Abstract
The relationship between consumption of cruciferous vegetables (CV) and risk of gastric cancer has been investigated by many studies, but remains controversial. We carried out a meta‐analysis to summarize available evidence from epidemiological studies on this point. Relevant published reports of CV intake and gastric cancer were identified using MEDLINE (PubMed), EMBASE, and Web of Science databases through to the end of September 2012. We pooled the relative risk from individual studies using a fixed‐ or random‐effects model and carried out heterogeneity and publication bias analyses. Sixteen case–control and six prospective studies were included in our analysis. When all studies were pooled, we yielded a significantly inverse association between CV (relative risk = 0.81; 95% confidence interval, 0.75–0.88) intake and gastric cancer risk, with little heterogeneity (Q = 27.27, P = 0.292, I 2 = 12.0%). Specific analysis for cabbage intake yielded similar result. When separately analyzed, case–control studies of CV intake yielded significant results and the results of prospective studies showed borderline statistical significance. Moreover, significant results were consistent for high‐quality studies, for North American, European, and Asian studies, for studies on males, and for studies on non‐cardia gastric cancer. Findings from this meta‐analysis provide evidence that high intake of CV was inversely associated with the risk of gastric cancer and non‐cardia gastric cancer in humans. Further studies on other specific CV, food preparation methods, and stratified results by anatomic cancer site and histological type should be extended in the future.
Although gastric cancer incidence has decreased substantially in most parts of the world,1 this malignancy remains the fifth and second leading causes of cancer incidence and mortality worldwide, respectively, accounting for 8% of the total cancer cases and 10% of total cancer deaths in 2008.2 Primary prevention of gastric cancer is therefore a major public health priority. Although infection with Helicobacter pylori (Hp) is strongly implicated in gastric cancer etiology,3, 4 infecting 50% of the world's population, <5% of infected hosts will develop cancer, suggesting that such infection is not sufficient alone to cause this malignancy.5 The decline in gastric cancer incidence has been proposed to be partly attributable to the factors related to the increased use and availability of refrigeration, including the increased availability of fresh fruits and vegetables, and a decreased reliance on salted and preserved foods.6 Furthermore, several meta‐analyses and systematic reviews also provided evidence that fruit and vegetable intake has long been associated with a decreased risk of gastric cancer.7, 8 However, the current evidence does not clearly point out any specific group of vegetables that is responsible for the observed inverse associations, except Allium.9, 10
Cruciferous vegetables (CV) have been of specific interest due to their content, in particular, a variety of anticancer constituents such as glucosinolates, the precursors of isothiocyanates (ITC) as well as indole‐3‐carbinol (I3C), both of which may contribute to a reduced risk of gastric cancer. Experimental studies have indicated that ITC play a role in the induction of carcinogen‐detoxification phase 2 enzymes, arrest of cell cycle progression, and induction of apoptosis to protect against chemically induced tumors.11, 12 Evidence from animal studies has indicated oral I3C has been found to inhibit the development of cancer in a variety of animal models including gastric cancer.13 In addition, CV is a good source of dietary water‐soluble fiber which can prevent gastric cancer through its cleansing action, removing or diluting the carcinogens from the epithelial surface.14 A recent meta‐analysis also provided evidence that CV intake was inversely associated with colorectal cancer, another crucial gastrointestinal cancer.15
Although an inverse association between CV intake and gastric cancer risk is biologically plausible, many epidemiologic studies have been published from different countries reporting on the association between CV intake and risk of gastric cancer during the past few decades. Controversial results still exist and there has been no systematic or quantitative assessment of published findings on this topic. Therefore, we carried out a meta‐analysis of observational studies to summarize available evidence on this issue.
Methods
Search strategy
We carried out a comprehensive search of relevant published studies from database initiation until September 30, 2012 using the MEDLINE (PubMed), EMBASE, and ISI Web of Science databases limited to English language and studies of humans using the following search key words and medical subject heading terms: (Brassicaceae OR Brassica OR cruciferous vegetables OR broccoli OR cabbage OR cauliflower OR Brussels sprouts OR mustard plants OR sauerkraut OR cole slaw OR collards OR bok choy OR turnip greens OR vegetables) AND (stomach OR gastric) AND (cancer OR neoplasm OR carcinoma OR tumor). Furthermore, we searched the reference lists of all included studies for additional studies. A similar search strategy was used for a previous meta‐analysis of CV intake and colorectal cancer.15 We then followed standard criteria for carrying out and reporting meta‐analyses.16
Study selection
Published studies were included if they: (i) used a case–control or prospective study design; (ii) evaluated the association between CV intake and gastric cancer risk; and (iii) presented odds ratio (OR), relative risk (RR), or hazard ratio estimates with 95% confidence intervals (CI), standard errors, or data necessary to calculate these. When multiple publications from the same study were available, we used the publication with the largest number of cases and most applicable information. We excluded studies on gastric cancer mortality, studies that did not provide risk estimates, and duplicate publications in our analysis.
Data abstraction and quality assessment
For each eligible study, two investigators (Q.‐J.W. and Y.Y.) independently carried out the eligibility evaluation, data abstraction, and quality assessment. Any disagreements were further discussed and resolved by consensus. Data abstracted from each study included were: the first author's last name; year of publication; study design; the country in which the study was carried out; study sample size (numbers of case patients and control subjects or cohort size); duration years of follow‐up for cohort studies; measures and types of CV and intake categories; study‐specific adjusted ORs or RRs with their 95% CIs for the highest versus lowest category of CV intake (if multiple estimates were available, we abstracted the estimate that adjusted for the most covariates); and factors controlled for by matching or in the multivariable model.
To assess the study quality, a 10‐star system on the basis of the Newcastle–Ottawa Scale15, 17 was used in this meta‐analysis. The full score was 10 and a high quality study was defined as one with quality scores ≥7.
Statistical analysis
The study‐specific adjusted RRs were used as the measure of association across studies. Because the absolute risk of gastric cancer is low, we assumed that estimates of ORs from case–control studies and risk, rate, or hazard ratios from cohort studies were all valid estimates of the RR; we therefore report all results as the RR for simplicity. Boeing et al.18 presented individual risk estimates of cabbage and cauliflower intake separately but did not report the effect of total CV intake. In this situation, the study‐specific effect size in overall analysis was calculated by pooling the risk estimates of the various CV types using the inverse variance method.15, 19 For studies that reported results separately for cardia and non‐cardia or intestinal and diffuse gastric cancer, but not combined, we pooled the results using a fixed‐effects model to obtain an overall combined estimate before combining with the rest of the studies. For studies not using the category with the lowest consumption as the reference, we used the effective count method proposed by Hamling et al.20 to recalculate the RR using the stratum with the lowest consumption as the reference.
The possible heterogeneity in results across studies was examined by using the Cochran Q and I 2 statistics.19 For the Q statistic, a P‐value < 0.1 was considered to be representative of statistically significant heterogeneity. The I 2 represents the proportion of total variation contributed by between‐study variation.19 The summary estimate based on the random effects model (DerSimonian and Laird method)21 or fixed effects model (the inverse variance method) was reported when substantial heterogeneity was detected or not. We used these two effects models to calculate summary RRs and 95% CI for the highest versus the lowest categories of CV intake for the analysis. Due to the limited number of studies on broccoli, cauliflower, or other specific CV intake and gastric cancer, summary estimates were just calculated for CV and cabbage intakes, respectively. Heterogeneity between subgroups was evaluated by meta‐regression. Subgroup analyses were carried out based on study quality, study design (prospective versus case–control studies), type of controls in case–control study (population‐based versus hospital‐based controls), geographic location (Europe, North America, and Asia), gender (males versus females), Lauren's classification (intestinal versus diffuse cancer), and anatomic subsite (cardia versus non‐cardia gastric cancer). Moreover, we also stratified the meta‐analysis by potentially important confounders and risk factors. Finally, we carried out sensitivity analyses excluding one study at a time to explore whether the results were strongly influenced by a specific study.
Publication bias was evaluated using Egger's linear regression method,22 Begg's rank correlation method,23 and funnel plots. A P‐value < 0.05 for Egger's or Begg's tests was considered representative of significant statistical publication bias. Statistical analyses were carried out with Stata (version 11.2; StataCorp, College Station, TX, USA). P‐values were two‐sided with a significance level of 0.05.
Results
Search of published reports
We identified 1172 potentially relevant articles from our search of the three databases. Of these, 1139 articles were excluded after the first screening based on abstracts or titles, leaving 33 articles for full‐text review. Hand searching of the bibliographic references of these articles identified three additional articles,24, 25, 26 for a total of 36 articles for full‐text review. Figure 1 shows a flow diagram, identifying the relevant studies. On this review, three articles were excluded because of duplicate reports from the same study population, eight articles were excluded because they did not report usable or sufficient data of risk estimates, and two articles were excluded because they reported cancer mortality instead of incidence. After exclusion, the remaining 22 articles18, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 were included in this meta‐analysis.
Figure 1.
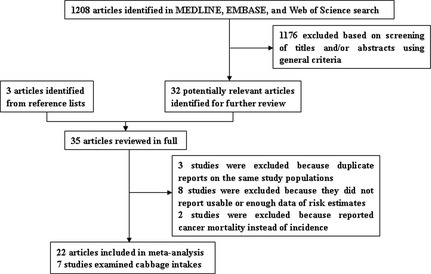
Selection of studies assessing the association between cruciferous vegetable consumption and gastric cancer risk for inclusion in meta‐analysis.
Study characteristics and quality assessment
Characteristics of the 22 included articles are shown in Table S1. All of these articles, which included 7594 cases and 1 399 379 non‐cases, were published between 1985 and 2012, consisting of six prospective studies (five cohort studies27, 28, 29, 30, 31 and one nested case–control study35) and 16 case–control studies.18, 32, 33, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Of the six prospective studies, three were carried out in the Europe,27, 28, 31 two in the USA,29, 35 and one in China,30 Sample sizes of prospective studies ranged from 800635 to 521 457,28 and the number of gastric cancer cases varied from 11135 to 616.31
Of the 16 case–control studies,18, 32, 33, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 three were carried out in the USA,32, 39, 44 two each in China,34, 40 Spain,36, 37 Italy,33, 47 Japan,43, 45 and Poland,18, 46 and one each in Korea,38 Sweden,41 and Uruguay.42 The number of patients enrolled in these studies ranged from 9139 to 741,18 and the number of controls varied from 13239 to 36 490.45 Controls were drawn from the general population in five studies37, 40, 41, 44, 46 and hospitals in 11 studies.18, 32, 33, 34, 36, 38, 39, 42, 43, 45, 47
Study‐specific quality scores are summarized in Tables S2 and S3. The range of quality scores was from 4 to 8; the median score was 7. The median scores of prospective and case–control studies were 7 and 6, respectively. High‐quality studies (i.e., those studies that had at least a score of 7) included all prospective and five case–control studies.40, 41, 43, 44, 46
Cruciferous vegetables
In a fixed‐effect pooled analysis of these studies, high CV intake (comparing the highest with the lowest category) was associated with a reduced risk of gastric cancer (RR = 0.81; 95% CI = 0.75–0.88) (Table 1). There was little heterogeneity for the all studies (Q = 27.27, P = 0.292, I 2 = 12.0%). No publication bias was observed by the funnel plot (Fig. S1), Egger's regression test (P = 0.668), or by Begg's rank correlation test (P = 0.870).
Table 1.
Summary risk estimates of the association between cruciferous vegetable (CV) consumption and gastric cancer risk
| No. of studies | Summary RR (95% CI) | Q statistic | I‐square value (%) | P h * | P h ** | |
|---|---|---|---|---|---|---|
| Overall studies | ||||||
| CV | 22 | 0.81 (0.75–0.88) | 27.27 | 12.0 | 0.292 | – |
| Cabbage | 7 | 0.68 (0.58–0.80) | 5.77 | 0 | 0.449 | |
| Subgroup analyses for CV | ||||||
| High quality studies (scores ≥7) | 11 | 0.84 (0.76–0.93) | 8.29 | 0 | 0.824 | 0.437 |
| Study design | ||||||
| Prospective studies | 6 | 0.89 (0.77–1.02) | 3.42 | 0 | 0.755 | 0.179 |
| Case–control studies | 16 | 0.78 (0.71–0.86) | 21.75 | 21.8 | 0.195 | |
| Type of control subjects | ||||||
| Population based | 5 | 0.74 (0.64–0.87) | 3.65 | 0 | 0.724 | 0.491 |
| Hospital based | 11 | 0.81 (0.72–0.90) | 17.44 | 42.7 | 0.065 | |
| Geographic location | ||||||
| Europe | 11 | 0.82 (0.74–0.92) | 11.70 | 14.6 | 0.305 | 0.877 |
| North America | 5 | 0.82 (0.70–0.95) | 5.36 | 6.8 | 0.373 | |
| Asia | 6 | 0.79 (0.69–0.92) | 10.05 | 30.3 | 0.186 | |
| Gender | ||||||
| Male | 4 | 0.78 (0.64–0.96) | 3.03 | 1.0 | 0.387 | 0.787 |
| Female | 4 | 0.82 (0.66–1.00) | 1.54 | 0 | 0.673 | |
| Lauren's classification | ||||||
| Intestinal | 3 | 0.77 (0.56–1.05) | 3.15 | 36.5 | 0.207 | 0.469 |
| Diffuse | 3 | 0.93 (0.66–1.30) | 0.21 | 0 | 0.900 | |
| Anatomic subsite | ||||||
| Cardia | 4 | 0.84 (0.65–1.09) | 4.51 | 33.5 | 0.211 | 0.914 |
| Non‐cardia | 6 | 0.86 (0.74–0.99) | 7.16 | 2.3 | 0.412 | |
| Adjustment for confounders or risk factors | ||||||
| Vegetables/fruit intake | ||||||
| Yes | 4 | 0.88 (0.55–1.42) | 9.62 | 68.8 | 0.022 | 0.431 |
| No | 18 | 0.81 (0.75–0.88) | 17.96 | 0 | 0.590 | |
| Smoking status | ||||||
| Yes | 15 | 0.81 (0.74–0.89) | 11.92 | 0 | 0.750 | 0.977 |
| No | 7 | 0.87 (0.68–1.11) | 16.04 | 56.3 | 0.025 | |
| Alcohol drinking | ||||||
| Yes | 7 | 0.81 (0.72–0.91) | 6.02 | 0.3 | 0.421 | 0.894 |
| No | 15 | 0.83 (0.75–0.92) | 22.01 | 22.8 | 0.184 | |
| Salted food | ||||||
| Yes | 2 | 0.83 (0.63–1.11) | 0.92 | 0 | 0.339 | 0.867 |
| No | 20 | 0.82 (0.76–0.89) | 27.24 | 19.2 | 0.202 | |
| Total energy intake | ||||||
| Yes | 13 | 0.86 (0.78–0.95) | 13.12 | 0 | 0.593 | 0.076 |
| No | 9 | 0.76 (0.67–0.86) | 12.54 | 36.2 | 0.129 | |
| Education/socioeconomic status | ||||||
| Yes | 12 | 0.85 (0.77–0.94) | 16.87 | 17.0 | 0.263 | 0.256 |
| No | 10 | 0.79 (0.70–0.89) | 10.41 | 13.5 | 0.319 | |
| Subgroup analyses for cabbage | ||||||
| High quality studies (scores ≥7) | 2 | 0.77 (0.56–1.06) | 0.27 | 0 | 0.607 | 0.426 |
| Study design | ||||||
| Prospective studies | 2 | 0.77 (0.56–1.06) | 0.27 | 0 | 0.607 | 0.426 |
| Case–control studies | 5 | 0.66 (0.55–0.79) | 4.76 | 15.9 | 0.313 | |
*P‐value for heterogeneity within each subgroup; **P‐value for heterogeneity between subgroups with meta‐regression analysis. –, not applicable, CI, confidence interval; RR, relative risk.
Cabbage
Two cohort27, 28 and five case–control studies18, 34, 37, 38, 45 investigated the association between cabbage intake and gastric cancer risk. In a fixed‐effect pooled analysis of these studies, high cabbage intake (comparing the highest with the lowest category) was associated with a decreased risk of gastric cancer (RR = 0.68; 95% CI = 0.58–0.80) (Table 1, Fig. 2). There was no heterogeneity among the seven studies (Q = 5.77, P = 0.449, I 2 = 0%), and no publication bias was found by the funnel plot (Fig. S2), Egger's regression test (P = 0.125), or Begg's rank correlation test (P = 0.293).
Figure 2.
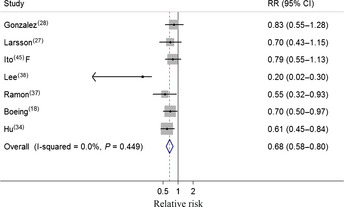
Forest plot (fixed effect model) of cabbage consumption and gastric cancer risk . Squares indicate study‐specific relative risks (RR) (size of the square reflects the study‐specific statistical weight); horizontal lines indicate 95% confidence interval (CI); diamond indicates the summary RR estimate with its 95% CI. F, females.
Subgroup and sensitivity analyses
In subgroup analyses of CV intake and gastric cancer, all strata showed inverse associations, but some associations were not statistically significant (Table 1). Similar results were observed for cabbage intake. Compared with the significant result in case–control studies (Fig. 3), prospective studies yielded a borderline statistical significance inverse relationship between CV intake and gastric cancer risk (Fig. 4). Furthermore, there is no evidence of significant heterogeneity between subgroups with meta‐regression analyses.
Figure 3.

Forest plot (fixed effect model) of cruciferous vegetable consumption and gastric cancer risk in case–control studies. Squares indicate study‐specific relative risks (RR) (size of the square reflects the study‐specific statistical weight); horizontal lines indicate 95% confidence interval (CI); diamond indicates the summary RR estimate with its 95% CI. F, females; M, males.
Figure 4.
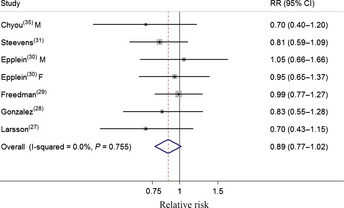
Forest plot (fixed effect model) of cruciferous vegetable consumption and gastric cancer risk in prospective studies. Squares indicate study‐specific relative risks (RR) (size of the square reflects the study‐specific statistical weight); horizontal lines indicate 95% confidence interval (CI); diamond indicates the summary RR estimate with its 95% CI. F, females; M, males.
In a sensitivity analysis of CV intake and gastric cancer risk, we sequentially removed one study at a time and re‐analyzed the data. The 22 study‐specific RRs ranged from a low of 0.80 (95% CI = 0.74–0.86) after omission of the study by Freedman et al.29 to a high of 0.83 (95% CI = 0.77–0.90) after omission of the study by Hu et al.,34 but were similar in general. Meanwhile, we removed three studies18, 33, 37 in which RRs and 95% CIs were not reported but calculated from raw data. The result from this analysis (RR = 0.82; 95% CI = 0.75–0.89) was similar. Like CV intake, similar sensitivity analyses for cabbage did not significantly change the results (data not shown). Furthermore, we chose not using the inverse variance method to pool the risk estimates of the various CV types; the result was also shown to be robust (RR = 0.82; 95% CI = 0.76–0.89).
Discussion
Overall, an increase in CV consumption was associated with 11% and 22% (comparing the highest with the lowest category) decreased risks of gastric cancer in prospective studies and case–control studies, respectively (Table 1). Significant results were consistent for high‐quality studies, for North American, European, and Asian studies, for studies on males, and for studies on non‐cardia gastric cancer. Additionally, specific analysis for cabbage intake yielded similar inverse association (Table 1, Fig. 2).
Our meta‐analysis supports an inverse association between intake of CV and gastric cancer risk. This kind of association is biologically plausible. In addition to containing a variety of bioactive components such as folate, vitamin C, tocopherols, and carotenoids,48 CV is good sources of glucosinolates which can be hydrolyzed into biologically active compounds (ITCs and I3C). Experimental studies have suggested that ITCs or I3C can not only confer protection against certain cancers by modulating the activity of enzymes involved in detoxifying carcinogens and metabolizing sex hormones,49 but inhibit migration and invasion through the several signaling pathways (e.g., nuclear factor‐κB, protein kinase C) in human gastric cells.50, 51 Furthermore, water‐soluble fiber contained in CV can delay the absorption of starch, thus reducing the glycemic load, which is related to the risk of gastric cancer through excess circulating insulin and stimulating mitogenic and cancer‐promoting insulin‐like growth factors.52
It is interesting and important to look into Lauren's subtype and anatomic site of gastric cancers, as evidence is growing that there are differences in risk factors between these cancers.6, 53, 54 Some studies also reported that infection with Hp is a risk factor for non‐cardia gastric cancer and might protect against cardia cancer. However, a meta‐analysis of the few nested case–control studies that specifically addressed this issue failed to confirm either an increase or a decrease in risk.4 Findings also suggested a strong influence of environmental or lifestyle risk factors on intestinal‐type cancers.6 Due to the limited number of studies, we only yielded a significant result between CV intake and non‐cardia gastric cancer with little heterogeneity. Similar, we found no significant results either in intestinal‐type or diffuse‐type which might also be ascribed to the same reason. These results should be interpreted cautiously and whether the association differs according to anatomic site or Lauren's subtype of gastric cancer warrants further study. Higher intake of CV might also be associated with other health behaviors (e.g., higher levels of physical activity,29, 30 lower intakes of alcohol and salted food,27, 30 and lower rates of smoking.29, 30 A meta‐analysis is not able to solve problems with confounding factors that could be inherent in the included studies. However, the majority of these studies have adjusted for major confounding factors, which should reduce the potential bias due to these lifestyle factors. In addition, the results generally showed an inverse association in the subgroup analyses when we stratified the results according to these confounding factors (Table 1).
The association between CV consumption and gastric cancer risk was statistically significantly stronger in case–control studies than in prospective studies. It is possible that the relationships reported from case–control studies might have been overstated due to recall or selection bias which was inherent in the original studies and possible prediagnostic early symptoms in cancer patients may have led to changes in dietary habits. The time interval between the period covered by the dietary assessment and diagnosis of the disease was usually 1–2 years (recent diet) in case–control studies (e.g., Bosetti et al.,47 Gonzalez et al.36), although it was as large as over 10 years (current diet at the time of subject recruitment) in prospective studies (e.g., Chyou et al.,35 Steevens et al.31). Furthermore, in our meta‐analysis, heterogeneity was more often present within case–control or hospital‐based case–control studies than within prospective or population‐based case–control studies (Table 1), which could be explained to some extent by the different study methodologies. The studies also varied in the number of potential confounding factors for which they had adjusted. Some prospective studies27, 28, 29, 31 published in recent years provided detailed information of adjustment for confounders, whereas some early case–control studies18, 32, 33, 37 adjusted for fewer factors.
A strength of this study is the large sample size, with 7594 cases and 1 399 379 non‐cases. This has the advantage of increasing the statistical power to detect the putative association between CV intake and gastric cancer, although compared to CV, fewer studies and cases (seven included studies with 2289 cases and 641 378 non‐cases) were included in the subgroup analyses of cabbage intake. Another strength is the thorough statistical analyses considering a number of subgroups depend on the relatively large number of included studies. Several potential limitations also should be considered. First, only one included study provided information on Hp infection,28 an important gastric cancer risk factor. However, studies that have stratified by Hp status have generally found no evidence of effect modification55, 56, 57 or have found the suggestion that vegetables are more protective for individuals positive for the bacteria.28 Thus, by including Hp‐negative individuals in this study, results may have been biased toward the null. Second, we could not exclude potential biases due to not only the misclassification of CV intake, because most studies used food frequency questionnaire as dietary assessment, but also the methods used to report CV intake and the range between lowest and highest categories among these included studies were different. Moreover, of the five prospective studies included in the meta‐analysis, none of them accounted for the changes of dietary intake over time, and in all studies, the dietary assessment was only made at baseline. The inherent measurement error in these assessments could cause bias to the results. Last but not least, as with any meta‐analysis, possible publication bias can be a problem in combined analyses of published reports, but we found no evidence of such bias in our analysis.
In conclusion, our meta‐analysis showed that higher intake of CV is associated with a lower risk of gastric cancer, especially cancer of non‐cardia. More in‐depth studies, particularly prospective studies, are warranted to evaluate more detailed results, including other specific vegetables within the CV family, stratified results by histological type, anatomic cancer site, food preparation methods, or adjustment for potential confounders.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Funnel plot corresponding to the fixed‐effects meta‐analysis of the relationship between cruciferous vegetable intake and gastric cancer risk.
Fig. S2. Funnel plot corresponding to the fixed‐effects meta‐analysis of the relationship between cabbage intake and gastric cancer risk.
Table S1. Characteristics of studies of cruciferous vegetable consumption and gastric cancer risk.
Table S2. Methodological quality of prospective studies included in the meta‐analysis.
Table S3. Methodological quality of case–control studies included in the meta‐analysis.
Acknowledgments
This work was supported by the fund of the State Key Project Specialized for Infectious Diseases of China (Grant Nos. 2008ZX10002‐015 and 2012ZX10002008‐002).
(Cancer Sci 2013; 104: 1067–1073)
References
- 1. Bertuccio P, Chatenoud L, Levi F et al Recent patterns in gastric cancer: a global overview. Int J Cancer 2009; 125: 666–73. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 3. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Schistosomes, Liver Flukes and Helicobacter pylori, vol. 61 Lyon, France: IARC Press, 1994. [PMC free article] [PubMed] [Google Scholar]
- 4. Helicobacter and Cancer Collaborative Group . Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001; 49: 347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: a meta‐analysis. J Natl Cancer Inst 2006; 98: 1078–87. [DOI] [PubMed] [Google Scholar]
- 6. Shibata A, Parsonnet J. Stomach cancer In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention, 3rd edn New York, NY: Oxford University Press, 2006; 707–20. [Google Scholar]
- 7. Gonzalez CA. Vegetable, fruit and cereal consumption and gastric cancer risk. IARC Sci Publ 2002; 156: 79–83. [PubMed] [Google Scholar]
- 8. Lunet N, Lacerda‐Vieira A, Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta‐analysis of cohort studies. Nutr Cancer 2005; 53: 1–10. [DOI] [PubMed] [Google Scholar]
- 9. Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta‐analysis. Gastroenterology 2011; 141: 80–9. [DOI] [PubMed] [Google Scholar]
- 10. World Cancer Research Fund/American Insitute for Cancer Research . Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: American Insitute for Cancer Research, 2007. [Google Scholar]
- 11. Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 2007; 55: 224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets 2010; 11: 652–66. [DOI] [PubMed] [Google Scholar]
- 13. Grubbs CJ, Steele VE, Casebolt T et al Chemoprevention of chemically‐induced mammary carcinogenesis by indole‐3‐carbinol. Anticancer Res 1995; 15: 709–16. [PubMed] [Google Scholar]
- 14. Bravi F, Scotti L, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Dietary fiber and stomach cancer risk: a case–control study from Italy. Cancer Causes Control 2009; 20: 847–53. [DOI] [PubMed] [Google Scholar]
- 15. Wu QJ, Yang Y, Vogtmann E et al Cruciferous vegetables intake and the risk of colorectal cancer: a meta‐analysis of observational studies. Ann Oncol 2013; 24: 1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC et al Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O'Connell D et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 3, 2012.
- 18. Boeing H, Jedrychowski W, Wahrendorf J, Popiela T, Tobiasz‐Adamczyk B, Kulig A. Dietary risk factors in intestinal and diffuse types of stomach cancer: a multicenter case–control study in Poland. Cancer Causes Control 1991; 2: 227–33. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 20. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27: 954–70. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 22. Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–101. [PubMed] [Google Scholar]
- 24. Inoue M, Tajima K, Kobayashi S et al Protective factor against progression from atrophic gastritis to gastric cancer–data from a cohort study in Japan. Int J Cancer 1996; 66: 309–14. [DOI] [PubMed] [Google Scholar]
- 25. Risch HA, Jain M, Choi NW et al Dietary factors and the incidence of cancer of the stomach. Am J Epidemiol 1985; 122: 947–59. [DOI] [PubMed] [Google Scholar]
- 26. Kneller RW, McLaughlin JK, Bjelke E et al A cohort study of stomach cancer in a high‐risk American population. Cancer 1991; 68: 672–8. [DOI] [PubMed] [Google Scholar]
- 27. Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 2006; 15: 1998–2001. [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez CA, Pera G, Agudo A et al Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC‐EURGAST). Int J Cancer 2006; 118: 2559–66. [DOI] [PubMed] [Google Scholar]
- 29. Freedman ND, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and gastric cancer risk in a large United States prospective cohort study. Cancer Causes Control 2008; 19: 459–67. [DOI] [PubMed] [Google Scholar]
- 30. Epplein M, Shu XO, Xiang YB et al Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women's and Men's Health studies. Am J Epidemiol 2010; 172: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer 2011; 129: 2681–93. [DOI] [PubMed] [Google Scholar]
- 32. Correa P, Fontham E, Pickle LW, Chen V, Lin YP, Haenszel W. Dietary determinants of gastric cancer in south Louisiana inhabitants. J Natl Cancer Inst 1985; 75: 645–54. [PubMed] [Google Scholar]
- 33. La Vecchia C, Negri E, Decarli A, D'Avanzo B, Franceschi S. A case–control study of diet and gastric cancer in northern Italy. Int J Cancer 1987; 40: 484–9. [DOI] [PubMed] [Google Scholar]
- 34. Hu JF, Zhang SF, Jia EM et al Diet and cancer of the stomach: a case–control study in China. Int J Cancer 1988; 41: 331–5. [DOI] [PubMed] [Google Scholar]
- 35. Chyou PH, Nomura AM, Hankin JH, Stemmermann GN. A case–cohort study of diet and stomach cancer. Cancer Res 1990; 50: 7501–4. [PubMed] [Google Scholar]
- 36. Gonzalez CA, Sanz JM, Marcos G et al Dietary factors and stomach cancer in Spain: a multi‐centre case–control study. Int J Cancer 1991; 49: 513–9. [DOI] [PubMed] [Google Scholar]
- 37. Ramon JM, Serra L, Cerdo C, Oromi J. Dietary factors and gastric cancer risk. A case–control study in Spain. Cancer 1993; 71: 1731–5. [DOI] [PubMed] [Google Scholar]
- 38. Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case–control study in Korea. Int J Epidemiol 1995; 24: 33–41. [DOI] [PubMed] [Google Scholar]
- 39. Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case–control study in the U.S. Cancer 1997; 80: 1021–8. [PubMed] [Google Scholar]
- 40. Ji BT, Chow WH, Yang G et al Dietary habits and stomach cancer in Shanghai, China. Int J Cancer 1998; 76: 659–64. [DOI] [PubMed] [Google Scholar]
- 41. Ekstrom AM, Serafini M, Nyren O, Hansson LE, Ye W, Wolk A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population‐based case–control study in Sweden. Int J Cancer 2000; 87: 133–40. [PubMed] [Google Scholar]
- 42. De Stefani E, Correa P, Boffetta P et al Plant foods and risk of gastric cancer: a case–control study in Uruguay. Eur J Cancer Prev 2001; 10: 357–64. [DOI] [PubMed] [Google Scholar]
- 43. Hara M, Hanaoka T, Kobayashi M et al Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital‐based case–control study in Japan. Nutr Cancer 2003; 46: 138–47. [DOI] [PubMed] [Google Scholar]
- 44. Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, Stemmermann GN. Case–control study of diet and other risk factors for gastric cancer in Hawaii (United States). Cancer Causes Control 2003; 14: 547–58. [DOI] [PubMed] [Google Scholar]
- 45. Ito LS, Inoue M, Tajima K et al Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non‐differentiated subtypes. Ann Epidemiol 2003; 13: 24–31. [DOI] [PubMed] [Google Scholar]
- 46. Lissowska J, Gail MH, Pee D et al Diet and stomach cancer risk in Warsaw, Poland. Nutr Cancer 2004; 48: 149–59. [DOI] [PubMed] [Google Scholar]
- 47. Bosetti C, Filomeno M, Riso P et al Cruciferous vegetables and cancer risk in a network of case–control studies. Ann Oncol 2012; 23: 2198–203. [DOI] [PubMed] [Google Scholar]
- 48. Kurilich AC, Tsau GJ, Brown A et al Carotene, tocopherol, and ascorbate contents in subspecies of Brassica oleracea . J Agric Food Chem 1999; 47: 1576–81. [DOI] [PubMed] [Google Scholar]
- 49. Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev 1996; 5: 733–48. [PubMed] [Google Scholar]
- 50. Yang MD, Lai KC, Lai TY et al Phenethyl isothiocyanate inhibits migration and invasion of human gastric cancer AGS cells through suppressing MAPK and NF‐kappaB signal pathways. Anticancer Res 2010; 30: 2135–43. [PubMed] [Google Scholar]
- 51. Ho CC, Lai KC, Hsu SC et al Benzyl isothiocyanate (BITC) inhibits migration and invasion of human gastric cancer AGS cells via suppressing ERK signal pathways. Hum Exp Toxicol 2011; 30: 296–306. [DOI] [PubMed] [Google Scholar]
- 52. Augustin LS, Gallus S, Negri E, La Vecchia C. Glycemic index, glycemic load and risk of gastric cancer. Ann Oncol 2004; 15: 581–4. [DOI] [PubMed] [Google Scholar]
- 53. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 54. Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 2006; 20: 633–49. [DOI] [PubMed] [Google Scholar]
- 55. Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol 2003; 13: 162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Machida‐Montani A, Sasazuki S, Inoue M et al Association of Helicobacter pylori infection and environmental factors in non‐cardia gastric cancer in Japan. Gastric Cancer 2004; 7: 46–53. [DOI] [PubMed] [Google Scholar]
- 57. Epplein M, Nomura AM, Hankin JH et al Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case–control study in Hawaii. Cancer Causes Control 2008; 19: 869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Funnel plot corresponding to the fixed‐effects meta‐analysis of the relationship between cruciferous vegetable intake and gastric cancer risk.
Fig. S2. Funnel plot corresponding to the fixed‐effects meta‐analysis of the relationship between cabbage intake and gastric cancer risk.
Table S1. Characteristics of studies of cruciferous vegetable consumption and gastric cancer risk.
Table S2. Methodological quality of prospective studies included in the meta‐analysis.
Table S3. Methodological quality of case–control studies included in the meta‐analysis.


