Abstract
The kinesin superfamily (KIF) is a group of proteins that share a highly conserved motor domain. Except for some members, many KIF proteins have adenosine triphosphatase activity and microtubule‐dependent plus‐end motion ability. Kinesins participate in several essential cellular functions, including mitosis, meiosis and the transport of macromolecules. Increasing evidence indicates kinesin proteins play critical roles in the genesis and development of human cancers. Some kinesin proteins are associated with maligancy as well as drug resistance of solid tumor. Thus, targeting KIF therapy seems to be a promising anticancer strategy. Inhibitors of KIF such as kinesin spindle protein (KSP/Eg5) have entered clinical trials for monotherapy or in combination with other drugs, and kinesins other than Eg5 with various potential anticancer target characteristics are also constantly being discovered and studied. Here, we summarize the oncogenic roles of kinesin proteins and potential cancer therapy strategies that target KIF.
Kinesin was first isolated from squid nervous tissue in 1985.1 Kinesin proteins are present in all eukaryotes, and in humans more than 40 kinesin proteins have been identified to date and are classified into 14 families.2, 3 Kinesin superfamily (KIF) members share a highly conserved motor domain and many motor kinesins have adenosine triphosphatase activity and microtubule‐dependent plus‐end motion ability. The conserved motor domain enables motor binding and stepping across microtubules by converting the chemical energy of ATP hydrolysis into a mechanical force.4
The KIF proteins participate in multiple normal cellular biological activities including mitosis and intracellular transport of vesicles and organelles.5 In mitosis, the activities of microtubule motors on the spindle mircotubule are precisely regulated to ensure that mitotic events are orchestrated by exact sequence throughout the progression of mitosis. Kinesin motors function in interpolar microtubules at the metaphase, which lead to a balance of outward forces (Fig. 1, red arrows) and inward forces (Fig. 1, blue arrows). However, it has been shown that overexpression of some motor kinesins such as Eg5 generate additional outward forces during mitosis and, more importantly, induce premature sister chromatid separation and overshooting before anaphase (Fig. 1, middle row),6 One possible mechanism might be that excessive spindle separation leads to collapse of spindles and the formation of monopolar spindles, which further results in unequal distribution of genetic material in anaphase and eventually causes aneuploid daughter cells (Fig. 1).7 The aneuploid cells with gained or lost genetic material are believed to cause aggressive progression of cancer, for example, invasion and metastasis.8 In contrast, downregulation of some motor kinesins such as Eg5 or KIF20B leads to mitotic defects, including mitotic arrest, failure of spindle assembly or cytokinesis defect, which eventually trigger apoptosis through p53 or other signal pathways in certain tumor cell lines (Fig. 1, lower row).6, 9 Therefore, kinesin targeting therapy has been regarded as a promising anticancer strategy and the oncogenic roles of some KIF proteins are also well studied (Table 1).10, 32 Here, we discuss the characteristics of the malignant phenotype of KIF proteins and the potential targeting KIF strategies in cancer therapy.
Figure 1.
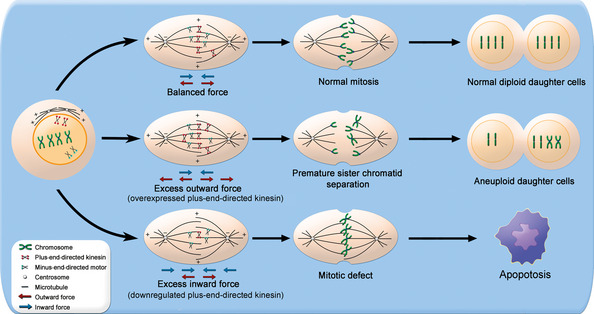
A model for the association between mitotic progression and motor kinesins. Before the nuclear envelope breakdown in prophase, bipolar assembly starts with the migration of centrosomes to opposing poles. Top row: kinesin motors function in interpolar microtubules at the metaphase, which lead to a balance of outward forces (red arrows) and inward forces (blue arrows), to ensure chromosome capture and attachment to spindle and prevent spindle elongation before anaphase. Middle row: a potential mechanism for overexpression of plus‐end‐directed kinesin motors such as Eg5 leads to spindle defects such as collapse of spindles and the formation of monopolar spindles, which might occur when anaphase begins. Bottom row: kinesin motors such as Eg5 or KIF20B downregulation leads to insufficient outward forces, which prevents elongation of spindle, and therefore induces mitotic defects and eventually apoptosis.
Table 1.
Kinesin family (KIF) members and their tumorigenic characteristics
| Kinesin member | KIF | Association with tumor |
|---|---|---|
| KIF5B | Kinesin‐1 | Overexpressed in cancers of the bladder, stomach, skin and breast10, 11 |
| KIF3A, KIF3B | Kinesin‐2 | Oncogenesis and metastasis of breast cancer and renal cell carcinoma12, 13 |
| KIF1B | Kinesin‐3 | Related to metastasis of nervous system tumor14 |
| KIF14 | Kinesin‐3 | Overexpression promotes the development of breast, lung and retinoblastoma tumors15, 16, 17 |
| KIF4A | Kinesin‐4 | Oncogenesis of cervical cancer and non‐small‐cell lung cancer18, 19 |
| KIF7 | Kinesin‐4 | Oncogenesis and metastasis of multiple cancers20, 21 |
| Eg5/KIF11 | Kinesin‐5 | Overexpresion promotes the developmemnt of multiple cancers |
| MPHOSPH1/KIF20B | Kinesin‐6 | Overexpressed in cancers of the bladder and colorectal9, 22 |
| MKLP1/KIF23 | Kinesin‐6 | Downregulation causes cytokinesis defect in tumor cells23 |
| MKLP2/KIF20A | Kinesin‐6 | Overexpressed in pancreatic ductaladenocarcinoma cells and downregulation inhibits the growth of gastric cancer cells24, 25 |
| CENP‐E/KIF10 | Kinesin‐7 | Downregulation inhibits the growth of multiple cancer cells26 |
| MCAK/KIF2C | Kinesin‐13 | Overexpressed in many kinds of cancers and linked to taxel resistance of tumor cells27, 28, 29 |
| KIF2A | Kinesin‐13 | Upregulation promotes the development of squamous cell carcinoma of the tongue30 |
| HSET/KIFC1 | Kinesin‐14 | Associated with brain metastasis of lung cancer31 |
| KIFC3 | Kinesin‐14 | Upregulation causes docetaxel resistance in breast cancer cells32 |
Kinesin Superfamily as Diagnostic and Prognostic Factors
A number of KIF proteins show aberrant overexpression in various cancer cells. Taniwaki et al.18 reported at least a fivefold upregulation of KIF4A, a member of the Kinesin‐4 family, in most cases of small‐cell lung cancer and in approximately 40% cases of non‐small‐cell lung cancer. This study also demonstrated a strong association of KIF4A overexpression with poor prognosis of non‐small‐cell lung cancer. Besides lung cancer, KIF4A is also overexpressed in cervical cancer19 and plays a key role in the oncogenesis of glioma, melanoma, breast cancer and bladder cancer, suggesting a prognostic value in clinic.33 However, some conflicting results have been reported recently. Gao et al.34 found downregulated KIF4 in gastric carcinoma and overexpression of KIF4 inhibits the proliferation of BGC cells as well as growth of xenograft tumor in vivo. In an earlier study, Mazumdar et al.35 also reported that multiple mitotic defects caused by loss of KIF4A might lead to carcinogenesis. Therefore, much work is still needed to better understand the complex roles KIF4 plays in cancer development and progression.
KIF14, a member of the Kinesin‐3 family, plays an important role in the cytokinesis of eukaryotic cells. This microtubule motor is amplified and upregulated in primary tumors including breast, lung and retinoblastoma cancer15, 36 and its expression level has been studied as a prognostic indicator in breast and lung cancer.15, 16 In lung cancer, KIF14 overexpression significantly decreased disease‐free survival and trended toward decreasing overall survival, suggesting KIF14 expression is independently prognostic for disease‐free survival in lung cancer.16
It has been indicated that MCAK, a Kinesin‐13 family member, participates in many essential aspects of mitosis.37 Recently, the tumorgenic effect of MCAK has received much attention.38 Nishidate et al.39 reported that MCAK is one of the multiple upregulated genes in a genome‐wide expression analysis of 81 breast cancer tissues. Further results revealed that MCAK expression can be suppressed by the expression of foreign p53.40 Upregulation of MACK was detected not only in breast cancer, but also in colorectal, gastric cancer and glioma tissues.27, 41 These data highlight that MCAK is aberrantly regulated in cancer cells, suggesting that overexpressed MCAK might play an oncogenic role in the development of cancer, particularly in breast, gastric and colorectal cancer.
Some other KIF proteins were also found specifically overexpressed in solid tumor tissues. For instance, KIF20B (also known as M‐phase phosphoprotein 1) is strongly overexpressed in bladder cancer tissues and downregulation of endogenous KIF20B leads to cytokinesis defect.22 Recently, we also reported that in multiple cancer cells, knockdown of KIF20B not only dramatically inhibits tumor cell growth, but also causes mitotic arrest, senescence and postmitotic apoptosis.9
Kinesin Superfamily are Involved in Malignancy
It has been reported that highly expressed MCAK is associated with invasiveness and metastasis in colorectal cancer.28 Compared with paired corresponding normal tissues, MCAK expression is significantly elevated both at the mRNA and protein levels in colorectal cancer tissues, and more importantly, overexpressed MCAK expression levels are firmly associated with lymph node metastasis, venous invasion, peritoneal dissemination and Dukes' classification, as well as a poor survival rate.28 Elevated expression of MCAK is also observed in gastric cancer42 and further studies indicate MCAK upregulation is tightly associated with lymphatic invasion, lymph node metastasis and a poor prognosis in gastric cancer patients.42 In addition, it is noted that inactivating mutations of the tumor suppressor adenomatous polyposis coli (APC) happen in more than 80% cases of colorectal cancer.43 The APC protein directly interacts with end‐binding protein 1 (EB1), a microtubules (MT) plus‐end tracking protein, which facilitates MT growth by increasing rescue frequency and stabilizing catastrophe of plus‐ends.44 Interestingly, MCAK protein was also reported to co‐localize with EB1 at MT plus‐ends.45, 46 Thus, it is of great interest to investigate how plus‐end tracking proteins interacting with elevated MCAK show an effect on the MT cytoskeleton and cell motility in APC‐absent colon cancer cells.38
KIF2A, like MCAK/KIF2C, belongs to the Kinesin‐13 family. KIF2A is classified as a MT depolymerase that depolymerizes MT from the end.47 It specifically localizes to centrosomes during mitosis and is necessary for bipolar spindle assembly and normal mitosis completion.47 Recently, Wang et al.30 reported that KIF2A is upregulated in squamous cell carcinoma of the oral tongue (SCCOT) tissues against paired adjacent tissues and an increased level of KIF2A is also strongly associated with lymph node metastasis and tumor clinical stage. Further results from a transwell chamber assay showed that Tca8113 cells transfected with KIF2A‐siRNA had decreased migratory ability compared with nonsense‐siRNA‐transfected cells,30 which suggests KIF2A overexpression is associated with the progression, invasion and metastasis of SCCOT and therefore might be used as a predictor for prognosis. In another study, Li et al.48 reported that MicroRNA 183 (miR‐183) directly inhibits the expression of KIF2A, and targeting of KIF2A by miR‐183 in HeLa cells increased the formation of cells with monopolar spindles. Intriguingly, the authors also found that transfection with miR‐183 of HeLa cells led to a significant decrease in the capacities of cell invasion and migration, suggesting that KIF2A plays an important role in the invasion and metastasis of cancer cells.48
KIF14 also plays an important role in the malignancy of various solid tumors, including retinoblastoma, breast, lung, pancreatic, laryngeal and ovarian carcinoma.15, 16, 36, 49, 50 In lung cancer, an elevated KIF14 level is associated with decreased survival of patients.17 In breast cancer, the level of KIF14 increases significantly with the fraction of tumor‐positive nodes and percent invasive cells.15 In a study of ovarian cancer, enhanced expression of KIF14 in tumors independently predicted a worse outcome and increased rates of recurrence.49 Results from a recent study in pancreatic carcinoma indicated significant upregulation of KIF14 and Rho‐GDP dissociation inhibitor beta (ARHGDIbeta) mRNA levels in patients with pancreatic cancer and, more importantly, both proteins were critically involved in perineural invasion, which is a common and key feature of pancreatic cancer and directly correlates with a poor prognosis.50 Further study indicated that knockdown of KIF14 and ARHGDIbeta resulted in altered perineural invasion of pancreatic tumor cells.50 All of this work provides novel insights into the molecular determinants of malignancy of human solid carcinoma.
Involvement of KIF in Taxane Resistance
Systemic chemotherapy of cancer has been significantly improved over the past few decades with the introduction of new drugs, especially taxanes, which have a long record of clinical success and are routinely used for a wide range of solid tumors.51 However, drug resistance, as manifested by relapse and cancer progression, still remains a major challenge. Various mechanisms in acquired or secondary taxane resistance have been reported,52, 53 and recent studies demonstrated that some kinesin proteins also play critical roles in taxane resistance.32, 54 In breast cancer, De et al.32 identified KIFC3 as the gene responsible for docetaxel resistance of cancer cells. They found that overexpression of KIFC3, KIFC1 and KIF5A increased resistance of breast cancer cells to docetaxel through opposing the microtubule stabilizing effect of docetaxel. Similar results were also recently reported on the relationship between taxane resistance in basal‐like breast cancer and kinesins, in which Tan et al.54 found kinesin proteins are overexpressed in docetaxel and paclitaxel‐resistant NCI‐60 cells. These specific KIF proteins include KIFC3, KIF5A and KIF12 and overexpression of these kinesins increases resistance to docetaxel but not anthracyclines or vincristine. It was further demonstrated that the ATP‐binding domain of kinesin is essential for resistance to docetaxel.54 These results highlight the potential opportunity for sequential or synergistic modulation of taxane resistance in breast cancer using selective KIF inhibitors.
Intriguingly, MCAK not only has a tight relationship with malignancy progression, but also with taxane resistance. Ganguly et al.29 reported that MCAK plays a key role in microtubule detachment and is responsible for the resistance to paclitaxel. Further results demonstrated that depletion of MCAK increased the sensitivity of mutant paclitaxel‐resistant cells to paclitaxel by reversing the aberrantly high frequency of microtubule detachment, indicating the relationship between aberrant expression of MCAK and drug resistance of cancer cells.29
Clinical Trials of Inhibitors Targeting KIF
Among KIF proteins, Eg5 is a plus end‐directed motor of the Kinesin‐5 subfamily. It functions in the early stages of mitosis and is responsible for centrosome separation and bipolar spindle assembly, which are essential for proper segregation of chromosomes.55 A large body of evidence suggests that Eg5 is upregulated in tumor cells compared with normal cells.56 Inhibition of Eg5 causes mitotic arrest with a monopolar spindle, with no effect on non‐proliferating cells.57, 58 The first identified Eg5 selective inhibitor is monastrol (Fig. 2)59 and several new potent Eg5 inhibitors for cancer treatment are currently under development (Table 2, Fig. 2).60 Ispinesib (SB‐715992) by Cytokinetics (South San Francisco, CA, USA) and GlaxoSmithKline (London, UK) was the first kinesin inhibitor to enter clinical trials.61 Presently, this quinazolinone derivative represents the most studied Eg5 inhibitor and is now in a phase II clinical trial.62, 63, 64, 65 The phase I studies for solid tumors indicated ispinesib is well tolerated with an acceptable safety profile and no indications of neurotoxicity.62 However, recent results from the phase II trial in 15 patients with metastatic hepatocellular carcinoma showed no conclusive evidence of benefit with ispinesib monotherapy.63 Other results of ispinesib from recurrent or metastatic squamous cell carcinoma of the head and neck, melanoma, colorectal cancer, ovarian cancer and renal cell carcinoma are similar in suggesting a lack of clinical efficacy.56, 64, 65
Figure 2.
Structure of kinesin protein inhibitors in clinical development.
Table 2.
Kinesin inhibitors in clinical trials
| Target | Drug | Company | Clinical phase | Trial number |
|---|---|---|---|---|
| Eg5 | Ispinesib (SB‐715992) | Cytokinetics | II | A: 0; C: 15 |
| SB‐743921 | Cytokinetics | I/II | A: 0; C: 2 | |
| ARRY‐520 | Array BioPharma (Boulder, CO, USA) | I/II | A: 3; C: 2 | |
| AZD4877 | Astra Zeneca (London, UK) | I | A: 0; C: 2 | |
| MK0731 | Merck & Co. | I | A: 0; C: 1 | |
| Litronesib (LY2523355) | Kyowa Hakko Kirin & Eli Lilly | I/II | A: 3; C: 3 | |
| ARQ 621 | ArQule (Woburn, MA, USA) | I | A: 0; C: 1 | |
| 4SC‐205 | 4SC AG (Planegg‐Martinsried, Germany) | I | A: 1; C: 0 | |
| CENP‐E | GSK923295 | GlaxoSmithKline | I | A: 1; C: 0 |
Adapted from information obtained from www.cancer.gov. A, active clinical trial; C, completed clinical trial.
Much attention has been directed towards the structural variations of ispinesib, for example, the replacement of the quinazolinone core. A variety of 6,6, 5,6 and 6,5 heterocyclic/carbocyclic fused ring systems have been used, such as SB‐743921 (Table 2, Fig. 2). Other series of Eg5 inhibitors include dihydropyrroles/dihydropyrazoles and dihydrothiadiazoles/dihydrooxadiazoles such as MK0731 (Merck & Co., Whitehouse Station, NJ, USA) and Litronesib (Kyowa Hakko Kirin [Tokyo, Japan] and Eli Lilly [Indianapolis, IN, USA]) (Table 2, Fig. 2). To test the clinical efficacy of these kinesin inhibitors, three ongoing phase I and II clinical trials of Eg5 inhibitors ARRY‐520 and Litronesib are currently being carried out for advanced cancer patients; after the phase I trials were completed, no further continuous clinical studies for AZD4877 and MK0731 have been carried out to date (Table 2).66, 67 Some of these Eg5 inhibitors showed great efficacy in preclinical models of human solid tumors;66, 67, 68, 69, 70 however, more trials are still needed to test their efficacy in clinic.
Moreover, toxicological side‐effects of Eg5 inhibitors have been observed. The most prevalent dose‐limiting toxicity for all inhibitors is neutropenia; other toxicities include leukopenia, elevation of aspartate and alanine aminotransferase, hyperbilirubinemia and hyponatremia.66, 67, 68, 69, 70 Other common grade three or four Common Terminology Criteria for Adverse Events (version 3)71 toxicities seen at or close to the maximum tolerated dose include anemia, fatigue and nausea/vomiting. Serious side‐effects therefore remain a major challenge for clinical use of Eg5 inhibitors.72
Another kinesin protein, centromeric protein E (CENP‐E/KIF10), which is also a component of the mitotic checkpoint that catalyzes congression of chromosomes at the spindle equator before biorientation, is currently under evaluation as an inhibition target in clinical trials. Two known CENP‐E‐specific inhibitors include the allosteric inhibitor GSK923295 and the lead compound syntelin.72, 73, 75 Besides its potent antitumor effects on xenografts,74 results from clinical trials of GSK923295 are particularly encouraging, with one patient displaying a partial response and one‐third of patients indicating stable disease in accordance with response evaluation criteria in solid tumors with a low incidence of myelosuppression and neuropathy.73
Future Cancer Therapy Strategy Targeting KIF
In a classic point of view, drugs that specifically inhibit KIF have no or little effect on MT, thus avoiding the neurotoxicity that encumbers MT‐targeting agents such as taxanes and vinca alkaloids.56 However, Komlodi‐Pasztor et al.76, 77 took the clinical disappointment of mitosis‐specific suppressors, such as kinesin spindle protein (KSP/Eg5) inhibitors, as evidence that mitosis is not a suitable target for cancer therapy in clinic. According to the authors, an interesting explanation to the rather disappointing clinical trials of Eg5 inhibitors such as ispinesib is that the doubling time of human tumors is much longer than xenografts in preclinical trials, which suggests a much small proportion of mitotic cells in human tumors compared with animal xenografts.77 Specific antimitotic drugs, such as ispinesib, are therefore unlikely to efficiently target tumor tissues. Based on this hypothesis, targeting the more rapidly growing leukemias and lymphomas with Eg5 inhibitors might represent a better alternative clinical strategy.77
Another challenge for the development of clinical KIF inhibitors is drug resistance. Resistance to chemotherapeutic drugs is a major obstacle in treating cancer that encumbers the efficacy of cytostatic drugs. The cause of drug resistance is complicated and the expression of efflux pumps and antiapoptotic proteins might be a major mechanism.52, 53 Some KIF proteins, such as KIFC3 and MCAK, might be involved in drug resistance.29, 32 The strategy of targeting KIF combined with chemotherapy might therefore provide an alternative way to treat cancer when serious chemotherapeutic drug resistance exists. Moreover, expression of antiapoptotic proteins of cancer cells can also generate drug resistance. Liu et al.78 observed that suppression of Eg5 by monastrol arrests mitosis and induces apoptosis in myeloma cells, but upregulates the antiapoptotic protein heat‐shock protein 70 (Hsp70), which might block apoptosis in tumor cells.79 Thus, this finding suggests a combination of Eg5 inhibitors with agents that abrogate Hsp70 induction is a promising therapy strategy for myeloma.
Moreover, we recently reported a strategy that combined the downregulation of KIF20B with the expression of foreign interleukin‐24 (a member of the IL‐10 family of cytokines), which is a tumor suppressor gene that shows potent antitumor ability against various cancer cells,80 strongly enhanced the antitumor effects of KIF20B inhibition in xenografts in vivo.9 Intriguingly, another recent study also indicated that CENP‐E can be regulated by KIF18A, another kinesin protein.81 These results imply that particular kinesin proteins can not just be viewed as independent elements to be targeted, but rather as a whole which are regulated by mitotic proteins including other KIF, which suggests monotherapy of KIF inhibitor might be insufficient to achieve efficient antitumor effects. Thus, further study focusing on the molecular network of kinesin regulation and exploring novel combined treatments with other anticancer therapeutics that is capable of bringing synergistic or potent antitumor effects for KIF inhibitors is necessary.
Disclosure Statement
The authors have no conflicts of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81172971, 81202557 and 81222043) and China Postdoctor Science Foundation (2012M510180).
(Cancer Sci, doi: 10.1111/cas.12138, 2013)
References
- 1. Vale RD, Reese TS, Sheetz MP. Identification of a novel force‐generating protein, kinesin, involved in microtubule‐based motility. Cell 1985; 42: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence CJ, Dawe RK, Christie KR et al A standardized kinesin nomenclature. J Cell Biol 2004; 167: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA 2001; 98: 7004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diefenbach RJ, Mackay JP, Armati PJ, Cunningham AL. The C‐terminal region of the stalk domain of ubiquitous human kinesin heavy chain contains the binding site for kinesin light chain. Biochemistry 1998; 37: 16663–70. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein LS, Philp AV. The road less traveled: emerging principles of kinesin motor utilization. Annu Rev Cell Dev Biol 1999; 15: 141–83. [DOI] [PubMed] [Google Scholar]
- 6. Castillo A, Morse HC 3rd, Godfrey VL, Naeem R, Justice MJ. Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res 2007; 67: 10138–47. [DOI] [PubMed] [Google Scholar]
- 7. Wordeman L. How kinesin motor proteins drive mitotic spindle function: lessons from molecular assays. Semin Cell Dev Biol 2010; 21: 260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oki E, Hisamatsu Y, Ando K, Saeki H, Kakeji Y, Maehara Y. Clinical aspect and molecular mechanism of DNA aneuploidy in gastric cancers. J Gastroenterol 2012; 47: 351–8. [DOI] [PubMed] [Google Scholar]
- 9. Liu XR, Cai Y, Cao X et al A new oncolytic adenoviral vector carrying dual tumour suppressor genes shows potent anti‐tumour effect. J Cell Mol Med 2012; 16: 1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dyrskjot L, Kruhoffer M, Thykjaer T et al Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res 2004; 64: 4040–8. [DOI] [PubMed] [Google Scholar]
- 11. Richardson AL, Wang ZC, de Nicolo A et al X chromosomal abnormalities in basal‐like human breast cancer. Cancer Cell 2006; 9: 121–32. [DOI] [PubMed] [Google Scholar]
- 12. Jimbo T, Kawasaki Y, Koyama R et al Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol 2002; 4: 323–7. [DOI] [PubMed] [Google Scholar]
- 13. Lukong KE, Richard S. Breast tumor kinase BRK requires kinesin‐2 subunit KAP3A in modulation of cell migration. Cell Signal 2008; 20: 432–42. [DOI] [PubMed] [Google Scholar]
- 14. Yeh IT, Lenci RE, Qin Y et al A germline mutation of the KIF1B beta gene on 1p36 in a family with neural and nonneural tumors. Hum Genet 2008; 124: 279–85. [DOI] [PubMed] [Google Scholar]
- 15. Corson TW, Gallie BL. KIF14 mRNA expression is a predictor of grade and outcome in breast cancer. Int J Cancer 2006; 119: 1088–94. [DOI] [PubMed] [Google Scholar]
- 16. Corson TW, Zhu CQ, Lau SK, Shepherd FA, Tsao MS, Gallie BL. KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clin Cancer Res 2007; 13: 3229–34. [DOI] [PubMed] [Google Scholar]
- 17. Corson TW, Huang A, Tsao MS, Gallie BL. KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene 2005; 24: 4741–53. [DOI] [PubMed] [Google Scholar]
- 18. Taniwaki M, Takano A, Ishikawa N et al Activation of KIF4A as a prognostic biomarker and therapeutic target for lung cancer. Clin Cancer Res 2007; 13: 6624–31. [DOI] [PubMed] [Google Scholar]
- 19. Narayan G, Bourdon V, Chaganti S et al Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosom Cancer 2007; 46: 373–84. [DOI] [PubMed] [Google Scholar]
- 20. Tremblay MR, Lescarbeau A, Grogan MJ et al Discovery of a potent and orally active hedgehog pathway antagonist (IPI‐926). J Med Chem 2009; 52: 4400–18. [DOI] [PubMed] [Google Scholar]
- 21. Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK. Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene 2009; 28: 3468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanehira M, Katagiri T, Shimo A et al Oncogenic role of MPHOSPH1, a cancer‐testis antigen specific to human bladder cancer. Cancer Res 2007; 67: 3276–85. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi S, Fusaki N, Ohta S et al Downregulation of KIF23 suppresses glioma proliferation. J Neurooncol 2012; 106: 519–29. [DOI] [PubMed] [Google Scholar]
- 24. Taniuchi K, Nakagawa H, Nakamura T et al Down‐regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res 2005; 65: 105–12. [PubMed] [Google Scholar]
- 25. Yan GR, Zou FY, Dang BL et al Genistein‐induced mitotic arrest of gastric cancer cells by downregulating KIF20A, a proteomics study. Proteomics 2012; 12: 2391–9. [DOI] [PubMed] [Google Scholar]
- 26. Schafer‐Hales K, Iaconelli J, Snyder JP et al Farnesyl transferase inhibitors impair chromosomal maintenance in cell lines and human tumors by compromising CENP‐E and CENP‐F function. Mol Cancer Ther 2007; 6: 1317–28. [DOI] [PubMed] [Google Scholar]
- 27. Bie L, Zhao G, Wang YP, Zhang B. Kinesin family member 2C (KIF2C/MCAK) is a novel marker for prognosis in human gliomas. Clin Neurol Neurosurg 2012; 114: 356–60. [DOI] [PubMed] [Google Scholar]
- 28. Ishikawa K, Kamohara Y, Tanaka F et al Mitotic centromere‐associated kinesin is a novel marker for prognosis and lymph node metastasis in colorectal cancer. Br J Cancer 2008; 98: 1824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ganguly A, Yang H, Cabral F. Overexpression of mitotic centromere‐associated kinesin stimulates microtubule detachment and confers resistance to paclitaxel. Mol Cancer Ther 2011; 10: 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang CQ, Qu X, Zhang XY et al Overexpression of Kif2a promotes the progression and metastasis of squamous cell carcinoma of the oral tongue. Oral Oncol 2010; 46: 65–9. [DOI] [PubMed] [Google Scholar]
- 31. Grinberg‐Rashi H, Ofek E, Perelman M et al The expression of three genes in primary non‐small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res 2009; 15: 1755–61. [DOI] [PubMed] [Google Scholar]
- 32. De S Cipriano R, Cipriano R, Jackson MW, Stark GR. Overexpression of kinesins mediates docetaxel resistance in breast cancer cells. Cancer Res 2009; 69: 8035–42. [DOI] [PubMed] [Google Scholar]
- 33. Rouam S, Moreau T, Broet P. Identifying common prognostic factors in genomic cancer studies: a novel index for censored outcomes. BMC Bioinformatics 2010; 11: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao J, Sai N, Wang C et al Overexpression of chromokinesin KIF4 inhibits proliferation of human gastric carcinoma cells both in vitro and in vivo . Tumour Biol 2011; 32: 53–61. [DOI] [PubMed] [Google Scholar]
- 35. Mazumdar M, Lee JH, Sengupta K, Ried T, Rane S, Misteli T. Tumor formation via loss of a molecular motor protein. Curr Biol 2006; 16: 1559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Madhavan J, Coral K, Mallikarjuna K et al High expression of KIF14 in retinoblastoma: association with older age at diagnosis. Invest Ophthalmol Vis Sci 2007; 48: 4901–6. [DOI] [PubMed] [Google Scholar]
- 37. Maney T, Hunter AW, Wagenbach M, Wordeman L. Mitotic centromere‐associated kinesin is important for anaphase chromosome segregation. J Cell Biol 1998; 142: 787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanhaji M, Friel CT, Wordeman L, Louwen F, Yuan J. Mitotic centromere‐associated kinesin (MCAK): a potential cancer drug target. Oncotarget 2011; 2: 935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishidate T, Katagiri T, Lin ML et al Genome‐wide gene‐expression profiles of breast‐cancer cells purified with laser microbeam microdissection: identification of genes associated with progression and metastasis. Int J Oncol 2004; 25: 797–819. [PubMed] [Google Scholar]
- 40. Shimo A, Tanikawa C, Nishidate T et al Involvement of kinesin family member 2C/mitotic centromere‐associated kinesin overexpression in mammary carcinogenesis. Cancer Sci 2008; 99: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gnjatic S, Cao Y, Reichelt U et al NY‐CO‐58/KIF2C is overexpressed in a variety of solid tumors and induces frequent T cell responses in patients with colorectal cancer. Int J Cancer 2010; 127: 381–93. [DOI] [PubMed] [Google Scholar]
- 42. Nakamura Y, Tanaka F, Haraguchi N et al Clinicopathological and biological significance of mitotic centromere‐associated kinesin overexpression in human gastric cancer. Br J Cancer 2007; 97: 543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagase H, Nakamura Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat 1993; 2: 425–34. [DOI] [PubMed] [Google Scholar]
- 44. Komarova Y, de Groot CO, Grigoriev I et al Mammalian end binding proteins control persistent microtubule growth. J Cell Biol 2009; 184: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moore AT, Rankin KE, von Dassow G et al MCAK associates with the tips of polymerizing microtubules. J Cell Biol 2005; 169: 391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee T, Langford KJ, Askham JM, Bruning‐Richardson A, Morrison EE. MCAK associates with EB1. Oncogene 2008; 27: 2494–500. [DOI] [PubMed] [Google Scholar]
- 47. Ganem NJ, Compton DA. The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J Cell Biol 2004; 166: 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Targeting of integrin beta1 and kinesin 2alpha by microRNA 183. J Biol Chem 2010; 285: 5461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Theriault BL, Pajovic S, Bernardini MQ, Shaw PA, Gallie BL. Kinesin family member 14: an independent prognostic marker and potential therapeutic target for ovarian cancer. Int J Cancer 2012; 130: 1844–54. [DOI] [PubMed] [Google Scholar]
- 50. Abiatari I, DeOliveira T, Kerkadze V et al Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol Cancer Ther 2009; 8: 1494–504. [DOI] [PubMed] [Google Scholar]
- 51. Kavallaris M. Microtubules and resistance to tubulin‐binding agents. Nat Rev Cancer 2010; 10: 194–204. [DOI] [PubMed] [Google Scholar]
- 52. Zelnak A. Overcoming taxane and anthracycline resistance. Breast J 2010; 16: 309–12. [DOI] [PubMed] [Google Scholar]
- 53. Morris PG, Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res 2008; 14: 7167–72. [DOI] [PubMed] [Google Scholar]
- 54. Tan MH, De S, Bebek G et al Specific kinesin expression profiles associated with taxane resistance in basal‐like breast cancer. Breast Cancer Res Treat 2012; 131: 849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus‐end‐directed microtubule motor. Nature 1992; 359: 540–3. [DOI] [PubMed] [Google Scholar]
- 56. Sarli V, Giannis A. Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res 2008; 14: 7583–7. [DOI] [PubMed] [Google Scholar]
- 57. Blangy A, Lane HA, d'Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin‐related motor essential for bipolar spindle formation in vivo . Cell 1995; 83: 1159–69. [DOI] [PubMed] [Google Scholar]
- 58. Weil D, Garcon L, Harper M, Dumenil D, Dautry F, Kress M. Targeting the kinesin Eg5 to monitor siRNA transfection in mammalian cells. Biotechniques 2002; 33: 1244–8. [DOI] [PubMed] [Google Scholar]
- 59. Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype‐based screen. Science 1999; 286: 971–4. [DOI] [PubMed] [Google Scholar]
- 60. Huszar D, Theoclitou ME, Skolnik J, Herbst R. Kinesin motor proteins as targets for cancer therapy. Cancer Metastasis Rev 2009; 28: 197–208. [DOI] [PubMed] [Google Scholar]
- 61. Sakowicz R, Finer JT, Beraud C et al Antitumor activity of a kinesin inhibitor. Cancer Res 2004; 64: 3276–80. [DOI] [PubMed] [Google Scholar]
- 62. Blagden SP, Molife LR, Seebaran A et al A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br J Cancer 2008; 98: 894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Knox JJ, Gill S, Synold TW et al A phase II and pharmacokinetic study of SB‐715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168). Invest New Drugs 2008; 26: 265–72. [DOI] [PubMed] [Google Scholar]
- 64. Tang PA, Siu LL, Chen EX et al Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs 2008; 26: 257–64. [DOI] [PubMed] [Google Scholar]
- 65. Lee CW, Belanger K, Rao SC et al A phase II study of ispinesib (SB‐715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs 2008; 26: 249–55. [DOI] [PubMed] [Google Scholar]
- 66. Kantarjian HM, Padmanabhan S, Stock W et al Phase I/II multicenter study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of AZD4877 in patients with refractory acute myeloid leukemia. Invest New Drugs 2012; 30: 1107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holen KD, Belani CP, Wilding G et al A first in human study of SB‐743921, a kinesin spindle protein inhibitor, to determine pharmacokinetics, biologic effects and establish a recommended phase II dose. Cancer Chemother Pharmacol 2011; 67: 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gerecitano JF, Stephenson JJ, Lewis NL et al A Phase I trial of the kinesin spindle protein (Eg5) inhibitor AZD4877 in patients with solid and lymphoid malignancies. Invest New Drugs 2013; 31: 355–62. [DOI] [PubMed] [Google Scholar]
- 69. Holen K, DiPaola R, Liu G et al A phase I trial of MK‐0731, a kinesin spindle protein (KSP) inhibitor, in patients with solid tumors. Invest New Drugs 2012; 30: 1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Khoury HJ, Garcia‐Manero G, Borthakur G et al A phase 1 dose‐escalation study of ARRY‐520, a kinesin spindle protein inhibitor, in patients with advanced myeloid leukemias. Cancer 2012; 118: 3556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Common Terminology Criteria for Adverse Events (version 3) : Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. March 31, 2003. [Published 09 Aug 2006.] Available from URL: http://ctep.cancer.gov.
- 72. Gomez HL, Philco M, Pimentel P et al Phase I dose‐escalation and pharmacokinetic study of ispinesib, a kinesin spindle protein inhibitor, administered on days 1 and 15 of a 28‐day schedule in patients with no prior treatment for advanced breast cancer. Anticancer Drugs 2012; 23: 335–41. [DOI] [PubMed] [Google Scholar]
- 73. Chung V, Heath EI, Schelman WR et al First‐time‐in‐human study of GSK923295, a novel antimitotic inhibitor of centromere‐associated protein E (CENP‐E), in patients with refractory cancer. Cancer Chemother Pharmacol 2012; 69: 733–41. [DOI] [PubMed] [Google Scholar]
- 74. Lock RB, Carol H, Morton CL et al Initial testing of the CENP‐E inhibitor GSK923295A by the pediatric preclinical testing program. Pediatr Blood Cancer 2012; 58: 916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ding X, Yan F, Yao P et al Probing CENP‐E function in chromosome dynamics using small molecule inhibitor syntelin. Cell Res 2010; 20: 1386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Komlodi‐Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol 2011; 8: 244–50. [DOI] [PubMed] [Google Scholar]
- 77. Komlodi‐Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res 2012; 18: 51–63. [DOI] [PubMed] [Google Scholar]
- 78. Liu M, Aneja R, Liu C et al Inhibition of the mitotic kinesin Eg5 up‐regulates Hsp70 through the phosphatidylinositol 3‐kinase/Akt pathway in multiple myeloma cells. J Biol Chem 2006; 281: 18090–7. [DOI] [PubMed] [Google Scholar]
- 79. Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti‐apoptotic function downstream of caspase‐3‐like proteases. EMBO J 1998; 17: 6124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu X, Cao X, Wei R et al Gene‐viro‐therapy targeting liver cancer by a dual‐regulated oncolytic adenoviral vector harboring IL‐24 and TRAIL. Cancer Gene Ther 2012; 19: 49–57. [DOI] [PubMed] [Google Scholar]
- 81. Huang Y, Yao Y, Xu HZ, Wang ZG, Lu L, Dai W. Defects in chromosome congression and mitotic progression in KIF18A‐deficient cells are partly mediated through impaired functions of CENP‐E. Cell Cycle 2009; 8: 2643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


