Abstract
A phase II study of preoperative chemoradiation (CRT) with S‐1 plus oxaliplatin in patients with locally advanced rectal cancer was conducted. The total radiotherapy dose was 50.4 Gy. Chemotherapy consisted of oxaliplatin 50 mg/m2 on days 1, 8, 22 and 29 and S‐1 80 mg/m2 per day on days 1–14 and 22–35. The tumor apparent diffusion coefficient (ADC) was measured using diffusion‐weighted magnetic resonance imaging (DW‐MRI) before and after CRT. Total mesorectal excision was performed within 6 ± 2 weeks. The primary end‐point was the pathological complete response (pCR) rate. A total of 38 patients were enrolled. The pCR rate was 22.9% (8/35; 95% CI, 10.9–42.1), and 10 (28.6%) patients showed near‐total tumor regression. There was no grade 4 adverse event, and grade 3 adverse events included leukopenia (5.4%), diarrhea (5.4%), anorexia (2.7%) and nausea (2.7%). The tumor ADC was calculated in 38 patients (including those who participated in the phase I study). The post‐CRT ADC (P = 0.037) and the percentage change in ADC (P = 0.026) were significantly correlated with pathological response. In conclusion, preoperative CRT with S‐1 plus oxaliplatin showed promising results in pathological responses and favorable toxicity profiles. (Cancer Sci 2013; 104: 111–115)
The treatment of locally advanced rectal cancer (LARC) has progressed significantly over the past several years. Radiation therapy, before or after surgery, significantly reduced local recurrence rates in stage II or III rectal cancer, and the addition of fluoropyrimidines, either 5‐fluorouracil (5‐FU) or capecitabine, afforded further benefits in local control.1, 2 Preoperative chemoradiation therapy (CRT) had been superior to postoperative CRT in terms of local control, sphincter preservation and toxicity.3, 4, 5 Therefore, preoperative CRT with fluoropyrimidines has become accepted as the standard treatment modality for patients with LARC.
To improve outcomes, the addition of other agents to fluoropyrimidine‐based CRT has been investigated. Oxaliplatin, when combined with fluoropyrimidines, improved overall survival (OS) and disease‐free survival (DFS) of patients with completely resected stage II or III colon cancer.6, 7 In addition, oxaliplatin was associated with radiation sensitization in a preclinical study, suggesting that this drug might be a good candidate for chemoradiation regimens in patients with rectal cancer.8
Oral fluoropyrimidine is an attractive alternative to 5‐FU, because it is easier to use and patients are constantly exposed to the drug. S‐1, a novel fluoropyrimidine, was designed to enhance antitumor activity of 5‐FU and to reduce gastrointestinal (GI) toxicity.9 In addition, this drug showed antitumor activity when used as a radiosensitizer in human colon cancer xenografts.10 We previously performed a phase I study to determine the recommended dose of S‐1, in combination with oxaliplatin, for preoperative CRT in patients with LARC. We found that the recommended dose of S‐1 was 80 mg/m2 per day on days 1–14 and 22–35, combined with oxaliplatin 50 mg/m2 on days 1, 8, 22 and 29.11
Patients who showed good pathological responses after preoperative CRT were found to have favorable long‐term outcomes.12 In selected patients showing complete pathological responses to preoperative CRT, local excision has not compromised survival.13 Furthermore, Habr‐Gama et al. reported that patients with radiological and clinical evidence of complete responses after preoperative CRT had excellent long‐term outcomes regardless of surgery.14 Therefore, it is important to assess the response to preoperative CRT in patients who would benefit from less invasive surgery. Diffusion‐weighted magnetic resonance imaging (DW‐MRI) is a non‐invasive method for obtaining information about microscopic structures through the detection of water mobility in biological tissue, and this can be quantified by the apparent diffusion coefficient (ADC).15 By detecting tumor microstructural changes, DW‐MRI can be used to predict early response to treatment and to differentiate between viable and necrotic or inflammatory tissue.16 Several recent studies have investigated the ability of DW‐MRI to predict the efficacy of preoperative CRT in patients with LARC.17, 18, 19
The aim of this multicenter phase II study was to assess the efficacy and safety of preoperative CRT with oxaliplatin and S‐1 in patients with LARC. We also evaluated the clinical utility of tumor ADC, as measured using DW‐MRI, to monitor response to preoperative CRT.
Patients and Methods
Patient population
Patients with locally advanced, non‐metastatic and histologically confirmed adenocarcinoma of the rectum were enrolled. All patients had a distal tumor margin located 0–12 cm from the anal verge by digital rectal examination; had a clinically T3 or T4 lesion, or involvement of regional nodes (N+), as determined using MRI with or without endorectal ultrasound; were >18 years; had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1; and had not been prescribed any prior anticancer therapy. Patients were excluded if they had lack of integrity of the upper GI track that might compromise the absorption of S‐1, or any condition indicating unsuitability for CRT.
The study was approved by the Institutional Review Board of each participating center. All patients provided written informed consent.
Treatment
The treatment scheme is shown in Fig. 1. Preoperative radiotherapy was delivered at a total dose of 50.4 Gy within 6 weeks; 45 Gy in 25 fractions to the pelvis and 5.4 Gy in a three‐fraction boost to the primary tumor. Details regarding the radiotherapy simulation, beam weight and radiation field have been described previously.11
Figure 1.
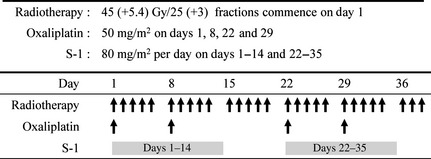
Treatment scheme.
Concurrent chemotherapy consisted of oxaliplatin (50 mg/m2) on days 1, 8, 22 and 29, and oral S‐1 (80 mg/m2 per day) on days 1–14 and 22–35. Compliance to S‐1 was monitored by counting the remaining pills at each outpatient visit. Surgery was performed within 6 ± 2 weeks of the completion of planned CRT. Total mesorectal excision was always considered the first choice of surgical treatment.
Patients were scheduled to start postoperative chemotherapy, consisting of oxaliplatin 130 mg/m2 on day 1 and S‐1 80 mg/m2 per day on days 1–14 of every 21‐day cycle, within 4–6 weeks after surgery. A total of six cycles was planned.
Dose modification
Treatment‐related adverse events were evaluated according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute, version 3.0. If grade 3 toxicity was encountered, chemotherapy was stopped but radiotherapy was continued. If grade 4 toxicity was evident, both chemotherapy and radiotherapy were interrupted. Dose reduction was required after the appearance of grade 3 or 4 toxicity. Chemotherapy, with a 25% reduction in the doses of both oxaliplatin and S‐1, was recommenced when toxicity had recovered to grade 1 or less. If treatment was delayed for longer than 3 weeks, the patient was withdrawn from the study.
Assessments of efficacy and toxicity
The primary end‐point was the pathological complete response (pCR) rate and the secondary end‐points included rates of sphincteric preservation, R0 resection, downstaging and safety. Pretreatment evaluation included full medical history, physical examination, routine laboratory test, pelvic MRI with or without endorectal ultrasound, computed tomography (CT) scan of the abdomen and chest, and chest X‐ray. Toxicity assessments were performed every week during treatment. After the completion of planned CRT, an additional pelvic MRI was recommended but not required.
Pathological responses were classified according to Dworak's classification.20 The definition of a positive margin was the presence of tumor within 1 mm or less of the circumferential margin. A pCR was defined as tumor regression grade (TRG) 4. Tumor downstaging was considered appropriate when the postoperative pathological stage (pT) was lower than the pre‐CRT clinical stage (cT).
DW images analysis
Images from the DW–MRI were analyzed in patients enrolled in both phase I and II studies who underwent pelvic MRI before and after CRT. Images from the DW–MRI and ADC maps were acquired using a b value of 0 and 1000 s/mm2 applied in direction x, y and z. Pre‐ and post‐CRT ADC for individual lesions were obtained for the regions of interest, which was drawn along the border of tumor on each ADC map, and percentage changes in ADC (ΔADC) after CRT were calculated. Mean ADC values obtained before and after CRT, and mean ΔADC were correlated with pathological findings after surgery.
Statistics
According to Fleming's one‐stage phase II design, 33 patients were required to accept the hypothesis that the pCR rate was greater than 25% with 80% power and to reject the hypothesis that the pCR rate was less than or equal to 10% with 5% significance. Assuming that 10% of patients would not be assessable, we planned to include at least 37 patients in the present study. Patient characteristics and toxicities were evaluated using descriptive methods. Pre‐ and post‐CRT tumor ADC and ΔADC in the pCR and non‐pCR groups were compared using the Mann–Whitney U‐test.
Results
Patient characteristics
From January 2009 to January 2010, 38 patients were enrolled. Patient characteristics are summarized in Table 1. Of the 38 patients, 31 (81.6%) had a clinical T3 lesion and 34 (89.5%) had node‐positive disease. The median distance from the anal verge to the lower border of tumor was 5.0 cm (range, 2.0–10.0 cm) and 23 (60.5%) tumors were located within 5.0 cm of the anal verge.
Table 1.
Patient characteristics
| Characteristics | n = 38 (%) |
|---|---|
| Gender | |
| Male | 22 (57.9) |
| Female | 16 (42.1) |
| Median age (range) (years) | 54 (28–67) |
| ECOG PS | |
| 0 | 3 (7.9) |
| 1 | 35 (92.1) |
| Clinical T stage | |
| cT2 | 7 (18.4) |
| cT3 | 31 (81.6) |
| cN0 | 4 (10.5) |
| cN+ | 34 (89.5) |
| Tumor location | |
| ≤5 cm from anal verge | 23 (60.5) |
| >5 cm from anal verge | 15 (39.5) |
| Mean ± SD (cm) | 5.6 ± 2.4 |
| Median (cm) (range) | 5.0 (2.0–10.0) |
| Tumor differentiation | |
| Well differentiated | 10 (26.3) |
| Moderately differentiated | 27 (71.1) |
| Signet ring cell carcinoma | 1 (2.6) |
| Initial Hb level (g/dL) | |
| Mean ± SD | 13.2 ± 1.5 |
| Median initial CEA level (ng/mL) (range) | 2.49 (0.60–26.20) |
CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; PS, performance status; SD, standard deviation.
Dose intensity and toxicity
Of the 38 patients, 37 commenced planned CRT, but one patient withdrew consent before starting CRT. Thirty‐five patients completed the planned CRT; one patient withdrew consent after the second week due to persistent grade 3 diarrhea, and another patient stopped chemotherapy after the second week due to grade 3 anorexia and nausea, but continued with planned radiotherapy. Thirty‐six patients (94.7%) received full‐dose radiotherapy. The relative dose intensities of S‐1 and oxaliplatin were 97.5% (546.1 mg/m2 per week) and 96.6% (48.3 mg/m2 per week), respectively.
Toxicity profiles during chemoradiation are shown in Table 2. No grade 4 toxicity was observed. Two patients experienced grade 3 leukopenia, but no other grade 3 hematological toxicities were observed. Grade 3 non‐hematological toxicities included diarrhea (5.4%), anorexia (2.7%) and nausea (2.7%). Grade 3 anorexia and nausea occurred simultaneously in one patient, causing discontinuation of chemotherapy.
Table 2.
Toxicity profiles (toxicity grades according to CTC‐AE of the National Cancer Institute, version 3.0 [per person]) during treatment
| Preoperative chemoradiation (n = 37;%) | Postoperative chemotherapy (n = 33;%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Hematological toxicity | ||||||||
| Leukopenia | 6 (16.2) | 10 (27.0) | 2 (5.4) | 0 | 11 (33.3) | 13 (39.4) | 3 (9.1) | 0 |
| Neutropenia | 6 (16.2) | 8 (21.6) | 0 | 0 | 10 (30.3) | 10 (30.3) | 9 (27.3) | 0 |
| Anemia | 19 (51.4) | 1 (2.7) | 0 | 0 | 22 (66.7) | 5 (15.2) | 0 | 0 |
| Thrombocytopenia | 22 (59.5) | 0 | 0 | 0 | 20 (50.6) | 4 (12.1) | 3 (9.1) | 0 |
| Febrile neutropenia | – | – | 0 | 0 | – | – | 1 (3.0) | 0 |
| Non‐hematological toxicity | ||||||||
| Anorexia | 20 (54.1) | 0 | 1 (2.7) | 0 | 18 (54.5) | 6 (18.2) | 0 | 0 |
| Nausea | 24 (64.9) | 0 | 1 (2.7) | 0 | 16 (48.5) | 2 (6.1) | 1 (3.0) | 0 |
| Vomiting | 5 (13.5) | 2 (5.4) | 0 | 0 | 4 (12.1) | 1 (3.0) | 1 (3.0) | 0 |
| Stomatitis | 10 (27.0) | 0 | 0 | 0 | 10 (30.3) | 2 (6.1) | 0 | 0 |
| Constipation | 12 (32.4) | 1 (2.7) | 0 | 0 | 1 (3.0) | 0 | 0 | 0 |
| Diarrhea | 13 (35.1) | 5 (13.5) | 2 (5.4) | 0 | 2 (6.1) | 7 (21.2) | 0 | 0 |
| Sensory neuropathy | 23 (62.2) | 0 | 0 | 0 | 24 (72.7) | 3 (9.1) | 1 (3.0) | 0 |
| Hand–foot syndrome | 1 (2.7) | 0 | 0 | 0 | 3 (9.1) | 1 (3.0) | 0 | 0 |
| AST/ALT elevation | 11 (29.0) | 0 | 0 | 0 | 8 (24.2) | 0 | 1 (3.0) | 0 |
| Hyperbilirubinemia | 8 (21.6) | 4 (10.8) | 0 | 0 | 9 (27.3) | 4 (12.1) | 1 (3.0) | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTC‐AE, Common Terminology Criteria for Adverse Events; –, not applicable.
Surgical procedure and pathological responses
Of the 37 patients who received CRT, 35 underwent surgery; one patient withdrew consent after the second week and another patient completed CRT but refused surgery. Six (17.1%) patients underwent abdominoperineal resection (APR) and 29 (82.9%) underwent sphincter‐saving operations. Of the 22 patients who had rectal cancer within 5 cm of the anal verge, 17 (77.3%) underwent sphincter‐saving operations. The median time from the end of CRT to surgery was 6.9 weeks (range, 1.7–8.7 weeks). Three patients underwent surgery out of the predefined range of duration; one received APR earlier, in 1.7 weeks, because of a vesicorectal fistula, and two received surgical resections in 8.1 and 8.7 weeks, which corresponded to 1‐ and 5‐day delays because of the surgeon's schedule and hospital working days. R0 resection was achieved in 33 (94.3%) patients. Perioperative complications occurred in three patients; one patient developed a vesicorectal fistula, which was treated surgically, another patient had post‐operative ileus, and the third patient experienced anastomosis site leakage.
The clinical (c) and pathological (p) stages are shown in Table 3. Because two patients did not undergo surgery, the pathological stage was evaluable in 35 patients. Tumor downstaging was observed in 21 patients (60.0%). Eight patients (22.9%) achieved primary tumor pCR (pT0) and 22 (63.9%) achieved nodal pCR (pN0).
Table 3.
Pathological staging compared with clinical staging at baseline (n = 35)
| Pathological T stage | Pathological N stage | |||||||
|---|---|---|---|---|---|---|---|---|
| pT0 | pT1 | pT2 | pT3 | pT4 | pN0 | pN1 | pN2 | |
| Clinical T | ||||||||
| cT2 | 0 | 1 | 5 | 0 | 0 | – | – | – |
| cT3 | 8 | 2 | 10 | 9 | 0 | – | – | – |
| Total | 8 (22.9%) | 3 | 15 | 9 | 0 | – | – | – |
| Clinical N | ||||||||
| cN0 | – | – | – | – | – | 3 | 1 | 0 |
| cN+ | – | – | – | – | – | 19 | 12 | 0 |
| Total | – | – | – | – | – | 22 (62.9%) | 13 | 0 |
–, not applicable.
According to Dworak's classification, total tumor regression (TRG 4) occurred in eight of 35 (22.9%) patients, near total regression (TRG 3) in 10 (28.6%) patients, moderate regression (TRG 2) in 15 (42.9%) patients and minimal regression (TRG 1) in two (5.4%) patients. Therefore, the primary end‐point, pCR rate, was 22.9% (95% CI, 10.9–42.1).
Postoperative chemotherapy, follow up and recurrence
Of the 35 patients who underwent surgery, 33 received postoperative chemotherapy. One patient refused postoperative chemotherapy and another patient, who developed vesicorectal fistula after surgery, was removed from the study. The median time from surgery to chemotherapy was 3.9 weeks (range, 2.6–10.0 weeks). Of the 33 patients, 29 (82.9%) patients completed all six cycles of postoperative chemotherapy, with or without dose reduction. The relative dose intensities of S‐1 and oxaliplatin were 80.0% (896 mg/m2 per cycle) and 78.0% (102 mg/m2 per cycle), respectively. One patient was lost to follow up after one cycle, one patient withdrew consent after two cycles and another patient ceased chemotherapy after five cycles because of persistent grade 3 neutropenia and thrombocytopenia. The other toxicity profiles are summarized in Table 2.
One patient experienced disease recurrence after two cycles of postoperative chemotherapy. The time to recurrence after surgery was 5.6 weeks, and the postoperative TNM stage and TRG were pT2N1 and TRG 3, respectively. On 31 January 2011, at a median follow‐up time of 14.4 months (range, 1.2–19.3 months), only one patient experienced disease recurrence and all patients were still alive.
DW image analysis
DW‐MRI was performed before and after CRT on 24 of the 38 patients in this phase II study and 14 of the 15 patients in our previous phase I study. Therefore, of our total 50 patients, 38 were evaluable in terms of tumor ADC. The pre‐ and post‐CRT mean tumor ADC and ΔADC are summarized in Table 4. After CRT, nine patients (two in phase I and seven in phase II) had attained pCR, whereas 29 (12 in phase I and 17 in phase II) did not. The mean initial tumor ADC did not differ between the pCR and non‐pCR groups (1.17 × 10−3 mm2/s ± 0.45 vs 1.17 × 10−3 mm2/s ± 0.46, P = 0.972), but the mean tumor ADC after CRT was significantly higher in the pCR group than in the non‐pCR group (1.52 × 10−3 mm2/s ± 0.46 vs 1.07 × 10−3 mm2/s ± 0.58, P = 0.037). Moreover, the tumor ADC after CRT significantly increased by 44.5% in the pCR group, although it decreased by 7.6% in the non‐pCR group (P = 0.026).
Table 4.
Mean tumor ADC and change in ADC pre‐ and post‐chemoradiation (mean ± SD [95% CI])
| pCR (n = 9) | Non‐pCR (n = 29) | P value | |
|---|---|---|---|
| Pre‐CRT ADC (x10−3 mm2/s) | 1.17 ± 0.45 (0.81–1.51) | 1.17 ± 0.46 (1.00–1.34) | 0.972 |
| Post‐CRT ADC (x10−3 mm2/s) | 1.52 ± 0.46 (1.18–1.89) | 1.07 ± 0.58 (0.85–1.29) | 0.037 |
| ADC change (%) | 44.5 ± 68.7 (−8.6–97.1) | −7.6 ± 46.6 (−25.3–10.2) | 0.026 |
ADC, apparent diffusion coefficient; CI, confidence interval; CRT, chemoradiotherapy; pCR, pathological complete response; SD, standard deviation.
Discussion
This multicenter phase II study evaluated the efficacy and safety of the new preoperative chemoradiation regimen with S‐1 plus oxaliplatin in patients with LARC and showed that this combination was effective and safe with manageable toxicity profiles. We also found that tumor ADC, measured using DW‐MRI after CRT, and ΔADC could be a potential candidate for predicting pathological responses.
Protracted infusion of 5‐FU during radiation has been shown to yield superior DFS and OS compared with use of intermittent bolus 5‐FU. This might reflect a prolonged tumor cell exposure to the drug or the use of higher doses of 5‐FU.21 However, prolonged infusion can be inconvenient for patients and has been associated with development of common catheter‐related complications, including infection and thrombosis. Daily administration of oral fluoropyrimidine during concomitant radiotherapy might be more beneficial than protracted infusion of 5‐FU, because the pharmacokinetics of oral fluoropyrimidine is proven to be similar to that of continuous infusion of 5‐FU. S‐1, a novel oral fluoropyrimidine, was developed to improve tumor selective cytotoxicity and to reduce GI toxicity of 5‐FU.10 Lesser GI toxicity suggests that S‐1 might be a useful component of preoperative chemoradiation regimens, because diarrhea is the most frequent toxicity during CRT in patients with LARC.9
In an effort to improve outcomes, several studies have evaluated the efficacy and toxicity of preoperative CRT with 5‐FU, given either intravenously or administered orally, plus oxaliplatin in patients with LARC.22, 23 The Cancer and Leukemic Group B 89901 trial, which explored the utility of adding oxaliplatin to a continuous infusion of 5‐FU during radiation, showed that this regimen was effective, with a pCR rate of 25%, but more toxic than when 5‐FU was used alone; grade 3 or 4 diarrhea occurred in 38% of patients and only 56% completed six cycles of chemotherapy.22 Subsequently, a German multicenter phase II trial showed that the combination of oxaliplatin and capecitabine was well tolerated (grade 3 or 4 diarrhea in 12% and grade 3 or 4 sensory neuropathy in 18%) and produced a pCR rate of 17%.23 Similarly, we observed a pCR rate of 22.9%. Also, the incidence rate of grade 3 or 4 diarrhea and the rate of completing treatment were 5.4% and 95.1%, respectively. Our present findings indicate that S‐1, when combined with oxaliplatin, was at least equivalent in efficacy to that of other 5‐FU agents and was associated with lower rates of GI toxicity, including diarrhea.
Three randomized phase III studies, Action Clinique Coordonnées en cancérologie Digestive (ACCORD), STAR‐01 and CAO/ARO/AIO‐04 studies, compared preoperative chemoradiotherapy using both fluoropyrimidine and oxaliplatin with preoperative chemoradiotherapy using fluoropyrimidine alone.24, 25, 26 The ACCORD trial, which compared capecitabine plus oxaliplatin with capecitabine alone, showed no between‐group difference in the pCR rate, the primary end‐point (19.2% vs 13.9%, P = 0.09).24 Similarly, the STAR‐01 trial, which compared continuous infusional 5‐FU plus oxaliplatin with 5‐FU alone, showed no difference in the pCR rate (15% vs 16%, P = 0.982), but a longer follow up has been needed to assess OS, the primary end‐point. Although no difference in the pCR rate was evident, the percentage of patients with pathological M stage was significantly lower in the 5‐FU plus oxaliplatin group (2% vs 11%, P = 0.014), suggesting that addition of oxaliplatin to preoperative CRT affected the development of distant micrometastses.25 The CAO/ARO/AIO‐04 study also compared 5‐FU‐based preoperative CRT with or without the addition of oxaliplatin, and showed an improved pCR rate in patients receiving oxaliplatin plus 5‐FU‐based CRT (17% vs 13%, P = 0.038).26 However, the fact is that the role of oxaliplatin in preoperative CRT is still unclear; the current standard strategy is to combine fluoropyrimidine alone in preoperative radiotherapy for these patients.
Several recent studies have investigated the utility of tumor ADC, as determined using DW‐MRI, in predicting the response to preoperative CRT in patients with LARC.17, 18, 19 We found that the post‐CRT ADC was significantly higher in patients with a pCR compared with those with non‐pCR, but the pre‐CRT ADC did not differ between the two groups. These results were in contrast to those of previous studies.17, 18 Dzik‐Jurasz et al. first reported that a strong negative correlation between mean pretreatment ADC and the percentage change of tumor size after treatment.17 Subsequently, Sun et al. reported that the pre‐CRT ADC in the downstaged group was lower than that in the non‐downstaged group.18 However, more recently Kim et al. demonstrated that the post‐CRT ADC was significantly correlated with pCR. These discrepancies might be attributable to the use of different definitions of responder group (pCR or downstaging) and the relatively small size of the study populations.19
The percentage change in ADC has also been shown to be a useful predictor of pCR.18, 19 In the present study, the tumor ADC was increased in the pCR group after CRT, but fell in the non‐pCR group. Similarly, other studies found that the percentage change in ADC after CRT was significantly greater in the responder group, although the tumor ADC increased in all groups.18, 19 Together, these findings suggest that a change in ADC, as measured using DW‐MRI, could be a potential surrogate marker to predict the response to CRT.
In conclusion, we found that a new preoperative CRT regimen with S‐1 and oxaliplatin yielded results that compared favorably with the result of other studies of other preoperative CRT regimen in patients with LARC.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
Oxaliplatin and S‐1 were provided by Sanofi‐Aventis Korea (Seoul, Korea) and JEIL Pharm. Co. (Seoul, Korea) respectively. This study was supported by grants from the Korea Health 21 R&D Project, Ministry of Health & Welfare and Family Affairs, Republic of Korea (A062254 and A102065).
(Cancer Sci, doi: 10.1111/cas.12001, 2012)
Presented in part at the 2011 Gastrointestinal Cancer Symposium, San Francisco, CA, USA, January 22, 2011.
References
- 1. Colorectal Cancer Collaborative Group . Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001; 358: 1291–304. [DOI] [PubMed] [Google Scholar]
- 2. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005; 23: 5644–50. [DOI] [PubMed] [Google Scholar]
- 3. Sauer R, Becker H, Hohenberger W et al Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40. [DOI] [PubMed] [Google Scholar]
- 4. Gerard JP, Conroy T, Bonnetain F et al Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006; 24: 4620–5. [DOI] [PubMed] [Google Scholar]
- 5. Bosset JF, Collette L, Calais G et al Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–23. [DOI] [PubMed] [Google Scholar]
- 6. Andre T, Boni C, Mounedji‐Boudiaf L et al Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. [DOI] [PubMed] [Google Scholar]
- 7. Andre T, Boni C, Navarro M et al Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–16. [DOI] [PubMed] [Google Scholar]
- 8. Cividalli A, Ceciarelli F, Livdi E et al Radiosensitization by oxaliplatin in a mouse adenocarcinoma: influence of treatment schedule. Int J Radiat Oncol Biol Phys 2002; 52: 1092–8. [DOI] [PubMed] [Google Scholar]
- 9. Takechi T, Nakano K, Uchida J et al Antitumor activity and low intestinal toxicity of S‐1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol 1997; 39: 205–11. [DOI] [PubMed] [Google Scholar]
- 10. Saif MW, Syrigos KN, Katirtzoglou NA. S‐1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs 2009; 18: 335–48. [DOI] [PubMed] [Google Scholar]
- 11. Hong YS, Lee JL, Park JH et al Phase I study of preoperative chemoradiation with S‐1 and oxaliplatin in patients with locally advanced resectable rectal cancer. Int J Radiat Oncol Biol Phys 2011; 79: 684–9. [DOI] [PubMed] [Google Scholar]
- 12. Rodel C, Martus P, Papadoupolos T et al Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005; 23: 8688–96. [DOI] [PubMed] [Google Scholar]
- 13. Borschitz T, Wachtlin D, Mohler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2‐3 rectal cancer. Ann Surg Oncol 2008; 15: 712–20. [DOI] [PubMed] [Google Scholar]
- 14. Habr‐Gama A, Perez RO, Nadalin W et al Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long‐term results. Ann Surg 2004; 240: 711–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norris DG. The effects of microscopic tissue parameters on the diffusion weighted magnetic resonance imaging experiment. NMR Biomed 2001; 14: 77–93. [DOI] [PubMed] [Google Scholar]
- 16. Thoeny HC, De Keyzer F, Chen F et al Diffusion‐weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a‐4 phosphate after repeated administration. Neoplasia 2005; 7: 779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dzik‐Jurasz A, Domenig C, George M et al Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002; 360: 307–8. [DOI] [PubMed] [Google Scholar]
- 18. Sun YS, Zhang XP, Tang L et al Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion‐weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 2010; 254: 170–8. [DOI] [PubMed] [Google Scholar]
- 19. Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 2011; 21: 987–95. [DOI] [PubMed] [Google Scholar]
- 20. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997; 12: 19–23. [DOI] [PubMed] [Google Scholar]
- 21. O'Connell MJ, Martenson JA, Wieand HS et al Improving adjuvant therapy for rectal cancer by combining protracted‐infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994; 331: 502–7. [DOI] [PubMed] [Google Scholar]
- 22. Ryan DP, Niedzwiecki D, Hollis D et al Phase I/II study of preoperative oxaliplatin, fluorouracil, and external‐beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901. J Clin Oncol 2006; 24: 2557–62. [DOI] [PubMed] [Google Scholar]
- 23. Rodel C, Liersch T, Hermann RM et al Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 2007; 25: 110–17. [DOI] [PubMed] [Google Scholar]
- 24. Gerard JP, Azria D, Gourgou‐Bourgade S et al Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405‐Prodige 2. J Clin Oncol 2010; 28: 1638–44. [DOI] [PubMed] [Google Scholar]
- 25. Aschele C, Cionini L, Lonardi S et al Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR‐01 randomized phase III trial. J Clin Oncol 2011; 29: 2773–80. [DOI] [PubMed] [Google Scholar]
- 26. Rodel C, Liersch T, Becker H et al Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO‐04 randomised phase 3 trial. Lancet Oncol 2012; 13: 679–87. [DOI] [PubMed] [Google Scholar]


