may alter diagnostic techniques.165 Empiric treatment with echinocandins can also lead to the presence of primarily trophozoite life-forms due to selective targeting of the beta glucan synthetase in the cystic life-form.1,8,166–169 The increasing use of echinocandins is another reason that life-form specific genetic markers would be helpful for detection of trophozoites. Additional research into the impact of these and other host factors on detection may improve diagnostic testing in patient subgroups.
Geographic variations in the organism
As Pneumocystis jirovecii has spread around the world, it has evolved. Genetic polymorphisms in the organism may result in certain molecular methods such as PCR or LAMP being less effective for detection in certain geographic subpopulations. The impact of genetic variation on molecular diagnosis has not been demonstrated in the literature to date. We recommend that future studies compare the sensitivity and specificity of molecular assays in different geographic populations. If low rates of detection are identified, genomic sequencing to identify polymorphisms may aid in identification of better molecular assays.
Notably, genetic polymorphisms can also impact therapeutic response. Studies have shown an association of treatment failure, high fungal burden, and severe cases of PCP with certain single-nucleotide polymorphisms/haplotypes in genes such as DHFR and DHPS, the enzymes targeted by trimethoprim-sulfamethoxazole and dapsone.170–177 As it is not feasible to routinely culture Pneumocystis jirovecii to identify therapeutic sensitivities, it may be valuable to incorporate methods of assessing for therapeutic response into molecular diagnostic panels in populations with high rates of resistance. Some commercial PCR platforms are already testing for the mtLSU gene along with DHPS and DHFR mutations.178 We recommend that future studies assess for impact of genotype on phenotype to identify clinically relevant genetic markers that may be useful to include in diagnostic testing.
Heterogeneity of studies
We recommend that manuscripts developing new diagnostic methods for PCP adhere to reporting of inclusion of sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio. These measures should be included for standardization of technique quality. Positive and negative predictive value should not be included unless the manuscripts explicitly state the prevalence of PCP in their population. In many populations, the prevalence of PCP is very low (<5%), so the likelihood that a negative test result represents a true negative test is high. In these populations, tests with only moderate sensitivity will still have high negative predictive values, which is misleading. In general, inclusion of prevalence rates would be very helpful for physicians to identify diagnostic techniques that apply to their patient population.
There are a wide range of definitions of PCP being utilized, which increases study heterogeneity and negatively impacts the ability to compare studies. Guideline-based definitions of proven PCP should be utilized such as the one provided by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group: detection of the organism in tissue, BALF, or sputum using conventional or immunofluorescent stains.179 New methods should always be compared to this gold standard.
Limited clinician recognition of PCP
Few studies have assessed knowledge of clinicians with regard to PCP diagnosis.180 The delays in PCP diagnosis in the non-HIV patient population are testaments to the fact that not enough providers are considering PCP as part of the differential diagnosis or performing appropriate diagnostic tests.17,31,36–41 While the diagnostic research going on in the field is excellent, it is unlikely to make a meaningful difference if clinicians do not include PCP in the differential diagnosis or know what diagnostic tools are most appropriate. Improving clinician awareness of PCP and these methods is vital as early diagnosis and treatment have been shown to lead to better patient outcomes.17,31,38–41,66,181,182
Conclusions
PCP is a diagnostic challenge, but there are a variety of promising new techniques that can increase our ability to detect the organisms in less invasive patient samples. While these new techniques face multiple barriers to incorporation, we believe that these barriers can be addressed by further identification of phenotypes that progress to PCP and diagnostic cut-offs for colonization, generation of life-form specific markers, comparison of commercial PCR assays, investigation of cost-effective point of care options for resource-poor environments, evaluation of host factors such as HIV status that may impact diagnosis, and identification of markers of genetic diversity that may be useful in diagnostic panels. Additionally, performance of high quality studies and education of physicians will be crucial to improve diagnostic methods for PCP and ultimately to improve patient outcomes.
Contributor Information
Marjorie Bateman, Center for Translational Research in Infection and Inflammation, Tulane University School of Medicine, New Orleans, LA 70122, USA.
Rita Oladele, Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Nigeria.
Jay K Kolls, Center for Translational Research in Infection and Inflammation, Tulane University School of Medicine, New Orleans, LA 70122, USA.
Funding
This work was supported in part by a NIH grant to JKK, R01-AI120033.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Thomas CF Jr., Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004; 350: 2487–2498. [DOI] [PubMed] [Google Scholar]
- 2. Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis. 2002; 8: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet North Am Ed. 2019; 394: 757–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovacs JA, Masur H.. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009; 301: 2578–2585. [DOI] [PubMed] [Google Scholar]
- 5. de Souza W, Benchimol M. Basic biology of Pneumocystis carinii: a mini review. Mem Inst Oswaldo Cruz. 2005; 100: 903–908. [DOI] [PubMed] [Google Scholar]
- 6. Martinez A, Aliouat el M, Standaert-Vitse A et al.. Ploidy of cell-sorted trophic and cystic forms of Pneumocystis carinii. PLoS One. 2011; 6: e20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma L, Cissé OH, Kovacs JA. A molecular window into the biology and epidemiology of Pneumocystis spp. Clin Microbiol Rev. 2018; 31: e00009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cushion MT, Linke MJ, Ashbaugh A et al.. Echinocandin treatment of pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS One. 2010; 5: e8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez A, Halliez MC, Aliouat el M et al.. Growth and airborne transmission of cell-sorted life cycle stages of Pneumocystis carinii. PLoS One. 2013; 8: e79958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Itatani CA, Marshall GJ. Ultrastructural morphology and staining characteristics of Pneumocystis carinii in situ and from bronchoalveolar lavage. J Parasitol. 1988; 74: 700–712. [PubMed] [Google Scholar]
- 11. Kohout E, Post C, Azadeh B et al.. Immunoglobulin levels in infantile pneumocystosis. J Clin Pathol. 1972; 25: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes WT, Price RA, Kim HK et al.. Pneumocystis carinii pneumonitis in children with malignancies. J Pediatr. 1973; 82: 404–415. [DOI] [PubMed] [Google Scholar]
- 13. Morris A, Lundgren JD, Masur H et al.. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004; 10: 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes WT, McNabb PC, Makres TD, Feldman S. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1974; 5: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teshale EH, Hanson DL, Wolfe MI et al.. Reasons for lack of appropriate receipt of primary Pneumocystis jirovecii pneumonia prophylaxis among HIV-infected persons receiving treatment in the United States: 1994–2003. Clin Infect Dis. 2007; 44: 879–883. [DOI] [PubMed] [Google Scholar]
- 16. Fei MW, Sant CA, Kim EJ et al.. Severity and outcomes of Pneumocystis pneumonia in patients newly diagnosed with HIV infection: an observational cohort study. Scand J Infect Dis. 2009; 41: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bienvenu AL, Traore K, Plekhanova I et al.. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016; 46: 11–17. [DOI] [PubMed] [Google Scholar]
- 18. Azoulay É, Bergeron A, Chevret S et al.. Polymerase chain reaction for diagnosing Pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009; 135: 655–661. [DOI] [PubMed] [Google Scholar]
- 19. Azoulay E, Bergeron A, Chevret S et al.. Polymerase chain reaction for diagnosing Pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009; 135: 655–661. [DOI] [PubMed] [Google Scholar]
- 20. Elsegeiny W, Eddens T, Chen K, Kolls JK. Anti-CD20 antibody therapy and susceptibility to Pneumocystis pneumonia. Infect Immun. 2015; 83: 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fillatre P, Decaux O, Jouneau S et al.. Incidence of Pneumocystis jirovecii pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014; 127: 1242.e11-1242.e17. [DOI] [PubMed] [Google Scholar]
- 22. Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J. 2016; 2016: 2464791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget. 2017; 8: 59729–59739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iriart X, Bouar ML, Kamar N, Berry A. Pneumocystis pneumonia in solid-organ transplant recipients. J Fungi. 2015; 1: 293–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iikuni N, Kitahama M, Ohta S et al.. Evaluation of Pneumocystis pneumonia infection risk factors in patients with connective tissue disease. Mod Rheumatol. 2006; 16: 282–288. [DOI] [PubMed] [Google Scholar]
- 26. Teichtahl AJ, Morrisroe K, Ciciriello S et al.. Pneumocystis jirovecci pneumonia in connective tissue diseases: comparison with other immunocompromised patients. Semin Arthritis Rheum. 2015; 45: 86–90. [DOI] [PubMed] [Google Scholar]
- 27. Wang EH, Partovi N, Levy RD et al.. Pneumocystis pneumonia in solid organ transplant recipients: not yet an infection of the past. Transpl Infect Dis. 2012; 14: 519–525. [DOI] [PubMed] [Google Scholar]
- 28. Fillatre P, Decaux O, Jouneau S et al.. Incidence of Pneumocystis jirovecii pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014; 127: 1242.e11-7. [DOI] [PubMed] [Google Scholar]
- 29. Roblot F, Godet C, Le Moal G et al.. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002; 21: 523–531. [DOI] [PubMed] [Google Scholar]
- 30. Kofteridis DP, Valachis A, Velegraki M et al.. Predisposing factors, clinical characteristics and outcome of Pneumonocystis jirovecii pneumonia in HIV-negative patients. J Infect Chemother. 2014; 20: 412–416. [DOI] [PubMed] [Google Scholar]
- 31. Roux A, Canet E, Valade S et al.. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. 2014; 20: 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su YS, Lu JJ, Perng CL, Chang FY. Pneumocystis jirovecii pneumonia in patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect. 2008; 41: 478–482. [PubMed] [Google Scholar]
- 33. Wickramasekaran RN, Jewell MP, Sorvillo F, Kuo T. The changing trends and profile of pneumocystosis mortality in the United States, 1999–2014. Mycoses. 2017; 60: 607–615. [DOI] [PubMed] [Google Scholar]
- 34. Limper AH, Offord KP, Smith TF, Martin WJ 2nd. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989; 140: 1204–1209. [DOI] [PubMed] [Google Scholar]
- 35. Oren I, Hardak E, Finkelstein R, Yigla M, Sprecher H. Polymerase chain reaction-based detection of Pneumocystis jirovecii in bronchoalveolar lavage fluid for the diagnosis of pneumocystis pneumonia. Am J Med Sci. 2011; 342: 182–185. [DOI] [PubMed] [Google Scholar]
- 36. Alanio A, Desoubeaux G, Sarfati C et al.. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect. 2011; 17: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 37. Louis M, Guitard J, Jodar M et al.. Impact of HIV infection status on interpretation of quantitative PCR for detection of Pneumocystis jirovecii. J Clin Microbiol. 2015; 53: 3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKinnell JA, Cannella AP, Kunz DF et al.. Pneumocystis pneumonia in hospitalized patients: a detailed examination of symptoms, management, and outcomes in human immunodeficiency virus (HIV)-infected and HIV-uninfected persons. Transpl Infect Dis. 2012; 14: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ainoda Y, Hirai Y, Fujita T, Isoda N, Totsuka K. Analysis of clinical features of non-HIV Pneumocystis jirovecii pneumonia. J Infect Chemother. 2012; 18: 722–728. [DOI] [PubMed] [Google Scholar]
- 40. Asai N, Motojima S, Ohkuni Y et al.. Early diagnosis and treatment are crucial for the survival of Pneumocystis pneumonia patients without human immunodeficiency virus infection. J Infect Chemother. 2012; 18: 898–905. [DOI] [PubMed] [Google Scholar]
- 41. Li M-C, Lee N-Y, Lee C-C et al.. Pneumocystis jirovecii pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J Microbiol Immunol Infect. 2014; 47: 42–47. [DOI] [PubMed] [Google Scholar]
- 42. Karstaedt AS, Grannum S. Pneumocystis carinii pneumonia in patients with AIDS in South Africa. Trans R Soc Trop Med Hyg. 2001; 95: 40–41. [DOI] [PubMed] [Google Scholar]
- 43. Kaplan JE, Masur H, Holmes KK. Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons — 2002: Recommendations of the US Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Rep. 2002; 51: 1–52. [PubMed] [Google Scholar]
- 44. Limper AH, Knox KS, Sarosi GA et al.. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011; 183: 96–128. [DOI] [PubMed] [Google Scholar]
- 45. Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014; 2014: Cd005590. [DOI] [PubMed] [Google Scholar]
- 46. Kaplan JE, Benson C, Holmes KK et al.. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009; 58: 1–207; quiz CE1-4. [PubMed] [Google Scholar]
- 47. Oladele RO, Otu AA, Richardson MD, Denning DW. Diagnosis and management of Pneumocystis pneumonia in resource-poor settings. J Health Care Poor Underserved. 2018; 29: 107–158. [DOI] [PubMed] [Google Scholar]
- 48. Limper AH. Diagnosis of Pneumocystis carinii pneumonia: does use of only bronchoalveolar lavage suffice? Mayo Clin Proc. 1996; 71: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 49. Tang F-F, Zhao X-S, Xu L-P et al.. Utility of flexible bronchoscopy with polymerase chain reaction in the diagnosis and management of pulmonary infiltrates in allogeneic HSCT patients. Clin Transplant. 2018; 32: e13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu Y, Ling G, Qiang C et al.. PCR diagnosis of Pneumocystis pneumonia: a bivariate meta-analysis. J Clin Microbiol. 2011; 49: 4361–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PLoS One. 2013; 8: e73099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Summah H, Zhu YG, Falagas ME, Vouloumanou EK, Qu JM. Use of real-time polymerase chain reaction for the diagnosis of Pneumocystis pneumonia in immunocompromised patients: a meta-analysis. Chin Med J. 2013; 126: 1965–1973. [PubMed] [Google Scholar]
- 53. McTaggart LR, Wengenack NL, Richardson SE. Validation of the MycAssay Pneumocystis kit for detection of Pneumocystis jirovecii in bronchoalveolar lavage specimens by comparison to a laboratory standard of direct immunofluorescence microscopy, real-time PCR, or conventional PCR. J Clin Microbiol. 2012; 50: 1856–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang L, Hecht FM, Stansell JD et al.. Suspected Pneumocystis carinii pneumonia with a negative induced sputum examination: is early bronchoscopy useful? Am J Respir Crit Care Med. 1995; 151: 1866–1871. [DOI] [PubMed] [Google Scholar]
- 55. Cruciani M, Marcati P, Malena M et al.. Meta-analysis of diagnostic procedures for Pneumocystis carinii pneumonia in HIV-1-infected patients. Eur Respir J. 2002; 20: 982–989. [DOI] [PubMed] [Google Scholar]
- 56. Rohner P, Jacomo V, Studer R, Schrenzel J, Graf JD. Detection of Pneumocystis jirovecii by two staining methods and two quantitative PCR assays. Infection. 2009; 37: 261–265. [DOI] [PubMed] [Google Scholar]
- 57. Wang Y, Doucette S, Qian Q, Kirby JE. Yield of primary and repeat induced sputum testing for Pneumocystis jirovecii in human immunodeficiency virus-positive and -negative patients. Arch Pathol Lab Med. 2007; 131: 1582–1584. [DOI] [PubMed] [Google Scholar]
- 58. Jiancheng W, Minjun H, Yi-jun A et al.. Screening Pneumocystis carinii pneumonia in non-HIV-infected immunocompromised patients using polymerase chain reaction. Diagn Microbiol Infect Dis. 2009; 64: 396–401. [DOI] [PubMed] [Google Scholar]
- 59. Caliendo AM, Hewitt PL, Allega JM et al.. Performance of a PCR assay for detection of Pneumocystis carinii from respiratory specimens. J Clin Microbiol. 1998; 36: 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cartwright CP, Nelson NA, Gill VJ. Development and evaluation of a rapid and simple procedure for detection of Pneumocystis carinii by PCR. J Clin Microbiol. 1994; 32: 1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pinlaor S, Mootsikapun P, Pinlaor P et al.. PCR diagnosis of Pneumocystis carinii on sputum and bronchoalveolar lavage samples in immuno-compromised patients. Parasitol Res. 2004; 94: 213–218. [DOI] [PubMed] [Google Scholar]
- 62. Revathy M, Therese KL, Bagyalakshmi R et al.. Application of real time polymerase chain reaction targeting kex 1 gene & its comparison with the conventional methods for rapid detection of Pneumocystis jirovecii in clinical specimens. Indian J Med Res. 2014; 140: 406–413. [PMC free article] [PubMed] [Google Scholar]
- 63. Pennington K, Wilson J, Limper AH, Escalante P. Positive Pneumocystis jirovecii sputum PCR results with negative bronchoscopic PCR results in suspected Pneumocystis pneumonia. Can Respir J. 2018; 2018: 6283935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matos O, Costa MC, Lundgren B et al.. Effect of oral washes on the diagnosis of Pneumocystis carinii pneumonia with a low parasite burden and on detection of organisms in subclinical infections. Eur J Clin Microbiol Infect Dis. 2001; 20: 573–575. [DOI] [PubMed] [Google Scholar]
- 65. Hviid CJ, Lund M, Sorensen A et al.. Detection of Pneumocystis jirovecii in oral wash from immunosuppressed patients as a diagnostic tool. PLoS One. 2017; 12: e0174012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Halsema C, Johnson L, Baxter J et al.. Short communication: Diagnosis of Pneumocystis jirovecii pneumonia by detection of DNA in blood and oropharyngeal wash, compared with sputum. AIDS Res Hum Retroviruses. 2016; 32: 463–466. [DOI] [PubMed] [Google Scholar]
- 67. Helweg-Larsen J, Jensen JS, Benfield T et al.. Diagnostic use of PCR for detection of Pneumocystis carinii in oral wash samples. J Clin Microbiol. 1998; 36: 2068–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fischer S, Gill VJ, Kovacs J et al.. The use of oral washes to diagnose Pneumocystis carinii pneumonia: a blinded prospective study using a polymerase chain reaction-based detection system. J INFECT DIS. 2001; 184: 1485–1488. [DOI] [PubMed] [Google Scholar]
- 69. Fraczek MG, Ahmad S, Richardson M et al.. Detection of Pneumocystis jirovecii by quantitative real-time PCR in oral rinses from Pneumocystis pneumonia asymptomatic human immunodeficiency virus patients. J Mycol Med. 2019; 29: 107–111. [DOI] [PubMed] [Google Scholar]
- 70. Juliano JJ, Barnett E, Parobek CM et al.. Use of oropharyngeal washes to diagnose and genotype Pneumocystis jirovecii. Open Forum Infect Dis. 2015; 2: ofv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Samuel CM, Whitelaw A, Corcoran C et al.. Improved detection of Pneumocystis jirovecii in upper and lower respiratory tract specimens from children with suspected Pneumocystis pneumonia using real-time PCR: a prospective study. BMC Infect Dis. 2011; 11: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morrow BM, Hsaio NY, Zampoli M, Whitelaw A, Zar HJ. Pneumocystis pneumonia in South African children with and without human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Pediatr Infect Dis J. 2010; 29: 535–539. [DOI] [PubMed] [Google Scholar]
- 73. Miyawaki H, Fujita J, Hojo S et al.. Detection of Pneumocystis carinii sequences in serum by polymerase chain reaction. Respir Med. 1996; 90: 153–157. [DOI] [PubMed] [Google Scholar]
- 74. Atzori C, Lu JJ, Jiang B et al.. Diagnosis of Pneumocystis carinii pneumonia in AIDS patients by using polymerase chain reactions on serum specimens. J Infect Dis. 1995; 172: 1623–1626. [DOI] [PubMed] [Google Scholar]
- 75. Schluger N, Godwin T, Sepkowitz K et al.. Application of DNA amplification to pneumocystosis: presence of serum Pneumocystis carinii DNA during human and experimentally induced Pneumocystis carinii pneumonia. J Exp Med. 1992; 176: 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang D, Hu Y, Li T, Rong HM, Tong ZH. Diagnosis of Pneumocystis jirovecii pneumonia with serum cell-free DNA in non-HIV-infected immunocompromised patients. Oncotarget. 2017; 8: 71946–71953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tamburrini E, Mencarini P, Visconti E et al.. Detection of Pneumocystis carinii DNA in blood by PCR is not of value for diagnosis of P. carinii pneumonia. J Clin Microbiol. 1996; 34: 1586–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lipschik GY, Gill VJ, Lundgren JD et al.. Improved diagnosis of Pneumocystis carinii infection by polymerase chain reaction on induced sputum and blood. Lancet North Am Ed. 1992; 340: 203–206. [DOI] [PubMed] [Google Scholar]
- 79. Rabodonirina M, Cotte L, Boibieux A et al.. Detection of Pneumocystis carinii DNA in blood specimens from human immunodeficiency virus-infected patients by nested PCR. J Clin Microbiol. 1999; 37: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ascioglu S, Rex JH, de Pauw B et al.. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002; 34: 7–14. [DOI] [PubMed] [Google Scholar]
- 81. De Pauw B, Walsh TJ, Donnelly JP et al.. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McMullan BJ, Halliday C, Sorrell TC et al.. Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS One. 2012; 7: e49541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dufresne SF, Datta K, Li X et al.. Detection of urinary excreted fungal galactomannan-like antigens for diagnosis of invasive aspergillosis. PLoS One. 2012; 7: e42736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jarvis JN, Percival A, Bauman S et al.. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011; 53: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lindsley MD, Mekha N, Baggett HC et al.. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis. 2011; 53: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Salonen J, Lehtonen OP, Terasjarvi MR, Nikoskelainen J. Aspergillus antigen in serum, urine and bronchoalveolar lavage specimens of neutropenic patients in relation to clinical outcome. Scand J Infect Dis. 2000; 32: 485–490. [DOI] [PubMed] [Google Scholar]
- 87. Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol. 2004; 42: 4873–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Robert-Gangneux F, Belaz S, Revest M et al.. Diagnosis of Pneumocystis jirovecii pneumonia in immunocompromised patients by real-time PCR: a 4-year prospective study. J Clin Microbiol. 2014; 52: 3370–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Procop GW, Haddad S, Quinn J et al.. Detection of Pneumocystis jirovecii in respiratory specimens by four staining methods. J Clin Microbiol. 2004; 42: 3333–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kaur R, Wadhwa A, Bhalla P, Dhakad MS. Pneumocystis pneumonia in HIV patients: a diagnostic challenge till date. Med Myco. 2015; 53: 587–592. [DOI] [PubMed] [Google Scholar]
- 91. Tiley SM, Marriott DJ, Harkness JL. An evaluation of four methods for the detection of Pneumocystis carinii in clinical specimens. Pathology. 1994; 26: 325–328. [DOI] [PubMed] [Google Scholar]
- 92. Raab SS, Cheville JC, Bottles K, Cohen MB. Utility of gomori methenamine silver stains in bronchoalveolar lavage specimens. Mod Pathol. 1994; 7: 599–604. [PubMed] [Google Scholar]
- 93. Mishra M, Thakar YS, Akulwar SL, Tankhiwale NS, Powar RM. Detection of Pneumocystis carinii in induced sputum samples of HIV positive patients. Indian J Med Microbiol. 2006; 24: 149–150. [DOI] [PubMed] [Google Scholar]
- 94. Stratton N, Hryniewicki J, Aarnaes SL et al.. Comparison of monoclonal antibody and calcofluor white stains for the detection of Pneumocystis carinii from respiratory specimens. J Clin Microbiol. 1991; 29: 645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nato F, Contini C, Zamora-Zavala C et al.. Production and characterization of monoclonal antibodies to human Pneumocystis carinii for the diagnosis of P. carinii pneumonia. Eur J Med. 1992; 1: 132–138. [PubMed] [Google Scholar]
- 96. Cregan P, Yamamoto A, Lum A et al.. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990; 28: 2432–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ng VL, Yajko DM, McPhaul LW et al.. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990; 28: 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Orholm M, Holten-Andersen W, Lundgren JD. Improved detection of Pneumocystis carinii by an immunofluorescence technique using monoclonal antibodies. Eu J Clin Microbiol Infect Dis. 1990; 9: 880–885. [DOI] [PubMed] [Google Scholar]
- 99. Gupta R, Mirdha BR, Guleria R et al.. Improved detection of Pneumocystis jirovecii infection in a tertiary care reference hospital in India. Scand J Infect Dis. 2007; 39: 571–576. [DOI] [PubMed] [Google Scholar]
- 100. Aderaye G, Woldeamanuel Y, Asrat D et al.. Evaluation of toluidine blue O staining for the diagnosis of Pneumocystis jirovecii in expectorated sputum sample and bronchoalveolar lavage from HIV-infected patients in a tertiary care referral center in Ethiopia. Infection. 2008; 36: 237–243. [DOI] [PubMed] [Google Scholar]
- 101. Alli OA, Ogbolu DO, Ademola O, Oyenike MA. Molecular detection of Pneumocystis jirovecii in patients with respiratory tract infections. North Am J Med Sci. 2012; 4: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Halford JA, Shield PW, Wright RG. The value of direct fluorescent antibody (DFA) testing for the detection of Pneumocystis carinii in cytological specimens. Cytopathology. 1994; 5: 234–242. [DOI] [PubMed] [Google Scholar]
- 103. Armbruster C, Pokieser L, Hassl A. Diagnosis of Pneumocystis carinii pneumonia by bronchoalveolar lavage in AIDS patients: comparison of Diff-Quik, fungifluor stain, direct immunofluorescence test and polymerase chain reaction. Acta Cytol. 1995; 39: 1089–1093. [PubMed] [Google Scholar]
- 104. Ng VL, Virani NA, Chaisson RE et al.. Rapid detection of Pneumocystis carinii using a direct fluorescent monoclonal antibody stain. J Clin Microbiol. 1990; 28: 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Midgley J, Parsons PA, Shanson DC, Husain OA, Francis N. Monoclonal immunofluorescence compared with silver stain for investigating Pneumocystis carinii pneumonia. J Clin Pathol. 1991; 44: 75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wazir JF, Macrorie SG, Coleman DV. Evaluation of the sensitivity, specificity, and predictive value of monoclonal antibody 3F6 for the detection of Pneumocystis carinii pneumonia in bronchoalveolar lavage specimens and induced sputum. Cytopathology. 1994; 5: 82–89. [DOI] [PubMed] [Google Scholar]
- 107. Hauser PM, Bille J, Lass-Florl C et al.. Multicenter, prospective clinical evaluation of respiratory samples from subjects at risk for Pneumocystis jirovecii infection by use of a commercial real-time PCR assay. J Clin Microbiol. 2011; 49: 1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Alvarez-Martinez MJ, Miro JM, Valls ME et al.. Sensitivity and specificity of nested and real-time PCR for the detection of Pneumocystis jirovecii in clinical specimens. Diagn Microbiol Infect Dis. 2006; 56: 153–160. [DOI] [PubMed] [Google Scholar]
- 109. Gits-Muselli M, White PL, Mengoli C et al.. The fungal PCR initiative's evaluation of in-house and commercial Pneumocystis jirovecii qPCR assays: toward a standard for a diagnostics assay. Med Mycol. 2019. doi: 10.1093/mmy/myz115. [DOI] [PubMed] [Google Scholar]
- 110. Gupta R, Mirdha B, Guleria R et al.. Improved detection of Pneumocystis jirovecii infection in a tertiary care reference hospital in India. Scand J Infect Dis. 2007; 39: 571–576. [DOI] [PubMed] [Google Scholar]
- 111. Fillaux J, Malvy S, Alvarez M et al.. Accuracy of a routine real-time PCR assay for the diagnosis of Pneumocystis jirovecii pneumonia. J Microbiol Methods. 2008; 75: 258–261. [DOI] [PubMed] [Google Scholar]
- 112. Flori P, Bellete B, Durand F et al.. Comparison between real-time PCR, conventional PCR and different staining techniques for diagnosing Pneumocystis jirovecii pneumonia from bronchoalveolar lavage specimens. J Med Microbiol. 2004; 53: 603–607. [DOI] [PubMed] [Google Scholar]
- 113. Kawano S, Maeda T, Suzuki T et al.. Loop-mediated isothermal amplification with the procedure for ultra rapid extraction kit for the diagnosis of Pneumocystis pneumonia. J Infect Chemother. 2015; 21: 224–226. [DOI] [PubMed] [Google Scholar]
- 114. Nakashima K, Aoshima M, Ohkuni Y et al.. Loop-mediated isothermal amplification method for diagnosing Pneumocystis pneumonia in HIV-uninfected immunocompromised patients with pulmonary infiltrates. J Infect Chemother. 2014; 20: 757–761. [DOI] [PubMed] [Google Scholar]
- 115. Uemura N, Makimura K, Onozaki M et al.. Development of a loop-mediated isothermal amplification method for diagnosing Pneumocystis pneumonia. J Med Microbiol. 2008; 57: 50–57. [DOI] [PubMed] [Google Scholar]
- 116. Singh P, Singh S, Mirdha BR et al.. Evaluation of loop-mediated isothermal amplification assay for the detection of Pneumocystis jirovecii in immunocompromised patients. Mol Biol Int. 2015; 2015: 819091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alvarez-Barrientos A, Arroyo J, Cantón R, Nombela C, Sánchez-Pérez M. Applications of flow cytometry to clinical microbiology. Clin Microbiol Rev. 2000; 13: 167–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Barbosa J, Bragada C, Costa-de-Oliveira S et al.. A new method for the detection of Pneumocystis jirovecii using flow cytometry. Eur J Clin Microbiol Infect Dis. 2010; 29: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 119. Barbosa J, Costa-de-Oliveira S, Silva AT, Rodrigues AG, Pina-Vaz C. Specific detection of Pneumocystis jirovecii in clinical samples by flow cytometry. In: O'Connor L, Glynn B, Fungal Diagnostics: Methods and Protocols. Totowa, NJ: Humana Press; 2013: 203–211. [DOI] [PubMed] [Google Scholar]
- 120. Tomás AL, Cardoso F, Esteves F, Matos O. Serological diagnosis of pneumocystosis: production of a synthetic recombinant antigen for immunodetection of Pneumocystis jirovecii. Sci Rep. 2016; 6: 36287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Daly KR, Koch J, Levin L, Walzer PD. Enzyme-linked immunosorbent assay and serologic responses to Pneumocystis jiroveci. Emerg Infect Dis. 2004; 10: 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zheng M, Cai Y, Eddens T, Ricks DM, Kolls JK. Novel pneumocystis antigen discovery using fungal surface proteomics. Infect Immun. 2014; 82: 2417–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Blount RJ, Jarlsberg LG, Daly KR et al.. Serologic responses to recombinant Pneumocystis jirovecii major surface glycoprotein among Ugandan patients with respiratory symptoms. PLoS One. 2012; 7: e51545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Elvin K, Bjorkman A, Heurlin N et al.. Seroreactivity to Pneumocystis carinii in patients with AIDS versus other immunosuppressed patients. Scand J Infect Dis. 1994; 26: 33–40. [DOI] [PubMed] [Google Scholar]
- 125. Crothers K, Daly KR, Rimland D et al.. Decreased serum antibody responses to recombinant pneumocystis antigens in HIV-infected and uninfected current smokers. Clin Vaccine Immunol. 2011; 18: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Peglow SL, Smulian AG, Linke MJ et al.. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis. 1990; 161: 296–306. [DOI] [PubMed] [Google Scholar]
- 127. Daly K, Koch J, Respaldiza N et al.. Geographical variation in serological responses to recombinant Pneumocystis jirovecii major surface glycoprotein antigens. Clin Microbiol Infect. 2009; 15: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Walzer PD, Djawe K, Levin L et al.. Long-term serologic responses to the Pneumocystis jirovecii major surface glycoprotein in HIV-positive individuals with and without P. jirovecii infection. J Infect Dis. 2009; 199: 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Daly KR, Koch JV, Shire NJ, Levin L, Walzer PD. Human immunodeficiency virus-infected patients with prior Pneumocystis pneumonia exhibit increased serologic reactivity to several major surface glycoprotein clones. CVI. 2006; 13: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tipirneni R, Daly KR, Jarlsberg LG et al.. Healthcare worker occupation and immune response to Pneumocystis jirovecii. Emerg Infect Dis. 2009; 15: 1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Onishi A, Sugiyama D, Kogata Y et al.. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jirovecii pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012; 50: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Karageorgopoulos DE, Qu JM, Korbila IP et al.. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013; 19: 39–49. [DOI] [PubMed] [Google Scholar]
- 133. Li WJ, Guo YL, Liu TJ, Wang K, Kong JL. Diagnosis of Pneumocystis pneumonia using serum (1-3)-beta-D-Glucan: a bivariate meta-analysis and systematic review. J Thoracic Dis. 2015; 7: 2214–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Alanio A, Hauser PM, Lagrou K et al.. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016; 71: 2386–2396. [DOI] [PubMed] [Google Scholar]
- 135. Tasaka S, Hasegawa N, Kobayashi S et al.. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest. 2007; 131: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 136. Passos AIM, Dertkigil RP, Ramos MC et al.. Serum markers as an aid in the diagnosis of pulmonary fungal infections in AIDS patients. Braz J Infect Dis. 2017; 21: 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Butt AA, Michaels S, Kissinger P. The association of serum lactate dehydrogenase level with selected opportunistic infections and HIV progression. Int J Infect Dis. 2002; 6: 178–181. [DOI] [PubMed] [Google Scholar]
- 138. Esteves F, Cale SS, Badura R et al.. Diagnosis of Pneumocystis pneumonia: evaluation of four serologic biomarkers. Clin Microbiol Infect. 2015; 21: 379.e1.e1-10. [DOI] [PubMed] [Google Scholar]
- 139. Esteves F, Lee CH, de Sousa B et al.. (1–3)-beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2014; 33: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 140. Vogel M, Weissgerber P, Goeppert B et al.. Accuracy of serum LDH elevation for the diagnosis of Pneumocystis jirovecii pneumonia. Swiss Med Wkly. 2011; 141: w13184. [DOI] [PubMed] [Google Scholar]
- 141. Nakamura H, Tateyama M, Tasato D et al.. Clinical utility of serum beta-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern Med. 2009; 48: 195–202. [DOI] [PubMed] [Google Scholar]
- 142. Merali S, Vargas D, Franklin M, Clarkson AB Jr. S-adenosylmethionine and Pneumocystis carinii. J Biol Chem. 2000; 275: 14958–14963. [DOI] [PubMed] [Google Scholar]
- 143. Skelly M, Hoffman J, Fabbri M et al.. S-adenosylmethionine concentrations in diagnosis of Pneumocystis carinii pneumonia. Lancet North Am Ed. 2003; 361: 1267–1268. [DOI] [PubMed] [Google Scholar]
- 144. Skelly MJ, Holzman RS, Merali S. S-adenosylmethionine levels in the diagnosis of Pneumocystis carinii pneumonia in patients with HIV infection. Clin Infect Dis. 2008; 46: 467–471. [DOI] [PubMed] [Google Scholar]
- 145. de Boer MG, Gelinck LB, van Zelst BD et al.. beta-D-glucan and S-adenosylmethionine serum levels for the diagnosis of Pneumocystis pneumonia in HIV-negative patients: a prospective study. J Infect. 2011; 62: 93–100. [DOI] [PubMed] [Google Scholar]
- 146. Alanio A, Bretagne S. Pneumocystis jirovecii detection in asymptomatic patients: what does its natural history tell us? F1000Res. 2017; 6: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012; 25: 297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Khodadadi H, Mirhendi H, Mohebali M et al.. Pneumocystis jirovecii colonization in non-HIV-infected patients based on nested-PCR detection in bronchoalveolar lavage smples. Iran J Public Health. 2013; 42: 298–305. [PMC free article] [PubMed] [Google Scholar]
- 149. Wang DD, Zheng MQ, Zhang N, An CL. Investigation of Pneumocystis jirovecii colonization in patients with chronic pulmonary diseases in the People's Republic of China. Int J Chron Obstruct Pulmon Dis. 2015; 10: 2079–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gutierrez S, Morilla R, Leon JA et al.. High prevalence of Pneumocystis jirovecii colonization among young HIV-infected patients. J Adolesc Health. 2011; 48: 103–105. [DOI] [PubMed] [Google Scholar]
- 151. Mori S, Cho I, Ichiyasu H, Sugimoto M. Asymptomatic carriage of Pneumocystis jirovecii in elderly patients with rheumatoid arthritis in Japan: a possible association between colonization and development of Pneumocystis jirovecii pneumonia during low-dose MTX therapy. Mod Rheumatol. 2008; 18: 240–246. [DOI] [PubMed] [Google Scholar]
- 152. Karstaedt AS, Grannum S. Pneumocystis carinii pneumonia in patients with AIDS in South Africa. Trans R Soc Trop Med Hyg. 2001; 95: 40–41. [DOI] [PubMed] [Google Scholar]
- 153. Fauchier T, Hasseine L, Gari-Toussaint M et al.. Detection of Pneumocystis jirovecii by quantitative PCR to differentiate colonization and pneumonia in immunocompromised HIV-positive and HIV-negative patients. J Clin Microbiol. 2016; 54: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Damiani C, Le Gal S, Goin N et al.. Usefulness of (1,3) ss-D-glucan detection in bronchoalveolar lavage samples in Pneumocystis pneumonia and Pneumocystis pulmonary colonization. J Mycol Med. 2015; 25: 36–43. [DOI] [PubMed] [Google Scholar]
- 155. Damiani C, Le Gal S, Da Costa C et al.. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1→3)-beta-D-glucan for differential diagnosis of Pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol. 2013; 51: 3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Matsumura Y, Ito Y, Iinuma Y et al.. Quantitative real-time PCR and the (1→3)-beta-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect. 2012; 18: 591–597. [DOI] [PubMed] [Google Scholar]
- 157. Hoarau G, Le Gal S, Zunic P et al.. Evaluation of quantitative FTD-Pneumocystis jirovecii kit for Pneumocystis infection diagnosis. Diagn Microbiol Infect Dis. 2017; 89: 212–217. [DOI] [PubMed] [Google Scholar]
- 158. Eddens T, Elsegeiny W, Ricks D et al.. Transcriptomic and proteomic approaches to finding novel diagnostic and immunogenic candidates in Pneumocystis. mSphere. 2019; 4: e00488–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cisse OH, Pagni M, Hauser PM. De novo assembly of the Pneumocystis jirovecii genome from a single bronchoalveolar lavage fluid specimen from a patient. mBio. 2012; 4: e00428–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cushion MT, Ashbaugh A, Hendrix K et al.. Gene expression of Pneumocystis murina after treatment with anidulafungin results in strong signals for sexual reproduction, cell wall integrity, and cell cycle arrest, indicating a requirement for ascus formation for proliferation. Antimicrob Agents Chemother. 2018; 62: e02513–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cooley L, Dendle C, Wolf J et al.. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014; 44: 1350–1363. [DOI] [PubMed] [Google Scholar]
- 162. Lackner M, Lass-Florl C. Commercial molecular tests for fungal diagnosis from a practical point of view. Methods Mol Biol. 2017; 1508: 85–105. [DOI] [PubMed] [Google Scholar]
- 163. Harris JR, Marston BJ, Sangrujee N, DuPlessis D, Park B. Cost-effectiveness analysis of diagnostic options for Pneumocystis pneumonia (PCP). PLoS One. 2011; 6: e23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Baughman RP. Technical aspects of bronchoalveolar lavage: recommendations for a standard procedure. Semin Respir Crit Care Med. 2007; 28: 475–485. [DOI] [PubMed] [Google Scholar]
- 165. Tamburrini E, Mencarini P, Visconti E et al.. Imbalance between Pneumocystis carinii cysts and trophozoites in bronchoalveolar lavage fluid from patients with pneumocystosis receiving prophylaxis. J Med Microbiol. 1996; 45: 146–148. [DOI] [PubMed] [Google Scholar]
- 166. Wyder MA, Johnston LQ, Kaneshiro ES. Evidence for DNA synthesis in Pneumocystis carinii trophozoites treated with the beta-1,3-glucan synthesis inhibitor pneumocandin L-693,989. J Eukaryot Microbiol. 2010; 57: 447–448. [DOI] [PubMed] [Google Scholar]
- 167. Huang YS, Liu CE, Lin SP et al.. Echinocandins as alternative treatment for HIV-infected patients with Pneumocystis pneumonia. AIDS. 2019; 33: 1345–1351. [DOI] [PubMed] [Google Scholar]
- 168. Sun P, Tong Z.. Efficacy of caspofungin, a 1,3-beta-D-glucan synthase inhibitor, on Pneumocystis carinii pneumonia in rats. Med Mycol. 2014; 52: 798–803. [DOI] [PubMed] [Google Scholar]
- 169. Powles MA, Liberator P, Anderson J et al.. Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother. 1998; 42: 1985–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Singh Y, Mirdha BR, Guleria R et al.. Genetic polymorphisms associated with treatment failure and mortality in pediatric Pneumocystosis. Sci Rep. 2019; 9: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Esteves F, de Sousa B, Calderon EJ et al.. Multicentre study highlighting clinical relevance of new high-throughput methodologies in molecular epidemiology of Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2016; 22: 566.e9 e9-566.e19. [DOI] [PubMed] [Google Scholar]
- 172. Ponce CA, Chabe M, George C et al.. High prevalence of Pneumocystis jirovecii dihydropteroate synthase gene mutations in patients with a first episode of Pneumocystis pneumonia in Santiago, Chile, and clinical response to trimethoprim-sulfamethoxazole therapy. Antimicrob Agents Chemother. 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Rabodonirina M, Vaillant L, Taffe P et al.. Pneumocystis jirovecii genotype associated with increased death rate of HIV-infected patients with pneumonia. Emerg Infect Dis. 2013; 19: 21–28; quiz 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Singh Y, Mirdha BR, Guleria R et al.. Circulating genotypes of Pneumocystis jirovecii and its clinical correlation in patients from a single tertiary center in India. Eur J Clin Microbiol Infect Dis. 2017; 36: 1635–1641. [DOI] [PubMed] [Google Scholar]
- 175. Esteves F, Gaspar J, De Sousa B et al.. Clinical relevance of multiple single-nucleotide polymorphisms in Pneumocystis jirovecii pneumonia: development of a multiplex PCR-single-base-extension methodology. J Clin Microbiol. 2011; 49: 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Esteves F, Gaspar J, Marques T et al.. Identification of relevant single-nucleotide polymorphisms in Pneumocystis jirovecii: relationship with clinical data. Clin Microbiol Infect. 2010; 16: 878–884. [DOI] [PubMed] [Google Scholar]
- 177. Valerio A, Tronconi E, Mazza F et al.. Genotyping of Pneumocystis jirovecii pneumonia in Italian AIDS patients: clinical outcome is influenced by dihydropteroate synthase and not by internal transcribed spacer genotype. J Acquir Immun Defic Syndr. 2007; 45: 521–528. [DOI] [PubMed] [Google Scholar]
- 178. Montesinos I, Delforge ML, Ajjaham F et al.. Evaluation of a new commercial real-time PCR assay for diagnosis of Pneumocystis jirovecii pneumonia and identification of dihydropteroate synthase (DHPS) mutations. Diagn Microbiol Infect Dis. 2017; 87: 32–36. [DOI] [PubMed] [Google Scholar]
- 179. Donnelly JP, Chen SC, Kauffman CA et al.. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Curtis JR, Paauw DS, Wenrich MD, Carline JD, Ramsey PG. Ability of primary care physicians to diagnose and manage Pneumocystis carinii pneumonia. J Gen Intern Med. 1995; 10: 395–399. [DOI] [PubMed] [Google Scholar]
- 181. Gracia JD, Miravitlles M, Mayordomo C et al.. Empiric treatments impair the diagnostic yield of BAL in HIV-positive patients. Chest. 1997; 111: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 182. Hardak E, Neuberger A, Yigla M et al.. Outcome of Pneumocystis jirovecii pneumonia diagnosed by polymerase chain reaction in patients without human immunodeficiency virus infection. Respirology. 2012; 17: 681–686. [DOI] [PubMed] [Google Scholar]