Abstract
Lymph node metastasis is the most important prognostic factor of endometrial cancer. However, effective therapy has not been established against lymph node metastasis. In this study, we explored the efficacy of gene therapy targeting lymph node metastasis of endometrial cancer by suppressing the action of vascular endothelial growth factor (VEGF)‐C through soluble VEGF receptor‐3 (sVEGFR‐3) expression. For this purpose, we first conducted a model experiment by introducing sVEGFR‐3 cDNA into an endometrial cancer cell line HEC1A and established HEC1A/sVEGFR‐3 cell line with high sVEGFR‐3 expression. The conditioned medium of HEC1A/sVEGFR‐3 cells inhibited lymphatic endothelial cell growth in vitro, and sVEGFR‐3 expression in HEC1A cells suppressed in vivo lymph node and lung metastases without inhibiting the growth of a subcutaneously inoculated tumor. To validate the therapeutic efficacy, adeno‐associated virus vectors encoding sVEGFR‐3 were injected into the skeletal muscle of mice with lymph node metastasis. Lymph node and lung metastases of HEC1A cells were completely suppressed by the muscle‐mediated expression of sVEGFR‐3 using adeno‐associated virus vectors. These results suggest the possibility of gene therapy against lymph node and lung metastases of endometrial cancer by using muscle‐mediated expression of sVEGFR‐3.
Endometrial cancer is the most commonly encountered gynecologic malignancy and the fourth most common malignant tumor in the USA.1 Because this cancer is often detected at an early stage while it is still confined to the uterus, the overall survival rate exceeds 80%.1 However, the prognosis of advanced endometrial cancer remains poor.2, 3 Although various attempts have been made to treat advanced endometrial cancer, including surgery, radiotherapy and multidrug chemotherapy, satisfactory progress has not been achieved. In fact, overall treatment results in endometrial cancer have not improved over the past 30 years.1 The most important prognostic factor in endometrial cancer is extra‐uterine spread, especially lymph node and lung metastases.4 Therefore, to improve the prognosis of endometrial cancer, it is necessary to develop effective therapy against such advanced conditions.
Factors related to lymphangiogenesis and lymph node metastasis have been clarified recently. Among these factors, vascular endothelial growth factor (VEGF)‐C, which is the natural ligand for VEGF receptor‐3 (VEGFR‐3), is one of the most important. VEGF‐C binds to VEGFR‐3 and induces its tyrosine autophosphorylation. VEGF‐C is specific to the lymphatic vascular system and mediates lymphangiogenesis.5 In malignant tissues, the tumor cells and stromal cells promote VEGF‐C secretion, thereby inducing lymphangiogenesis and lymph node metastasis.6 The soluble form of VEGFR‐3 (sVEGFR‐3) is a potent inhibitor of VEGF‐C signaling, which inhibits fetal lymphangiogenesis and induces regression of already formed lymphatic vessels.7 Therefore, lymph node metastasis of a malignant tumor may be controlled by the action of sVEGFR‐3.
Recently, we developed a murine model for lymph node metastasis using orthotopic injection of an endometrial cancer cell line.8 Based on the study, we sought to investigate the efficacy of sVEGFR3 by its constitutive expression. For this purpose, the adeno‐associated virus (AAV) vector is appropriate. The AAV is a widely–used vector derived from a nonpathogenic virus, and long‐term transgene expression can be obtained following intramuscular injection.9 We have reported the efficacy of muscle‐mediated soluble Flt‐1 expression using AAV vectors in both subcutaneous and intraperitoneally disseminated ovarian cancer.10 In this study, we explored the efficacy of gene therapy against metastases of endometrial cancer by muscle‐mediated expression of sVEGFR‐3 using AAV vectors.
Materials and Methods
Cells and plasmids
The human endometrial cancer cell line HEC1A11 was obtained from the Japanese Collection of Research Bioresources, where the cell line is authenticated by the Multiplex‐PCR method using short tandem repeats.12 The HEC1A was cultured in DMEM/F12 (GIBCO, Grand Island, NY, USA) supplemented with 10% inactivated FCS, 100 U/mL of penicillin and 100 μg/mL of streptomycin (GIBCO) at 37°C in a 5% CO2 atmosphere. Human neonatal dermal lymphatic endothelial cells (LEC) were purchased from AngioBio (Del Mar, CA, USA) and maintained in EGM‐MV2 BulletKit (Cambrex, East Rutherford, NJ, USA) supplemented with 10% inactivated FCS at 37°C in a 5% CO2 atmosphere. All cell lines were maintained for less than 3 months after resuscitation. The cDNA of sVEGFR‐3 was cloned by PCR using a human lung cDNA library (Stratagene, La Jolla, CA, USA) as a template, with the primers previously described.7 Cloned sVEGFR‐3 cDNA was inserted into the multi‐cloning site (MCS) of pSecTagHygroB vector (Stratagene) to generate a VEGFR‐3‐expression plasmid. A luciferase (LUC)‐encoding plasmid was also constructed and used as a control vector. p2ITR‐sVEGFR‐3 is an sVEGFR‐3 expression plasmid prepared by incorporating human sVEGFR‐3 cDNA into the EcoRI site of pAAV‐MCS (Stratagene).
Development of stably transduced cells
Either an sVEGFR3‐expression or a LUC‐expression plasmid was introduced into the HEC1A cells using the standard calcium phosphate precipitation method.13 After transfection, the cells were cultured and selected in the presence of 200 μg/mL of hygromycin B (Invitrogen, Carlsbad, CA, USA). After 4 weeks, the hygromycin B‐resistant HEC1A/sVEGFR‐3 and HEC1A/LUC cell lines were established and maintained thereafter in the presence of 200 μg/mL of hygromycin B.
Adeno‐associated virus vector production
Adeno‐associated virus vectors were produced by triple‐plasmid transfection to 293 cells (Stratagene) using p2ITR‐sVEGFR‐3, the helper plasmid for adenovirus genes,14 and the helper plasmid for AAV1.15, 16 A plasmid encoding human coagulation factor IX (hfIX) gene was used to prepare the control AAV vector.17 The vector stocks were purified using cesium chloride density‐gradient ultracentrifugation, and the titer was determined by dot blot and real‐time PCR, as described previously.18 The primers were designed to amplify the cytomegalovirus promoter sequence, and the forward and reverse primers were 5′‐GTA TTT ACG GTA AAC TGC CCA CTT‐3′ and 5′‐AGT CCC ATA AGG TCA TGT ACT GG‐3′, respectively.
Vascular endothelial growth factor‐C and soluble vascular endothelial growth factor receptor‐3 quantitation
HEC1A, HEC1A/LUC and HEC1A/sVEGFR‐3 cells were inoculated in 10‐cm dishes and cultured in a 10% FCS‐supplemented DMEM/F12 medium. When the cells grew to approximately 80% confluence, the culture supernatant was replaced with serum‐free culture medium. After 48 h, the culture supernatant was recovered. The concentration of VEGF‐C in the supernatant of HEC1A was determined using a Quantikine Human VEGF‐C enzyme‐linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA). The concentrations of sVEGFR‐3 in the supernatant of HEC1A, HEC1A/LUC and HEC1A/sVEGFR3 were determined by DuoSet Human VEGFR‐3 (R&D Systems).
Lymphatic endothelial cells proliferation assay
Conditioned media were generated by culturing 1 × 106 HEC1A/LUC cells or HEC1A/sVEGFR‐3 cells in 2 mL of serum‐free DMEM/F12 for 48 h. LEC (5 × 103/well) were plated in 96‐well plates in EGM‐MV2 plus 5% FCS containing 50% of either conditioned medium with 100 ng/mL VEGF‐C (R&D Systems). LEC proliferation was assessed by a colorimetric assay using Cell Proliferation Kit II (XTT; Boehringer Mannheim Biochemica, Mannheim, Germany) 48 h after plating.
Tumor cell transduction model: subcutaneously inoculated tumor growth
Four to six‐week‐old female BALB/c nude mice (Japan Clea Laboratories, Tokyo, Japan) were used in the experiment. HEC1A/LUC or HEC1A/sVEGFR‐3 cells (5 × 106) were subcutaneously transplanted into the backs of the mice, and tumor sizes were measured once a week using a micrometer caliper. Tumor volume was calculated using the formula: volume = (short diameter)2 × (long diameter) × 0.5.19
Evaluation of metastasis
HEC1A/LUC or HEC1A/sVEGFR‐3 cells (5 × 106) were injected into the uterine cavities of pentobarbital sodium‐anesthetized, laparotomized mice, as described previously.8 After 8 weeks, metastatic lesions were thoroughly investigated and counted.
Therapeutic model using adeno‐associated virus vector: evaluation of metastasis in orthotopically inoculated model
HEC1A cells (5 × 106) were injected into the uterine cavities of pentobarbital sodium‐anesthetized, laparotomized mice, as described previously.8 At the same time AAV1‐hfIX or AAV1‐sVEGFR‐3 vector (2.5 × 1012 genome copy) was injected into the hind‐limb skeletal muscles of the mice. Eight weeks after injection, the metastatic changes were extensively investigated and numbers of enlarged lymph nodes and lung metastases were counted.
Statistical analysis
Intergroup differences were tested for significance using Student's t‐test. A P‐value <0.05 was considered significant.
Results
Detection of vascular endothelial growth factor‐C and soluble vascular endothelial growth factor receptor‐3 in culture supernatants
The concentration of VEGF‐C in the culture supernatant of HEC1A cells was 235 ± 12 pg/mL. In the culture supernatant of HEC1A/sVEGFR‐3 cells, 45.0 ± 3.2 pg/mL of sVEGFR‐3 was detected, but no sVEGFR‐3 was detected in the culture supernatant of either HEC1A or HEC1A/LUC cells.
Inhibitory effects of soluble vascular endothelial growth factor receptor‐3 on in vitro lymphatic endothelial cells growth
The effect of the sVEGFR‐3 expression of HEC1A/sVEGFR‐3 cells on the action of VEGF‐C was estimated using in vitro cultures of LEC. The number of LEC in EGM‐MV2 culture medium, including 100 ng/mL recombinant VEGF‐C plus 50% conditioned medium from HEC1A/sVEGFR‐3 cells, was significantly smaller than that in the control (Fig. 1a–c, P < 0.01). We concluded that the mitogenic effect of VEGF‐C on LEC was abrogated by the presence of sVEGFR‐3 in the HEC1A/sVEGFR‐3 conditioned medium.
Figure 1.
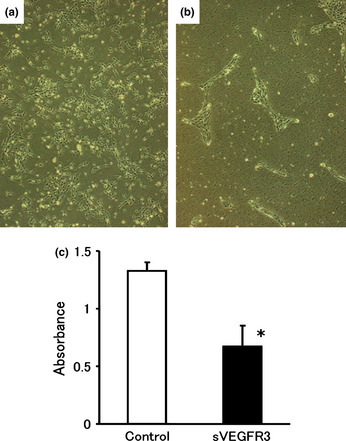
Suppression of vascular endothelial growth factor (VEGF)‐C‐driven lymphatic endothelial cell (LEC) proliferation by conditioned medium of soluble vascular endothelial growth factor receptor‐3 (sVEGFR‐3)‐expressing cells. Cells were plated at 5 × 103 cells/well in 96‐well plates, and 50% sVEGFR‐3‐conditioned medium or luciferase‐conditioned medium was added with 100 ng/mL recombinant human VEGF‐C. The number of LEC with 50% sVEGFR‐3‐conditioned medium (b) was clearly smaller than that with control (a). The cells were counted by colorimetric assay 48 h after plating. Each bar represents the mean ± SD. (*P < 0.01) (c).
Tumor cell transduction model: subcutaneously inoculated tumor growth
The tumor growth curves of HEC1A/LUC and HEC1A/sVEGFR‐3 show no significant differences between the two groups (Fig. 2). This indicates that expression of sVEGFR‐3 did not affect the growth of subcutaneously inoculated tumors.
Figure 2.
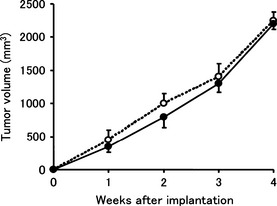
The tumor growth curves of HEC1A/luciferase and HEC1A/vascular endothelial growth factor receptor‐3. Tumor cells were subcutaneously injected into the backs of mice, and the sizes of tumors were measured every week. There were no significant differences between the two groups. Each bar represents the mean ± SD.
Lymph node metastasis
The effects of sVEGFR‐3 gene expression on lymph node metastasis in vivo are shown (Fig. 3a–c). The mean number of lymph node metastases 8 weeks after injection was 1.0 ± 0.7 in the control group, but no lymph node metastases were observed in the HEC1A/sVEGFR‐3‐injected group (Fig. 3c), indicating that sVEGFR‐3 inhibited lymph node metastasis of the HEC1A cells.
Figure 3.
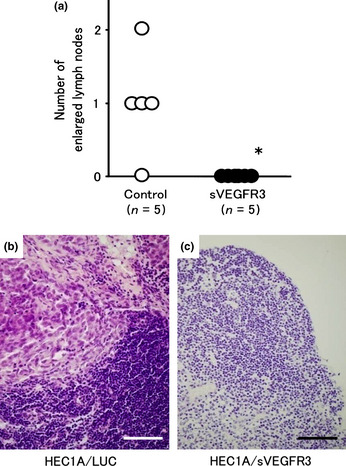
(a) The number of lymph node metastases 8 weeks after injection of HEC1A/luciferase and HEC1A/vascular endothelial growth factor receptor‐3 (sVEGFR‐3) cells. Lymph node metastases were observed in the control group (b), while no lymph node metastases were observed in the HEC1A/sVEGFR‐3‐injected group (c). The mean number of lymph node metastases was 1.0 ± 0.7 in the control group, while no lymph node metastases were observed in the HEC1A/sVEGFR‐3‐injected group. Bars represent 100 μm.
Lung metastasis
After thorough investigation for metastasis, we noticed lung metastasis in these animals. Therefore, we focused on the number of lung metastases along with the number of lymph node metastases. The effects of sVEGFR‐3 gene expression on lung metastasis in vivo are summarized in Figure 4. The mean number of lung metastases 8 weeks after injection was 3.8 ± 0.8 in the control group, but no lung metastases were observed in the HEC1A/sVEGFR‐3‐injected group, indicating that sVEGFR‐3 completely inhibited lung metastasis of the HEC1A cells.
Figure 4.
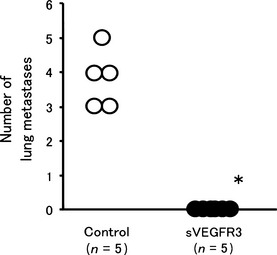
The number of lung metastases 8 weeks after injection of HEC1A/luciferase and HEC1A/vascular endothelial growth factor receptor‐3 (sVEGFR‐3) cells. The mean number of lung metastases 8 weeks after injection was 3.8 ± 0.8 in the control group, while no lung metastases were observed in the HEC1A/sVEGFR‐3‐injected group.
Therapeutic model using adeno‐associated virus vector
The efficacy of muscle‐mediated sVEGFR‐3 expression was evaluated in lymph node and lung metastases models using HEC1A cells. As shown in Figure 5, the mean number of lymph node metastases 8 weeks after injection of HEC1A cells was 2.4 ± 0.5 in the control group, while no lymph node metastases were observed in the AAV1‐sVEGFR‐3‐injected group. Moreover, the mean number of lung metastases 8 weeks after injection of HEC1A cells was 5.7 ± 2.1 in the control group, while no lung metastases were observed in the AAV1‐sVEGFR‐3‐injected group (Fig. 6a–c). Thus, we observed a significant therapeutic effect in both lymph node and lung metastases.
Figure 5.
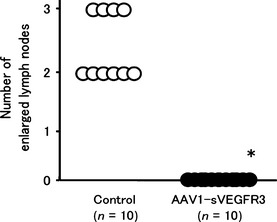
The number of lymph node metastases 8 weeks after injection of HEC1A cells in the mice that had received intramuscular injections of AAV1‐sVEGFR‐3 or control vector. The mean number of lymph node metastases was 2.4 ± 0.5 in the control group, while no lymph node metastases were observed in the AAV1‐sVEGFR‐3‐injected group. sVEGFR‐3, vascular endothelial growth factor receptor‐3.
Figure 6.
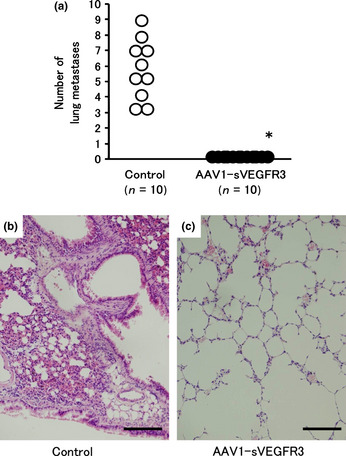
Lung metastases 8 weeks after injection of HEC1A cells in the mice that had received intramuscular injections of AAV1‐sVEGFR‐3 or control vector. The mean number of lung metastases was 5.7 ± 2.1 in the control group, while no lung metastases were observed in the AAV1‐sVEGFR‐3‐injected group (a). Lung metastases were observed in the control group (b), while no lung metastases were observed in the AAV1‐sVEGFR‐3‐injected group (c). Bars represent 100 μm. sVEGFR‐3, vascular endothelial growth factor receptor‐3.
Discussion
In this study, we explored the possibility of gene therapy targeted at lymph node and lung metastasis using muscle‐mediated expression of sVEGFR‐3 as a new treatment modality for advanced endometrial cancer. Our results show that sVEGFR‐3 in the conditioned medium of sVEGFR‐3‐transduced endometrial cancer cells inhibited LEC growth in vitro, and sVEGFR‐3 expression in endometrial cancer cells suppressed in vivo lymph node and lung metastases, although it did not inhibit the growth of subcutaneously inoculated tumors. In addition, lymph node and lung metastases of endometrial cancer cells were suppressed by muscle‐mediated expression of sVEGFR‐3 using AAV vectors.
Lymph node metastasis is the most important prognostic factor in endometrial cancer.3, 20 In this study, we tested the efficacy of a gene therapy strategy using sVEGFR‐3 to suppress lymph node metastasis through VEGF‐C inhibition. We first introduced sVEGFR‐3 cDNA into an endometrial cancer cell line HEC1A and established a cell line (HEC1A/sVEGFR‐3) with high expression of sVEGFR‐3 to investigate its function. The growth of HEC1A/sVEGFR‐3 did not show any difference in either in vitro cell proliferation (data not shown) or in vivo tumor expansion (Fig. 2), showing that overexpression of sVEGFR‐3 did not influence the spread of endometrial cancer per se. In contrast, in the lymph node metastasis model using HEC1A/sVEGFR‐3, lymph node metastasis was completely suppressed. Moreover, in this model, lung metastasis was also completely eliminated. Thus, we demonstrated that expression of sVEGFR‐3 could control lymph node and lung metastases of endometrial cancer. Because the expression of sVEGFR‐3 did not influence the growth of HEC1A cells, its action was confined to the suppression of lymph node metastasis. The VEGF‐C in culture supernatant is thought to be derived from HEC1A cells, and accumulated during cell culture. In fact, we demonstrated various but similar concentrations of VEGF‐C in tumor cell culture supernatant.8 In contrast, soluble VEGFR3 was demonstrated specifically to the cells transduced by sVEGFR3 gene, as shown in the Results section.
Based on these findings, we aimed to establish a gene therapy using sVEGFR‐3. For this purpose, we compared the utility of candidate vectors to attain this goal. Non‐viral vectors are easier to prepare, and may be safer, but the efficacy is much weaker than viral vectors. Successful in vivo delivery has been limited.21. As for the viral vectors, many successful outcomes in clinical trials have been reported.22 For the current study, we chose an AAV1‐based vector, as it appears to be the most efficient in muscle transduction.9, 15, 23 The result was that both lymph node and lung metastases of endometrial cancer cells were completely suppressed by muscle‐mediated expression of sVEGFR‐3. These results suggest the possibility of gene therapy targeting lymph node and lung metastases of endometrial cancer by muscle‐mediated expression of sVEGFR‐3. In the case of AAV vectors, a couple of weeks may be necessary for maximal transgene expression.22, 24, 25, 26 Nonetheless, as sufficient levels of expression can last over the observation period of 8 weeks, a significant outcome was obtained even when the vector was administered simultaneously to the tumor inoculation. In this study, we selected muscle tissue for gene expression. For the clinical translation, other tissues may be more appropriate: for example, liver can be efficiently targeted by AAV8 vector26 and adipose tissue can be targeted by AAV1 vectors.24
This study aimed to suppress actions of VEGF‐C through the expression of a soluble form of its receptor, sVEGFR3. Therefore, lymph node metastasis was suppressed as a result of lymphangiogenesis inhibition. However, because this treatment did not suppress primary tumor growth, it may be necessary to combine it with other treatment modalities such as surgery and chemotherapy in clinical practice. One recent study utilized chemotherapeutic reagents in addition to gene therapy using soluble VEGF receptors for prolonged survival in mice.27
Recently, we developed a lymph node metastasis model using orthotopic injection of endometrial cancer.8 In that report, only lymph node metastasis was noted. During the current series of experiments, metastatic foci of the lungs could barely be recognized from the surface, and we noticed lung metastasis after extensive microscopic examination. Therefore, the number of metastasis was counted based on the microscopic examination of the tissue sections. As for lung metastasis, both lymphatic and hematogenous routes are known. It is not easy to determine the route of metastasis solely by histopathological examination. Nonetheless, we assume that in human endometrial cancer, the main route of lung metastasis is lymphatic as lymph node metastasis is detected in more than half of the patients with lung metastasis.28 Also, in the present study, lung metastasis of endometrial cancer was completely suppressed as a result of controlling lymph node metastasis with sVEGFR‐3.
In this study, we demonstrated the efficacy of sVEGFR‐3 at one vector dose. As both lymph node and lung metastasis were completely eliminated, there is a possibility that the therapeutic efficacy can be demonstrated at lower vector doses. In addition, in our observation, no side effects were noted in the mice, including in behavior, body weight and muscle tissue. However, for application to human therapy, these points need to be clarified in more detail.
Few studies concerning molecular‐targeted therapy or gene therapy for endometrial cancer have been reported. Gene therapy against lymph node and lung metastases, which we propose here, could be beneficial for patients with advanced endometrial cancer. New therapeutic modalities, including this one, are expected to result in improved outcomes for endometrial cancer.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported by Grants‐in‐Aid for Scientific Research (17016067 and 21591248) and the Support Program for Strategic Research Infrastructure from the Japanese Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare, Japan.
(Cancer Sci 2013; 104: 1107–1111)
References
- 1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–36. [DOI] [PubMed] [Google Scholar]
- 2. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet 2005; 366: 491–505. [DOI] [PubMed] [Google Scholar]
- 3. Wolfson AH, Sightler SE, Markoe AM et al The prognostic significance of surgical staging for carcinoma of the endometrium. Gynecol Oncol 1992; 45: 142–6. [DOI] [PubMed] [Google Scholar]
- 4. Lurain J. Uterine cancer In: Berec J, ed. Novak's Gynecology, 12th edn Baltimore: Williams & Wilkins, 1996; 1057–77. [Google Scholar]
- 5. Makinen T, Veikkola T, Mustjoki S et al Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF‐C/D receptor VEGFR‐3. EMBO J 2001; 20: 4762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skobe M, Hawighorst T, Jackson DG et al Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 7. Makinen T, Jussila L, Veikkola T et al Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor‐3. Nat Med 2001; 7: 199–205. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi K, Saga Y, Mizukami H et al Development of a mouse model for lymph node metastasis with endometrial cancer. Cancer Sci 2011; 102: 2272–7. [DOI] [PubMed] [Google Scholar]
- 9. Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno‐associated virus vectors. Gene Ther 2008; 15: 858–63. [DOI] [PubMed] [Google Scholar]
- 10. Takei Y, Mizukami H, Saga Y et al Suppression of ovarian cancer by muscle‐mediated expression of soluble VEGFR‐1/Flt‐1 using adeno‐associated virus serotype 1‐derived vector. Int J Cancer 2007; 120: 278–84. [DOI] [PubMed] [Google Scholar]
- 11. Kuramoto H, Tamura S, Notake Y. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am J Obstet Gynecol 1972; 114: 1012–9. [DOI] [PubMed] [Google Scholar]
- 12. Tanabe H, Takada Y, Minegishi D, Kurematsu M, Masui T, Mizusawa H. Cell line individualization by STR multiplex system in the cell bank found cross contamination between ECV304 and EJ‐1/T24. Tiss Cult Res Commun 1999; 18: 329–38. [Google Scholar]
- 13. Takei Y, Saga Y, Mizukami H et al Overexpression of PTEN in ovarian cancer cells suppresses i.p. dissemination and extends survival in mice. Mol Cancer Ther 2008; 7: 704–11. [DOI] [PubMed] [Google Scholar]
- 14. Matsushita T, Elliger S, Elliger C et al Adeno‐associated virus vectors can be efficiently produced without helper virus. Gene Ther 1998; 5: 938–45. [DOI] [PubMed] [Google Scholar]
- 15. Mochizuki S, Mizukami H, Kume A et al Adeno‐associated virus (AAV) vector‐mediated liver‐ and muscle‐directed transgene expression using various kinds of promoters and serotypes. Gene Ther Mol Biol 2004; 8: 9–18. [Google Scholar]
- 16. Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno‐associated virus type 1. J Virol 1999; 73: 3994–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogura T, Mizukami H, Mimuro J et al Utility of intraperitoneal administration as a route of AAV serotype 5 vector‐mediated neonatal gene transfer. J Gene Med 2006; 8: 990–7. [DOI] [PubMed] [Google Scholar]
- 18. Ishiwata A, Mimuro J, Mizukami H et al Liver‐restricted expression of the canine factor VIII gene facilitates prevention of inhibitor formation in factor VIII‐deficient mice. J Gene Med 2009; 11: 1020–9. [DOI] [PubMed] [Google Scholar]
- 19. Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia‐inducible transcription. Nat Med 2000; 6: 1335–40. [DOI] [PubMed] [Google Scholar]
- 20. Lurain JR, Rice BL, Rademaker AW, Poggensee LE, Schink JC, Miller DS. Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol 1991; 78: 63–9. [PubMed] [Google Scholar]
- 21. Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther 2012; 20: 1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giacca M, Zacchigna S. Virus‐mediated gene delivery for human gene therapy. J Control Release 2012; 161: 377–88. [DOI] [PubMed] [Google Scholar]
- 23. Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno‐associated viral serotype vectors. Mol Ther 2000; 2: 619–23. [DOI] [PubMed] [Google Scholar]
- 24. Mizukami H, Mimuro J, Ogura T et al Adipose tissue as a novel target for in vivo gene transfer by adeno‐associated viral vectors. Hum Gene Ther 2006; 17: 921–8. [DOI] [PubMed] [Google Scholar]
- 25. Flotte TR, Trapnell BC, Humphries M et al Phase 2 clinical trial of a recombinant adeno‐associated viral vector expressing alpha1‐antitrypsin: interim results. Hum Gene Ther 2011; 22: 1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nathwani AC, Tuddenham EG, Rangarajan S et al Adenovirus‐associated virus vector‐mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sopo M, Anttila M, Sallinen H et al Antiangiogenic gene therapy with soluble VEGF‐receptors ‐1, ‐2 and ‐3 together with paclitaxel prolongs survival of mice with human ovarian carcinoma. Int J Cancer 2012; 131: 2394–401. [DOI] [PubMed] [Google Scholar]
- 28. Otsuka I, Ono I, Akamatsu H, Sunamori M, Aso T. Pulmonary metastasis from endometrial carcinoma. Int J Gynecol Cancer 2002; 12: 208–13. [DOI] [PubMed] [Google Scholar]


