Abstract
The purpose of this study was to investigate the value of post‐operative radiotherapy in the treatment of pT3N0M0 breast cancer after mastectomy. We analyzed the clinical data of 1390 patients with pT1‐3N0M0 breast cancer who were admitted and treated from 1998 to 2007 at the Sun Yat‐sen University Cancer Center. All patients underwent mastectomy and did not receive radiotherapy. The locoregional recurrence‐free survival, distant metastasis‐free survival and overall survival of different T stages of breast cancer were compared. The median follow‐up duration was 72 months. The 10‐year locoregional recurrence‐free survival patients with pT1N0, pT2N0 and pT3N0 breast cancers were 95.3, 91.9 and 93.6%, respectively (χ 2 = 2.550, P = 0.279). The 10‐year distant metastasis‐free survival rates of patients with pT1N0, pT2N0 and pT3N0 breast cancers were 88.1%, 81.0% and 78.4%, respectively (χ 2 = 8.254, P = 0.016). The 10‐year overall survival rates of patients with pT1N0, pT2N0 and pT3N0 breast cancers were 91.9%, 83.5% and 73.0%, respectively (χ 2 = 12.403, P = 0.002). Univariate analyses failed to identify any prognostic factors for locoregional recurrence in pT3N0 patients. Multivariate analysis showed that the T stage had no effect on locoregional recurrence. The locoregional recurrence rate in patients with pT3N0M0 breast cancer who underwent mastectomy and did not receive postoperative radiotherapy was not higher than that in patients with pT1‐2N0M0 breast cancer who received the same treatment, suggesting that routine adjuvant post‐operative radiotherapy should not be recommended in this patient population.
Breast‐conserving surgery and mastectomy are two of the standard surgical options for primary treatment of invasive breast cancers. Randomized trials have shown that patients with axillary lymph node‐positive breast cancer benefit from post‐operative radiotherapy,1, 2, 3 but the value of post‐operative adjuvant radiotherapy for axillary lymph node‐negative breast cancer remains controversial, particularly for pT3N0M0 patients. Existing clinical guidelines vary in therapeutic protocols for pT3N0M0 breast cancer patients. The American Society of Clinical Oncology recommended in 2001 that post‐operative radiotherapy should be applied to patients with pT3N0M0.4 Some studies published by European, American and Chinese authors also support the use of post‐operative radiotherapy for pT3N0M0 patients.5, 6 However, the American College of Radiology expert panel for breast cancer recommended in 2009 that post‐operative radiotherapy should be used for pT3N1M0 but not for pT3N0M0 patients.7 The National Comprehensive Cancer Network (NCCN) guidelines listed pT3N0M0 as an absolute indication for post‐operative radiotherapy prior to 2010, but the wording has changed to “consider” since 2011. Therefore, further studies are required to evaluate the value of post‐mastectomy radiotherapy in pT3N0M0 breast cancers.
This study aimed to explore the value of post‐mastectomy radiotherapy in patients with pT3N0M0 breast cancer undergoing mastectomy by comparing the locoregional recurrence and survival rates in patients with pT3N0M0 and pT1‐2N0M0 breast cancers who underwent total mastectomy and did not receive post‐operative radiation therapy.
Materials and Methods
Study subjects and inclusion criteria
We retrospectively analyzed the clinicopathological data of 3759 patients treated at the Sun Yat‐sen University Cancer Center from January 1998 to December 2007. The subjects were selected based on the following criteria: female; unilateral breast cancer; completion of mastectomy and axillary lymph node dissection; post‐mastectomy pathological stage of pT1‐3N0M0 according to the 2009 Union for International Cancer Control/American Joint Committee on Cancer diagnosis criteria; complete dissection of tumor without positive surgical margins as indicated by pathological results; no pre‐operative adjuvant therapy or post‐operative adjuvant radiotherapy; and no serious accompanying disease. A total of 1390 patients were included in the study. All patients provided written informed consent for use of their clinicopathological data for research and the study was approved by the Ethics Committee of the Sun Yat‐sen University Cancer Center.
Clinical data and treatment protocols
The median age of onset of breast cancer was 47 years (19–90 years). All patients underwent mastectomy and axillary lymph node dissection after a definite diagnosis was made. The median size of the primary tumor was 2.5 cm (0.5–10 cm). There were 557 cases (40.1%), 764 cases (55.0%) and 69 cases (4.9%) of pT1N0, pT2N0 and pT3N0, respectively. The median numbers of axillary lymph nodes removed in the patients with pT1N0, pT2N0 and pT3N0 breast cancers were 13 (1–34), 14 (1–36) and 12 (4–28), respectively. The following prognostic factors were evaluated: age (<35 years of age vs ≥35 years of age); menstruation status (pre‐menopausal versus post‐menopausal); estrogen receptor (ER) status (negative versus positive); progesterone receptor (PR) status (negative versus positive); human epidermal growth factor receptor 2 (Her2) status (negative versus positive); number of axillary lymph nodes removed (<10 vs ≥10); adjuvant chemotherapy regimen (cyclophosphamide, methotrexate and fluorouracil [CMF] regimen versus taxane‐based and/or anthracycline‐based regimen). The clinical and pathological characteristics of the patients are shown in Table 1.
Table 1.
Patient and tumor characteristics (n = 1390)
| Characteristics | T1 | T2 | T3 | P | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| 1390 | 557 (40.1) | 764 (55.0) | 69 (4.9) | ||
| Age (years) | |||||
| <35 | 110 (7.9) | 35 (6.3) | 69 (9.0) | 6 (8.7) | 0.183 |
| ≥35 | 1280 (92.1) | 522 (93.7) | 695 (91.0) | 63 (91.3) | |
| Menopausal status | |||||
| Premenopausal | 896 (64.5) | 352 (63.2) | 494 (64.7) | 50 (72.5) | 0.312 |
| Postmenopausal | 494 (35.5) | 205 (36.8) | 270 (35.3) | 19 (27.5) | |
| ER status | |||||
| Negative | 564 (40.6) | 195 (35.0) | 331 (43.3) | 38 (55.1) | <0.001 |
| Positive | 826 (59.4) | 362 (65.0) | 433 (56.7) | 31 (44.9) | |
| PR status | |||||
| Negative | 500 (36.0) | 180 (32.3) | 287 (37.6) | 33 (47.8) | 0.016 |
| Positive | 890 (64.0) | 377 (67.7) | 477 (62.4) | 36 (52.2) | |
| Her2 status | |||||
| Negative | 1057 (76.0) | 436 (78.3) | 583 (76.3) | 38 (55.1) | <0.001 |
| Positive | 333 (34.0) | 121 (21.7) | 181 (23.7) | 31 (44.9) | |
| Chemotherapy | |||||
| CMF | 236 (17.0) | 88 (15.8) | 135 (17.7) | 13 (18.8) | 0.870 |
| Taxane and/or anthracycline | 881 (83.0) | 345 (84.2) | 488 (82.3) | 48 (81.2) | |
CMF, cyclophosphamide, methotrexate and fluorouracil; ER, estrogen receptor; PR, progesterone receptor.
After surgery, 1117 patients (80.4%) received adjuvant chemotherapy, of which 236 cases (17.0%) were treated with CMF chemotherapy and 881 cases (63.4%) received anthracycline‐based and/or taxane‐based chemotherapy. The median number of chemotherapy cycles was 6 (1–8 cycles). Adjuvant chemotherapy was not carried out in 273 patients (19.6%). Endocrine therapies were adopted in 971 cases (69.9%), including all the ER‐positive and/or PR‐positive patients as well as some patients with negative receptor statuses. Herceptin‐targeted therapy was applied in eight patients with Her2‐positive tumors.
Follow up and method
All patients were followed up at the outpatient clinic or by phone consultation. The first day after surgery was the starting date of the follow up. The primary study endpoints included locoregional recurrence‐free survival (LRFS; LRFS ± DMFS), distant metastasis‐free survival (DMFS) and overall survival (OS). Locoregional recurrence referred to recurrent lesions in the ipsilateral chest wall and lymph nodes in the supra‐clavicular and infra‐clavicular fossa, the axillary area and the internal mammary region, confirmed by biopsy, the absence of distant metastasis, or occurrence of distant metastasis after at least 3 months. Distant metastasis refers to the spread of cancer to distant sites from locoregional recurrence sites, which was confirmed by two types of medical imaging tests, and pathology, if necessary. The mortality endpoint was defined as breast cancer‐related death.
Statistical analysis
All data were analyzed using the spss 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). The Kaplan–Meier method was used to generate the survival curves and to compare the survival rates. The differences between groups were evaluated using the log‐rank test. Cox regression analysis was used for both univariate and multivariate analysis. The statistical significance level was set at 0.05.
Results
Overall survival
The median follow‐up duration was 72 months (3–156 months). Locoregional recurrence occurred in 70 patients. There were 36 cases (51.4%) of relapse within the chest wall only, 19 cases (27.1%) in the supra‐clavicular fossa only, four cases (5.7%) in the axillary only, two cases (2.9%) in the infra‐clavicular fossa only, and nine cases (12.9%) with relapses in more than two of the abovementioned locations. The 5‐ and 10‐year LRFS rates were 95.2% and 93.3%, respectively. The median recurrence time was 29.6 months (5–98 months), with 35.2 months (9–79 months), 28.8 months (5–98 months) and 21.1 months (3–31 months) for patients with T1, T2 and T3 tumors, respectively. Distant metastasis occurred in 161 cases. The 5‐ and 10‐year DMFS rates were 90.2% and 83.8%, respectively. The 5‐ and 10‐year disease‐free survival rates were 88.5% and 81.6%, respectively. A total of 119 patients died. The 5‐ and 10‐year OS rates were 93.5% and 86.0%, respectively.
Survival of different T stages and prognosis analysis
There were no significant differences in LRFS among patients with pT1N0M0, pT2N0M0 and pT3N0M0 tumors. The corresponding 5‐ and 10‐year LRFS rates were 95.7 and 95.3%, 94.9 and 91.9%, and 93.6 and 93.6%, in each patient group with pT1N0M0, pT2N0M0 and pT3N0M0 tumors, respectively (x 2 = 2.550, P = 0.279; Fig. 1). The 5‐ and 10‐year DMFS rates in the three patient groups were 91.6 and 88.1, 89.5 and 81.0%, and 87.0 and 78.4%, respectively (x 2 = 8.254, P = 0.016; Fig. 2). The 5‐ and 10‐year OS rates in the three patient groups were 94.4 and 91.9%, 93.4 and 83.5%, and 86.5 and 73.0%, respectively (x 2 = 12.403, P = 0.002; Fig. 3). The Cox univariate analysis (Tables 2, 3) failed to identify any prognostic factors for LRFS in patients with pT3N0 tumors, but the results showed that the number of lymph nodes removed was a prognostic factor for improved OS. Hormone receptor status, age, number of lymph nodes removed and chemotherapy regimen were found to be prognostic factors for LRFS and better OS rates in patients with pT1N0M0 and pT2N0M0 tumors. The Cox multivariate analysis (Table 4) showed that age, instead of T stage, affected locoregional recurrence. Age, T stage and chemotherapy regimen were found to be independent prognostic factors for better OS.
Figure 1.
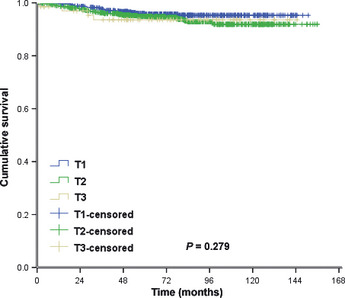
Locoregional recurrence‐free survival.
Figure 2.
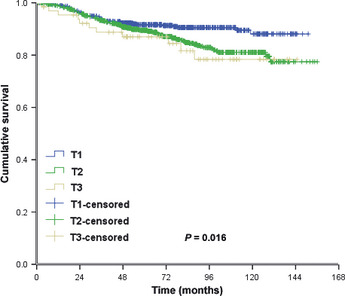
Distant recurrence‐free survival.
Figure 3.
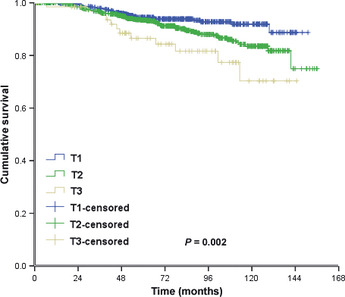
Overall survival.
Table 2.
Univariate analysis of the impact of locoregional recurrence‐free survival according to different pT stage
| Characteristics | T1 | T2 | T3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||||
| ≥35 vs <35 | 0.395 (0.117–1.336) | 0.135 | 0.370 (0.178–0.771) | 0.008* | 0.410 (0.179–1.335) | 0.668 |
| Menopausal status | ||||||
| Pre versus post | 0.834 (0.340–2.045) | 0.691 | 0.784 (0.410–1.499) | 0.462 | 0.029 (0.001–27.21) | 0.450 |
| ER status | ||||||
| Positive/unknown versus negative | 0.595 (0.340–2.045) | 0.225 | 0.596 (0.329–1.079) | 0.088 | 0.417 (0.043–4.006) | 0.448 |
| PR status | ||||||
| Positive/unknown versus negative | 0.383 (0.166–0.887) | 0.025* | 0.541 (0.300–0.978) | 0.042* | 2.746 (0.286–26.406) | 0.382 |
| Her2 status | ||||||
| Positive/unknown versus negative | 1.438 (0.563–3.676) | 0.448 | 0.558 (0.236–1.321) | 0.184 | 0.326 (0.034–3.144) | 0.332 |
| Nodes removed (n) | ||||||
| ≥10 vs <10 | 0.848 (0.313–2.299) | 0.746 | 0.466 (0.244–0.890) | 0.021* | 0.257 (0.036–1.827) | 0.175 |
| Chemotherapy | ||||||
| Taxane and/or anthracycline versus CMF | 0.633 (0.243–1.651) | 0.350 | 0.749 (0.356–1.576) | 0.446 | 0.248 (0.035–1.769) | 0.164 |
*P < 0.05, indicates a significant difference. CI, confidence interval; CMF, cyclophosphamide, methotrexate and fluorouracil; ER, estrogen receptor; HR, hazards ratio; PR, progesterone receptor.
Table 3.
Univariate analysis of the impact of overall survival according to different pT stages
| Characteristics | T1 | T2 | T3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||||
| ≥35 vs <35 | 0.632 (0.193–2.072) | 0.449 | 0.657 (0.326–1.321) | 0.238 | 0.653 (0.142–3.008) | 0.584 |
| Menopausal status | ||||||
| Pre versus post | 0.917 (0.444–1.892) | 0.814 | 1.561 (0.986–2.472) | 0.058 | 1.028 (0.275–3.851) | 0.967 |
| ER status | ||||||
| Positive/unknown versus negative | 0.383 (0.192–0.765) | 0.007* | 0.688 (0.436–1.086) | 0.108 | 0.302 (0.081–1.135) | 0.076 |
| PR status | ||||||
| Positive/unknown versus negative | 0.414 (0.208–0.822) | 0.012* | 0.579 (0.367–0.915) | 0.019* | 0.363 (0.108–1.217) | 0.101 |
| Her2 status | ||||||
| Positive/unknown versus negative | 2.027 (0.978–4.201) | 0.057 | 1.218 (0.703–2.098) | 0.486 | 0.886 (0.271–2.893) | 0.841 |
| Nodes removed (n) | ||||||
| ≥10 vs <10 | 1.403 (0.541–3.634) | 0.486 | 0.554 (0.331–0.926) | 0.024* | 0.226 (0.072–0.706) | 0.010* |
| Chemotherapy | ||||||
| Taxane and/or anthracycline versus CMF | 1.327 (0.524–3.360) | 0.550 | 0.468 (0.264–0.830) | 0.009* | 0.704 (0.192–2.573) | 0.595 |
*P < 0.05, indicates a significant difference. CI, confidence interval; CMF, cyclophosphamide, methotrexate and fluorouracil; ER, estrogen receptor; HR, hazards ratio; PR, progesterone receptor.
Table 4.
Multivariate Cox regression analysis for locoregional recurrence‐free survival and overall survival
| Characteristic | LRFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||
| ≥35 vs <35 | 0.431 | 0.212–0.875 | 0.020* | 0.491 | 0.266–0.907 | 0.023* |
| Menopausal status | ||||||
| Pre versus post | 0.926 | 0.492–1.746 | 0.813 | 1.269 | 0.784–2.053 | 0.333 |
| Tumor stage | ||||||
| T2 versusT1 | 1.110 | 0.635–1.941 | 0.715 | 1.105 | 0.695–1.757 | 0.673 |
| T3 versusT1 | 1.356 | 0.456–4.039 | 0.584 | 2.372 | 1.169–4.814 | 0.017 |
| ER status | ||||||
| Positive/unknown versus negative | 0.600 | 0.293–1.231 | 0.164 | 0.685 | 0.381–1.234 | 0.208 |
| PR status | ||||||
| Positive/unknown versus negative | 0.772 | 0.375–1.591 | 0.483 | 0.731 | 0.404–1.320 | 0.298 |
| Her2 status | ||||||
| Positive/unknown versus negative | 0.642 | 0.323–1.278 | 0.207 | 1.157 | 0.705–1.899 | 0.563 |
| Nodes removed (n) | ||||||
| ≥10 vs <10 | 0.668 | 0.364–1.227 | 0.194 | 0.685 | 0.421–1.112 | 0.126 |
| Chemotherapy | ||||||
| Taxane and/or anthracycline versus CMF | 0.677 | 0.379–1.209 | 0.188 | 0.593 | 0.372–0.945 | 0.025* |
*P < 0.05, indicates a significant difference. CI, confidence interval; CMF, cyclophosphamide, methotrexate and fluorouracil; ER, estrogen receptor; HR, hazard ratio; LRFS, locoregional recurrence‐free survival; OS, overall survival; PR, progesterone receptor.
Discussion
Adjuvant post‐operative radiotherapy is currently recommended after mastectomy for pT3N0M0 breast cancer based on the results of the Danish Breast Cancer Cooperative Group 82b and 82c trials.1, 2 In the present study, we found that the locoregional recurrence rates were similar in patients with pT3N0 and pT1‐2N0 tumors. The 10‐year locoregional recurrence rates in both groups were less than 10%. Our results also showed no increase in the locoregional recurrence rate with enlargement of the primary tumor, whereas the OS of the patients was affected primarily by distant failure.
At present, research is focused on the effect of positive lymph node status on local recurrence in patients with large tumors (≥5 cm), instead of the increased locoregional recurrence caused by the large tumors themselves. The American College of Radiology expert panel for breast cancer has recommended radiotherapy for only the patients with pT3N1M0, but not those with pT3N0M0, tumors. Nagao et al.7 report that in patients with tumor sizes ≥5 cm, the locoregional recurrence rate was lower in axillary lymph node‐negative patients than in lymph node‐positive patients (1.6 vs 10.1%).8 Our study compared the locoregional recurrence rate in patients with pT1N0 M0, pT2N0M0 and pT3N0M0 tumors and found that the size of primary tumor did not have any effect on the locoregional recurrence rate. The 10‐year locoregional recurrence rates were less than 10% in all groups, which is consistent with the report of Mignano et al.9 In addition, in a study reported by Goulart et al.10 on the effect of adjuvant post‐operative radiotherapy in axillary lymph node‐negative breast cancer patients with tumor sizes ≥5 cm, the 10‐year locoregional recurrence rate was 2.3 and 8.9% (P = 0.2), and the 10‐year breast cancer survival rate was 85.8 and 74.6% (P = 0.24) in the radiotherapy and non‐radiotherapy groups, respectively.
Two database analyses of Surveillance, Epidemiology and End Results showed that post‐operative radiotherapy was unable to improve the survival rate, with only one subgroup analysis suggesting that radiotherapy may increase the 10‐year OS (70.7 vs 58.4%, P < 0.001) in patients over 50 years.11, 12 Goulart et al.10 show that recurrence in the non‐radiotherapy group occurs primarily in patients with pathologic stage III tumors who did not receive endocrine therapies. In a study conducted by Hamamoto et al. (2010), the locoregional recurrence rate was only 7% in patients with pT3N0 tumors, and the 8‐year locoregional recurrence rate was significantly higher in patients ≤40 years than in those >40 years (27 vs 2%, P = 0.0135), suggesting that adjuvant post‐operative radiotherapy may be appropriate for some pT3N0 patients with a high risk of recurrence.13 However, the risk factors vary in different studies. The present study did not identify any risk factor for recurrence. Therefore, further studies are needed to identify risk factors for recurrence in specific subsets of patients.
Based on the data from the above studies, the locoregional recurrence rate is not high in pT3N0 patients, and post‐operative radiotherapy cannot enhance the survival rate. This might explain why the NCCN changed the wording from “indication” to “recommendation” for radiotherapy for patients with pT3N0M0 tumors. Although significant progress has been made in modern radiation therapy, long‐term cardiopulmonary function impairment induced by radiotherapy after breast cancer surgery should not be ignored. The 10‐year locoregional recurrence rate was <10% in patients with pT3N0M0 tumors in the present study. Following the recommendation that post‐operative radiotherapy should be performed in patients with a locoregional recurrence rate >20%,14 adjuvant post‐operative radiotherapy should not be recommended for patients with pT3N0M0 breast cancers. However, our study was a retrospective analysis with a limited number of study subjects. Large randomized clinical trials are still needed to determine the exact value of post‐operative radiotherapy for pT3N0M0 breast cancer patients.
The adjuvant chemotherapy and endocrine therapy regimens of the Danish Breast Cancer Cooperative Group 82b and 82c trials are currently inadequate for invasive breast cancers.1, 2 Taghian et al.15 applied adjuvant chemotherapy and endocrine therapies, achieving a 10‐year locoregional recurrence rate of 7.0 and 7.2% in patients with tumor size <5 cm and >5 cm, respectively (P = 0.9). Mignano et al.9 report that no locoregional recurrence was found in patients who received adjuvant chemotherapy. In this study, the majority of patients with pT3N0 were treated with taxane‐based and/or anthracycline‐based chemotherapy, and the 10‐year locoregional recurrence rate was only 6.4%. Treatment failure was mostly due to distant metastasis that affected the OS. Therefore, adjuvant treatment may exert more significant beneficial effects in pT3N0M0 patients with the advance in such treatment.
In summary, our results show that the locoregional recurrence rate in patients with pT3N0M0 breast cancer who underwent mastectomy and did not receive post‐mastectomy radiotherapy was not higher than that in pT1‐2N0M0 patients who received the same treatment, suggesting that it might be inappropriate to routinely recommend adjuvant post‐operative therapy in this patient population. However, large randomized clinical trials are needed to confirm this conclusion.
Disclosure Statement
The authors have no conflict of interest to declare.
(Cancer Sci 2013; 104: 599–603)
References
- 1. Overgaard M, Hansen PS, Overgaard J et al Postoperative radiotherapy in high‐risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997; 337: 949–55. [DOI] [PubMed] [Google Scholar]
- 2. Overgaard M, Jensen MB, Overgaard J et al Postoperative radiotherapy in high‐risk postmenopausal breast‐cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8. [DOI] [PubMed] [Google Scholar]
- 3. Ragaz J, Olivotto IA, Spinelli JJ et al Locoregional radiation therapy in patients with high‐risk breast cancer receiving adjuvant chemotherapy: 20‐year results of the British Columbia randomized trial. J Natl Cancer Inst 2005; 97: 116–26. [DOI] [PubMed] [Google Scholar]
- 4. Recht A, Edge SB, Solin LJ et al Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001; 19: 1539–69. [DOI] [PubMed] [Google Scholar]
- 5. Ceilley E, Jagsi R, Goldberg S et al Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys 2005; 61: 365–73. [DOI] [PubMed] [Google Scholar]
- 6. Hui Z, Li Y, Yu Z, Liao Z. Survey on use of postmastectomy radiotherapy for breast cancer in China. Int J Radiat Oncol Biol Phys 2006; 66: 1135–42. [DOI] [PubMed] [Google Scholar]
- 7. Taylor ME, Haffty BG, Rabinovitch R et al ACR appropriateness criteria on postmastectomy radiotherapy expert panel on radiation oncology‐breast. Int J Radiat Oncol Biol Phys 2009; 73: 997–1002. [DOI] [PubMed] [Google Scholar]
- 8. Nagao T, Kinoshita T, Tamura N, Hojo T, Morota M, Kagami Y. Locoregional recurrence risk factors and the impact of postmastectomy radiotherapy on patients with tumors 5 cm or larger. Breast Cancer 2012, (in press). [DOI] [PubMed] [Google Scholar]
- 9. Mignano JE, Gage I, Piantadosi S, Ye X, Henderson G, Dooley WC. Local recurrence after mastectomy in patients with T3pN0 breast carcinoma treated without postoperative radiation therapy. Am J Clin Oncol 2007; 30: 466–72. [DOI] [PubMed] [Google Scholar]
- 10. Goulart J, Truong P, Woods R, Speers CH, Kennecke H, Nichol A. Outcomes of node‐negative breast cancer 5 centimeters and larger treated with and without postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2011; 80: 758–64. [DOI] [PubMed] [Google Scholar]
- 11. Yu JB, Wilson LD, Dasgupta T, Castrucci WA, Weidhaas JB. Postmastectomy radiation therapy for lymph node‐negative, locally advanced breast cancer after modified radical mastectomy: analysis of the NCI Surveillance, Epidemiology, and End Results database. Cancer 2008; 113: 38–47. [DOI] [PubMed] [Google Scholar]
- 12. McCammon R, Finlayson C, Schwer A, Rabinovitch R. Impact of postmastectomy radiotherapy in T3N0 invasive carcinoma of the breast: a Surveillance, Epidemiology, and End Results database analysis. Cancer 2008; 113: 683–9. [DOI] [PubMed] [Google Scholar]
- 13. Hamamoto Y, Ohsumi S, Aogi K et al Identification of candidates for postmastectomy radiotherapy in patients with pT3N0M0 breast cancer. Breast Cancer 2012, (in press). [DOI] [PubMed] [Google Scholar]
- 14. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the treatment of primary breast cancer. J Natl Cancer Inst 1998; 90: 1601–8. [DOI] [PubMed] [Google Scholar]
- 15. Taghian AG, Jeong JH, Mamounas EP et al Low locoregional recurrence rate among node‐negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol 2006; 24: 3927–32. [DOI] [PubMed] [Google Scholar]


