Abstract
Preoperative chemoradiotherapy has been shown to improve the outcome of patients with esophageal cancer, but because response to this therapy varies, it is desirable to identify in advance individuals who would be unlikely to benefit, in order to avoid unnecessary adverse drug effects. The serum profiles of 84 cytokines and related proteins were determined in 37 patients with esophageal squamous cell carcinoma who received identical neoadjuvant preoperative chemoradiotherapy regimens and underwent surgical resection. Histological response to this therapy was assessed in surgically resected specimens. The serum soluble interleukin‐6 receptor (sIL6R) level was significantly higher in 30 patients who failed to achieve a histological complete response (P = 0.005). Multivariate analysis revealed that the increased level of sIL6R was one of several significant independent predictors of an unfavorable outcome (hazard ratio, 2.87; P = 0.017). The increased level of this cytokine in patients who did not obtain a complete response was reproducibly observed in an independent cohort of 34 patients. Esophageal squamous cell carcinoma patients with an increased serum level of sIL6R are predicted to respond poorly to preoperative chemoradiotherapy, therefore, their exclusion from this treatment may be considered. Persistent systemic inflammation is implicated as a possible mechanism of resistance to this therapy.
Esophageal cancer is one of the leading causes of cancer mortality worldwide, accounting for >300 000 deaths annually.1 Surgical resection is one of the most reliable methods for local management of the disease, but lymph node metastasis and invasion to neighboring organs, such as the lung, trachea, and large vessels, often hamper curative resection of the tumors. To improve resectability, various trials of PCRT have been attempted,2, 3, 4, 5, 6 and a recent large randomized phase III clinical trial clearly indicated that it improved the overall survival of patients with potentially curable esophageal or esophagogastric‐junction cancer.7
However, PCRT does not always improve the survival of patients with ESCC. We previously showed an unfavourable outcome of patients with ESCC that did not response to PCRT.3 Similar results have been reported by other investigators.8, 9, 10 The combination of chemotherapy with radiation enhances the degree of toxicity.11, 12 Therefore, if PCRT proves ineffective, patients potentially would merely have suffered more severe adverse events without receiving any of the anticipated benefits. For this reason, development of a new diagnostic method that would reliably predict the response of every patient to the treatment is clearly desirable.11
The biological behavior of cancer may be determined, or at least influenced, by the tissue microenvironment. Cytokine gene expression signatures in non‐cancerous tissues have been reported to predict the outcome of patients with hepatocellular carcinoma and lung adenocarcinoma.13, 14 Chemoradiation induces cancer cell death through tumor antigen‐specific T‐cell responses.15
Based on these observations, we assumed that a certain type of host reaction might influence the efficacy of chemoradiotherapy. Here we report the comprehensive profiling of serum cytokines in ESCC patients who received an identical protocol of neoadjuvant PCRT, which revealed a correlation between serum sIL6R and the histological response to PCRT that to our knowledge has not been reported previously.
Materials and Methods
Serum samples
Serum samples from a total of 218 ESCC patients in three retrospective cohorts (PCRT‐discovery [n = 37], PCRT‐validation [n = 34], and PCT [n = 100]) and two prospective cohorts (prospective PCRT [n = 26] and prospective PCT [n = 21]) were analyzed.
The diagnosis of primary squamous cell carcinoma was confirmed histologically in all cases by pretreatment endoscopic biopsy. Patients were staged clinically according to the International Union Against Cancer's TNM classification of malignant tumors (6th edition).16 Serum samples were collected before the initiation of any treatment and kept frozen until analysis. Patients received PCRT (PCRT‐discovery, PCRT‐validation, and prospective PCRT Cohorts) or preoperative combinational chemotherapy (PCT and prospective PCT Cohorts) and underwent standard esophagectomy and lymphadenectomy with curative intent. Histological responses to treatments were classified into Grades 0–3 (G0, G1, G2, and G3) according to the 9th edition of the Japanese Classification of Esophageal Cancer (Table S1).17
Individuals who had previously undergone therapy for esophageal cancer or chemoradiotherapy for other malignancies, or had histories of other active malignancies, were excluded. This study was carried out with approval from the Internal Review Boards on ethical issues of TMU and the NCC. The therapy protocols used for the various cohorts (1–5) were as follows:
PCRT‐discovery Cohort was a retrospective cohort of 37 stage II–IVa ESCC patients randomly selected from among those who consecutively received neoadjuvant PCRT at TMU between 2000 and 2005 (Table 1).3, 18 The PCRT consisted of low‐dose CDDP (5 mg/m2/day, 5 days weekly for 4 weeks; total 100 mg/m2) and 5‐FU (350 mg/m2/day, 5 days weekly for 4 weeks; total 7000 mg/m2) plus concurrent radiation (10‐MV linear accelerator, 2 Gy/day, 5 days weekly for 4 weeks; total 40 Gy). Surgical resection was carried out 4 weeks after the completion of PCRT.
PCRT‐validation Cohort was a retrospective cohort comprising 34 serum samples obtained from the remaining stage II–III ESCC patients and those who received neoadjuvant PCRT using the same protocol as that for the PCRT‐discovery Cohort at TMU between 2006 and 2009 (Table 1).3, 18
Prospective PCRT Cohort was a prospective cohort of serum samples from 26 stage II–III (excluding T4) patients who were enrolled in a phase II clinical trial of neoadjuvant PCRT at the National Cancer Center Hospital and the National Cancer Center Hospital East between 2010 and 2011.19 These patients underwent two courses of protracted infusion of 5‐FU (1000 mg/m2/day) on days 1–4 and days 29–32, and 2‐h infusions of CDDP on days 1 and 29 (75 mg/m2), along with adequate hydration and antiemetic coverage, plus concurrent irradiation (1.8 Gy/day). Surgical resection was carried out 6–8 weeks after the completion of PCRT.
PCT Cohort was a retrospective cohort of serum samples from 100 stage II–III (excluding T4) patients who received neoadjuvant combinational chemotherapy at the NCC between 2003 and 2010. The combinational chemotherapy comprised 5‐FU (800 mg/m2/day) on days 1–5 and days 22–26, and 2‐h infusions of CDDP (80 mg/m2) on days 1 and 22, with adequate hydration and antiemetic coverage. The treatment was repeated three times at 3‐week intervals.
Prospective PCT Cohort was a prospective cohort comprising serum samples from 21 stage II–III (excluding T4) patients who were enrolled in a phase II clinical trial of neoadjuvant combinational chemotherapy at the National Cancer Center Hospital between 2009 and 2010. The chemotherapy regimen consisted of docetaxel (70 mg/m2) on day 1, 5‐FU (750 mg/m2/day) on days 1–5, and 2‐h infusions of CDDP (70 mg/m2) on day 1, with adequate hydration and antiemetic coverage. The treatment was repeated three times at 3‐week intervals.
Table 1.
Clinicopathological characteristics of esophageal squamous cell carcinoma patients in preoperative chemoradiotherapy (PCRT)‐discovery and PCRT‐validation cohorts
| Variable | PCRT‐discovery cohort | PCRT‐validation cohort | ||||
|---|---|---|---|---|---|---|
| G3 (n = 7) | G2 and G1 (n = 30) | P‐value* | G3 (n = 4) | G2 and G1 (n = 30) | P‐value* | |
| Age | ||||||
| <65 years | 4 (54%) | 21 (70%) | 0.66 | 3 (75%) | 14 (47%) | 0.60 |
| ≥65 years | 3 (43%) | 9 (30%) | 1 (25%) | 16 (53%) | ||
| Gender | ||||||
| Male | 5 (71%) | 27 (70%) | 0.23 | 4 (100%) | 23 (77%) | 0.56 |
| Female | 2 (29%) | 3 (10%) | 0 | 7 (23%) | ||
| Tumor location† | ||||||
| Ce | 0 | 3 (10%) | 0.37 | 0 | 1 (3%) | 1.00 |
| Te | 6 (86%) | 27 (90%) | 4 (100%) | 29 (97%) | ||
| Ae | 1 (14%) | 0 | 0 | 0 | ||
| Clinical stage‡ | ||||||
| II | 0 | 4 (13%) | 0.30 | 1 (25%) | 3 (10%) | 0.41 |
| III | 7 (100%) | 20 (67%) | 3 (75%) | 27 (90%) | ||
| IVa | 0 | 6 (20%) | 0 | 0 | ||
| CRP§ | ||||||
| <0.3 mg/dL | 4 (57%) | 15 (52%) | 0.57 | |||
| ≥0.3 mg/dL | 3 (43%) | 14 (48%) | ||||
Histological responses graded (G1–G3) according to the Japanese Classification of Esophageal Cancer (9th edition). *P‐values were calculated using Fisher's extact t‐test. †Tumor location was classified according to the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus (9th edition).17 ‡Clinical stage was classified according to the International Union Against Cancer's TNM Classification of Malignant Tumors (6th edition).16 §C‐reactive protein (CRP) data were not available for one patient. Ae, Abdominal esophagus; Ce, cervical esophagus; PCRT, preoperative chemoradiotherapy; Te, thoracic esophagus.
Multiplexed immunobead‐based assay
The levels of 84 cytokines (listed in Table S2) were measured in the sera of the 37 patients in the PCRT‐discovery Cohort using nine multiplex kits: an acute‐phase 4‐plex panel (Invitrogen, Carlsbad, CA, USA); an SAA human single‐plex beads kit (Invitrogen); MAP human serum adipokine panel A (Millipore, Billerica, MA, USA); MAP human serum adipokine panel B (Millipore); MAP human soluble cytokine receptor premix 14‐plex (Millipore); the MAP human soluble cytokine/chemokine panel (Millipore); cytokine assay human (Panomics, Fremont, CA, USA); human adhesion molecular multianalyte profile base kit (R&D Systems, Minneapolis, MN, USA); and the human obesity multianalyte profiling kit (R&D Systems). The assays were carried out by investigators who were unaware of the clinical data.
Enzyme‐linked immunosorbent assay
The level of serum sIL6R was measured using the ELISA sIL6R assay kit (R&D Systems). The level of c‐reactive protein (CRP) was measured using the high‐sensitivity ELISA CRP assay kit (Siemens, Munich, Germany). The assays were carried out by investigators who were unaware of the clinical data.
Immunohistochemistry
Formalin‐fixed paraffin‐embedded biopsy specimens of the prospective PCRT Cohort were cut into 4‐μm‐thick sections. The sections were immunostained with a rabbit mAb against phosphorylated STAT3 protein at the tyrosine 705 (Y705) residue (Cell Signaling Technology, Boston, MA, USA), as described previously.18, 20
Statistical analysis
Survival curves covering the period from the date of surgery to the date of death or last follow‐up were plotted using the Kaplan–Meier method, and differences between the curves were assessed with the log–rank test. Student's t‐tests and the Cox proportional hazards regression model were carried out using the StatFlex statistics package (version 5.0) (Atiteck, Osaka, Japan) and tools available in the R statistical package (http://www.r-project.org/).18, 21, 22 Differences having P‐values of <0.05 were considered to be statistically significant.
Results
Circulating cytokines associated with response to PCRT
The levels of 84 cytokines in serum samples from 37 patients that were obtained prior to PCRT (PCRT‐discovery Cohort) were measured using the multiplexed immunobead‐based assay. All of the patients received the same protocol of chemoradiotherapy (CDDP plus 5‐FU and concurrent irradiation) and underwent esophagectomy. Histological examination of the resected specimens revealed that viable tumor cells had completely disappeared in seven (19%) patients (pathological complete response or G3), whereas residual viable tumor cells were detected in the remaining 30 (G1 or G2). No case was graded as G0 (no recognizable effect). There was no significant difference in age, gender, tumor location, clinical stage, or serum CRP level between these two sets of 7 and 30 patients (Table 1). The seven patients who obtained a G3 response showed a markedly favorable postsurgical outcome in comparison with the G1 and G2 cases (P = 0.014, log–rank test) (Fig. 1a).
Figure 1.
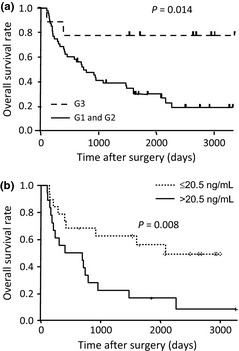
Distinct outcomes of esophageal squamous cell carcinoma (ESCC) patients who achieved a pathological complete response to preoperative chemoradiotherapy (PCRT). (a) Kaplan–Meier estimates of overall postsurgical survival for seven ESCC patients of the PCRT‐discovery Cohort who achieved a complete pathological response (G3) to PCRT and 30 patients who did not (G2 and G1). (b) Kaplan–Meier estimates of overall postsurgical survival for 18 ESCC patients of the PCRT‐discovery Cohort having a baseline level of soluble interleukin‐6 receptor higher than 20.5 ng/mL (median value of 37 patients), and for the remaining 19 patients.
Through multiplex protein profiling we found that the baseline serum levels of six cytokines, including MIP1B (P = 0.002, t‐test), sIL6R (P = 0.005), MIP1A (P = 0.027), insulin (P = 0.031), interferon‐α2 (P = 0.048), and MMP3 (P = 0.049), were significantly decreased in the seven patients who showed a complete pathological response (Table 2). The differences did not remain statistically significant after Bonferroni adjustment for multiple testing,23 probably due to the small number of patients assessed, especially those who obtained a G3 response.
Table 2.
Six cytokines that differed significantly between esophageal squamous cell carcinoma patients whose histological response to preoperative chemoradiotherapy was graded G3, and those whose response was graded G1/2
| Cytokines | G1 and G2 (n = 30) | G3 (n = 7) | P‐value* | ||
|---|---|---|---|---|---|
| Average (pg/mL) | SEM | Average (pg/mL) | SEM | ||
| MIP1B | 84.7 | 10.1 | 40.3 | 8.3 | 0.002 |
| sIL6R | 22528.6 | 1210.6 | 16520.7 | 1432.4 | 0.005 |
| MIP1A | 31.7 | 7.8 | 9.2 | 5.6 | 0.027 |
| Insulin | 2.1 | 0.6 | 0.6 | 0.0 | 0.031 |
| IFNA2 | 386.1 | 172.6 | 25.7 | 25.5 | 0.048 |
| MMP3 | 46396.2 | 5057.2 | 34817.6 | 2565.2 | 0.049 |
Histological responses graded according to the Japanese Classification of Esophageal Cancer (9th edition). *P‐values were calculated using Student's t‐test. INFA2, interferon‐α2; MIP1A, macrophage inflammatory protein α1; MIP1B, macrophage inflammatory protein 1‐β; SEM, standard error of the mean; sIL6R, soluble interleukin‐6 receptor.
Correlation coefficient analysis revealed no significant mutual association (correlation coefficient ≥0.70) among the six cytokines (Table S3).
Association of high sIL6R with poorer OS
To further select the most critical factor, the 37 patients in the PCRT‐discovery Cohort were classified into two groups according to their levels of each cytokine, and OS was compared between the groups. Among the six cytokines, we found that only the serum level of sIL6R was significantly associated with patient outcome. Nineteen patients with sIL6R higher than 20.5 ng/mL (median value for 37 patients) had significantly worse OS than the remaining 18 (P = 0.008, log–rank test) (Fig. 1b). We adopted here the median values for the 37 patients in order to avoid introducing any selection bias.
Univariate analysis by the Cox proportional hazards model (Table 3) revealed that only clinical stage (specifically, stages III–IV vs stage II; hazard ratio, 3.06; P = 0.008) and serum sIL6R (>20.5 ng/mL vs ≤20.5 ng/mL; hazard ratio, 3.20; P = 0.008) were significantly correlated with patient outcome. Multivariate analysis indicated that sIL6R (hazard ratio, 2.87; P = 0.017) and clinical stage (hazard ratio, 2.50; P = 0.034) were independent predictors (Table 3).
Table 3.
Cox regression model analysis of prognostic significance
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age | ||||||
| ≥65 years/<65 years | 1.31 | 0.57–2.96 | 0.521 | – | – | – |
| Gender | ||||||
| Male/female | 0.31 | 0.07–1.33 | 0.115 | – | – | – |
| Tumor location† | ||||||
| Ce and Ut/Mt, Lt, and Ae | 0.70 | 1.54–3.65 | 0.472 | – | – | – |
| Clinical stage‡ | ||||||
| III and IVa/I and II | 3.06 | 1.33–7.02 | 0.008 | 2.50 | 1.10–6.52 | 0.034 |
| Serum sIL6R | ||||||
| >20.5 ng/mL/≤20.5 ng/mL | 3.20 | 1.34–7.53 | 0.008 | 2.87 | 1.20–6.85 | 0.017 |
†Tumor location was classified according to the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus (9th edition).17 ‡Clinical stage was classified according to the International Union Against Cancer's TNM Classification of Malignant Tumors (6th edition).16 Ae, abdominal esophagus; Ce, cervical esophagus; CI, confidence interval; HR, hazard ratio; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; sIL6R, soluble interleukin‐6 receptor; Ut, upper thoracic esophagus.
Validation in an independent cohort
The relative unresponsiveness of individuals with a high sIL6R level to PCRT was further validated in the independent cohort of 30 patients who received the same PCRT protocol (PCRT‐validation Cohort). As the sIL6R level determined by a commercial ELISA kit was well correlated with that determined by the multiplexed immunobead‐based assay (R = 0.726, Pearson's correlation coefficient) (Fig. S1), we used the kit for further measurements, since sandwich ELISA is generally accepted as a standard protocol for various clinical tests.
Among the PCRT‐validation Cohort, 12% (4/34) of patients achieved a complete pathological response. There was no statistically significant difference in age, gender, tumor location, or clinical stage (Table 1), but the sIL6R level in the 4 patients who achieved a G3 response was significantly lower than in the remaining 34 patients (P = 0.040, t‐test) (Fig. 2a).
Figure 2.
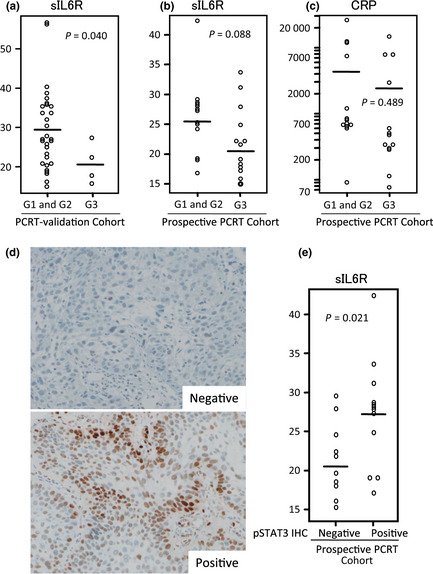
Independent retrospective and prospective validation. (a) Soluble interleukin‐6 receptor (sIL6R) level (ng/mL) in four esophageal squamous cell carcinoma (ESCC) patients who achieved a complete pathological response (G3) to preoperative chemoradiotherapy (PCRT) and 34 patients who did not (G1 and G2) in the PCRT‐validation Cohort. Bars indicate mean values (G1 and G2, 29.6 ng/mL; G3, 21.4 ng/mL). (b) sIL6R levels (ng/mL) in 13 ESCC patients who achieved a complete pathological response (G3) to PCRT and 13 patients who did not (G1 and G2) in the prospective PCRT Cohort. Bars indicate mean values (G1 and G2, 25.2 ng/mL; G3, 21.7 ng/mL). (c) C‐reactive protein (CRP) level (ng/mL) in 13 ESCC patients who achieved a complete pathological response (G3) to PCRT and 13 patients who did not (G1 and G2) in the prospective PCRT Cohort. Bars indicate mean values (G1 and G2, 4525.9 ng/mL; G3, 2859.7 ng/mL). (d) Representative phosphorylated signal transducer and activator of transcription‐3 (pSTAT3)‐negative (upper) and ‐positive (lower) cases. (e) sIL6R level (ng/mL) in 12 patients with ESCC positive for pSTAT3 and 10 patients with ESCC negative for pSTAT3. IHC, immunohistochemistry.
Validation in a prospective cohort
We have recently completed a phase II clinical trial in which the efficacy of a new protocol for neoadjuvant chemoradiotherapy for clinical stage II–III ESCC was evaluated.19 As a collateral study, we prospectively collected serum samples from patients participating in the trial (Prospective PCRT Cohort) and measured their levels of sIL6R and CRP.
Owing to the relatively intense PCRT protocol adopted in this clinical trial, 50% (13/26) of the patients achieved a G3 response. The level of sIL6R in those patients was lower than that in the remaining 13 who did not achieve a complete pathological response, with marginal statistical significance (P = 0.088) (Fig. 2b), but the CRP level did not show such a correlation (P = 0.489) (Fig. 2c).
To reveal the status of STAT3 signaling in patients with a high serum sIL6R level, the pretreatment tumor biopsy specimens from patients of the Prospective PCRT Cohort were immunostained with anti‐pSTAT3 antibody. Intense nuclear staining of pSTAT3 in more than 30% of the tumor area was evident in 50% (13/26) of the cases, and these were classified as positive (Fig. 2d). The level of sIL6R was found to be significantly higher in pSTAT immunohistochemistry‐positive cases than in negative cases (P = 0.021, t‐test) (Fig. 2e).
Soluble interleukin‐6 receptor is not a significant predictor of response to chemotherapy alone
Finally, we evaluated the sIL6R level in pretreatment serum samples from patients who received neoadjuvant combinational chemotherapy with CDDP/5‐FU (PCT Cohort, n = 100) and docetaxel/CDDP/5‐FU (prospective PCT Cohort, n = 21), and found that 7% (7/100) of patients in the PCT Cohort and 19% (4/21) of patients in the prospective PCT Cohort achieved a complete pathological G3 response.
There was no significant difference in sIL6R level between the seven individuals in the PCT Cohort who achieved a complete pathological response and the 93 individuals who did not (P = 0.345, t‐test) (Fig. 3a), or between the four individuals in the PCT Cohort who achieved a complete pathological response and the 17 individuals who did not (Fig. 3b) (P = 0.915), indicating that sIL6R is a biomarker that predicts response to chemoradiotherapy, but not to chemotherapy. Consistently, the serum level of sIL6R had no significant correlation with the OS of patients in the PCT Cohort (Fig. 3c) (P = 0.865, log–rank test). These findings suggested that the pathways of tumor cell killing in response to chemotherapy and radiotherapy might differ.
Figure 3.
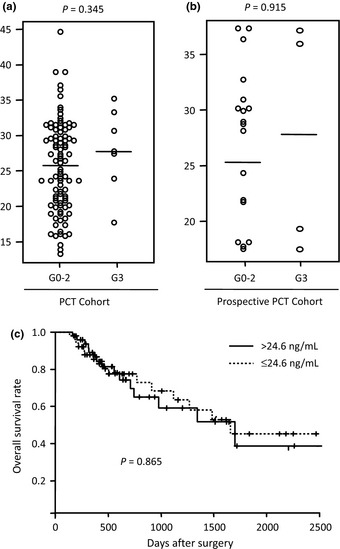
Soluble interleukin‐6 receptor (sIL6R) is not a significant predictor of response to chemotherapy alone. (a) sIL6R levels in seven esophageal squamous cell carcinoma (ESCC) patients who achieved a complete pathological response (G3) to preoperative chemotherapy (PCT) and 93 patients who did not (G0–2) in the PCT Cohort. Bars indicate mean values (G0–2, 25.6 pg/mL; G3, 27.9 pg/mL). (b) sIL6R level in 4 ESCC patients who achieved a complete pathological response (G3) to preoperative chemotherapy and 18 patients who did not (G0–2) in the prospective PCT Cohort. Bars indicate mean values (G0–2, 26.9 pg/mL; G3, 28.4 pg/mL). (c) Kaplan–Meier estimates of overall postsurgical survival of 49 ESCC patients with a serum level of sIL6R higher than 24.6 ng/mL (mean value of 100 patients) and the remaining 51 patients in the PCT Cohort.
Discussion
In the present study we showed for the first time that an increased serum level of sIL6R was correlated with a relatively poor response to PCRT in patients with ESCC. Several reports have documented the prognostic significance of serum CRP and IL6, but no biomarker that can predict the efficacy of PCRT for ESCC has yet been found.24, 25 The absence of any significant correlation with OS or pathological complete response in patients receiving chemotherapy alone (Fig. 3) indicated that sIL6R is not a prognostic biomarker. Squamous cell carcinoma is the predominant histological type of esophageal cancer in Asian countries26 and accounts for >90% of surgical cases in Japan. It is generally accepted that the effects of PCRT are more apparent among patients with squamous cell carcinoma than in those with adenocarcinoma.7, 11
Interleukin‐6 receptor (also known as CD126) is a cell membrane cognate receptor for the inflammatory cytokine IL6.27 Soluble IL6R is the alternatively spliced or proteolytically cleaved form of IL6R that lacks the transmembrane domain. Secreted sIL6R binds to IL6, and the sIL6R/IL6 complex evokes intracellular trans‐signaling through binding to the gp130 receptor (CD130) expressed on the surface of various cells.28 Soluble gp130, or sgp130, a soluble form of gp130,29 competes with gp130 for binding to sIL6R/IL6. We measured the serum levels of IL6 and sgp130 in the PCRT‐discovery Cohort, but found that they were not significantly correlated with response to PCRT (data not shown). A high level of sIL6R has been reported in patients with several chronic inflammatory and autoimmune diseases, as well as various malignancies.27 Secretion and expression of IL6 and IL6R have been reported in esophageal cancer cells,30, 31 but the source of the increased sIL6R production in patients with esophageal cancer remains undetermined.
Although we were unable to reproduce those previous results in the present study cohorts (Fig. 2c and data not shown), some reports have documented that serum CRP and VEGF are correlated with the sensitivity of ESCC to PCRT.31, 32, 33 C‐reactive protein is produced by hepatocytes in response to inflammatory cytokines, such as IL6. The production of VEGF is also regulated by IL6 signaling.34 As well as sIL6R, we found that the levels of five cytokines (MIP1A, MIP1B, interferon‐α2, insulin, and MMP3) were also significantly increased in patients who failed to achieve a complete pathological response (Table 2). Both MIP1A (also known as CCL3) and MIP1B (also known as CCL4) are cytokines released from macrophages in response to inflammatory stimuli. Alternatively, an increase in the levels of a variety of cytokines is considered to reflect a persistent systemic inflammatory status.
Suchi et al.35 reported that overexpression of IL6 in ESCC cells induced resistance to cisplatin. The sIL6R/IL6 signal is transduced into the nucleus through JAK‐mediated activation of STAT1/3.27 Efimova et al.36 reported that radiation‐resistant human squamous cell carcinoma cells showed constitutive activation of the STAT1 pathway. Leu et al.37 reported that IL6 inhibited apoptosis in human esophageal carcinoma cells through activation of the STAT3 and MAPK pathways. A small‐molecule inhibitor of JAK2 (TG101209) has been reported to affect the expression of survivin, thus sensitizing lung cancer cells to radiation.38 Here we observed that the nuclear expression of pSTAT3 was correlated with an increased level of circulating sIL6R (Fig. 2e). Based on these experimental and clinical findings, it would appear that active sIL6R/IL6/JAK/STAT signaling may be involved in the resistance of ESCC to PCRT.
However, there were certain limitations to this study in the context of potential clinical application. We observed that a substantial proportion of patients with a low level of sIL6R did not achieve a G3 response. Although patients with a high level of sILR6 may be potentially excluded from PCRT, we were unable to determine a safe cut‐off value, mainly because of the small number of cases examined and the differences in therapy protocols among the cohorts. A substantially high proportion of patients in the Prospective PCRT Cohort achieved a complete response, and this included some patients with a high sIL6R level; this outcome was probably attributable to the intense nature of the therapy protocol. It will be necessary to carry out a large prospective study to evaluate the clinical applicability of our present findings. We are now planning a phase III clinical trial to compare the efficacies of preoperative combinational chemotherapy and chemoradiotherapy for ESCC.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- 5‐FU
5‐fluorouracil
- CDDP
cis‐diamminedichloro‐platinum (II) (cisplatin)
- CRP
c‐reactive protein
- ESCC
esophageal squamous cell carcinoma
- G0, G1, G2, G3
grades 0, 1, 2, 3
- IL6
interleukin‐6
- MIP1A
macrophage inflammatory protein α1
- MIP1B
macrophage inflammatory protein β1
- NCC
National Cancer Center
- OS
overall survival
- PCRT
preoperative chemoradiotherapy
- PCT
preoperative chemotherapy
- pSTAT3
phosphorylated STAT3
- (s)gp130
(soluble)gp130
- sIL6R
soluble interleukin‐6 receptor
- STAT(1/3)
signal transducer and activator of transcription(‐1/‐3)
- TMU
Tokyo Medical University
- VEGF
vascular endothelial growth factor
Supporting information
Fig. S1. Comparison of the multiplex bead‐based assay and ELISA. The soluble interleukin‐6 receptor level of 37 patients in the preoperative chemoradiotherapy (PCRT)‐discovery Cohort was determined by the multiplex bead‐based assay (x‐axis) and ELISA (y‐axis).
Table S1. Pathological criteria for evaluation of therapeutic efficacy.
Table S2. List of 84 cytokines and related proteins analyzed by the multiplexed immunobead‐based assay.
Table S3. Correlation coefficients for six cytokines.
Acknowledgments
This work was supported by a Grant‐in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the National Cancer Center Research and Development Fund (23‐A‐38, and 23‐A‐11), and the Program for Promotion of Fundamental Studies in Health Sciences conducted by the National Institute of Biomedical Innovation of Japan. We thank Drs H. Fujiwara (Kyoto Prefectural University of Medicine, Kyoto, Japan) and S. Kikuchi (Hyogo College of Medicine, Nishinomiya, Japan) for helpful discussions.
(Cancer Sci 2013; 104: 1045–1051)
References
- 1. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001; 37(Suppl. 8): S4–66. [DOI] [PubMed] [Google Scholar]
- 2. Fiorica F, Di Bona D, Schepis F et al Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta‐analysis. Gut 2004; 53: 925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osaka Y, Takagi Y, Tsuchida A et al Concurrent preoperative chemoradiotherapy for stage III or IV esophageal squamous carcinoma. Oncol Rep 2004; 12: 1121–6. [PubMed] [Google Scholar]
- 4. Lee JL, Park SI, Kim SB et al A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 2004; 15: 947–54. [DOI] [PubMed] [Google Scholar]
- 5. Burmeister BH, Smithers BM, Gebski V et al Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005; 6: 659–68. [DOI] [PubMed] [Google Scholar]
- 6. Tepper J, Krasna MJ, Niedzwiecki D et al Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26: 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Hagen P, Hulshof MC, van Lanschot JJ et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 8. Berger AC, Farma J, Scott WJ et al Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005; 23: 4330–7. [DOI] [PubMed] [Google Scholar]
- 9. Kim MK, Kim SB, Ahn JH et al Treatment outcome and recursive partitioning analysis‐based prognostic factors in patients with esophageal squamous cell carcinoma receiving preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2008; 71: 725–34. [DOI] [PubMed] [Google Scholar]
- 10. Donahue JM, Nichols FC, Li Z et al Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009; 87: 392–8; discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007; 25: 4110–7. [DOI] [PubMed] [Google Scholar]
- 12. Geh JI. The use of chemoradiotherapy in oesophageal cancer. Eur J Cancer 2002; 38: 300–13. [DOI] [PubMed] [Google Scholar]
- 13. Budhu A, Forgues M, Ye QH et al Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006; 10: 99–111. [DOI] [PubMed] [Google Scholar]
- 14. Seike M, Yanaihara N, Bowman ED et al Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst 2007; 99: 1257–69. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki Y, Mimura K, Yoshimoto Y et al Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012; 72: 3967–76. [DOI] [PubMed] [Google Scholar]
- 16. Sobin LH. TNM Classification of Malignant Tumours, 6th edn. New York: Wiley‐Liss, 2002; 99–103. [Google Scholar]
- 17. The Japanese Society for Esophageal Disease . Guidlines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus, 9th edn. In: Isono K, ed. Tokyo: Kanehara Shuppan, 2001. [Google Scholar]
- 18. Kikuchi S, Honda K, Tsuda H et al Expression and gene amplification of actinin‐4 in invasive ductal carcinoma of the pancreas. Clin Cancer Res 2008; 14: 5348–56. [DOI] [PubMed] [Google Scholar]
- 19. Kojima T, Hashimoto J, Kato K et al Feasibility study of neoadjuvant chemoradiotherapy with cisplatin plus 5‐fluorouracil and elective nodal irradiation for stage II/III esophageal squamous cell carcinoma. J Clin Oncol 2012; 37(Suppl. 4): Abstract 130. [DOI] [PubMed] [Google Scholar]
- 20. Honda K, Yamada T, Endo R et al Actinin‐4, a novel actin‐bundling protein associated with cell motility and cancer invasion. J Cell Biol 1998; 140: 1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honda K, Yamada T, Hayashida Y et al Actinin‐4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology 2005; 128: 51–62. [DOI] [PubMed] [Google Scholar]
- 22. Honda K, Hayashida Y, Umaki T et al Possible detection of pancreatic cancer by plasma protein profiling. Cancer Res 2005; 65: 10613–22. [DOI] [PubMed] [Google Scholar]
- 23. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 1996; 86: 726–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zingg U, Forberger J, Rajcic B, Langton C, Jamieson GG. Association of C‐reactive protein levels and long‐term survival after neoadjuvant therapy and esophagectomy for esophageal cancer. J Gastrointest Surg 2010; 14: 462–9. [DOI] [PubMed] [Google Scholar]
- 25. Shimada H, Nabeya Y, Okazumi S et al Elevation of preoperative serum C‐reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol 2003; 83: 248–52. [DOI] [PubMed] [Google Scholar]
- 26. Yamaji T, Inoue M, Sasazuki S et al Fruit and vegetable consumption and squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer 2008; 123: 1935–40. [DOI] [PubMed] [Google Scholar]
- 27. Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 2001; 15: 43–58. [DOI] [PubMed] [Google Scholar]
- 28. Jones SA, Scheller J, Rose‐John S. Therapeutic strategies for the clinical blockade of IL‐6/gp130 signaling. J Clin Invest 2011; 121: 3375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garbers C, Thaiss W, Jones GW et al Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem 2011; 286: 42959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oka M, Iizuka N, Yamamoto K et al The influence of interleukin‐6 on the growth of human esophageal cancer cell lines. J Interferon Cytokine Res 1996; 16: 1001–6. [DOI] [PubMed] [Google Scholar]
- 31. Fujiwara H, Suchi K, Okamura S et al Elevated serum CRP levels after induction chemoradiotherapy reflect poor treatment response in association with IL‐6 in serum and local tumor site in patients with advanced esophageal cancer. J Surg Oncol 2011; 103: 62–8. [DOI] [PubMed] [Google Scholar]
- 32. Shimada H, Takeda A, Nabeya Y et al Clinical significance of serum vascular endothelial growth factor in esophageal squamous cell carcinoma. Cancer 2001; 92: 663–9. [DOI] [PubMed] [Google Scholar]
- 33. Shimada H, Kitabayashi H, Nabeya Y et al Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery 2003; 133: 24–31. [DOI] [PubMed] [Google Scholar]
- 34. Jee SH, Shen SC, Chiu HC, Tsai WL, Kuo ML. Overexpression of interleukin‐6 in human basal cell carcinoma cell lines increases anti‐apoptotic activity and tumorigenic potency. Oncogene 2001; 20: 198–208. [DOI] [PubMed] [Google Scholar]
- 35. Suchi K, Fujiwara H, Okamura S et al Overexpression of Interleukin‐6 suppresses cisplatin‐induced cytotoxicity in esophageal squamous cell carcinoma cells. Anticancer Res 2011; 31: 67–75. [PubMed] [Google Scholar]
- 36. Efimova EV, Liang H, Pitroda SP et al Radioresistance of Stat1 over‐expressing tumour cells is associated with suppressed apoptotic response to cytotoxic agents and increased IL6‐IL8 signalling. Int J Radiat Biol 2009; 85: 421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin‐6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen‐activated protein kinase pathways. Oncogene 2003; 22: 7809–18. [DOI] [PubMed] [Google Scholar]
- 38. Sun Y, Moretti L, Giacalone NJ et al Inhibition of JAK2 signaling by TG101209 enhances radiotherapy in lung cancer models. J Thorac Oncol 2011; 6: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of the multiplex bead‐based assay and ELISA. The soluble interleukin‐6 receptor level of 37 patients in the preoperative chemoradiotherapy (PCRT)‐discovery Cohort was determined by the multiplex bead‐based assay (x‐axis) and ELISA (y‐axis).
Table S1. Pathological criteria for evaluation of therapeutic efficacy.
Table S2. List of 84 cytokines and related proteins analyzed by the multiplexed immunobead‐based assay.
Table S3. Correlation coefficients for six cytokines.


