Abstract
We report an adult T‐cell leukemia/lymphoma patient suffering from Stevens–Johnson Syndrome (SJS) during mogamulizumab (humanized anti‐CCR4 monoclonal antibody) treatment. There was a durable significant reduction of the CD4+ CD25high FOXP3+ regulatory T (Treg) cell subset in the patient's PBMC, and the affected inflamed skin almost completely lacked FOXP3‐positive cells. This implies an association between reduction of the Treg subset by mogamulizimab and occurrence of SJS. The present case should contribute not only to our understanding of human pathology resulting from therapeutic depletion of Treg cells, but also alert us to the possibility of immune‐related severe adverse events such as SJS when using mogamulizumab. We are currently conducting a clinical trial of mogamulizumab for CCR4‐negative solid cancers (UMIN000010050), specifically aiming to deplete Treg cells.
Adult T‐cell leukemia/lymphoma (ATL) is an aggressive peripheral T‐cell neoplasm caused by HTLV‐1. The disease is resistant to conventional chemotherapeutic agents, and has a very poor prognosis.1 Mogamulizumab (KW‐0761) is a defucosylated humanized monoclonal antibody targeting CC chemokine receptor 4 (CCR4).2 A phase I clinical trial for relapsed CCR4‐positive peripheral T‐cell neoplasms, including ATL, and a phase II study for relapsed ATL have been conducted with mogamulizumab.3, 4 This agent was subsequently approved for the treatment of relapsed or refractory ATL in Japan, the first country in the world to do so, in March 2012. Mogamulizimab went on sale on 29 May 2012. The interim report for the post‐marketing surveillance from 29 May to 28 September 2012 revealed skin‐related severe adverse events (SAE), as defined by the Medical Dictionary for Regulatory Activities Terminology/Japan, in nine patients. Thus, during only the first 4 months of use, 9 skin‐related SAE, including 4 cases of Stevens–Johnson Syndrome (SJS)/toxic epidermal necrolysis (TEN) were reported, with 1 SJS/TEN fatality. These skin‐related, potentially fatal SAE are certainly a challenge to the free use of this agent and clearly require investigation. Therefore, here we report an informative ATL patient suffering from SJS on mogamulizumab treatment, focusing on the reduction of the regulatory T (Treg) cell subset (CD4+CD25highFOXP3+) caused by the antibody.
Case Report
A 71‐year old woman was admitted due to elevation of her lymphocyte count. She had been diagnosed as suffering from acute‐type ATL nearly 5 months prior to admission. She had received VCAP‐AMP‐VECP chemotherapy5 followed by oral sobuzoxane in another hospital, and achieved a transient partial remission. We started mogamulizumab to treat the flare‐up of ATL disease (Fig. 1). Grade 1 skin eruptions appeared around her neck after three antibody infusions. Because we were also giving her antibacterial (ciprofloxacin hydrochloride), fungal (itraconazole), pneumocystic (sulfamethoxazole‐trimethoprim) and viral (aciclovir) prophylaxes in addition to stomach medicine (lansoprazole), we judged the skin event to be due to drug eruption caused by one of these concomitant drugs. Therefore, we stopped all five, but continued with mogamulizumab. Despite their discontinuation and treatment with topical steroids, the skin rashes continued to worsen. We started the patient on 30 mg oral prednisolone, which improved the skin symptoms. The patient was then able to complete the eight planned infusions, and oral prednisolone was tapered off. She was discharged from hospital 8 days after her eighth infusion (day 65), and thereafter seen as an outpatient. However, she had to be readmitted as an emergency patient at day 75 because of fulminant skin rashes. These included erythemas, scale‐like plaques, vesicles, blisters and erosions over many areas of the body. Her lips were swollen and oral mucosa was erosive (Fig. 2a). Skin biopsy revealed marked liquefaction, degeneration and perivascular inflammation with dominant CD8‐positive cells but almost complete lack of FOXP3‐positive cells (Fig. 2b). We diagnosed her as a SJS, and immediately started steroid pulse therapy (methylprednisolone 500 mg/day ×3 days), followed by oral prednisolone. Her skin and mucosal lesions improved gradually, and became inactive. At the same time, her general condition improved. Thus, we again tapered the steroid dose, and she was discharged at day 144. However, she had to come back yet again as an emergency patient on day 151 for the same reason as before, with fulminant skin rashes. We prescribed her mini‐steroid pulse therapy (methylprednisolone 125 mg/day ×1 day), followed by oral prednisolone. Once more, her skin lesions improved gradually. Over this whole period, complete ATL remission was maintained by mogamulizumab. The HTLV‐1 provirus load in PBMC pre‐treatment, and at days 121 and 162 was 750.1, 0.0 (under the limit of detection) and 0.8 copies/1000 cells, respectively. These post‐treatment values are strikingly low, considering that median HTLV‐1 load in asymptomatic carriers reported by other investigators is 18.0 copies/1000 cells.6
Figure 1.
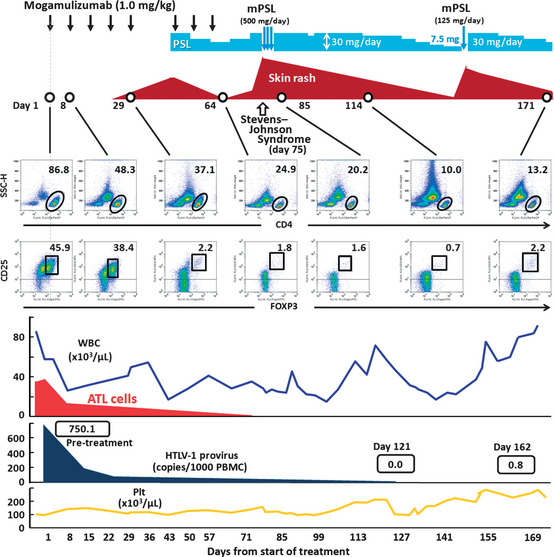
Clinical course of an ATL patient receiving mogamulizumab monotherapy. ATL; adult T‐cell leukemia/lymphoma; mPSL, methyl‐prednisolone; Plt, platelet PSL; prednisolone; WBC, white blood cell.
Figure 2.
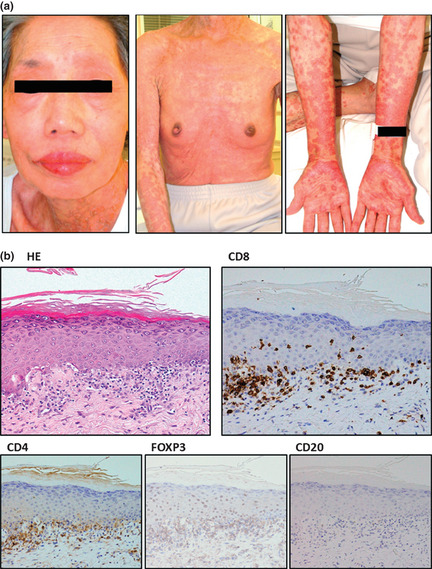
(a) Macroscopic observations of the patient's skin on the day she was diagnosed with Stevens–Johnson Syndrome. (b) Corresponding skin biopsy showing liquefaction, degeneration and perivascular inflammation with dominant CD8‐positive cells but almost no FOXP3‐positive cells.
We also analyzed CD4, CD25 and FOXP3 expression by PBMC during and after antibody treatment (Fig. 1, middle panels). Before treatment, the majority of the patient's PBMC consisted of CD4‐positive and CD25‐positive ATL cells. Just before the 5th antibody infusion (day 29), around the time when her skin rash first appeared, the proportion of CD25highFOXP3+/CD4+ cells was markedly reduced, to 2.2%. This is low even compared to healthy individuals (CD25highFOXP3+/CD4+ cells, mean 3.3%, median 3.3%, range 2.6–4.4%) (Fig. 3). Around the time of SJS onset, the proportion of cells in the Treg subset was further reduced. The proportion of CD25highFOXP3+/CD4+ cells at days 64, 85 and 114 was 1.8%, 1.6% and 0.7%, respectively. The striking reduction of the Treg subset persisted until 4 months after the last of the eight antibody infusions (day 171).
Figure 3.
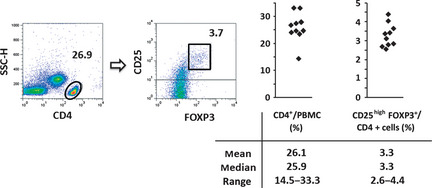
CD4+ CD25high FOXP3+ regulatory T cells in PBMC from healthy volunteers (n = 10).
Discussion
Drugs often induce adverse cutaneous reactions of varying severity, ranging from simple uncomplicated eruptions to potentially fatal eruptions, such as SJS and TEN, within the spectrum of severe adverse reactions affecting skin and mucosa. Although many factors that might cause variability in the clinical course of such adverse reactions have been suggested, it remains unknown which factors are predominantly involved in these processes. The most prevalent severe drug eruptions are thought to be mediated by drug‐reactive T‐cells,7 although we also need to be aware of the alternative view that severe drug eruptions are due to a dysregulated immune system. In this regard, an effect mediated by Treg cells is a likely candidate in severe drug eruptions. Indeed, it is reported that Treg cells can prevent experimentally‐induced epidermal injury mimicking TEN in an animal model.8 Furthermore, Takahashi et al. (9) report that Treg cell function is profoundly impaired in patients with TEN.9 Consistent with these reports, a marked reduction of the Treg subset was observed in the present case.
Mogamulizumab is the first therapeutic agent targeting CCR4, which is expressed on Treg cells,10, 11 to receive marketing approval anywhere in the world. The reduction of the Treg subset seen here was not specific to the present case, but is commonly observed in ATL patients receiving mogamulizumab. In fact, skin rashes were observed as a frequent non‐hematologic adverse event (AE) (63%), mostly occurring after the fourth or subsequent infusions in the phase II study.4 The present case was one of these patients. It has been reported that alterations in CD4+CD25+FOXP3+ Treg cell frequencies and/or function may contribute to various types of autoimmune diseases.12 Because the CCR4 molecule aids lymphocyte skin‐specific homing,13 it is not unexpected that skin rashes, which could be an immune‐related AE, will be frequently observed in ATL patients receiving mogamulizumab. Because it is an urgent issue to identify which factors determine the severity of immune‐related skin disorders associated with mogamulizumab treatment, further investigation on this matter are clearly warranted.
However, reduction of Treg cells is a promising strategy for boosting antitumor immunity in cancer patients, because these cells are increased in the tumor microenvironment and may play an important role in tumor escape from host immunity in several different types of cancer.14, 15 Thus, reduction of Treg cells by mogamulizumab in cancer patients would have both potential benefits leading to enhanced antitumor immunity, but also pose risks of autoimmune disease. The skin‐related SAE, including SJS/TEN, are representative of the latter. Currently, several clinical trials of mogamulizumab are being conducted worldwide, not only for ATL, but also other types of lymphoma. In addition, we are currently conducting a clinical trial of mogamulizumab for CCR4‐negative solid cancers (UMIN000010050), specifically aiming to deplete Treg cells. Therefore, it is a matter of some urgency to establish the safest and most effective treatment strategies for using mogamulizumab not only in ATL patients but also other types of cancer, to maximize benefit and minimize risk.
In summary, the present case should contribute not only to our understanding of human pathology resulting from therapeutic depletion of Treg cells, but also alert us to the possibility of immune‐related SAE, such as SJS/TEN, when using mogamulizumab.
Disclosure Statement
Nagoya City University Graduate School of Medical Sciences has received research grant support from Kyowa Hakko Kirin for works provided by Takashi Ishida. Takashi Ishida received honoraria from Kyowa Hakko Kirin for his works. Shiro Akinaga is an employee of Kyowa Hakko Kirin. No other conflict of interest relevant to this article is reported.
Acknowledgments
The authors thank the husband of the patient for consenting to the publication of her clinical details. The present study was supported by Grants‐in‐Aid for Young Scientists (A) (No. 22689029), Scientific Research (B) (No. 22300333), and Scientific Support Programs for Cancer Research (No. 221S0001) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant‐in‐Aid from the National Cancer Center Research and Development Fund (No. 23‐A‐17), and Health and Labour Sciences Research Grants (H22‐Clinical Cancer Research‐general‐028 and H23‐Third Term Comprehensive Control Research for Cancer‐general‐011) from the Ministry of Health, Labour and Welfare, Japan.
(Cancer Sci 2013; 104: 647–650)
References
- 1. Ishida T, Ueda R. Antibody therapy for Adult T‐cell leukemia‐lymphoma. Int J Hematol 2011; 94: 443–52. [DOI] [PubMed] [Google Scholar]
- 2. Ishii T, Ishida T, Utsunomiya A et al Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clin Cancer Res 2010; 16: 1520–31. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto K, Utsunomiya A, Tobinai K et al Phase I study of KW‐0761, a defucosylated humanized anti‐CCR4 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. J Clin Oncol 2010; 28: 1591–8. [DOI] [PubMed] [Google Scholar]
- 4. Ishida T, Joh T, Uike N et al Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase ii study. J Clin Oncol 2012; 30: 837–42. [DOI] [PubMed] [Google Scholar]
- 5. Tsukasaki K, Utsunomiya A, Fukuda H et al VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 2007; 25: 5458–64. [DOI] [PubMed] [Google Scholar]
- 6. Sonoda J, Koriyama C, Yamamoto S et al HTLV‐1 provirus load in peripheral blood lymphocytes of HTLV‐1 carriers is diminished by green tea drinking. Cancer Sci 2004; 95: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nassif A, Bensussan A, Boumsell L et al Toxic epidermal necrolysis: effector cells are drug‐specific cytotoxic T cells. J Allergy Clin Immunol 2004; 114: 1209–15. [DOI] [PubMed] [Google Scholar]
- 8. Azukizawa H, Sano S, Kosaka H, Sumikawa Y, Itami S. Prevention of toxic epidermal necrolysis by regulatory T cells. Eur J Immunol 2005; 35: 1722–30. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol 2009; 182: 8071–9. [DOI] [PubMed] [Google Scholar]
- 10. Iellem A, Mariani M, Lang R et al Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 2001; 194: 847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishida T, Ishii T, Inagaki A et al Specific recruitment of CC chemokine receptor 4‐positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res 2006; 66: 5716–22. [DOI] [PubMed] [Google Scholar]
- 12. Michels‐van Amelsfort JM, Walter GJ, Taams LS. CD4+ CD25+ regulatory T cells in systemic sclerosis and other rheumatic diseases. Expert Rev Clin Immunol 2011; 7: 499–514. [DOI] [PubMed] [Google Scholar]
- 13. Campbell JJ, Haraldsen G, Pan J et al The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999; 400: 776–80. [DOI] [PubMed] [Google Scholar]
- 14. Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 2012; 13: e32–42. [DOI] [PubMed] [Google Scholar]
- 15. Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci 2011; 102: 44–50. [DOI] [PubMed] [Google Scholar]


