Abstract
Prostate cancer cells include a small population of cancer stem‐like cells (CSCs)/cancer‐initiating cells (CICs) that have roles in initiation and progression of the cancer. Recently, we isolated prostate CSCs/CICs as aldehyde dehydrogenase 1‐highh (ALDH1high) cells using the ALDEFLUOR assay; however, the molecular mechanisms of prostate CSCs/CICs are still elusive. Prostate CSCs/CICs were isolated as ALDH1high cells using the ALDEFLUOR assay, and the gene expression profiles were analyzed using a cDNA microarray and RT‐PCR. We found that prostate CSCs/CICs expressed higher levels of growth factors including hepatocyte growth factor (HGF). Hepatocyte growth factor protein expression was confirmed by enzyme linked immunosorbent assay and Western blotting. On the other hand, c‐MET HGF receptor was expressed in both CSCs/CICs and non‐CSCs/CICs at similar levels. Hepatocyte growth factor and the supernatant of myofibloblasts derived from the prostate augmented prostasphere formation in vitro, and prostasphere formation was inhibited by an anti‐HGF antibody. Furthermore, c‐MET gene knockdown by siRNA inhibited the prostasphere‐forming ability in vitro and tumor‐initiating ability in vivo. Taken together, the results indicate that HGF secreted by prostate CSCs/CICs and prostate myofibroblasts has a role in the maintenance of prostate CSCs/CICs in an autocrine and paracrine fashion.
Prostate cancer is one of the common and lethal cancers in males. Although there are some curative treatments for early‐stage prostate cancer, there is no effective treatment for advanced metastatic prostate cancer. Cancer stem‐like cells (CSCs)/cancer‐initiating cells (CICs) have high tumor‐initiating ability and are resistant to chemotherapy and radiotherapy, and CSCs/CICs are therefore thought to be responsible for cancer recurrence after treatment and for distant metastasis.1, 2
Prostate cancer contains a small population of CSCs/CICs, and we previously described the successful isolation of prostate CSCs/CICs from the human prostate carcinoma cell line 22Rv1 by using aldehyde dehydrogenase (ALDH) activity.3 In addition, we isolated prostate CSC/CIC‐specific genes, including HGF and IGF1, by microarray screening and RT‐PCR analysis. In a physiological condition, hepatocyte growth factor (HGF) is secreted by mesenchymal cells and promotes epithelial cell growth in a paracrine fashion. However, signaling of HGF and its receptor c‐MET is activated in cancer cells and is related to cancer cell growth, cell motility and matrix invasion, and HGF/c‐MET signaling can therefore be a reasonable target of cancer therapy.4 In this study, we confirmed HGF protein secretion from prostate CSCs/CICs, and we investigated the HGF/c‐MET signaling function in maintenance of prostate CSCs/CICs.
Materials and Methods
Cell culture and ALDEFLUOR assay
The human prostate cancer cell line 22Rv1 was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), and the cells were cultured in DMEM (Life Technologies, Grand Island, NY, USA) supplemented with 10% FCS. Prostate myofibroblast cells (WPMY‐1) were purchased from ATCC and were cultured in DMEM supplemented with 5% of FCS. The cells were kept in a 37°C incubator with humidified air and 5% CO2. The culture medium was changed twice a week. ALDEFLUOR assay was performed as described previously.3 ALDH1high and ALDH1low cells were defined as those in the previous report.3
RNA preparation and reverse transcription‐polymerase chain reaction
Isolation of total RNA and RT‐PCR analysis were performed as described previously.3 Primer pairs used for RT‐PCR analysis were 5′‐GGGCTGAAAAGATTGGATCA‐3′ and 5′‐TTGTATTGGTGGGTGCTTCA‐3′ for HGF with an expected PCR product size of 245 base pairs (bp), 5′‐CAGGCAGTGCAGCATGTAGT‐3′ and 5′‐GATGATTCCCTCGGTCAGAA‐3′ for MET (hepatocyte growth factor receptor) with an expected PCR product size of 201 bp, and 5′‐ACCACAGTCCATGCCATCAC‐3′ and 5′‐TCCACCACCCTGTTGCTGTA‐3′ for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) with an expected product size of 452 bp.
Enzyme‐linked immunosorbent assay
Sorted ALDH1high and ALDH1low cells (10 000 cells each) were incubated in 150 μL DMEM + 10% FCS media in each well of a 96‐well plate for 48 h. Enzyme linked immunosorbent assay was performed for detection of HGF in each cell culture supernatant (HGF Human ELISA Kit, Abcam, Cambridge, UK). The absorbance was measured at 450 nm. Data were obtained from seven independent samples, and the data were statistically analyzed.
Western blotting and immunohistochemical staining
Western blotting was performed as described previously.5 Briefly, 5 × 104 of ALDH1high cells and ALDH1low cells were lysed in 100 μL of SDS sample buffer. Anti‐ALDH1 mouse monoclonal antibody (clone: 44/ALDH; BD Biosciences, San Jose, CA, USA) was used at 1000‐times dilution. Anti‐HGF goat polyclonal antibody (R&D Systems, Minneapolis, MN, USA) and anti‐MET goat polyclonal antibody (R&D Systems) were used at 1000‐times dilution. Anti‐β‐Actin mouse monoclonal antibody (Sigma, St. Louis, MO, USA) was used at 2000‐times dilution. Anti‐mouse IgG + IgM and anti‐goat IgG and IgM second antibodies (KPL) were uses at 2000‐times dilution. The membrane was visualized with Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA) according to the manufacturer's protocol, and pictures were taken by Odyssey Fc Imaging System (LI‐COR, Lincoln, NE, USA).
Immunohistochemical staining using formalin‐fixed paraffin‐embedded sections of surgically resected prostate carcinoma was performed as described previously.6 Anti‐ALDH1 mouse antibody was used at 250‐times dilution. Anti‐HGF mouse monoclonal antibody (Abcam) was used at 10 μg/mL. Anti‐SOX2 rabbit polyclonal antibody (Invitrogen, Palo Alto, CA, USA) was used at 250‐times dilution. Peroxidase‐labeled goat anti‐rabbit polyclonal antibody (Nichirei, Tokyo, Japan) was used as manufacturer's protocol and visualized by 3,3′‐diaminobenzidine tetrachloride (DAB). Alkaline phosphatase‐labeled goat anti‐mouse polyclonal antibody (Nichirei) was used according to the manufacturer's protocol and visualized by New Fuchsin (Nichirei). Nucleus brown staining was judged as positive staining for SOX2, and cytoplasm red staining was judged as positive staining for ALDH1 and HGF.
Prostasphere formation assay
Isolated ALDH1high and ALDH1low cells were seeded into an Ultra‐Low Attachment Surface culture six‐well plate (CORNING, Tewksbury, MA, USA) at a concentration of 8 × 103/well and cultured in DMEM/F12 medium (Invitrogen, San Diego, CA, USA) supplemented with 20 ng/mL of epidermal growth factor (EGF; Sigma‐Aldrich, St. Louis, MO, USA), 10 ng/mL of basic fibroblast growth factor (bFGF; Sigma‐Aldrich), 10 ng/mL of HGF (Sigma‐Aldrich) and 10 ng/mL IGF‐1 (Sigma‐Aldrich). The morphology of the cells was assayed and pictures were taken under a light microscope every day. For inhibition of HGF, anti‐HGF antibody was supplemented at a concentration of 20 ng/mL. Anti‐HLA class I antibody (clone: W6/32) was used as a negative control. Prostate myofibroblast culture supernatant was obtained from myofibroblasts cultured in serum‐free DMEM media for 2 days. The myofibroblast supernatant was supplemented in a sphere culture condition at 50% (v/v).
MET mRNA knockdown by siRNA
A MET gene knockdown experiment was performed using small interfering RNA (siRNA). MET siRNA duplex was obtained from Validated Stealth RNAi siRNA systems (Oligo ID; HSS106479; Invitrogen). Negative control siRNA was obtained from Invitrogen. 22Rv1 cells were seeded into a 24‐well plate, and transfections were carried out using Lipofectamine RNAi max (Invitrogen) in Opti‐MEM according to the manufacturer's instructions.
Analysis of cell growth
Negative control cells and MET knocked‐down 22Rv1 cells were each seeded into a six‐well plate at 5 × 104 cells per well. After incubation for 24, 48 and 72 h, the cells were removed by trypsin and viable cell numbers were determined using Countess (Life Technologies).
Xenograft transplantation into NOD/SCID mice
Experiments using animals were done in accordance with the institutional guidelines for the use of laboratory animals. Bulk and MET knocked‐down cells were re‐suspended at concentrations of 1 × 103 cells in 100 μL of PBS and Matrigel (BD Biosciences) mixture (1:1). The cells were injected subcutaneously into the right and left mid‐back areas of anesthetized 6‐week‐old male non‐obese diabetic/severe combined immunodeficiency (NOD/SCID) mice. The progression of cancer cell growth was monitored weekly, and mice underwent autopsy at 50 days after cell injection.
Statistical analysis
Data are presented as means ± SD from at least three independent experiments. Statistical analysis of the data was performed by Student's t‐test. P‐values of ≤0.05 were considered statistically significant.
Results
Preferential growth factor gene expressions in prostate cancer stem cells
We previously reported the successful isolation of prostate CSCs/CICs from the prostate carcinoma cell line 22Rv1 by the ALDEFLUOR assay as ALDHhigh cells, and we found that more than 200 genes were overexpressed in prostate CSCs/CICs compared with expression levels in non‐CSCs/CICs.3 The overexpressed genes included the growth factors IGF1 and HGF. Activation of c‐MET, the receptor for HGF, is related to a stem‐like phenotype in prostate cancer cells,7 and we therefore further analyzed the function of HGF in this study. Upregulation of the expression of IGF1 and HGF was confirmed by RT‐PCR analysis, whereas the expression levels of MET showed no significant difference in ALDHhigh and ALDHlow cells (Fig. 1A). Hepatocyte growth factor protein expression level was higher in ALDH1high cells than in ALDH1low cells, whereas MET protein expression level were similar in ALDH1high cells and ALDH1low cells (Fig. 1B). Stem cell markers including SOX2, POU5F1 and NANOG were described to be overexpressed in prostate CSCs/CICs.8 To address expressions of ALDH1 and HGF in stem cells of prostate cancer tissue, we performed double immunohistochemical staining using anti‐ALDH1 antibody, anti‐HGF antibody and anti‐SOX2 antibody as a prostate CSC/CIC marker. Part of SOX2‐positive prostate adenocarcinoma cells exhibited positive expression of ALDH1 and HGF (Fig. 1C). Thus ALDH1 and HGF proteins were expressed in prostate CSCs/CICs in primary prostate cancer tissue.
Figure 1.
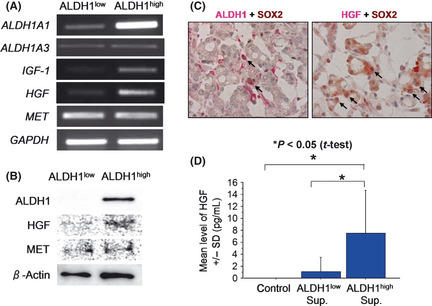
Expression of growth factors and receptors in prostate cancer stem‐like cells (CSCs)/cancer‐initiating cells (CICs). (A) Reverse transcription‐polymerase chain reaction (RT‐PCR) of HGF,IGF1 and MET in ALDH1high cells and ALDH1low cells. HGF,IGF1 and MET mRNA expression was evaluated by RT‐PCR. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as a positive control. (B) Western blot analysis of ALDH1, hepatocyte growth factor (HGF) and c‐MET. Western blot analysis using 5 × 104 ALDH1high cells and ALDH1low cells was performed. Anti‐β‐Actin was used as an internal control. (C) ALDH1 and HGF expression in primary prostate CSCs/CICs. Prostate adenocarcinoma tissue was stained by anti‐SOX2 and anti‐ALDH1 (left panel) and anti‐SOX2 and anti‐HGF (right panel). Nucleus brown staining was judged as positive staining for SOX2, and cytoplasm red staining was judged as positive staining for ALDH1 and HGF. Arrows indicate SOX2 and ALDH1 double positive cells and SOX2 and HGF double positive cells. Magnification, ×400. (D) Hepatocyte growth factor secretion by ALDH1high cells and ALDH1low cells. Isolated ALDH1high cells and ALDH1low cells were incubated in a 96‐well plate for 2 days. Then HGF was evaluated by enzyme linked immunosorbent assay (ELISA). Control represents only culture medium. Asterisks represents statistically significant difference (P < 0.05, t‐test).
Prostate cancer stem cells secrete a growth factor, HGF
Hepatocyte growth factor protein secretion by ALDHhigh cells was investigated by ELISA. The mean HGF level in ALDHhigh cell culture medium was 7.499 pg/mL, whereas HGF level in ALDHlow cell culture medium was 1.082 pg/mL (Fig. 1D), and the difference was statistically significant (P < 0.05).
Growth factors enhance prostasphere formation
Non‐adherent sphere‐forming assays are increasingly being used to evaluate stem cell phenotypes in normal tissues as well as putative CSCs/CICs. We previously reported that ALDH1high cells have higher sphere‐forming ability than that of ALDH1low cells using EGF and bFGF.3 In the present study, we found that growth factors (HGF and IGF1) are overexpressed in ALDH1high cells compared with the expression in ALDH1low cells, and we therefore investigated the effects of growth factors on sphere‐forming ability of prostate carcinoma cells. Eight thousand prostate carcinoma cells were incubated in ultra‐low attachment surface culture dishes in serum‐free culture media with or without additional growth factors (IGF1 and HGF). The addition of HGF onto EGF and bFGF enhanced sphere formation, whereas the addition of IGF1 onto EGF and bFGF did not enhance sphere formation. The addition of both HGF and IGF1 resulted in maximum sphere formation of ALDH1high cells (Fig. 2A). Growth factors increased sphere formation of both ALDH1high and ALDH1low cells, and ALDH1high cells cultured with growth factors showed the highest sphere formation efficiency (Fig. 2B,C).
Figure 2.
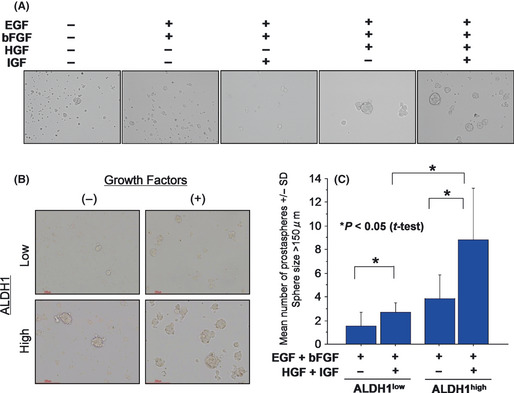
Growth factors enhance prostasphere formation in vitro. (A) Prostasphere formation was increased by hepatocyte growth factor (HGF). Prostasphere formation was performed in serum‐free Dulbecco's modified eagle medium (DMEM)/F12 media. Growth factors were added to the prostasphere culture. Representative pictures of prostaspheres are shown. Magnification, ×100. (B) Representative pictures of prostaspheres formed by ALDH1high cells and ALDH1low cells. ALDH1high cells and ALDH1low cells were cultured with or without HGF and IGF1. Representative pictures are shown. Magnification ×100. (C) Prostasphere formation of ALDH1high cells and ALDH1low cells with or without growth factors (HGF and IGF1). ALDH1high cells and ALDH1low cells were cultured in serum‐free media with or without HGF and IGF1. The numbers of prostaspheres were evaluated at day 3. Asterisks represents statistically significant difference (P < 0.05, t‐test).
Since ALDH1high cells secrete HGF, we hypothesized that HGF secreted from ALDH1high cells has a role in sphere formation of ALDH1high cells in an autocrine fashion. We therefore added anti‐HGF mAb to the sphere culture supplemented with only EGF and bFGF. The anti‐HGF mAb significantly suppressed sphere formation of ALDH1high cells (Fig. 3).
Figure 3.
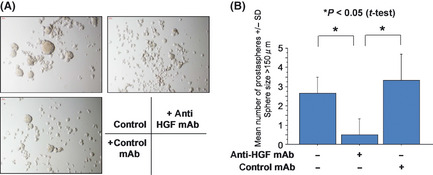
Prostasphere formation was inhibited by anti‐hepatocyte growth factor (HGF) antibody. ALDH1high cells were cultured in Dulbecco's modified eagle medium (DMEM)/F12 medium supplemented with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). Anti‐HGF antibody and control antibody were added to the culture. Representative pictures are shown (A). Magnification, ×100. The numbers of prostaspheres were evaluated at day 3 (B). Asterisks represents statistically significant difference (P < 0.05, t‐test).
Since HGF is secreted from mesenchymal cells, we hypothesized that HGF secreted from stromal cells also has a role in the maintenance of prostate CSCs/CICs. To address this question, myofibroblasts derived from the prostate were cultured, and the culture supernatant was added to ALDH1high cells. The myofibloblast supernatant increased the sphere formation of ALDH1high cells, which was inhibited by anti‐HGF mAb (Fig. 4). These results indicate that HGF derived from both ALDH1high cells and stromal cells has a role in the maintenance of prostate CSCs/CICs.
Figure 4.
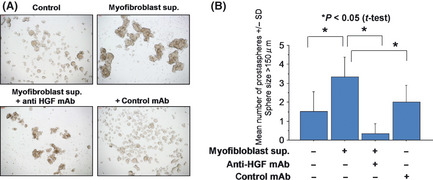
Prostasphere formation was increased by supernatant of prostate myofibroblasts. ALDH1high cells were cultured in Dulbecco's modified eagle medium (DMEM)/F12 medium supplemented with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). Fifty percent (v/v) of myofibroblast culture supernatant was added to culture. Anti‐HGF antibody and control antibody were added to the culture. Representative pictures are shown (A). Magnification, ×100. The numbers of prostaspheres were evaluated at day 3 (B). Asterisks represents statistically significant difference (P < 0.05, t‐test).
c‐MET has a role in the maintenance of prostate cancer stem cells both in vitro and in vivo
To confirm the roles of HGF in cell growth and tumorigenicity, we performed gene‐knockdown studies using siRNA of c‐MET, the HGF receptor (Fig. 5A). Gene knockdown of c‐MET mRNA did not affect the rate of ALDH1high cells, whereas it suppressed cell growth in vitro and sphere formation (Fig. 5B–D).
Figure 5.
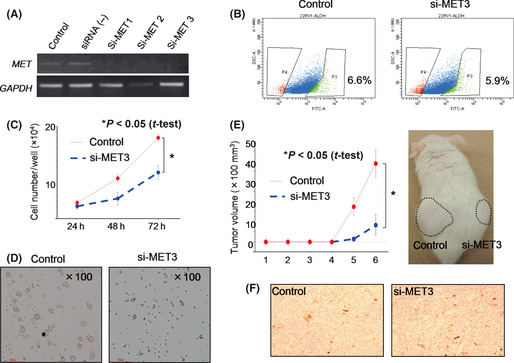
c‐MET has a role in the maintenance of prostate cancer stem‐like cells (CSCs)/cancer‐initiating cells (CICs). (A) Reverse transcription‐polymerase chain reaction (RT‐PCR) of MET knockdown cells. MET‐specific siRNA and control siRNA were transfected into 22Rv1 cells. Two days after transfection, total RNA was purified and analyzed by RT‐PCR. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as an internal control. (B) ALDEFLUOR assay of MET knockdown cells. MET siRNA3‐ and control siRNA‐transfected 22Rv1 cells were analyzed by ALDEFLUOR assay. Percentages indicate rates of ALDH1high cells. (C) MET gene knockdown inhibited cell growth in vitro. MET siRNA3‐ and control siRNA‐transfected 22Rv1 cells were seeded into a six‐well plate, and cell numbers were counted at 24, 48 and 72 h. An asterisk represents a statistically significant difference (P < 0.05, t‐test). (D) MET gene knockdown inhibited prostasphere formation in vitro. MET siRNA3‐ and control siRNA‐transfected 22Rv1 cells were seeded into an Ultra‐Low Attachment plate and cultured for 3 days. (E) MET gene knockdown inhibited tumor initiation in vivo. MET siRNA3‐ and control siRNA‐transfected 22Rv1 cells were transplanted into male NOD/SCID mice subcutaneously. Tumor growth was measured every week. Tumor growth curve and a representative picture are shown. An asterisk represents a statistically significant difference (P < 0.05, t‐test). (F) ALDH1 expression in MET knockdown tumor. ALDH1 expression was investigated by immunohistochemical staining. Representative pictures are shown. Magnification, ×100.
To evaluate the role of HGF in tumorigenicity, 1 × 103 negative control and siRNA‐transfected 22Rv1 cells were injected into the backs of NOD/SCID mice (n = 4). The tumors derived from siRNA‐transfected 22Rv1 cells were significantly smaller than those derived from negative control cells (P < 0.05, Fig. 5E), whereas ALDH1 expression was not suppressed by c‐MET siRNA (Fig. 5F). These results indicate that MET signaling has a role in the maintenance of prostate CSCs/CICs, whereas it has no role in the expression of ALDH1.
Discussion
Hepatocyte growth factor is a paracrine factor produced by cells of mesenchymal origin, while the HGF receptor, c‐MET, is expressed by epithelial and endothelial cells.9 Hepatocyte growth factor is a heterodimeric protein comprised of a 55–60‐kDa α chain and a 32–34‐kDa β chain linked by a single disulfide bond. c‐MET is a tyrosine kinase receptor with a single transmembrane spanning region and a conserved tyrosine kinase domain. c‐MET is translated as a single polypeptide chain that is proteolytically cleaved to form an approximately 145‐kDa β heavy chain and an approximately 35‐kDa α light chain linked by a single disulfide bond. Signal transduction by HGF leads to a variety of biological responses including proliferation, migration and morphogenesis. Hepatocyte growth factor plays important roles in the progression of many invasive and metastatic cancers.10 The interaction between tumor cells and their surrounding stromal environment remains a crucial factor governing tumor invasion and metastasis.
c‐MET receptor tyrosine kinase had been shown to be involved in tumor proliferation and progression, and overexpression of c‐MET has been shown to be associated with advanced prostate cancer.11, 12, 13, 14, 15 These findings suggested that the strategy of inhibiting the activation of both HGF/c‐MET and androgen receptor (AR) signaling pathways should be considered in the treatment of advanced prostate cancer. It has recently been reported that c‐MET inhibitors demonstrated anti‐proliferative efficacy when combined with androgen ablation therapy for advanced prostate cancer.16
It has been reported that c‐MET was overexpressed in immature DU145 prostate carcinoma cells and that c‐MET signaling has a role in induction of a stem‐like cell phenotype.5 On the other hand, c‐MET was expressed in both ALDH1high cells and ALDH1low cells derived from 22Rv1 prostate carcinoma cells at similar levels, whereas its ligand HGF was overexpressed in ALDH1high cells.3 ALDH1high cells secrete a larger amount of HGF than do ALDH1low cells, and HGF together with IGF1 enhanced the efficiency of sphere formation in vitro. Gene knockdown of c‐MET inhibited cell growth in vitro and abrogated the sphere‐forming ability and tumor‐initiating ability in NOD/SCID mice. Therefore, our results indicate that activation of c‐MET signaling has a role in the maintenance of prostate CSCs/CICs and that c‐MET activation might be caused by HGF secretion from prostate CSCs/CICs, not by overexpression of c‐MET.
Hepatocyte growth factor/c‐MET signaling is related to epithelial‐mesenchymal transition (EMT), anoikis and dissemination in gastric carcinoma cells, and high expression levels of HGF and c‐MET in gastric carcinoma specimens are related to poor prognosis.17 In colon carcinoma cells, HGF secreted by stromal fibroblasts is required for nuclear localization of β‐catenin, which is essential for maintenance of colon CSCs/CICs.18 Cancer‐associated fibroblasts (CAFs) are a candidate of HGF secretion, and we actually confirmed that the culture supernatant of myofibroblasts derived from the prostate enhanced prostasphere formation. Therefore, we hypothesize that both prostate CSCs/CICs and fibroblasts secrete HGF and that HGF has a role in maintenance of prostate CSCs/CICs in autocrine and paracrine fashions. Hepatocyte growth factor may also enable non‐CSCs/CICs to obtain malignant phenotypes including stemness in a paracrine fashion through activating the Wnt/β‐catenin signal (Fig. 6).
Figure 6.
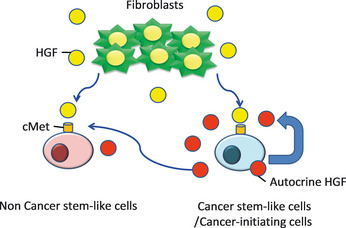
Model of the maintenance of prostate cancer stem‐like cells (CSCs)/cancer‐initiating cells (CICs)through hepatocyte growth factor (HGF)/c‐MET signaling.
In summary, HGF/c‐MET signaling has a major role in the maintenance of prostate CSCs/CICs. Prostate CSCs/CICs secrete HGF, and HGF may stimulate c‐MET expressed on prostate CSCs/CICs in an autocrine fashion. Blocking HGF/c‐MET autocrine activation might be a promising approach to target prostate CSCs/CICs.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant Nos. 16209013, 17016061, 15659097 and 24592399) for Practical Application Research from the Japan Science and Technology Agency, and for Cancer Research (15‐17 and 19‐14) from the Ministry of Health, Labor and Welfare of Japan, Ono Cancer Research Fund (to N. S.) and Takeda Science Foundation (to Y. H.). This work was supported in part by the National Cancer Center Research and Development Fund (23‐A‐44).
(Cancer Sci 2013; 104: 431–436)
References
- 1. Park CY, Tseng D, Weissman IL. Cancer stem cell‐directed therapies: recent data from the laboratory and clinic. Mol Ther 2009; 17: 219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirohashi Y, Torigoe T, Inoda S et al Immune response against tumor antigens expressed on human cancer stem‐like cells/tumor‐initiating cells. Immunotherapy 2010; 2: 201–11. [DOI] [PubMed] [Google Scholar]
- 3. Nishida S, Hirohashi Y, Torigoe T et al Gene expression profiles of prostate cancer stem cells isolated by ALDH activity assay. J Urol 2012; 188: 294–9. [DOI] [PubMed] [Google Scholar]
- 4. Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer 2010; 46: 1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inoda S, Hirohashi Y, Torigoe T et al Cep55/c10orf3, a tumor antigen derived from a centrosome residing protein in breast carcinoma. J Immunother 2009; 32: 474–85. [DOI] [PubMed] [Google Scholar]
- 6. Michifuri Y, Hirohashi Y, Torigoe T et al High expression of ALDH1 and SOX2 diffuse staining pattern of oral squamous cell carcinomas correlates to lymph node metastasis. Pathol Int 2012; 62: 684–9. [DOI] [PubMed] [Google Scholar]
- 7. van Leenders GJ, Sookhlall R, Teubel WJ et al Activation of c‐MET induces a stem‐like phenotype in human prostate cancer. PLoS ONE 2011; 6: e26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo . Cancer Res 2007; 67: 4807–15. [DOI] [PubMed] [Google Scholar]
- 9. Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c‐met proto‐oncogene product. Biochim Biophys Acta 1993; 1155: 357–71. [DOI] [PubMed] [Google Scholar]
- 10. Peruzzi B, Bottaro DP. Targeting the c‐Met signaling pathway in cancer. Clin Cancer Res 2006; 12: 3657–60. [DOI] [PubMed] [Google Scholar]
- 11. Bottaro DP, Rubin JS, Faletto DL et al Identification of the hepatocyte growth factor receptor as the c‐met proto‐oncogene product. Science 1991; 251: 802–4. [DOI] [PubMed] [Google Scholar]
- 12. Knudsen BS, Edlund M. Prostate cancer and the met hepatocyte growth factor receptor. Adv Cancer Res 2004; 91: 31–67. [DOI] [PubMed] [Google Scholar]
- 13. Humphrey PA, Zhu X, Zarnegar R et al Hepatocyte growth factor and its receptor (c‐MET) in prostatic carcinoma. Am J Pathol 1995; 147: 386–96. [PMC free article] [PubMed] [Google Scholar]
- 14. Kasai S, Sugimura K, Matsumoto K, Nishi N, Kishimoto T, Nakamura T. Hepatocyte growth factor is a paracrine regulator of rat prostate epithelial growth. Biochem Biophys Res Commun 1996; 228: 646–52. [DOI] [PubMed] [Google Scholar]
- 15. Knudsen BS, Gmyrek GA, Inra J et al High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002; 60: 1113–7. [DOI] [PubMed] [Google Scholar]
- 16. Tu WH, Zhu C, Clark C, Christensen JG, Sun Z. Efficacy of c‐Met inhibitor for advanced prostate cancer. BMC Cancer 2010; 10: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toiyama Y, Yasuda H, Saigusa S et al Co‐expression of hepatocyte growth factor and c‐Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c‐Met signaling in gastric cancer. Int J Cancer 2011; 130: 2912–21. [DOI] [PubMed] [Google Scholar]
- 18. Vermeulen L, De Sousa E, Melo F, van der Heijden M et al Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12: 468–76. [DOI] [PubMed] [Google Scholar]


