Graphical abstract
Keywords: COVID-19, IBD, Colitis, ICU, Gut microbiome, Probiotics
Highlights
-
•
Gut microbial diversity dysbiosis leads to increased death in COVID-19.
-
•
High death tool in COVID-19 is due to microbiome disturbance in co-morbid patients.
-
•
More deaths in ICU Individual seems to be because of microbiome dysbiosis.
-
•
Probiotics can be a Supplementary Therapy to decrease death tool in COVID-19.
Abstract
In December 2019, a pneumonia outbreak of unknown etiology was reported which caused panic in Wuhan city of central China, which was later identified as Coronavirus disease (COVID-19) caused by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the Chinese Centre for Disease Control and Prevention (CDC) and WHO. To date, the SARS-CoV-2 spread has already become a global pandemic with a considerable death toll. The associated symptoms of the COVID-19 infection varied with increased inflammation as an everyday pathological basis. Among various other symptoms such as fever, cough, lethargy, gastrointestinal (GI) symptoms included diarrhea and IBD with colitis, have been reported. Currently, there is no sole cure for COVID-19, and researchers are actively engaged to search out appropriate treatment and develop a vaccine for its prevention. Antiviral for controlling viral load and corticosteroid therapy for reducing inflammation seems to be inadequate to control the fatality rate. Based on the available related literature, which documented GI symptoms with diarrhea, inflammatory bowel diseases (IBD) with colitis, and increased deaths in the intensive care unit (ICU), conclude that dysbiosis occurs during SARS−COV-2 infection as the gut-lung axis cannot be ignored. As probiotics play a therapeutic role for GI, IBD, colitis, and even in viral infection. So, we assume that the inclusion of studies to investigate gut microbiome and subsequent therapies such as probiotics might help decrease the inflammatory response of viral pathogenesis and respiratory symptoms by strengthening the host immune system, amelioration of gut microbiome, and improvement of gut barrier function.
1. COVID-19 prelude
The recent ongoing global pandemic, COVID-19, caused by novel SARS-CoV-2, first appeared in Wuhan, Hubei province of China. In December 2019, the seafood and wet animal wholesale market were considered a hot zone of the epidemic eruption. It was soon identified as a highly contagious and infectious disease with an exponential increase in daily cases. COVID-19 mainly affects the respiratory system causing acute and severe respiratory infection manifesting pneumonia as a classic clinical characteristic [1]. SARS-CoV-2 infection's pathogenesis is based on the increased production of inflammatory cytokines, leading to lung injury, organ failure, and subsequent death [2]. In general, corticosteroid therapy is employed to control inflammatory diseases [3] and acute respiratory distress syndrome (ARDS) in clinical settings. However, it is either ineffective or avoided due to side effects in COVID-19. Therefore, it was not recommended by WHO [3,4]. Vaccine development takes a long way to be practically available for use in clinical settings [[5], [6], [7], [8], [9]]. Despite respiratory distress as a significant symptom of COVID-19, this viral disease is associated with some non-classical symptoms affecting other organs such as gastrointestinal infection (GIT) [10,11] and diarrhea [12] along with ulcerative colitis [13]. Fascinatingly, patients with GIT symptoms had more severe respiratory disorders [14,15], which might be associated with microbial dysbiosis, where a decline in beneficial microbes Lactobacillus and Bifidobacterium was found [16].
On the other hand, the richness of Clostridium hathewayi, Clostridium ramosum, and Coprobacillus were positively correlated while that of Faecalibacterium prausnitzii was inversely correlated with the severity of the disease [17]. Furthermore, gut dysbiosis and strains such as Clostridium hathewayi, Clostridium ramosum, and Coprobacillus directly link with diarrhea, colitis, IBD, inflammation, etc [[18], [19], [20]]. Also, the increased death prevalence in the COVID-19 ICU patients seems correlated with the increased, antibiotics use and ICU. associated gut microbial dysbiosis [21,22]. Therefore, the current scenario demands a detailed investigation of novel therapeutic approaches toward gut microbiome dysbiosis, boosting the gut microbiome associated immune system, decreasing related symptoms, and viral infection progression would be vital. Manipulating gut microbiota with probiotics as a supplementary therapy to enhance the immune system presents a brighter option with reduced side effects and promising effectiveness by revitalizing the own defence mechanism. However, probiotics' mechanism is scarce and requires extensive research for future acceptance and approval in the case of COVID-19 [5,23]. In this review, we are highlighting the possible mechanisms of actions to expand our understanding and knowledge of gut microbiome link with various symptoms of COVID-19 comorbidities for future research and possible use of probiotics as s supplementary therapeutic in COVID-19 prevention strategies.
2. Role of probiotics in different diseases
Probiotics are live microorganisms or components of dead bacteria that are safe and free of vectors, which, when administered in specific doses, can confer health benefits by inducing resistance against antibiotics, xenobiotics, and pathogenicity or toxicity factors by strengthening immunity through the route of the gut. Strains of diverse microbiota likewise, Bifidobacterium, Enterococcus, Lactobacillus, Saccharomyces boulardii, and Escherichia coli Nissle 1917, Lactococcus, Leuconostoc, Pediococcus, and Streptococcus are widely used as probiotics [24,25] for the amelioration of GIT associated disorders including acute, nosocomial and antibiotic-associated diarrhea, Clostridium difficile–associated diarrhea, inflammatory bowel disorders in adults, and allergic disorders like atopic dermatitis and allergic rhinitis in infants [26,27]. Interestingly, probiotics' role is not limited to the disorders mentioned above; instead, its functional importance is emerging. The researchers show more great interest in identifying the vast therapeutic benefits that probiotics can achieve for treating different diseases. There is a growing field of research towards the possible use of probiotics as co-adjuvants in treating metabolic disorders such as type 2 diabetes, obesity, metabolic syndrome, and non-alcoholic fatty liver disease. However, probiotics' mechanisms of action have not been studied well in different disease conditions and are still in infancy but hold great promise as probiotics' actions are diverse and heterogeneous [28].
Respiratory tract infections (RTI) are the most prevalent diseases globally, in children, elderly, and immunocompromised individuals. Various studies have revealed the use of probiotics in the treatment of RTI by decreasing the incidence and severity of the diseases [29]. Respiratory conditions including chronic lung disorder; asthma [30], bacterial infections; tuberculosis [31], chronic obstructive pulmonary disease (COPD), cystic fibrosis, and viral infection such as influenza are all associated with intestinal manifestations and alterations in the composition of gut microbiota, suggesting the correlation between lungs and gut [32]. The use of probiotics has been studied preclinically and is found to be beneficial in asthma models [33]. The administration of Lactobacillus casei or L. rhamnosus and Bifidobacterium Bifidum were found to decrease the viral titers and alleviate the symptoms in respiratory influenzas virus infection [32,34]. Similarly, nasal administration of Lactobacillus rhamnosus provided protection against respiratory syncytial virus infection in mice models [30]. Hence, probiotic treatment might have beneficial effects by reducing disease-induced inflammation and fortifying mucosal immunity, thereby controlling the spread of viral infections [32].
3. Probiotics-novel approach to boost immunity in viral infections
Considering the ineffectiveness or side effects of the available therapies in treating COVID-19, additional supplements with a proven record of amelioration of inflammation may be considered. One such therapy includes the supplementation of probiotics might be helpful as of its effectiveness in previous viral diseases such as in influenzas through boosting immunity [35,36]. In addition, probiotic ameliorate pathogenicity by modulating monocyte chemoattractant protein-1 (MCP-1), which is SARS-2 virus linked mediator and subsequent amelioration of inflammation [35,37]. Similarly, probiotics may act as a modulator of dysbiosis caused by viral infection [38,39], which seems to have promising effects in COVID-19 as well, especially in the attenuation of GIT symptoms. Probiotics may lessen the extent of disease severity by balancing the gut microbiome, which might have valuable outcomes due to its role in the gut–lung axis communication [[40], [41], [42]], and vitamin A regulation [43], which is directly linked with the immune system [44]. More recently, Barcik and his co-worker associated gut microbiota dysbiosis with dysregulation of microbiota-related immunological processes and subsequent onset of various respiratory disease [45].
Consequently, microbiome analysis must be included in clinics as a diagnostic test as previous viral infections have caused dysbiosis [46]. Probiotic use might be helpful in SARS-CoV-2 specifically as its effect on GIT, lungs [47], and kidneys have been documented, which has a strong association with microbiome [40,48]. Gut microbiome analysis and evaluation in COVID-19 individuals, as well as the addition of probiotics in various food items in patients’ daily diets, might be useful because of its proven record of anti-inflammatory and antiviral properties in multiple trials and studies [49,50], which some are listed (Sup Table 1).
4. Recent evidence of probiotics in COVID-19 - preclinical and clinical studies
Among various therapeutic options against COVID-19, probiotics were also proposed by the National Health Commission of China and the National Administration of Traditional Chinese Medicine [51,52]. Interestingly, many clinical studies using Traditional Chinese Medicine have been listed in China's clinical studies registry, but few with focusing the gut microbiome. Although the reinforcement of gut microbiota might not directly affect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the gut-lung cross-talk has been implicated in the pathogenesis of some respiratory diseases [53]. Around 58–71 % of patients suffering from COVID-19 were treated with antibiotics [52], of which 2–36 % of patients suffered from diarrhea, which might be associated with administrated antibiotics and subsequent dysbiosis [54]. This led to the suggestion that strengthening the colonic flora might ameliorate vulnerability to secondary infections, and antibiotics induced diarrhea can be managed using probiotics [55,56]. During the COVID-19 pandemic in China, studies showed that 2–47 % of critically ill patients require mechanical ventilation [57,58].
In contrast, a clinical trial concluded that the supplementary of probiotics treatment containing Lactobacillus rhamnosus GG, live Bacillus subtilis, and Enterococcus faecalis, developed significantly less ventilator-associated pneumonia compared with those without probiotic supplementation [59]. There is currently only very limited data available on the COVID-19 induced disturbance in gut microbiota and the possible probiotics role (Table 1 ). A small case study from a Chinese researcher reported that some COVID-19 patients suffered from microbial dysbiosis with decreased Lactobacillus and Bifidobacterium - the non-pathogenic bacteria yet, preliminary animal studies did not show any correlation between the presence of Lactobacillus acidophilus and Bacillus clausii with the reduction of expression of coronavirus receptor in the murine small intestine as compared with control and post-Salmonella infection models [16,60]. Therefore, irrational use of conventional probiotics should be disregarded in COVID-19 unless we further expand our understanding of SARS-CoV-2 pathogenesis and its effect on gut microbiota and then explore various probiotics' roles in combating COVID-19 with valid evidence. It is hoped that manipulating the gut microbiota might play fruitful roles as a therapeutic strategy of COVID-19 and its comorbidities [53]. However, the therapeutic strength of probiotics in lowering the risk of mortality in COVID-19 patients is uncertain due to limitation of available clinical data, and so there is a need of prompt studies for probiotic validation (Table 2 ).
Table 1.
Studies which focus on gut microbiome and probiotics in COVID-19.
| S.No | Study Title | Article Type | Refs. |
|---|---|---|---|
| 1 | Targeting the gut–lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection | Review | [157] |
| 2 | Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives | Review | [158] |
| 3 | Traditional Chinese medicine for COVID-19 treatment | Review | [159] |
| 4 | Gut microbiota and Covid-19- possible link and implications | Review | [160] |
| 5 | Probiotics and COVID-19 : one size does not fit all | Review | [161] |
| 6 | Potential Effects Immunomodulators on Probiotics in COVID-19 Preventing Infection in the Future. A Narrative Review | Review | [162] |
| 7 | The small intestine, an underestimated site of SARS-CoV-2 infection: from Red Queen effect to probiotics | Review | [60] |
| 8 | Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles | Review | [163] |
| 9 | Antiviral effects of probiotic metabolites on COVID-19 | Express Communication | [164] |
| 10 | Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic | Review | [165] |
| 11 | Nutrition Therapy in Critically Ill Patients With Coronavirus Disease 2019 | Review | [166] |
| 12 | Approach to Nutrition in Cancer Patients in the Context of the Coronavirus Disease 2019 (COVID-19) Pandemic: Perspectives | Review | [167] |
| 13 | Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review | Review | [168] |
| 14 | Dietary recommendations during the COVID-19 pandemic | Review | [169] |
| 15 | Can Probiotics and Diet Promote Beneficial Immune Modulation and Purine Control in Coronavirus Infection? | Review | [170] |
| 16 | Should Probiotics, honey, and escin be used in the prevention or treatment of COVID-19? | Review | [171] |
Table 2.
Registered Clinical Trials (https://clinicaltrials.gov/; https://clinicaltrials.gov/ct2/who_table) focusing on probiotics role in COVID-19.
| S.No | Clinical Trial Title | Clinical Trial No | Country | Trial Completion Status (Y/N) |
|---|---|---|---|---|
| 1 | Study to Evaluate the Effect of a Probiotic in COVID-19 | NCT04390477 | Spain | N |
| 2 | Evaluation of the Probiotic Lactobacillus Coryniformis K8 on COVID-19 Prevention in Healthcare Workers | NCT04366180 | Spain | N |
| 3 | Efficacy of Intranasal Probiotic Treatment to Reduce Severity of Symptoms in COVID19 Infection | NCT04458519 | Canada | N |
| 4 | Oxygen-Ozone as Adjuvant Treatment in Early Control of COVID-19 Progression and Modulation of the Gut Microbial Flora (PROBIOZOVID) | NCT04366089 | Italy | N |
| 5 | Reduction of COVID 19 Transmission to Health Care Professionals | NCT04462627 | Belgium | N |
| 6 | Synbiotic Therapy of Gastrointestinal Symptoms During Covid-19 Infection (SynCov) | NCT04420676 | Austria | N |
| 7 | Effect of Lactobacillus on the Microbiome of Household Contacts Exposed to COVID-19 | NCT04399252 | United States | N |
| 8 | Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19 | NCT04334980 | United States | N |
| 9 | A prospective, multicenter, open-label, randomized, parallel-controlled trial for probiotics to evaluate efficacy and safety in patients infected with 2019 novel coronavirus pneumonia (COVID-19) | ChiCTR2000029974 | China | N |
| 10 | A clinical study for probiotics in the regulation of intestinal function and microflora structure of novel coronavirus pneumonia (COVID-19) | ChiCTR2000029999 | China | N |
| 11 | Stress-reduction Using Probiotics to Promote Ongoing Resilience Throughout COVID-19 for Healthcare Workers (SUPPORT COVID-19 Healthcare Workers) | ACTRN12620000480987p | New Zealand | N |
| 12 | Application of Regulating Intestinal Flora in the Treatment of Severe Novel Coronavirus Pneumonia (COVID-19) | ChiCTR2000029849 | China | N |
| 13 | Bacteriotherapy in the Treatment of COVID-19 (BACT-ovid) | NCT04368351 | Italy | N |
| 14 | Clinical Trial to Evaluate the Efficacy of Food Supplement Manremyc® Against SARS- COV-2 Infection (COVID-19) in Healthcare Workers | NCT04452773 | Spain | N |
| 15 | A pilot, multiple dose study to evaluate the efficacy and safety of MRx-4DP0004 in hospitalised patients with symptoms of COVID-19 (SARS-CoV-2 infection) | eudract_number:2020-001597-30 | United Kingdom | N |
| 16 | Immunological efficacy of lactic acid bacteria for COVID-19 | R000046202 | Japan | N |
| 17 | Evaluation of the effect of taking Newgen beta-gluten probiotic composite powder to nutrition intervention of patients with novel coronavirus pneumonia (COVID-19) | ChiCTR2000030897 | China | N |
| 18 | Clinical trial for the washed microbiota transplantation in the treatment of novel coronavirus pneumonia (COVID-19) patients suspected with gut microbiota dysbiosis | ChiCTR2000032737 | China | N |
5. COVID-19 associated diarrhea and probiotic use
A recent study of H1N1 influenza A virus patient was found with GIT signs and symptoms such as diarrhea and abdominal pain, which demonstrated that they should be followed for GIT symptoms even though the disease is respiratory [61]. Similarly, 30 % of patients with the Middle East Respiratory Syndrome (MERS) and 10.6 % of SARS-Cov-1 patients have been found to suffer from diarrhea [62]. Likewise, SARS-CoV-2 has also been found to cause diarrhea [47,63,64]. In another study, 43.8 % of COVID-19 individuals were found with diarrhea like symptoms [65]. Current therapies such as corticosteroids; antivirals such as chloroquine and hydroxychloroquine, Lopinavir/Ritonavir, Remdesivir, Nelfinavir, Ribavirin nucleoside analog, plasma therapy, Tocilizumab antibody, Baricitinib, Nitazoxanide; immunomodulator such as interferons along with other drugs such as Arbidol, Favipiravir have been reviewed in detail in COVID-19 which in some way or the other have either side effects or ineffective or need further validation [66]. Therefore, to date, there is no sole treatment for COVID-19. Studies have indicated gut microbial dysbiosis in viral diseases and antiviral therapies [67,68] that might also be the case in COVID-19.
In most cases, precisely the GIT symptoms of diarrhea are closely associated with altering gut microbial diversity and alleviated with probiotics use as described previously [69,70]. However, the gut microbiome's role during COVID-19 infection and its improvement through probiotics and prebiotics have not been much focused [71]. Therefore, improving the gut microbial diversity with already available probiotics as an additive and supplementary treatment [72] might help combat SARS-CoV-2 associated diarrhea.
6. IBD in COVID-19 and possible probiotic role
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD), and ulcerative colitis (UC), is a condition in which the gastrointestinal immune system responds inappropriately due to various triggering factors, including gut microbial dysbiosis [73,74]. IBD is commonly managed with immunosuppressing medications to alleviate inflammation. It is known that over 1 million residents in the USA and 2.5 million in Europe are estimated to have IBD [75]. Only 44 % of them have been to a clinic in the past three years. During the current coronavirus pandemic, an increased incidence of IBD has been documented [76]. As anxiety in IBD patients is more common because of social distancing and isolation in COVID-19 as described previously [77], it may worsen patients' condition, which might not be underestimated. Conventionally, the immunosuppressant is used to control inflammation in COVID-19, might not be helpful in IBD individuals as well but the therapeutic in use still not reliable completely as well comorbid individual with IBD might be at more high risk in COVID-19 case; as corticosteroids have been found to worsen the condition of IBD patients suffering from COVID-19 infection [78]. As discussed previously, gut microbiome dysbiosis may occur during viral disease, increasing the incidence of IBD and ulcerative colitis [79]. Probiotics have a proven record of alleviating IBD and colitis by modulating gut microbiome and inflammation; therefore, it might help supplementary therapy in COVID-19 [[80], [81], [82]]. Probiotics may work through its metabolites such as organic acids, bacteriocins, peptides, and multiple critical targets in various metabolic pathways, including inflammation, and may alleviate IBD [83].
7. An integrated mucosal immunity - gut-lung cross talk and probiotic role
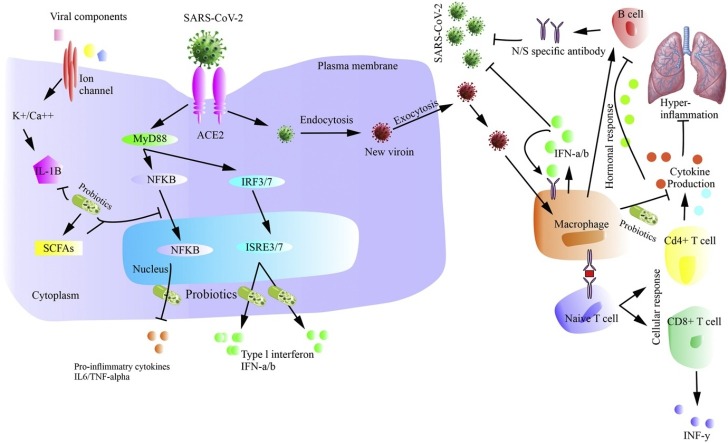
An essential concept of an integrated mucosal immunological system was developed in 1978, which suggested that our body's mucosal sites, including oral, respiratory, intestinal, and cervical, functioned as a unit and a system-wide organ to protect against pathogens. The idea of an integrated mucosal immunological system such as the lung-gut axis is supported by the fact that these two mucosal sites share the same embryonic origin and are similar structurally and functionally [84,85]. Still, the field is in its infancy, and it is required to understand the complexity and interactions of the mucosal immune system as an integrated global organ for future advances in understanding mucosal immunology and for future treatment of various chronic and inflammatory diseases alongside its association with gut [84,85]. As it is well known that a leaky gut due to dysbiosis may worsen the disease condition including lung disorder [86] as well as there might have more chances of infiltration of SARCoV-2 into the gut lumen and subsequently into the lymphatic system, which might cause infection in a secondary site and may increase the severity in COVID-19 [87]. Dysbiosis of the gut and lung microbiota occurs in various acute pulmonary diseases [[88], [89], [90], [91]]. Additionally, corticosteroids may induce dysbiosis as well [92], which are commonly used in COVID-19. More recently, Fanos and co-workers hypothesized that gut microbiota influences angiotensin-converting enzyme 2 (ACE2) at the intestinal and pulmonary level so that probiotics may overcome tackle the disease severity [92]. The addition of probiotics to improve gut microbiome and lung microbiome [85,92], seems promising to overcome disease severity in COVID-19 in terms of modulation of lung and gut microbiome. Furthermore, microbial metabolites, such as short-chain fatty acids (SCFAs) concentration, influence airways disease, have been previously documented. Higher fibers containing diet were found to enhance SCFA and subsequent protection from allergic inflammation [93]. The addition of dietary supplements might be helpful in lung inflammation in COVID-19.
8. Possible mechanism of action of Probiotics in COVID-19
Probiotics play their role in combating various diseases in terms of enhancing epithelial barrier function [94], as anti-inflammatory [95] improving gut microbial diversity [96], as the antagonist, for various harmful bacterial strains in the gut [97], blocking or enhancing multiple signalling pathways such as NF-kB [98,99] and production of metabolites such as SCFAs which can work in lessening various diseases severity [100]. As evidenced, the COVID-19 pathogenesis includes disruption of the epithelial barrier, inflammation, and dysbiosis, which can be alleviated using probiotics supplementation. Moreover, there is an increased production of inflammatory mediators such as IL-6, TNF-a, C-reactive protein, IL-1b, IL2, IL7, IL10, GCSF, IP10, MCP1, MIP1A, and LDH observed in COVID-19 which were found responsible for acute respiratory distress syndrome (ARDS), arrhythmia, and shock [2]. A range of available probiotics works to decrease inflammation by alleviating these inflammatory mediators [[101], [102], [103]], which seems to be effective in COVID-19. Besides, the role of macrophages in hyper inflammation during COVID-19 pathogenesis cannot be ignored [101], which can also be lessened through the use of probiotics to some extent [102,103]. It is expected that supplementation with appropriate probiotics may colonize the gut with the selected therapeutic bacterial strain, which can then spread to other parts of the body or extra-digestive sites and may enhance immunity against viral infections. It can be assumed that perhaps these probiotics microorganisms or their metabolites are engulfed directly by dendritic cells (DC) and macrophages through the intestinal lumen by penetrating into the intestinal epithelium, thereby transporting bacteria to other areas via blood stream; as in the case of COVID-19 may exhibit its affect in lungs [104,105]. Furthermore, COVID-19 comorbidities can be ameliorated through probiotics miRNA’s modulation, as well as regulation of signalling pathways such as NF-kb and STAT1 [82,106,107].
9. COVID-19 ICU patients and probiotics
During the COVID-19 pandemic, most of the patients were kept in ICU. There have reports of increased use of antibiotics [108,109] during their stay. In contrast, the interplay between the gut and lung microbiome has been reported in a recent study during ARDS [110]. Studies have demonstrated an increased incidence of gut microbiome dysbiosis during ICU, leading to sepsis and death [111]. This dysbiosis may lead to various other dysfunctions such as IBD, disruption of the immune system, and, eventually, organ dysfunction [112]. In the case of COVID-19, it is speculated that the increased ratio of organ dysfunction might be associated with ICU induced gut microbial diversity [113] due to increased use of antibiotics in COVID-19 ICU individuals [114,115]. A recent review discourages the use of antibiotics in COVID-19 unless it is needed [116]. Studies show that probiotics strains Lactobacillus rhamnosus GG and Bifidobacterium longum were promising in reducing infection in ICU patients [117,118]. We assumed that the incidence of microbiome disturbance and subsequent organ failure in COVID-19 could be decreased with supplementation of probiotics as additional therapy as described previously [97,113]. Furthermore, minimizing the administration of corticosteroids and antibiotics [120] might be helpful as they are associated with dysbiosis.
10. Presence of ACE2 in probiotics strains and possible role in COVID-19
The SARCoV-2 entry comes upon its binding to human angiotensin-converting enzyme type 2 (ACE2) receptor on the surface of alveolar epithelial cells and subsequent inflammation, leading to high fever and lung injury. The main target organs of COVID-19 infection are primarily the lungs; however, the intestine is another viral target organ. It has been found that the receptors for SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2) is highly expressed on differentiated enterocytes and cause infection in proliferative and mature enterocytes in the human small intestinal organoid (hSIO) model [119]. This role in viral pathogenies has made ACE2 a potential target in COVID-19 prevention and control [120]. Recently, inhibitors such as angiotensin-converting enzyme inhibitor (ACEI) and angiotensin II type-1 receptor blocker (ARB), have been proposed to effectively bind to the ACE2 receptor, neutralizing the virus and subsequent protection from lung injury [121,122].
Furthermore, the administration of the soluble form of ACE2 may also slow down the viral entry into cells and reduce the viral spread and protect the lungs from injury [123]. Studies demonstrate for oral delivery of human ACE2 using probiotic specie, Lactobacillus paracasei (LP), as a live vector for an effective treatment against Diabetes in the mouse model of diabetic nephropathy. It has been found that LP can efficiently express codon-optimized ACE2 and showed increased ACE2 activities in serum and tissues. These results suggest that engineered probiotic species can be employed as a live vector for human ACE2 with enhanced tissue bioavailability [124]. A similar approach can be tested for possible effective treatment and prevention of aggravation of COVID-19 infection. The newly identified bacterial derived B38-CAP enzyme has been found similar to that of human ACE2-like enzyme. Its protective effects are suppressing hypertension and cardiac dysfunction and subsequent lung injury [125]. Use of this bacterial strain with B38-CAP enzyme or identification of other bacterial strains with similar properties might play a beneficial role in controlling/decreasing the severity and pathogenesis of COVID-19 while working as an alternative ACE2 receptor for SARS-CoV2 instead of human tissue.
11. Probiotics bacteriocins role in COVID-19
Some lactobacilli and bifidobacteria produce bacteriocins, peptides composed of 30–60 cationic amino acids with antimicrobial properties targeting energized membrane vesicles to disrupt the proton-motive force. These molecules are effective against a wide range of intestinal bacterial pathogens and viruses, such as rotavirus [26]. The bacteriocins' activity produced by various probiotic microorganisms [126] might help and assist in the treatment against COVID-19 infection. Additionally, there is an abundance of various harmful bacterial strains reported where various probiotics strains may work as an antagonist and decrease disease severity [127]. Furthermore, Lactobacillus and Bifidobacterium toxins are useful in multiple other viral infections [128,129]. A recent study reported a dysbiosis of fungal microbiome in COVID-19 disease [131], which might be lessened with probiotics that produce antifungal toxins [130,131].
12. Probiotics mucin production and COVID-19
The mucus is an extracellular secretion by mucous membranes lining the body cavities, including respiratory, digestive, and urogenital tracts; as a physical protective barrier, mucins-glycoproteins are the significant component. The latest research has shown a strong induction of COVID-19 viral replication via enterocytes [119]. However, goblet cells were found less infected in the human small intestinal organoid (hSIO) model [119]. This phenomenon can be related to mucus and mucin production, indicating that increased activity of goblet cells with induced mucin production may prevent virus invasion through the gut into the immune system [132]. Hence, mucin production might play a trapping and inhibiting role in viral replication in the intestine and prevent further aggravation and viral infection progression in the body [26]. Previously, It was shown that intestinal mucins from mice were effective in inhibiting some strains of rotavirus [104,133].
Moreover, mucin biopolymers have emerged to acts as broad-spectrum antiviral agents [134]. Suggesting that goblet cells and mucins production enhancement play a role in the host defence against rotavirus infection and seems promising in COVID-19. Probiotics promoting mucin and mucus production in the gut appear to, directly and indirectly, enhance gut immunity to the whole body’s immunity [135]. Lactobacillus spp were reported to induce intestinal mucins and strengthening the mucus layer and glycocalyx over the intestinal epithelium thereby occupying the intestinal binding sites and inhibiting pathogenic adherence, preventing inflammation [136,137]. Moreover, probiotics are also known to alter qualitative properties of mucins preventing adherence of pathogens [138]. Strains with high adherence capacity have also shown to enhance the immunoglobulin A response to rotavirus [104]. Altogether this shows that enhancement of mucins production, primarily through supplementary probiotics therapy, might help SARS-CoV-2 infection.
13. Modulation of immune system through probiotics
The gut microbiota interacts with immune cells such as dendritic cells (DCs), monocytes/macrophages, and lymphocytes to release various immunomodulatory molecules to stimulate the innate and adaptive immune system. Viral infections are known to trigger innate immune responses via interactions between intestinal epithelial cells (IECs) and immune cells and activation of pattern recognition receptors (PRRs) such as toll-like receptor 3 (TLR3), leading to secretion of proinflammatory mediators and increased local tissue damage and immunopathology [139]. Immunoregulatory probiotic or immunobiotic such as lactic acid bacteria (LAB), when administered, may interact with the intestinal epithelial cells (IECs) or immune cells associated with the lamina propria through toll-like receptors and induce the production of different cytokines or chemokines [140] which sends signals to other immune cells leading to the activation of the mucosal immune system, characterized by an increase in immunoglobulin A+ (IgA) cells of the intestine, bronchus and mammary glands, and the activation of T cells. Probiotics specifically activate regulatory T-cells that release IL-10 and favour IgG production instead of IgE [44]. An imbalance of effector T-helper (Th) and regulatory T-cells (T-regs) leads to impaired immune response [8].
Similarly, probiotics are known to modulate innate and adaptive antiviral immunity of the mucosal and systemic immune systems [143], maintain intestinal homeostasis during viral infections, and improve the resistance to viral infections, normalize gut permeability, and increase the production of virus-specific antibodies [141]. In one of the randomized controlled trial, children suffering from mucosal diseases such as diarrhea and bronchitis were administered with the probiotic L. rhamnoses’ CRL1505 in a yogurt formulation, which was found to improve the mucosal immunity and reduced the incidence and severity of intestinal and respiratory infection in children [43]. Similarly, probiotics role in immune system stimulation, decreasing frequency of infection and increased production of antiviral antibodies have been demonstrated in various studies [42,[142], [143], [144], [145]]. Considering the available evidence and facts, probiotic bacteria are emerging as a safe and natural strategy for various disease prevention and treatment and it may prove itself fruitful for COVID-19 as well [145].
14. Aging associated gut microbiome dysbiosis, Covid-19 and probiotic role
Aging is a combination of various processes disturbing various physiological processes such as genomic, metabolism, and immunological functions [146,147]. Aging is associated with an increased incidence of multiple diseases such as IBD, Diabetes, alzheimer, cancer, cardiovascular diseases [147]. Studies demonstrated an increased incidence of dysbiosis in aged individuals compared to the youngsters [151]; which may lead to clinical issues including inflammation and gut-associated diseases by causing alterations in older people [148]. So, it seems wise to target the gut microbiome in age-linked comorbid COVID-19 patients through probiotics by stabilizing the gut microbiome [149]. Additionally, it is well-established that the extensive use of drugs/antibiotics is closely connected with a disturbance in the gut microbiome composition and functions [150]. It is speculated that extensive antibiotic use and age-associated gut microbiome disturbance might have synergistically contributed to the increased fatality rate in COVID-19 [151,152]. Studies in mice demonstrated the alleviation of age-linked dysbiosis through probiotics [153]. Furthermore, antibiotics and age-linked dysbiosis seem to have been lessened through probiotics treatments [154]. A recent study demonstrated that the ACE2 receptor is the critical factor in fewer outcomes in aged comorbid patients, where gut microbiome amelioration through probiotics seems to play its role in decreasing the fatality rate [155,156].
Conclusion
Most hospitalised individuals rely on corticosteroids, antibiotics, and antiviral for systematic inflammation and associated lung injury. These therapies' side effects cannot be ignored, including disruption of the gut microbiome and affecting individuals' well-being with related comorbidities such as GI symptoms. Besides, patients with severe disease conditions in ICU associate with increased dysbiosis, leading to an increased death rate. Also, the link of gut dysbiosis with lungs microbiome cannot be ignored. On the other hand, gut microbial dysbiosis is also linked with an increased incidence of IBD, colitis, which might have increased the death toll and severity of the disease. Additional, disease management with the inclusion of microbial diversity screening as a routine test in infected individual and subsequent therapies with either probiotic, dietary fiber, prebiotics, or other supplements as food additives for microbiome improvement as well as alleviating GI symptoms might be useful in lessening inflammation, enhancing immune response, improving gut barrier function as well as probiotics antagonist effect against harmful bacterial strains which were found recently in COVID-19 Individuals. To date, there are ongoing clinical trials, but there are no or limited clinical trials with concluding outcomes to pave the way for specific probiotics strain use in SARS-CoV-2 infection. There is a need for prompt studies and trials for probiotics use in COVID-19, or the previously approved probiotic strains can be. There is enough evidence to consider probiotics and therapies such as dietary interventions, symbiotic, prebiotics, metabolome modulation, and fecal microbiota transplant as additional and supplement treatment along with other therapies in use.
Author contribution
Ahmad Ud Din and Maryam Mazhar contributed equally and wrote the first Draft of the Manuscript. Jianbo Wu supervised and critically revised. While all other authors contributed to revising, editing various sections, and helping in the final draft of the manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China Grant (81570263), which was awarded to Jianbo Wu and Southwest Medical University (SWMU) research start-up Grant (42-00040149 awarded to Ahmad Ud Din.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.biopha.2020.110947.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Wuhan, M.H. Commission . Wuhan Municipal Health Commission; 2019. Report of Clustering Pneumonia of Unknown Etiology in Wuhan City. [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization W.H. World Health Organization; 2020. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance. 25 January 2020. [Google Scholar]
- 4.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19(10):667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 6.Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- 7.Kaslow D.C. Certainty of success: three critical parameters in coronavirus vaccine development. NPJ Vaccines. 2020;5(1):42. doi: 10.1038/s41541-020-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 9.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 10.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., Yu G.D., Xu K.J., Wang X.Y., Gu J.Q., Zhang S.Y., Ye C.Y., Jin C.L., Lu Y.F., Yu X., Yu X.P., Huang J.R., Xu K.L., Ni Q., Yu C.B., Zhu B., Li Y.T., Liu J., Zhao H., Zhang X., Yu L., Guo Y.Z., Su J.W., Tao J.J., Lang G.J., Wu X.X., Wu W.R., Qv T.T., Xiang D.R., Yi P., Shi D., Chen Y., Ren Y., Qiu Y.Q., Li L.J., Sheng J., Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norsa L., Indriolo A., Sansotta N., Cosimo P., Greco S., D’Antiga L. Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z., Zhang Q., Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 13.Mazza S., Sorce A., Peyvandi F., Vecchi M., Caprioli F. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020;69(6):1148–1149. doi: 10.1136/gutjnl-2020-321183. [DOI] [PubMed] [Google Scholar]
- 14.Gou W., Fu Y., Yue L., Chen G.-d., Cai X., Shuai M., Xu F., Yi X., Chen H., Zhu Y.J. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv. 2020 [Google Scholar]
- 15.Wan Y., Li J., Shen L., Zou Y., Hou L., Zhu L., Faden H.S., Tang Z., Shi M., Jiao N., Li Y., Cheng S., Huang Y., Wu D., Xu Z., Pan L., Zhu J., Yan G., Zhu R., Lan P. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020;5(6):534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., Li J., Wang H., Yu L., Huang H., Qiu Y., Wei G., Fang Q., Zhou J., Sheng J., Liang T., Li L. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., Lai C.K.C., Chen Z., Tso E.Y.K., Fung K.S.C., Chan V., Ling L., Joynt G., Hui D.S.C., Chan F.K.L., Chan P.K.S., Ng S.C. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzoor S.E., McNulty C.A.M., Nakiboneka-Ssenabulya D., Lecky D.M., Hardy K.J., Hawkey P.M. Investigation of community carriage rates of Clostridium difficile and Hungatella hathewayi in healthy volunteers from four regions of England. J. Hosp. Infect. 2017;97(2):153–155. doi: 10.1016/j.jhin.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Du Z., Hudcovic T., Mrazek J., Kozakova H., Srutkova D., Schwarzer M., Tlaskalova-Hogenova H., Kostovcik M., Kverka M. Development of gut inflammation in mice colonized with mucosa-associated bacteria from patients with ulcerative colitis. Gut Pathog. 2015;7(1):32. doi: 10.1186/s13099-015-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino K., Imaeda H., Sakai S., Ohno M., Nishida A., Andoh A. The abundance of Clostridium hathewayi, a potent inducer of t helper 17 (Th17) cells, is associated with the disease severity of Crohn’s disease. Gastroenterology. 2017;152(5):S993. [Google Scholar]
- 21.McDonald D., Ackermann G., Khailova L., Baird C., Heyland D., Kozar R., Lemieux M., Derenski K., King J., Vis-Kampen C., Knight R., Wischmeyer P.E. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1(4) doi: 10.1128/mSphere.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franz C., Kitsios G., Alexander S., Fair K., Morris A., Girard T., McVerry B. What’S New in Non-Pulmonary Critical Care? American Thoracic Society; 2020. Acute brain dysfunction, host inflammation, and gut dysbiosis during critical illness: a prospective cohort study in mechanically ventilated adults, D23. pp. A6323-A6323. [Google Scholar]
- 23.Paknahad Z., Moravejolahkami A.R. Probiotics against viruses; COVID-19 is a paper tiger: a Systematic Review. Endocr. Metab. Immune Disord. Drug Targets. 2020 doi: 10.2174/1871530320666200917114033. [DOI] [PubMed] [Google Scholar]
- 24.Fontana L., Bermudez-Brito M., Plaza-Diaz J., Munoz-Quezada S., Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013;109(Suppl 2):S35–50. doi: 10.1017/S0007114512004011. [DOI] [PubMed] [Google Scholar]
- 25.Gibson G.R., Probert H.M., Loo J.V., Rastall R.A., Roberfroid M.B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 2004;17(2):259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 26.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of action of probiotics. Adv. Nutr. 2019;10(suppl_1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouwehand A.C., Salminen S., Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82(1–4):279–289. [PubMed] [Google Scholar]
- 28.Hur K.Y., Lee M.S. Gut microbiota and metabolic disorders. Diabetes Metab. J. 2015;39(3):198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Li X., Ge T., Xiao Y., Liao Y., Cui Y., Zhang Y., Ho W., Yu G., Zhang T. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95(31):e4509. doi: 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crother T., Arditi M. The microbiome in asthma. Curr. Opin. Pediatr. 2016;28(6):764–771. doi: 10.1097/MOP.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervantes J., Hong B.-y. The gut–lung axis in tuberculosis. Pathog. Dis. 2017;75(8):ftx097. [Google Scholar]
- 32.Marsland B.J., Trompette A., Gollwitzer E.S. The gut–lung axis in respiratory disease. Ann. Am. Thorac. Soc. 2015;12(Supplement 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 33.Ly N.P., Litonjua A., Gold D.R., Celedón J.C. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J. Allergy Clin. Immunol. 2011;127(5):1087–1094. doi: 10.1016/j.jaci.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang G., Liu Z.Q., Yang P.C. Treatment of allergic rhinitis with probiotics: an alternative approach. N. Am. J. Med. Sci. 2013;5(8):465–468. doi: 10.4103/1947-2714.117299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung Y.J., Lee Y.T., Ngo V.L., Cho Y.H., Ko E.J., Hong S.M., Kim K.H., Jang J.H., Oh J.S., Park M.K., Kim C.H., Sun J., Kang S.M. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017;7(1):17360. doi: 10.1038/s41598-017-17487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libertucci J., Young V.B. The role of the microbiota in infectious diseases. Nat. Microbiol. 2019;4(1):35–45. doi: 10.1038/s41564-018-0278-4. [DOI] [PubMed] [Google Scholar]
- 37.Chen M.F., Weng K.F., Huang S.Y., Liu Y.C., Tseng S.N., Ojcius D.M., Shih S.R. Pretreatment with a heat-killed probiotic modulates monocyte chemoattractant protein-1 and reduces the pathogenicity of influenza and enterovirus 71 infections. Mucosal Immunol. 2017;10(1):215–227. doi: 10.1038/mi.2016.31. [DOI] [PubMed] [Google Scholar]
- 38.Mathew S., Smatti M.K., Al Ansari K., Nasrallah G.K., Al Thani A.A., Yassine H.M. Mixed viral-bacterial infections and their effects on gut microbiota and clinical illnesses in children. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-018-37162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling Z., Jin C., Xie T., Cheng Y., Li L., Wu N. Alterations in the fecal microbiota of patients with HIV-1 infection: an observational study in a Chinese population. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019;20(10):1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 41.Dickson R.P., Huffnagle G.B. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr. Pharm. Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grizotte-Lake M., Zhong G., Duncan K., Kirkwood J., Iyer N., Smolenski I., Isoherranen N., Vaishnava S. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate Interleukin-22 activity and prevent microbial dysbiosis. Immunity. 2018;49(6):1103–1115. doi: 10.1016/j.immuni.2018.11.018. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin a in the immune system. J. Clin. Med. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barcik W., Boutin R.C.T., Sokolowska M., Finlay B.B. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52(2):241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groves H.T., Cuthbertson L., James P., Moffatt M.F., Cox M.J., Tregoning J.S. Respiratory disease following viral lung infection alters the murine gut microbiota. Front. Immunol. 2018;9(182):182. doi: 10.3389/fimmu.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang T., Richards E.M., Pepine C.J., Raizada M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018;14(7):442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kechaou N., Chain F., Gratadoux J.-J., Blugeon S., Bertho N., Chevalier C., Le Goffic R., Courau S., Molimard P., Chatel J.M., Langella P., Bermúdez-Humarán L.G. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl. Environ. Microbiol. 2013;79(5):1491–1499. doi: 10.1128/AEM.03075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forsythe P. Probiotics and lung diseases. Chest. 2011;139(4):901–908. doi: 10.1378/chest.10-1861. [DOI] [PubMed] [Google Scholar]
- 51.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q., Han Y., Hu B., Hu F., Li B.H., Li Y.R., Liang K., Lin L.K., Luo L.S., Ma J., Ma L.L., Peng Z.Y., Pan Y.B., Pan Z.Y., Ren X.Q., Sun H.M., Wang Y., Wang Y., Weng H., Wei C.J., Wu D.F., Xia J., Xiong Y., Xu H.B., Yao X.M., Yuan Y.F., Ye T.S., Zhang X.C., Zhang Y.W., Zhang Y.G., Zhang H.M., Zhao Y., Zhao M.J., Zi H., Zeng X.T., Wang Y.Y., Wang X.H. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y. National Health Commission, National Administration of Traditional Chinese Medicine, Infectious Microbes Diseases; 2020. Translation: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) [Google Scholar]
- 53.Mak J.W., Chan F.K., Ng S.C. Probiotics and COVID-19: one size does not fit all. J The Lancet Gastroenterology. 2020 doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giannelli F.R. Antibiotic-associated diarrhea. JAAPA. 2017;30(10):46–47. doi: 10.1097/01.JAA.0000524721.01579.c9. [DOI] [PubMed] [Google Scholar]
- 55.Barbut F., Meynard J.L. Managing antibiotic associated diarrhoea. BMJ. 2002;324(7350):1345–1346. doi: 10.1136/bmj.324.7350.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Issa I., Moucari R. Probiotics for antibiotic-associated diarrhea: do we have a verdict? World J. Gastroenterol. 2014;20(47):17788–17795. doi: 10.3748/wjg.v20.i47.17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., C. China Medical Treatment Expert Group for Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng J., Wang C.T., Zhang F.S., Qi F., Wang S.F., Ma S., Wu T.J., Tian H., Tian Z.T., Zhang S.L., Qu Y., Liu L.Y., Li Y.Z., Cui S., Zhao H.L., Du Q.S., Ma Z., Li C.H., Li Y., Si M., Chu Y.F., Meng M., Ren H.S., Zhang J.C., Jiang J.J., Ding M., Wang Y.P. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42(6):1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- 60.Feng Z., Wang Y., Qi W. The small intestine, an underestimated site of SARS-CoV-2 infection: from red queen effect to probiotics. Preprints. 2020 [Google Scholar]
- 61.Kumar D., Gupta G., Jhamb U. Gastrointestinal symptoms among hospitalized children admitted with h1n1 infection: a report from a tertiary hospital in north india. Gut. 2019 [Google Scholar]
- 62.Habib A.M.G., Ali M.A.E., Zouaoui B.R., Taha M.A.H., Mohammed B.S., Saquib N. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. BMC Infect. Dis. 2019;19(1):870. doi: 10.1186/s12879-019-4555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song Y., Liu P., Shi X., Chu Y., Zhang J., Xia J., Gao X., Qu T., Wang M. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69(6):1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 64.D’Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin. Gastroenterol. Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y. Gastrointestinal tract symptoms in coronavirus disease 2019: analysis of clinical symptoms in adult patients. BMJ. 2020 2020.03.23.20040279. [Google Scholar]
- 66.Barlow A., Landolf K.M., Barlow B., Yeung S.Y.A., Heavner J.J., Claassen C.W., Heavner M.S. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40(5):416–437. doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flygel T.T., Sovershaeva E., Claassen-Weitz S., Hjerde E., Mwaikono K.S., Odland J.O., Ferrand R.A., McHugh G., Gutteberg T.J., Nicol M.P., Cavanagh J.P., Flaegstad T., Team B.S. Composition of gut microbiota of children and adolescents with perinatal human immunodeficiency virus infection taking antiretroviral therapy in Zimbabwe. J. Infect. Dis. 2020;221(3):483–492. doi: 10.1093/infdis/jiz473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuddenham S.A., Koay W.L.A., Zhao N., White J.R., Ghanem K.G., Sears C.L., H.I.V.M.R.-a. Consortium The impact of human immunodeficiency virus infection on gut microbiota alpha-diversity: an individual-level meta-analysis. Clin. Infect. Dis. 2020;70(4):615–627. doi: 10.1093/cid/ciz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menees S., Chey W. The gut microbiome and irritable bowel syndrome. Gastroentrology. 2018 doi: 10.12688/f1000research.14592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gareau M.G., Sherman P.M., Walker W.A. Probiotics and the gut microbiota in intestinal health and disease, Nature reviews. Gastroenterol. Hepatol. (N Y) 2010;7(9):503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalantar-Zadeh K., Ward S.A., Kalantar-Zadeh K., El-Omar E.M. Considering the effects of microbiome and diet on SARS-CoV-2 infection: nanotechnology roles. ACS Nano. 2020;14(5):5179–5182. doi: 10.1021/acsnano.0c03402. [DOI] [PubMed] [Google Scholar]
- 72.Lourens-Hattingh A., Viljoen B.C. Yogurt as probiotic carrier food. Int. Dairy J. 2001;11(1–2):1–17. [Google Scholar]
- 73.Sartor R.B., Wu G.D. Roles for intestinal Bacteria, viruses, and Fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152(2):327–339. doi: 10.1053/j.gastro.2016.10.012. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan G.G. The global burden of IBD: from 2015 to 2025, Nature reviews. Gastroenterol. Hepatol. (N Y) 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 76.King D.S., Trudgill N.J., Adderley N.J. Editorial: increasing IBD prevalence and its complications in the context of the COVID-19 pandemic. Authors’ reply, Aliment Pharmacol Ther. 2020;51(12):1442–1443. doi: 10.1111/apt.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An P., Ji M., Ren H., Su J., Kang J., Yin A., Zhou Q., Shen L., Zhao L., Jiang X. Protection of 318 inflammatory bowel disease patients from the outbreak and rapid spread of COVID-19 infection in Wuhan, China. Lancet. 2020 [Google Scholar]
- 78.Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., Ng S.C., Rahier J.F., Reinisch W., Ruemmele F.M., Steinwurz F., Underwood F.E., Zhang X., Colombel J.F., Kappelman M.D. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491. doi: 10.1053/j.gastro.2020.05.032. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manichanh C., Borruel N., Casellas F., Guarner F. The gut microbiota in IBD, Nature reviews. Gastroenterol. Hepatol. (N Y) 2012;9(10):599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 80.Rachmilewitz D., Katakura K., Karmeli F., Hayashi T., Reinus C., Rudensky B., Akira S., Takeda K., Lee J., Takabayashi K., Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 81.De Greef E., Vandenplas Y., Hauser B., Devreker T., Veereman-Wauters G. Probiotics and IBD. Acta Gastroenterol. Belg. 2013;76(1):15–19. [PubMed] [Google Scholar]
- 82.Din A.U., Hassan A., Zhu Y., Zhang K., Wang Y., Li T., Wang Y., Wang G. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J. Nutr. Biochem. 2020;79:108353. doi: 10.1016/j.jnutbio.2020.108353. [DOI] [PubMed] [Google Scholar]
- 83.Kumar M., Nagpal R., Verma V., Kumar A., Kaur N., Hemalatha R., Gautam S.K., Singh B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013;71(1):23–34. doi: 10.1111/j.1753-4887.2012.00542.x. [DOI] [PubMed] [Google Scholar]
- 84.Keely S., Talley N.J., Hansbro P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5(1):7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tulic M., Piche T., Verhasselt V. Lung–gut cross‐talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin. Exp. Allergy. 2016;46(4):519–528. doi: 10.1111/cea.12723. [DOI] [PubMed] [Google Scholar]
- 86.Anand S., Mande S.S. Diet, microbiota and gut-lung connection. Front. Microbiol. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.AKTAŞ B., Aslim B. Gut-lung axis and dysbiosis in COVID-19. Turk. J. Biol. 2020;44(SI-1):265–272. doi: 10.3906/biy-2005-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L., Hao K., Yang T., Wang C. Role of the lung microbiome in the pathogenesis of chronic obstructive pulmonary disease. Chin. Med. J. (Engl) 2017;130(17):2107–2111. doi: 10.4103/0366-6999.211452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoggard M., Biswas K., Zoing M., Wagner Mackenzie B., Taylor M.W., Douglas R.G. International Forum of Allergy & Rhinology. Wiley Online Library; 2017. Evidence of microbiota dysbiosis in chronic rhinosinusitis; pp. 230–239. [DOI] [PubMed] [Google Scholar]
- 90.Shukla S.D., Budden K.F., Neal R., Hansbro P.M. Microbiome effects on immunity, health and disease in the lung. Clin. Transl. Immunology. 2017;6(3):e133. doi: 10.1038/cti.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samuelson D.R., Welsh D.A., Shellito J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fanos V., Pintus M.C., Pintus R., Marcialis M.A. Lung microbiota in the acute respiratory disease: from coronavirus to metabolomics. Journal of Pediatric Neonatal Individualized Medicine. 2020;9(1) [Google Scholar]
- 93.Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L.P., Harris N.L., Marsland B.J. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 94.Ohland C.L., Macnaughton W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298(6):G807–19. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 95.Quigley E.M. Therapies aimed at the gut microbiota and inflammation: antibiotics, prebiotics, probiotics, synbiotics, anti-inflammatory therapies. Gastroenterol. Clin. North Am. 2011;40(1):207–222. doi: 10.1016/j.gtc.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Uronis J.M., Arthur J.C., Keku T., Fodor A., Carroll I.M., Cruz M.L., Appleyard C.B., Jobin C. Gut microbial diversity is reduced by the probiotic VSL# 3 and correlates with decreased TNBS-induced colitis. Inflamm. Bowel Dis. 2011;17(1):289–297. doi: 10.1002/ibd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wischmeyer P.E., McDonald D., Knight R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’in critical illness. Curr. Opin. Crit. Care. 2016;22(4):347. doi: 10.1097/MCC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murch S.H. Toll of allergy reduced by probiotics. Lancet. 2001;357(9262):1057–1059. doi: 10.1016/S0140-6736(00)04305-1. [DOI] [PubMed] [Google Scholar]
- 99.Jobin C. Probiotics and Iletis: could augmentation of TNF/NF-κB activity be the answer? Gut Microbes. 2010;1(3):196–199. doi: 10.4161/gmic.1.3.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al Kassaa I. New Insights on Antiviral Probiotics. Springer; 2017. The antiviral activity of probiotic metabolites; pp. 83–97. [Google Scholar]
- 101.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rocha-Ramírez L.M., Hernández-Ochoa B., Gómez-Manzo S., Marcial-Quino J., Cárdenas-Rodríguez N., Centeno-Leija S., García-Garibay M. Evaluation of immunomodulatory activities of the heat-killed probiotic strain Lactobacillus casei IMAU60214 on macrophages in vitro. Microorganisms. 2020;8(1):79. doi: 10.3390/microorganisms8010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aarti C., Martina C., Khusro A. Antimycobacterium, anticancer, and antiviral properties of probiotics: an overview. Microb. Infec. Dis. 2020 [Google Scholar]
- 104.Juntunen M., Kirjavainen P.V., Ouwehand A.C., Salminen S.J., Isolauri E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 2001;8(2):293–296. doi: 10.1128/CDLI.8.2.293-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martín Ro., Langa S., Reviriego C., Jiménez E., Marín Ma.L., Olivares M., Boza J., Jiménez J., Fernández L., Xaus J., Rodríguez J.M. The commensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics. Trends Food Sci. Technol. 2004;15(3–4):121–127. [Google Scholar]
- 106.Resta–Lenert S., Barrett K.E. Probiotics and commensals reverse TNF-α–and IFN-γ–induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130(3):731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 107.Din A.U., Hassan A., Zhu Y., Yin T., Gregersen H., Wang G. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl. Microbiol. Biotechnol. 2019;103(23–24):9217–9228. doi: 10.1007/s00253-019-10142-4. [DOI] [PubMed] [Google Scholar]
- 108.Magill S.S., Edwards J.R., Beldavs Z.G., Dumyati G., Janelle S.J., Kainer M.A., Lynfield R., Nadle J., Neuhauser M.M., Ray S.M. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438–1446. doi: 10.1001/jama.2014.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., Reinhart K., E.I.G.o. Investigators International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 110.Dickson R.P., Singer B.H., Newstead M.W., Falkowski N.R., Erb-Downward J.R., Standiford T.J., Huffnagle G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016;1(10):16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimizu K., Ogura H., Hamasaki T., Goto M., Tasaki O., Asahara T., Nomoto K., Morotomi M., Matsushima A., Kuwagata Y., Sugimoto H. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig. Dis. Sci. 2011;56(4):1171–1177. doi: 10.1007/s10620-010-1418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klingensmith N.J., Coopersmith C.M. The gut as the motor of multiple organ dysfunction in critical illness. Crit. Care Clin. 2016;32(2):203–212. doi: 10.1016/j.ccc.2015.11.004. https://pubmed.ncbi.nlm.nih.gov/27016162/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morrow L.E., Wischmeyer P. Blurred lines: dysbiosis and probiotics in the ICU. Chest. 2017;151(2):492–499. doi: 10.1016/j.chest.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Cox M.J., Loman N., Bogaert D., O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1(1):e11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J., Tang Y., Ma Y., Zhou Q., Li W., Baskota M., Yang Y., Wang X., Li Q., Luo X., Fukuoka T., Ahn H.S., Lee M.S., Luo Z., Liu E., Chen Y., Evidence C.-, Recommendations Working G. Efficacy and safety of antibiotic agents in children with COVID-19: a rapid review. Ann. Transl. Med. 2020;8(10):619. doi: 10.21037/atm-20-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khailova L., Petrie B., Baird C.H., Dominguez Rieg J.A., Wischmeyer P.E. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manzanares W., Lemieux M., Langlois P.L., Wischmeyer P.E. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19(1):262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., Paul van Schayck J., Mykytyn A.Z., Duimel H.Q., van Donselaar E., Riesebosch S., Kuijpers H.J.H., Schipper D., van de Wetering W.J., de Graaf M., Koopmans M., Cuppen E., Peters P.J., Haagmans B.L., Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi: 10.1126/science.abc1669. (New York, N.Y.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu M., Wang T., Zhou Y., Zhao Y., Zhang Y., Li J. Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management. J. Transl. Int. Med. 2020:9–19. doi: 10.2478/jtim-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020;43(7):648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun M.L., Yang J.M., Sun Y.P., Su G.H. Inhibitors of RAS might Be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E014. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. [DOI] [PubMed] [Google Scholar]
- 123.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. J. Inten. Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verma A., Xu K., Du T., Zhu P., Liang Z., Liao S., Zhang J., Raizada M.K., Grant M.B., Li Q. Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol. Ther. Methods Clin. Dev. 2019;14:161–170. doi: 10.1016/j.omtm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Minato T., Nirasawa S., Sato T., Yamaguchi T., Hoshizaki M., Inagaki T., Nakahara K., Yoshihashi T., Ozawa R., Yokota S. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat. Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-14867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dobson A., Cotter P.D., Ross R.P., Hill C. Bacteriocin production: a probiotic trait? Appl. Environ. Microbiol. 2012;78(1):1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Valdés-Varela L., Gueimonde M., Ruas-Madiedo P. Updates on Clostridium Difficile in Europe. Springer; 2018. Probiotics for prevention and treatment of clostridium difficile infection; pp. 161–176. [DOI] [PubMed] [Google Scholar]
- 128.Mastromarino P., Cacciotti F., Masci A., Mosca L. Antiviral activity of Lactobacillus brevis towards herpes simplex virus type 2: role of cell wall associated components. Anaerobe. 2011;17(6):334–336. doi: 10.1016/j.anaerobe.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 129.An H.M., Lee D.K., Kim J.R., Lee S.W., Cha M.K., Lee K.O., Ha N.J. Antiviral activity of Bifidobacterium adolescentis SPM 0214 against herpes simplex virus type 1. Arch. Pharm. Res. 2012;35(9):1665–1671. doi: 10.1007/s12272-012-0918-9. [DOI] [PubMed] [Google Scholar]
- 130.Tkhruni F.N., Aghajanyan A.E., Balabekyan T.R., Khachatryan T.V., Karapetyan K.J. Characteristic of bacteriocins of Lactobacillus rhamnosus BTK 20-12 potential probiotic strain. Probiotics Antimicrob. Proteins. 2020;12(2):716–724. doi: 10.1007/s12602-019-09569-y. [DOI] [PubMed] [Google Scholar]
- 131.Bartkiene E., Lele V., Ruzauskas M., Domig K.J., Starkute V., Zavistanaviciute P., Bartkevics V., Pugajeva I., Klupsaite D., Juodeikiene G. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms. 2020;8(1):64. doi: 10.3390/microorganisms8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J.J., Khan W.I. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2013;2(1):55–70. doi: 10.3390/pathogens2010055. Basel, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen C.C., Baylor M., Bass D.M. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105(1):84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 134.Lieleg O., Lieleg C., Bloom J., Buck C.B., Ribbeck K. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules. 2012;13(6):1724–1732. doi: 10.1021/bm3001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pickard J.M., Zeng M.Y., Caruso R., Nunez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moran A.P., Gupta A., Joshi L. Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut. 2011;60(10):1412–1425. doi: 10.1136/gut.2010.212704. [DOI] [PubMed] [Google Scholar]
- 137.Bermudez-Brito M., Plaza-Diaz J., Munoz-Quezada S., Gomez-Llorente C., Gil A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012;61(2):160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 138.Kim J., Khan W. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2013 doi: 10.3390/pathogens2010055. (Basel, Switzerland) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Villena J., Vizoso-Pinto M.G., Kitazawa H. Intestinal innate antiviral immunity and immunobiotics: beneficial effects against rotavirus infection. Front. Immunol. 2016;7:563. doi: 10.3389/fimmu.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Galdeano C.M., Cazorla S.I., Dumit J.M.L., Vélez E., Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019;74(2):115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- 141.Dongarra M.L., Rizzello V., Muccio L., Fries W., Cascio A., Bonaccorsi I., Ferlazzo G. Mucosal immunology and probiotics. Curr. Allergy Asthma Rep. 2013;13(1):19–26. doi: 10.1007/s11882-012-0313-0. [DOI] [PubMed] [Google Scholar]
- 142.Pagnini C., Saeed R., Bamias G., Arseneau K.O., Pizarro T.T., Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc. Natl. Acad. Sci. 2010;107(1):454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jiang T., Zhang H., Xu X., Li H., Yang J. Mixed probiotics decrease the incidence of stage II-III necrotizing enterocolitis and death: a systematic review and meta-analysis. Microb. Pathog. 2020;138 doi: 10.1016/j.micpath.2019.103794. [DOI] [PubMed] [Google Scholar]
- 144.Al Kassaa I. New Insights on Antiviral Probiotics. 2016. Antiviral probiotics: a new concept in medical sciences; pp. 1–46. [Google Scholar]
- 145.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab. Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kenyon C.J. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 147.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim S., Jazwinski S.M. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–520. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buford T.W. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80. doi: 10.1186/s40168-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Biagi E., Candela M., Fairweather-Tait S., Franceschi C., Brigidi P. Ageing of the human metaorganism: the microbial counterpart. Age. 2012;34(1):247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iaccarino G., Grassi G., Borghi C., Ferri C., Salvetti M., Volpe M., Investigators S.-R. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the italian society of hypertension. Hypertension. 2020;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 152.Hall R.E., Jones C.I., Klenow P.J. National Bureau of Economic Research; 2020. Trading Off Consumption and covid-19 Deaths. [Google Scholar]
- 153.Lee H.J., Lee K.E., Kim J.K., Kim D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019;9(1):11814. doi: 10.1038/s41598-019-48342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vemuri R.C., Gundamaraju R., Shinde T., Eri R. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: antibiotics, probiotics or faecal microbiota transplantation? Benef. Microbes. 2017;8(2):179–192. doi: 10.3920/BM2016.0115. [DOI] [PubMed] [Google Scholar]
- 155.Viana S.D., Nunes S., Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities–Role of gut microbiota dysbiosis. Ageing Res. Rev. 2020 doi: 10.1016/j.arr.2020.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sundararaman A., Ray M., Ravindra P.V., Halami P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020;104(19):8089–8104. doi: 10.1007/s00253-020-10832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Conte L., Toraldo D.M. Targeting the gut–lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shinde T., Hansbro P.M., Sohal S.S., Dingle P., Eri R., Stanley R. Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms. 2020;8(6):921. doi: 10.3390/microorganisms8060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ren J.-l., Zhang A.-H., Wang X.-J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dhar D., Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mak J.W., Chan F.K., Ng S.C. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adnan M.L., Dewi M.D. Potential effects immunomodulators on probiotics in COVID-19 preventing infection in the future. A narrative review. Int. J. Med. Stud. 2020 [Google Scholar]
- 163.Kalantar-Zadeh K., Ward S.A., Kalantar-Zadeh K., El-Omar E.M. Considering the effects of microbiome and diet on SARS-CoV-2 infection: nanotechnology roles. ACS Nano. 2020 doi: 10.1021/acsnano.0c03402. [DOI] [PubMed] [Google Scholar]
- 164.Anwar F., Altayb H.N., Al-Abbasi F.A., Al-Malki A.L., Kamal M.A., Kumar V. Antiviral effects of probiotic metabolites on COVID-19. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1775123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baud D., Dimopoulou Agri V., Gibson G.R., Reid G., Giannoni E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front. Public Health. 2020;8:186. doi: 10.3389/fpubh.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition therapy in critically ill patients with coronavirus disease 2019. J. Parenter. Enter. Nutr. 2020;44(7):1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garofolo A., Qiao L., Maia-Lemos P.D.S. Approach to nutrition in Cancer patients in the context of the coronavirus disease 2019 (COVID-19) pandemic: perspectives. Nutr. Cancer. 2020:1–9. doi: 10.1080/01635581.2020.1797126. [DOI] [PubMed] [Google Scholar]
- 168.Infusino F., Marazzato M., Mancone M., Fedele F., Mastroianni C.M., Severino P., Ceccarelli G., Santinelli L., Cavarretta E., Marullo A.G.M., Miraldi F., Carnevale R., Nocella C., Biondi-Zoccai G., Pagnini C., Schiavon S., Pugliese F., Frati G., d’Ettorre G. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: a scoping review. Nutrients. 2020;12(6):1718. doi: 10.3390/nu12061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Faria Coelho-Ravagnani C., Corgosinho F.C., Sanches F.F.Z., Prado C.M.M., Laviano A., Mota J.F. Dietary recommendations during the COVID-19 pandemic. Nutr. Rev. 2020 doi: 10.1093/nutrit/nuaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morais A.H.A., Passos T.S., Maciel B.L.L., da Silva-Maia J.K. Can probiotics and diet promote beneficial immune modulation and purine control in coronavirus infection? Nutrients. 2020;12(6):1737. doi: 10.3390/nu12061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Joson M.V.A.S.G., Flores-Genuino R.N.S. 2020. Should Probiotics, Honey, and Escin Be Used in the Prevention or Treatment of COVID-19? [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.