Abstract
We aimed to assess the clinical efficacy of glutaraldehyde‐fixed human umbilical vein endothelial cell (HUVEC) vaccine for the treatment of patients with recurrent glioblastoma. Patients of a HUVEC vaccine group received intradermal injections of 5 × 107 HUVEC weekly during the first month, and every 2 weeks from the second month, until progression of the disease was observed. Salvage treatment consisted of multimodal chemotherapy, radiation, including gamma‐knife therapy, and/or repeated surgery, when feasible. Hazard ratios for death were calculated using a Cox model. A total of 17 patients with recurrent glioblastoma were enrolled in this study. All the patients received the initial treatment consisting of maximal safe surgical resection, followed by radiotherapy of 50–80 Gy or more, with concomitant and adjuvant chemotherapy consisting of temozolomide or nimustine (ACNU). A total of 352 vaccinations were performed for the patients of the HUVEC vaccine group (median number of vaccination = 11 doses; range 3–122 doses). The median progression‐free survival and overall survival were 5.5 and 11.4 months, respectively. The median overall survival from the diagnosis was 24.3 months. The HUVEC vaccine therapy significantly prolonged the tumor doubling time and contributed to reducing the tumor growth rate. Hematological adverse reactions due to chemotherapy were recognized: one patient experienced grade III leukocytopenia and one showed grade II lymphocytopenia. Associated with the HUVEC vaccine therapy, a delayed‐type hypersensitivity‐like skin reaction developed at the injection site. The HUVEC vaccine therapy effectively controlled disease progression, without evident adverse effects, except for a delayed‐type hypersensitivity‐like skin reaction at the injection site.
Glioblastoma (GBM) is one of the most devastating human tumors. Even with optimal surgical resection and standard chemoradiotherapy, GBM always recurs, and no specific treatment exists for recurrent GBM. Unfortunately, the median survival following recurrence is 5–7 months.1 GBM is a highly vascular tumor with high expression of vascular endothelial growth factor (VEGF).2, 3 Bevacizumab (BEV), a humanized monoclonal antibody to VEGF, which inhibits tumor angiogenesis, consequently decreasing the intratumoral blood flow,3, 4, 5, 6, 7 had been expected to reduce the volume of recurrent tumors at the time the drug was approved for clinical use and clinical studies were started. However, in patients with recurrent GBM, BEV has only limited clinical benefit. Although BEV causes a strong decrease of contrast enhancement on magnetic resonance images, vascular remodeling induced by BEV, which makes tumors more hypoxic and glycolytic,8, 9 might result in increased invasiveness of tumor cells into the normal brain tissue. Enhanced tumor cell infiltration after anti‐angiogenic treatment has been reported in other tumor models.4, 9, 10, 11
Human endothelial cells in culture share some properties with the angiogenic endothelium, such as the high expression of CD51 and CD105.12 We have been focusing on human umbilical vein endothelium cell (HUVEC), which, under culture with VEGF and basic‐fibroblast growth factor, has specific properties of angiogeneic endothelium, such as the high expression of the platelet endothelial cell adhesion molecule (CD31), integrin alpha‐V precursor (CD51) and endoglin (CD105), as confirmed by flow‐cytometry.12, 13 We have also confirmed the expression of these surface markers on the vascular endothelium of GBM and colorectal cancer. Based on these results, we tested and confirmed the effectiveness of glutaraldehyde‐fixed allogeneic endothelial cells as a vaccine against solid tumors (in an animal model). In addition, we commenced a clinical trial of allogeneic HUVEC as a vaccine for the treatment of angiogenic solid tumors (malignant brain tumors and colorectal cancer) in humans. Patients with recurrent malignant brain tumors had better clinical response than those with metastatic colorectal cancer. This might be mainly dependent on the smaller size of the targeted tumor lesions of the patients with malignant brain tumors.12 In the present study, we aim to assess the clinical efficacy of glutaraldehyde‐fixed HUVEC vaccine for the treatment of patients with recurrent GBM.
Materials and Methods
Study design
We investigated 25 consecutive patients with recurrent malignant gliomas. There was no limit on previous regimens or salvage treatments. The HUVEC vaccine therapy was approved in 2002 by The University of Tokyo Investigational Review Board (No. 506), according to the “Good Clinical Practice for Medical Devices” guidelines, as well as the “Pharmaceutical Affairs Act in Japan.” Informed consent, in accordance with the Declaration of Helsinki, was obtained from each patient or from a legally authorized representative before inclusion in the HUVEC vaccine therapy.
Patient population
The patient eligibility criterion was as follows: (i) presence of histopathologically‐confirmed glioblastoma; (ii) the standard of care involving surgery followed by chemoradiotherapy already performed; (iii) recurrence of the disease despite treatment; and (iv) no corticosteroid use at enrollment. There were no restrictions in regards to Karnofsky performance scale (KPS).
Vaccine preparation and treatment plan
HUVEC were isolated from healthy donors at delivery, with informed consent, and cultured on 0.1% gelatin (w/v)‐coated dishes in EC‐SFM (Life Technologies, Grand Island, NY, USA), as described in our previous manuscript. HUVEC were fixed with 0.025% glutaraldehyde (v/v) and stored at −80°C in single dose aliquots, containing 5 × 107 cells/mL in physiological saline for injection.12 The patients received intradermal injections of 1.5 mL vaccine in the upper arm weekly during the first month, and every 2 weeks subsequently, until progression of the disease was observed.
Efficacy and safety assessment
Patients who had received at least one dose of the HUVEC vaccine were included in the present study. MRI evaluations were performed at enrollment and every 3 months. Tumor progression was diagnosed based on the reports by neuroradiologists. Tumor response and safety were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)14 and the common terminology criteria for adverse events (NCI‐CTCAE version 4.0),15 respectively. Immune response was evaluated by examining patients' peripheral blood mononuclear cells and sera, which were taken monthly.
Statistical analysis
Progression‐free survival (PFS) was calculated from the start of HUVEC vaccine therapy to date of progression or last follow up, whereas overall survival time was defined as the time between HUVEC vaccine therapy and death or last follow up. For tumor doubling times, the following equation was used: Tumor doubling time = log2/3 × {(time between the HUVEC vaccine therapy and death or last follow up)/[log (the tumor diameter at each follow‐up period) − log (the residual tumor diameter after repeated resection)]}.16 A paired t‐test was performed to determine statistically significant differences between the two sets of measurements.
The Kaplan–Meier analysis was applied for the survival analyses, and statistical significance was calculated using the log‐rank test. With regard to survival time, multivariate analyses were performed using a forward Cox's proportional hazard model adjusted for the following 15 clinical variables: age, sex, tumor location (frontal/temporal/parietal/occipital), primary tumor diameter, extent of initial resection, initial radiation dose, time from initial resection to treatment, KPS at recurrence, repeat resection (yes/no), residual tumor diameter after repeat resection, recursive partitioning analysis classification (Class IV–VII) by Carson et al.,17 seeding at recurrence (yes/no), salvage radiotherapy (gamma knife/Boron neutron capture therapy/none), salvage chemotherapy (temozolomide and/or interferon‐beta/none), and cortical steroid use during the study (yes/no). A two‐sided P‐value <0.05 defined statistical significance in all models. All statistical analyses were conducted using IBM spss version 19.0 software (SPSS, IBM, Somers, NY, USA).
Results
Patient characteristics
The 25 consecutive patients were enrolled in the HUVEC vaccine therapy at the Department of Neurosurgery, the University of Tokyo Hospital, in the period between August 2002 and August 2011. Among them, eight patients were excluded because four had anaplastic glioma (World Health Organization grade III), including anaplastic oligodendroglioma, three had pontine glioma, and one had pineoblastoma. The median survival time for recurrent anaplastic oligodendroglioma was 14.5 months (95% CI, not calculated), and the median survival for recurrent pontine glioma was 10.0 months (95% CI, 6.5–13.5). Consequently, 17 patients with recurrent GBM (15 primary and 2 secondary) were enrolled in this study.
All patients had received the initial treatment consisting of maximal safe surgical resection, followed by radiotherapy of 50–80 Gy or more, with concomitant and adjuvant chemotherapy consisting of temozolomide or nimustine (ACNU); 12 received standard radiotherapy of 60 Gy, three received doses of 50–54 Gy and two received high‐dose radiotherapy of 80 Gy. All patients progressed in despite of previous temozolomide or nitorourea chemotherapy; 14 had the standard schedule of temozolomide (200 mg/m2 days 1–5, repeated every 28 days) and three received ACNU (ACNU 100 mg/body day 1, repeated every 2 months) or ACNU/VCR (ACNU 100 mg/body day 1 and VCR 1 mg/body day 8 and day 15, repeated every 2 months).
At recurrence, they received the currently available salvage treatment: chemotherapy, radiation consisting of gamma‐knife therapy or boron neutron capture therapy, and/or repeated operation when feasible. KPS was evaluated at recurrence: median KPS = 60%. No patient received prior BEV treatment. Corticosteroids were used by four patients during the study. The patient and tumor characteristics are outlined in Table 1.
Table 1.
Patient's characteristics
| Characteristics | Number of patients (n = 17) |
|---|---|
| Age, mean ± SD (years) | 46.9 ± 10.2 |
| <50 | 8 |
| >50 | 9 |
| Sex | |
| Women | 7 |
| Men | 10 |
| Tumor location | |
| Frontal | 8 |
| Parietal | 4 |
| Temporal | 3 |
| Occipital | 2 |
| Primary tumor diameter, mean ± SD (cm) | 4.1 ± 0.9 |
| <4 | 4 |
| >4 | 13 |
| Extent of initial resection, mean ± SD (%) | 75.9 ± 30.7 |
| >95 | 6 |
| <95 | 11 |
| Initial radiation dose, Gy | |
| 50−60 | 3 |
| 60 | 12 |
| 80 | 2 |
| Time from initial resection to treatment | |
| Median, months | 9.9 |
| 95% Confidence interval | 6.3–13.5 |
| <6 months | 6 |
| >6 months | 11 |
| KPS at recurrence | |
| 90–100 | 1 |
| 70–80 | 6 |
| 50–60 | 10 |
| Repeated resection at recurrence | |
| Yes | 6 |
| No | 11 |
| The diameter at the recurrence, mean ± SD (cm) | 3.4 ± 1.1 |
| The residual tumor diameter after repeated resection, mean ± SD (cm) | 2.5 ± 1.5 |
| <4 | 13 |
| >4 | 4 |
| Recursive partitioning analysis classification | |
| Class 4 | 1 |
| Class 5 | 9 |
| Class 6 | 4 |
| Class 7 | 3 |
| Seeding at recurrence | |
| Yes | 7 |
| No | 10 |
| Salvage radiotherapy | |
| Gamma knife | 4 |
| Born neutron capture therapy | 2 |
| None | 11 |
| Salvage chemotherapy | |
| Temozolomide and/or interferon‐beta | 7 |
| None | 10 |
| Corticosteroid use during the study | |
| Yes | 4 |
| No | 13 |
KPS, Karnofsky performance scale.
Immune response and clinical response
A total of 352 vaccinations were performed: median number of vaccination = 11 doses, range 3–122 doses. Immunological screening was performed to confirm the immune response against HUVEC. ELISA revealed specific immunoglobulin response against HUVEC membrane antigens. In addition, HUVEC‐specific CTL responses were detected using a gamma interferon enzyme‐linked immunospot assay 1 month or later after the start of the treatment. Patients' cellular effectors specifically lysed HUVEC, but not non‐endothelial control cells.12 Chromium‐release cytotoxicity assay revealed a specific cellular immune response against HUVEC (data not shown).12
Among 17 patients, progression disease occurred in four patients within 3 months (Fig. 1). An additional nine patients progressed slowly over several months. Three patients remained stable within 6 months and one patient had a partial response. The radiological response rate (cases with complete or partial response) was 5.9%.
Figure 1.
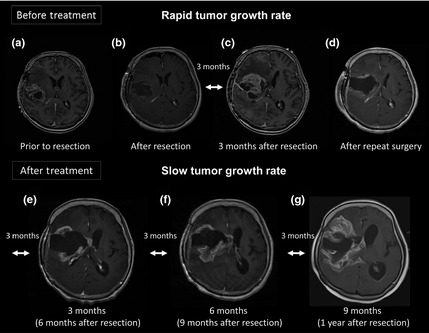
Serial magnetic resonance image of a patient who showed a rapid progression. The patient recurred within 3 months and repeated surgery was performed. Karnofsky performance scale at recurrence was 60%. After repeated surgery only the HUVEC vaccine therapy was continued. The tumor kept growing, but the tumor growth speed seemed to be slowed by the HUVEC vaccine therapy.
Progression‐free survival and tumor volume doubling time
The median progression‐free survival (PFS) was 5.5 months (95% CI, 3.1–7.9 months) (Fig. 2). The 6‐month PFS rate was 47.1% (95% CI, 25.5–69.7%), and the 12‐month PFS rate was 23.5% (95% CI, 9.1–48.6%). The mean tumor volume doubling time at recurrence was calculated as 25.3 ± 23.6 days (range, 3–77 days; median = 17 days, n = 17). After enrollment, measurements of the tumor volume doubling time were completed by 17 patients at 3‐month follow up, 16 patients at 6‐month follow up, nine patients at 9‐month follow up, and five patients at 12‐month follow up. The tumor volume doubling time at 3 months after enrollment was calculated to be fast (16–89 days) in 11 patients, slow (206–577 days) in five patients and negative (−94 days) in one patient, suggesting a shrinking tumor. The tumor doubling time, except for the shrinking tumor, was compared with that at recurrence: the mean tumor doubling time at 3‐month follow up = 134.7 ± 166.6 days (n = 16) versus the mean tumor doubling time at recurrence = 22.6 ± 21.6 days (n = 16); paired t‐test P = 0.012. The mean tumor doubling time after 6 months of treatment was also significantly elongated compared to that at recurrence: the mean tumor doubling time at 6‐month follow up = 169.5 ± 262.5 days (n = 15) versus the tumor doubling time at recurrence = 23.9 ± 21.7 days (n = 15); paired t‐test P = 0.047. After 6 months, however, the tumor volume doubling times were shortened in six patients. Fig.3 shows the time course of the mean diameter of recurrent tumors at each follow‐up period. The recurrent tumors were stable in size for 6 months after enrollment, which reflected the results of tumor volume doubling times.
Figure 2.
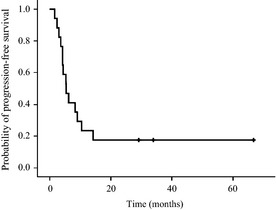
Kaplan–Meier progression‐free survival.
Figure 3.
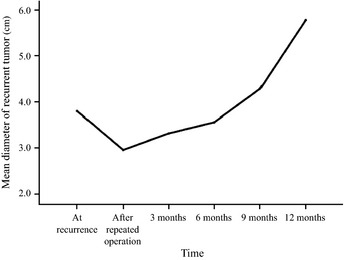
The time course of the mean diameter of the recurrent tumors.
Overall survival
The overall survival was 11.4 months (95% CI, 7.9–14.9 months) (Fig. 44). The 6‐month overall survival (OS) rate was 88.2% (95% CI, 63.2–97.0) and the 12‐month OS rate was 47.1% (95%CI, 25.5–69.7%). At the time of analysis, 14 patients had died of tumor progression and three patients were alive (29.2, 34.0 and 66.5 months since treatment started). One patient who survived more than 60 months after recurrence continued to receive the HUVEC vaccine therapy every month. The 5‐year OS rates were 17.6% (95% CI, 5.8–42.7%). The median time interval from the primary resection to the date of death or last follow up was 24.3 months (95% CI, 17.7–30.9).
Figure 4.
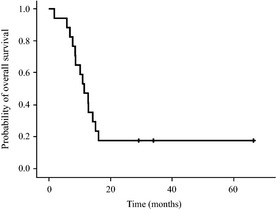
Kaplan–Meier overall survival.
Survival according to clinical variables was provided in Table 2. There was a statistically significant difference in OS between five patients <50 years of age and 12 patients >50 years of age: the median OS was 7.8 months (95% CI, 5.4–10.2 months) versus 14.2 months (95% CI, 9.8–18.6 months), respectively (P = 0.012). There was also a significant difference in OS between 11 patients with frontal or temporal tumors and six patients with parietal or occipital tumors: the median OS was 12.7 months (95% CI, 10.3–15.1 months) versus 6.9 months (95% CI, 4.8–9.0 months), respectively (P = 0.040). There were no significant differences in survival between six patients who were enrolled less than 6 months after the initial surgery and 11 patients who were enrolled at 6 months or more after the initial surgery (P = 0.8). There was also no difference in OS between four patients who used corticosteroids and 13 patients who did not (P = 0.8).
Table 2.
Survival according to clinical variables
| Variable | Number of patients | Overall survival | P | ||
|---|---|---|---|---|---|
| Median (95% CI) months | 6 months (95% CI) % | 12 months (95% CI) % | |||
| Age | |||||
| <50 years | 5 | 7.8 (5.4–10.2) | 75.0 (37.7–93.7) | 25.0 | 0.012 |
| >50 years | 12 | 14.2 (9.8–18.6) | 100 | (6.3–62.3) 55.6 (25.1–82.3) | |
| Location | |||||
| Frontal or temporal | 11 | 12.7 (10.3–15.1) | 100 | 58.3 (30.7–81.5) | 0.040 |
| Parietal or occipital | 6 | 6.9 (4.8–9.0) | 60.0 (20.0–90.0) | 20.0 (2.7–69.1) | |
| Disease progression | |||||
| <6 months pre‐enrollment | 6 | 8.7 (4.3–13.0) | 100 | 33.3 (8.3–73.2) | 0.8 |
| >6 months pre‐enrollment | 11 | 12.7 (9.8–15.6) | 81.8 (49.3–95.4) | 54.5 (26.8–79.7) | |
| Corticosteroid use | |||||
| Yes | 4 | 8.7 (0.0–20.9) | 75.0 (23.8–96.6) | 50.0 (12.3–87.7) | 0.8 |
| No | 13 | 11.4 (8.3–14.5) | 92.3 (60.9–98.9) | 46.2 (22.4–71.8) | |
Among the 15 clinical variables, however, multivariate survival analysis using Cox's proportional hazard model showed that age was the only clinical variable that lengthened overall survival: adjusted hazard ratio = 0.865, (95% CI, 0.785–0.952); P = 0.003. Seeding at the recurrence was not a prognostic factor (P = 0.4): seven patients with seeding = 12.7 months (95% CI, 6.0–19.4 months) versus 10.9 months (95%CI, 6.7–15.1 months) = 10 patients without seeding.
Safety
The regimen was well tolerated. Delayed‐type hypersensitivity (DTH)‐like skin reaction developed at the injection site in 14 of 17 patients. However, except for this kind of skin reaction, no other types of adverse effects associated with the HUVEC vaccine therapy were observed. Of 14 patients who underwent salvage chemotherapy consisting of temozolomide and interferon‐beta, one patient experienced grade III leukocytopenia, and one showed grade II lymphocytopenia: both continued the chemotherapy with a dose reduction of 25%. Other side effects, such as hemorrhage at the site of the tumor and a high level of protein in the urine, were not recognized.
Discussion
The HUVEC vaccine therapy was feasible for the patients with recurrent GBM and a low KPS score. In the present study, the radiological response rate was much lower (5.9%) than that for BEV (more than 50%),4 but the patients had relatively long survival time: the median PFS and OS were 5.5 and 11.4 months, respectively. The median OS from the diagnosis was 24.3 months. The median OS compares favorably with that reported for other salvage therapies. The median OS for patients with GBM treated with temozolomide at first relapse was approximately 8 months.18 Similarly, a phase II trial of BEV‐alone or the BEV‐plus‐irinotecan in patients with recurrent GBM demonstrated that the median OS was 9.2 and 8.7 months, respectively.5, 6, 7
According to the report by Carson, prognostic factors for recurrent GBM include age, KPS, corticosteroid use, and shorter time from original diagnosis to recurrence.17 In this study, age was the only prognostic factor, but the patients over age 50 survived longer than those under age 50. It could depend on the bias of patients' background: 10 patients (59%) had poor neurological function (KPS of 50‐60%), and four patients (24%) had used corticosteroids during the study.
Because patients with recurrent GBM were clinically deteriorated, and previously treated with multimodality therapy consisting of surgery and chemoradiotherapy, treatment options were limited to palliative surgery when feasible, radiotherapy, including stereotactic radiosurgery, and chemotherapy. Because immunotherapy is associated with low risk of toxicity, it is a promising treatment strategy for recurrent GBM. Wilms tumor 1 peptide vaccine therapy for patients with recurrent GBM showed promising results: the 6‐month PFS rate was 33.3% and the median OS was 9.2 months. This treatment, however, is limited to patients with the HLA‐A *2402 phenotype.19
We established the HUVEC vaccine therapy for patients with progressive malignancy.12 The HUVEC vaccine therapy was designed to target antigens specifically or preferentially on human tumor endothelium, such as CD31, integrin alpha v beta 3 and CD105: CD31 can be isolated from glioblastoma specimens;20 integrin alpha v beta 3 plays a key role in endothelial cell survival and migration during angiogenesis;21 and CD105 is considered an appropriate marker of tumor‐related angiogenesis and neovascularization.22 Therefore, different from single‐target therapies, such as “VEGF‐targeted therapy” or peptide‐based immunotherapy, HUVEC vaccine therapy is a multi‐targeted immunotherapy. Compared with single‐targeted therapies, multi‐targeted immunotherapy has the advantage of reducing the risk of resistance to therapy, as well as the in vivo selection of the ideal antigen to be exposed to the immune system by antigen‐presenting cells. The most important limiting factor of single peptide‐based immunotherapy is the use of peptides that are effectively presented only by specific human leukocyte antigen (HLA) subtypes, eliciting HLA‐restricted CTL responses. Thus, it cannot be applied to every patient, but to a limited population with a specific HLA subtype. In comparison, the HUVEC vaccine, which consists of whole cells expressing various kinds of angiogenic antigens (multi‐target), may allow the different antigen presenting cells expressing different HLA to “select” the best antigenic determinant from the antigen “repertoire” to be presented and to generate the anti‐angiogenic CTL. As evidence to support this theory, for at least 6 months after enrollment, the HUVEC vaccine therapy resulted in significant elongation of the tumor doubling time and delay of tumor progression in patients selected without consideration of their HLA subtypes (Fig. 2). Specific antibodies and cellular immunity reactive with HUVEC's membrane antigens were detected in monthly samples of the patients. At 1 month after enrollment, specific antibodies such as CD31, CD51, CD105 and CD146 were also detected. IFN‐γ secretion by patients' peripheral blood mononuclear cells was measured in the presence of HUVEC by enzyme‐linked immunospot.12 The evident reduction of the tumor growth rate, however, could be observed from the third month after the start of the HUVEC vaccine therapy.
In this study, hematological adverse reactions of grades 2–3 were recognized, but they were associated with the administration of chemotherapy. Although we continued the vaccination protocol for long periods of time with an expectation of inducing a long‐lasting immune response, no adverse effect caused by the HUVEC vaccine therapy was observed, except for a DTH‐like skin reaction at the injection site. In contrast, patients with recurrent GBM receiving BEV alone or in combination with irinotecan experienced grade 3 adverse events in 46.4 and 65.8% of cases, respectively, including hypertension, proteinuria, convulsion and neutropenia. Intracranial hemorrhage was also reported in 2 patients (2.4%) of a BEV‐alone group (grade 1) and in three patients (3.8%) of a BEV plus irinotecan group (grades 1, 2 and 4, respectively).6 In addition, BEV has been linked to increased invasiveness.11 Thus, the HUVEC vaccine seemed to be superior to BEV in terms of adverse reactions. The main difference between BEV and the HUVEC vaccine is that BEV targets one angiogenic factor, namely VEGF, whereas the HUVEC vaccine targets the angiogenic vascular endothelium itself, inducing humoral and cellular immune responses against it.
Cancer cells may acquire the ability to produce angiogenic factors other than VEGF, such as acidic or basic fibroblast growth factor, interleukin‐8, and epidermal growth factor, among others, overcoming the effect of BEV. Different from cancer cells, the HUVEC or angiogenic endothelial cells are normal cells, which rarely develop mutations. Because the HUVEC provide several targets, such as CD31 and CD105, different from the BEV, in which the only target is the VEGF, the HUVEC vaccine therapy might not increase the degree of invasiveness.
Once the immunity against tumor angiogenesis is stimulated, theoretically, it can be permanently reactivated by periodic vaccinations, and might be useful for the control of not only brain tumors, but also other solid tumors that also depend on angiogenesis for their growth and metastasis. Unfortunately, in the case of fast‐growing and large tumors, the vaccine is not successful in controlling the growth of tumor mass (Fig. 1). The HUVEC vaccine therapy against recurrent GBM, therefore, should be used in combination with other treatment modalities. In a pilot study including metastatic colorectal cancer as well as other malignant brain tumors, such as recurrent anaplastic oligodendroglioma and recurrent pineoblastoma, specific antibodies and cellular effectors against HUVEC membrane antigens were detected in all the patients.12 However, patients with malignant brain tumors had better clinical response than those with metastatic colorectal cancer, which might be mainly because of smaller lesions targeted. Different from patients with GBM, colorectal cancer patients were enrolled in the HUVEC vaccine protocol only after the relapse of all available treatment modalities. Thus, a similar trial could not be conducted for colorectal cancer. Patients with other tumor types have also been included, but only a few cases have been evaluated, without conclusive findings. Because the safety of this treatment modality could be proved, similar trials should be conducted to analyze the other solid tumor types that might benefit from its application.
In conclusion, the HUVEC vaccine therapy is a promising new treatment modality, without important adverse effects. Acquisition of resistance to multi‐target immunotherapy, namely the HUVEC vaccine therapy, seems to be less frequent. As a matter of course, a large‐scale prospective study will help confirm our present results.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
The authors thank Dr Yurai Okaji, Ms Madoka Nishimori, Ms Mika Matsuhashi, Mr Yutaka Nagura and Ms Michiru Kawabata from the Department of Transfusion Medicine (The University of Tokyo) for their kind advisory and technical assistance. This study was supported in part by the Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; in part by the Ministry of Health, Labor and Welfare of Japan; and in part by the Japan Society for the Promotion of Science.
(Cancer Sci, doi: 10.1111/cas.12055, 2012)
References
- 1. Cloughesy T. FDA accelerated approval benefits glioblastoma. Lancet Oncol 2010; 11: 1120. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. World Health Organization Classification of Tumors of the Central Nervous System. Lyon: IARC Press, 2007. p. 33–46. [Google Scholar]
- 3. Batchelor TT, Duda DG, di Tomaso E et al Phase II study of cediranib, an oral pan‐vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 2010; 28: 2817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott BJ, Quant EC, McNamara MB, Ryg PA, Batchelor TT, Wen PY. Bevacizumab salvage therapy following progression in high‐grade gliomas patients treated with VEGF receptor tyrosine kinase inhibitors. Neuro Oncol 2010; 12: 603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007; 25: 4722–9. [DOI] [PubMed] [Google Scholar]
- 6. Friedman HS, Prados MD, Wen PY et al Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009; 27: 4733–40. [DOI] [PubMed] [Google Scholar]
- 7. Xu T, Chen J, Lu Y, Wolff JEA. Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival‐gain analysis. BMC Cancer 2010; 10: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lucio‐Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 2009; 15: 4589–99. [DOI] [PubMed] [Google Scholar]
- 9. Pàez‐Ribes M, Allen E, Hudock J et al Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15: 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keunen O, Johansson M, Oudin A et al Anti‐VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A 2011; 108: 3749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pàez‐Ribes M, Allen E, Hudock J et al Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15: 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okaji Y, Tsuno NH, Tanaka M et al Pilot study of anti‐angiogenic vaccine using fixed whole endothelium in patients with progressive malignancy after failure of conventional therapy. Eur J Cancer 2008; 44: 383–90. [DOI] [PubMed] [Google Scholar]
- 13. Okaji Y, Tsuno NH, Saito S et al Vaccines targeting tumour angiogenesis – a novel strategy for cancer immunotherapy. Eur J Surg Oncol 2006; 32: 363–70. [DOI] [PubMed] [Google Scholar]
- 14. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors, European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 15. NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 data files. 2010. [Cited 17 May 2010.] Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 16. Collins VP, Loeffler RK, Tivey H. Observation on growth rates of human tumors. Am J Roentgenol 1956; 76: 988–1000. [PubMed] [Google Scholar]
- 17. Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol 2007; 25: 2601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yung WK, Albright RE, Olson J et al A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 2000; 83: 588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Izumoto S, Tsuboi A, Oka Y et al Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 2008; 108: 963–71. [DOI] [PubMed] [Google Scholar]
- 20. Ricci‐Vitiani L, Pallini R, Biffoni M et al Tumour vascularization via endothelial differentiation of glioblastoma stem‐like cells. Nature 2010; 468: 824–8. [DOI] [PubMed] [Google Scholar]
- 21. Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994; 264: 569–71. [DOI] [PubMed] [Google Scholar]
- 22. Nassiri F, Cusimano MD, Scheithauer BW et al Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res 2011; 31: 2283–90. [PubMed] [Google Scholar]


